Abstract
Background and Purpose
Oxytocin (OT) plays a major role in the control of male sexual responses. Notably, blockade of OT receptors has been reported to inhibit ejaculation in animals. The study aimed to investigate the action of a highly selective, non-peptide OT antagonist GSK557296 in a model of pharmacologically induced ejaculation in anaesthetized rats. The site of action was assessed by investigating different delivery routes for this compound.
Experimental Approach
Urethane-anaesthetized Wistar rats were implanted with a cerebral ventricle cannula for i.c.v. injections or with a subdural catheter for intrathecal (i.t.) GSK557296 injections. Occurrence of ejaculation was assessed following i.v. 7-hydroxy-2-(di-N-propylamino)tetralin (7-OH-DPAT), a dopamine D3 receptor agonist. In addition, seminal vesicle pressures (SVP) and bulbospongiosus muscle (BS) EMG were recorded as physiological markers of emission and expulsion phases of ejaculation respectively.
Key Results
Highest i.v. GSK557296 dose reduced occurrence of ejaculation and increases in SVP but had no effect on BS-EMG. I.c.v. GSK557296 dose dependently inhibited ejaculation, increases in SVP and BS contractions. At spinal thoracic level, GSK557296 dose dependently inhibited ejaculation and increases in SVP but BS-EMG was impaired only with the highest dose. When delivered at lumbar level, GSK557296 dose dependently inhibited ejaculation, increases in SVP and BS contractions.
Conclusions and Implications
In the 7-OH-DPAT-induced ejaculation model, GSK557296 acts peripherally and centrally to inhibit ejaculation with different modalities. Blockade of brain OT receptors seems to be the most effective mechanism of action. Targeting central OT receptors with highly selective antagonist seems a promising approach for the treatment of premature ejaculation.
Keywords: 7-OH-DPAT, bulbospongiosus muscle, intracerebroventricular, intrathecal, premature ejaculation, seminal vesicle
Introduction
Ejaculation, the final stage of the sexual response in the mammalian male, comprises two successive phases, emission and expulsion, each involving different pelvi-perineal anatomical structures (for review, Giuliano and Clément, 2005). These two phases are commanded by autonomic (sympathetic and parasympathetic) and somatic spinal centres, which act synchronically. Synchronization is essential for antegrade ejaculation to occur and is led by a group of lumbar spinal neurons forming a spinal generator of ejaculation (SGE; Truitt and Coolen, 2002; Borgdorff et al., 2008). The SGE is under a strong influence of peripheral and brain inputs that can be excitatory or inhibitory. Neurons belonging to a brain circuitry specifically controlling ejaculation have been identified in rodents (Coolen et al., 1998; Heeb and Yahr, 2001; Hamson and Watson, 2004), although their exact role and nature are not fully delineated. A great body of evidence indicates the fundamental role of serotonin and dopamine (DA) in the control of the ejaculatory response as well as an important contribution of several other neurotransmitters including the neuropeptide oxytocin (OT; reviewed in Giuliano and Clément, 2012).
A key role of central OT in the control of ejaculation has been demonstrated in rodents. When infused into the cerebral ventricle of a male rat free to copulate with a receptive female, OT facilitates ejaculatory behaviour by shortening ejaculation latency and post-ejaculatory refractory period (Arletti et al., 1985). Conversely, a potent OT antagonist impairs sexual performance by decreasing the intromission frequency and abolishing ejaculation at doses failing to modify any other behavioural parameters (Argiolas et al., 1988). I.c.v. delivery of a peptidergic OT receptor antagonist reversed ejaculation induced by the DA D3-preferring agonist 7-hydroxy-2-(di-N-propylamino)tetralin (7-OH-DPAT) in anaesthetized rat (Clément et al., 2008). Further supporting the crucial role of brain OT is the observation that OT neurons in hypothalamic nuclei are more intensely activated as ejaculatory performance increase (Pattij et al., 2005). In addition, exploration of the function of spinal OT receptors in the ejaculatory process showed that intrathecal (i.t.) administration of OT antagonist at lumbar (5th–6th segments; L5-L6) but not thoracic (12th–13th segments; T12-T13) level impaired emission phase of ejaculation (Clément et al., 2008).
The role of peripheral OT was also investigated. Ejaculation was reported facilitated in copulating male rats following systemic delivery of OT (Arletti et al., 1985; Stoneham et al., 1985). This could be explained by the pro-contractile activity of OT on smooth muscle cells of the seminal tract (Filippi et al., 2003). In rats and humans, a peak of plasma level of OT has been reported at the time of ejaculation (Stoneham et al., 1985; Carmichael et al., 1987; Murphy et al., 1987). However, the causative link between OT plasma level and ejaculation could not be established with OT release in systemic circulation, which may be a consequence of ejaculation. Moreover, recent experiments in rats do not support a major role for peripheral OT receptors in the occurrence of ejaculation in rat (Clément et al., 2008) and rabbit where the OT effect appears to be mediated by vasopressin 1A receptors (Gupta et al., 2008).
Given the evidence that central, and more particularly brain, OT plays a major role in ejaculation, it can be suggested that targeting CNS OT receptors may be an effective pharmacological approach for treating ejaculatory disorders. We notably suppose that blocking CNS OT receptors may represent a significant advance in the treatment of premature ejaculation.
The aim of the study was to assess the effects of the selective non-peptide OT antagonist GSK557296 (INN: Epelsiban; Figure 1) on ejaculation induced by 7-OH-DPAT in anaesthetized rat. A multi-centre randomized placebo-controlled phase II clinical trial evaluating the efficacy, safety, and tolerability of on demand GSK557296 in men with premature ejaculation has recently been conducted (R. Shinghal, A. Barnes, K. M. Mahar, B. Stier, L. Giancaterino, L. D. Condreay, L. Black, S. W. McCallum, submitted). Competitive binding experiments using isolated recombinant human OT receptors and in vivo functional assays in rat have demonstrated that GSK557296 is a highly selective antagonist for the OT receptor versus V1a (>50.000-fold), V1b (>63.000-fold) and V2 (>31.000-fold) vasopressin receptors (Borthwick et al., 2012). In the present study, physiological markers of emission [seminal vesicle pressure (SVP)] and expulsion [bulbospongiosus muscle (BS) EMG] were measured to assess the modalities of action of GSK557296. In addition, different routes of administration of GSK557296 were investigated to determine its site(s) of action.
Figure 1.
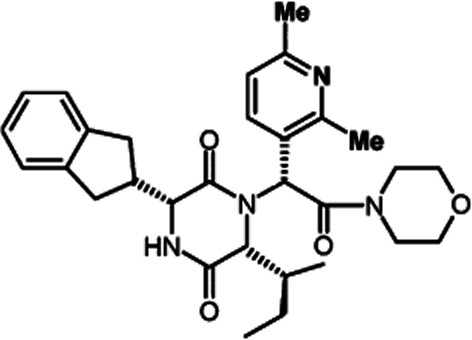
Skeletal formula of GSK557296.
Our data show that GSK557296 acts peripherally and centrally to inhibit ejaculation with different modalities in our experimental model.
The results of this study have been presented at the World Meeting on Sexual Medicine 2012 (26–30 August) in Chicago, IL, USA.
Methods
Animals
All animal studies were ethically reviewed and carried out in accordance with the European Directive 86/609/EEC and the GSK Policy on the Care, Welfare and Treatment of Animals. All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010). All efforts were undertaken to minimize the number of animals used and their suffering. All animals were housed under standard laboratory conditions (22 ± 1°C), on a 12 h light/dark cycle, and with food and water available ad libitum. A total of 91 sexually naive adult male Wistar rats (Charles River, L'Arbresle, France) weighing 235–290 g were used in the study.
Surgical preparation
Rats were anaesthetized with urethane (1.2 g·kg−1). Their temperature was maintained at 37°C using homeothermic blanket. The carotid artery was catheterized with polyethylene tubing filled with heparinized saline (50 IU·mL−1) to record BP via a pressure transducer (EM750, Elcomatic, Glasgow, UK). For i.v. injection, the jugular vein was catheterized with a polyethylene tube (0.5 mm) filled with NaCl 0.9% (w/v). SVP was measured with a catheter, filled with mineral oil, inserted in the seminal vesicle through the apex and connected to a pressure transducer. The BS was exposed via a perineal incision. Electrical activity of BS (BS EMG) was recorded by passing a Teflon-insulated stainless steel wire laterally throughout the muscles with two 1–2 mm pieces (separated by 1–2 mm) of insulation stripped off. Electrical signal from the BS muscles was amplified (DP-301, Warner Instrument, Phymep, Paris, France; gain, 10 000; low pass, 1 kHz; high pass, 10 Hz) before being digitized. SVP and BS EMG were recorded over 20 min after i.v. delivery of 7-OH-DPAT.
Intracerebroventricular cannula implantation
A cannula (26 gauge) was stereotactically placed into the cerebral ventricle (coordinates according to Paxinos & Watson Rat Brain Atlas: 0.5 mm posterior to bregma, 1 mm lateral to midline and 4 mm below the skull) and secured on the skull with zinc cement. The cannula was connected to a Hamilton syringe placed in a micropump allowing delivery of microvolume. At the end of the experimental session, methylene blue dye was injected through the cannula, and the brains, removed and grossly dissected, were inspected for the presence of blue dye in the ventricles.
Intrathecal catheter implantation
For i.t. catheter insertion, the rat's head was placed in a stereotaxic frame, and was rotated nose downwards. The catheter was a polyethylene tubing PE10 stretched to 150% of its original length in hot water, and cut to the required length so that its distal opening reached the targeted levels of the spinal cord (12th–13th thoracic; T12-T13 or 5th–6th lumbar; L5-L6 segments). The skin and neck muscles were incised and reclined. The atlanto-occipital membrane was opened and the catheter, filled with NaCl 0.9% (w/v), was carefully advanced in the caudal direction. The rostral free end of the catheter was secured with ligatures that closed the neck muscles and skin layers. The exact location of the caudal tip of the catheter was checked at the end of each experiment after sacrifice of the animal with an overdose of urethane and exposure of the spinal cord. Only rats with a catheter tip located at the level of T12-T13 or L5-L6 spinal segments were considered for the results.
Experimental design
Two doses of GSK557296 (3 and 12 mg·kg−1) were tested i.v. in separate groups. After a 5-min baseline period was obtained, i.v. delivery of GSK557296 followed; 10 min later, by i.v. 7-OH-DPAT was performed. Control rats were treated with vehicle i.v and then 10 min later by i.v. 7-OH-DPAT.
Two doses of GSK557296 (3.5 and 35 μg per rat) were tested i.c.v. in separate groups. After a 5 min baseline period was obtained, i.c.v. delivery of GSK557296 followed; 10 min later, by i.v. 7-OH-DPAT was performed. Control rats were treated with vehicle i.c.v and then 10 min later by i.v. 7-OH-DPAT.
Two doses of GSK557296 (3.5 and 35 μg per rat) were tested i.t. either at T12-T13 or L5-L6 segments in separate groups. After a 5 min baseline period was obtained, i.t. delivery of GSK557296 followed; 10 min later, by i.v. 7-OH-DPAT was performed. Control rats were treated with vehicle i.t. (T12-T13 or L5-L6 segments) and then 10 min later by i.v. 7-OH-DPAT.
Data analysis and statistics
Ejaculation, increases in SVP and BS rhythmic contractions were counted during the recording period. The latencies of the first ejaculation, as well as first SVP and BS responses, were also determined. Duration of clusters and frequency of bursts of BS contractions within a cluster as well as duration and amplitude of increases in SVP were calculated among the rats that displayed such responses and averaged for each experimental group. For each quantitative parameter of SVP and BS responses, individual means were obtained by including responses associated or not with ejaculation indifferently.
Results obtained from the different experimental groups were statistically compared using one-way anova (i.v., i.c.v. and i.t. studies) or t-test when only two groups were to be compared (i.c.v and i.t. studies). One-way anova was followed by Student-Newman-Keuls' test (SNK test).
Drugs
R(+)-7-hydroxy-(dipropylamino)tetralin (7-OH-DPAT; Sigma-Aldrich, Saint Quentin Fallavier, France) was dissolved each experimental day in NaCl 0.9% (w/v) at a concentration of 1 mg·mL−1 and injected i.v. in a volume of 1 mL·kg−1 over 2 min. GSK557296 (GlaxoSmithKline, Stevenage, UK) was dissolved each experimental day in 50 mM acetate buffer pH∼4 at the following concentrations: (i) 1 and 4 mg·mL−1 for i.v. study and (ii) 0.35 and 3.5 mg·mL−1 for i.c.v and i.t. studies. GSK557296 was injected i.v. in a volume of 3 mL·kg−1 over a 5 min period. GSK557296 was injected i.c.v and i.t. in a volume of 10 μL over a 5 min period.
Results
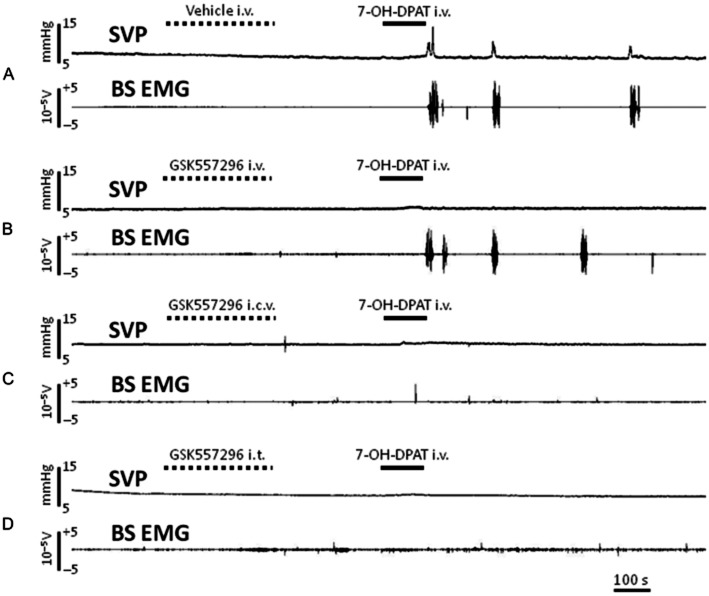
Whatever the route of administration used, GSK557296 had no effect per se on BP, SVP and BS EMG as observed during the 10 min interval before 7-OH-DPAT injection. In control rats treated with vehicle i.v., i.c.v. or i.t., 7-OH-DPAT i.v. was rapidly followed by temporally coordinated increases in SVP and BS activity with, in some cases, concomitant expulsion of sperm (see example of recordings on Figure 2).
Figure 2.
Samples of recording obtained in anaesthetized rats treated with vehicle i.v. (A), GSK557296 i.v. 12 mg·kg−1 (B), GSK557296 i.c.v. 35 μg (C), GSK557296 i.t. T12-T13 35 μg (D) prior to i.v. injection of the preferential DA D3 receptor agonist 7-OH-DPAT. Changes in SVP and activity on BS EMG are monitored throughout the experiment.
Effects of GSK557296 i.v
In the control group, ejaculation was observed in 6 out of 9 rats. This ratio was 7/10 and 3/9 in GSK557296 3 and 12 mg·kg−1 treatment groups respectively. The mean number of ejaculation was comparable between vehicle and 3 mg·kg−1 groups, whereas it was reduced by half in the group treated with GSK557296 12 mg·kg−1, although this did not reach statistical level of significance (one-way anova, F(2,28) = 1.4, P = 0.27; Table 1). No noticeable effect of the treatment was observed on the latency of the first ejaculation (Table 1). There was a dose-dependent decrease in the mean number of SVP responses following 7-OH-DPAT in i.v. GSK557296-treated rats (one-way anova, F(2,26) = 4.8, P = 0.017; Table 1). Post hoc test yielded a significant difference between vehicle and GSK557296 12 mg·kg−1 groups (SNK test, P < 0.05). Neither latency of the first SVP response nor mean duration of SVP peaks were modified in treated animals as compared to control group (Table 1). The mean amplitude of SVP peaks following 7-OH-DPAT was three times lower in i.v. 12 mg·kg−1 GSK557296-treated rats in comparison with vehicle, although this was not statistically different (one-way anova, F(2,20) = 2.0, P = 0.166; Table 1). The mean number of BS responses following 7-OH-DPAT was not modified in rats treated with GSK557296 i.v. (Table 1). Treatment had no effect on the other parameters of BS response (Table 1).
Table 1.
Effects of GSK557296 i.v. on ejaculation, and SVP and BS responses induced by 7-OH-DPAT
| GSK557296 i.v. (mg·kg−1) | |||
|---|---|---|---|
| Vehicle | 3 | 12 | |
| Ejaculation | |||
| Number | 0.8 ± 0.2 (9) | 0.9 ± 0.2 (10) | 0.4 ± 0.2 (9) |
| Latency first ejaculation(s) | 188 ± 31 (6) | 194 ± 40 (7) | 211 ± 19 (3) |
| SVP responses | |||
| Number | 4.9 ± 0.9 (8) | 3.3 ± 0.7 (10) | 1.3 ± 0.8 (9) |
| Latency first response (s) | 101 ± 23 (8) | 128 ± 42 (8) | 86 ± 10 (5) |
| Mean duration (s) | 13.3 ± 1.1 (8) | 13.4 ± 0.6 (8) | 15.0 ± 2.2 (5) |
| Mean amplitude (mmHg) | 4.6 ± 1.1 (8) | 5.5 ± 1.6 (8) | 1.4 ± 1.0 (5) |
| BS responses | |||
| Number | 4.8 ± 0.6 (9) | 3.4 ± 0.6 (10) | 4.0 ± 0.8 (9) |
| Latency first response (s) | 119 ± 27 (9) | 128 ± 37 (9) | 105 ± 19 (8) |
| Mean duration (s) | 13.2 ± 0.9 (9) | 13.9 ± 0.7 (9) | 14.8 ± 0.9 (8) |
| Mean frequency (s−1) | 0.95 ± 0.07 (9) | 0.98 ± 0.13 (9) | 1.00 ± 0.05 (8) |
The oxytocin antagonist (GSK557296) was injected i.v. 10 min prior to 7-OH-DPAT (i.v., 1 mg·kg−1). Ejaculation, seminal vesicle pressure (SVP) and bulbospongiosus muscle (BS) EMG were recorded and quantified over 20 min following 7-OH-DPAT delivery. The values are the means ± SEM of (n) rats. Statistics: one-way anova + Student-Newman-Keuls' post hoc test for comparisons. Bold figures indicate significant difference as compared to vehicle.
Effects of GSK557296 i.c.v
Ejaculation was observed in 2 out of 8 rats treated with vehicle i.c.v. In animals treated with GSK557296 3.5 μg i.c.v., ejaculation occurred in 1 out of 9 rats. None of the rat treated with GSK557296 35 μg i.c.v. displayed ejaculation (Table 2). A dose-dependent decrease in the mean number of 7-OH-DPAT-induced SVP responses was found in treated animals with GSK557296 i.c.v. when compared to controls (one-way anova, F(2,23) = 3.5, P = 0.049; Table 2). Inter-group a posteriori comparisons yielded a significant difference between vehicle and GSK557296 35 μg i.c.v.-treated rats (SNK test, P < 0.05). Regarding parameters of SVP responses, t-test was used to compare vehicle and GSK557296 3.5 μg groups since only one rat in the 35 μg group displayed SVP responses. No significant changes for SVP parameters were noticed in animals treated with GSK557296 3.5 μg i.c.v. as compared to controls (Table 2). I.c.v. GSK557296 exerted a dose-dependent inhibitory effect on the occurrence of BS responses elicited by 7-OH-DPAT (one-way anova, F(2,23) = 3.7, P = 0.043; Table 2). Statistical comparisons for BS response parameters using t-test did not yield a significant difference between GSK557296 3.5 μg and vehicle groups (Table 2).
Table 2.
Effects of GSK557296 i.c.v. on ejaculation, and SVP and BS responses induced by 7-OH-DPAT
| GSK557296 i.c.v. (μg per rat) | |||
|---|---|---|---|
| Vehicle | 3.5 | 35 | |
| Ejaculation | |||
| Number | 0.3 ± 0.2 (8) | 0.1 ± 0.1 (9) | 0 ± 0 (7) |
| Latency first ejaculation (s) | 218–233 (2) | 394 (1) | – |
| SVP responses | |||
| Number | 3.1 ± 1.1 (8) | 1.6 ± 0.7 (9) | 0.1 ± 0.1 (7) |
| Latency first response (s) | 151 ± 50 (5) | 116 ± 13 (4) | 96 (1) |
| Mean duration (s) | 15.8 ± 1.4 (5) | 14.7 ± 1.1 (4) | 13 (1) |
| Mean amplitude (mmHg) | 2.2 ± 0.5 (5) | 2.5 ± 1.6 (4) | 0.5 (1) |
| BS responses | |||
| Number | 3.1 ± 1.1 (8) | 1.4 ± 0.6 (9) | 0.1 ± 0.1 (7) |
| Latency first response (s) | 155 ± 50 (5) | 117 ± 13 (4) | 97 (1) |
| Mean duration (s) | 13.4 ± 1.3 (5) | 16.5 ± 1.3 (4) | 12.3 (1) |
| Mean frequency (s−1) | 1.10 ± 0.17 (5) | 0.98 ± 0.06 (4) | 1.30 (1) |
The oxytocin antagonist (GSK557296) was injected i.c.v. 10 min prior to 7-OH-DPAT (i.v., 1 mg·kg−1). Ejaculation, seminal vesicle pressure (SVP) and bulbospongiosus muscle (BS) EMG were recorded and quantified over 20 min following 7-OH-DPAT delivery. The values are the means ± SEM of (n) rats. Statistics: one-way anova + Student-Newman-Keuls' post hoc test for comparisons. Bold figures indicate significant difference as compared to vehicle.
Effects of GSK557296 i.t
In vehicle i.t.-treated animals, ejaculation was noted in 2 out of 7, and 2 out of 8 rats in i.t. injected at T12-T13 and L5-L6 segments respectively.
Treatment delivery at the T12-T13 segment
Ejaculation was abolished in rats treated with GSK557296 35 μg or 3.5 μg (Table 3). Occurrence of SVP responses induced by 7-OH-DPAT was also suppressed in GSK557296 35 μg rats. One-way anova analysis was not applicable to the entire set of data then t-test was used to compare T12-T13 GSK557296 3.5 μg and vehicle groups and did not yield any inter-group difference (Table 3). Delivered at the T12-T13 segment GSK557296 35 μg, i.t. abolished BS responses, whereas at 3.5 μg dose, the mean number of BS responses was unchanged (Table 3).
Table 3.
Effects of GSK557296 i.t. T12-T13 levels on ejaculation, and SVP and BS responses induced by 7-OH-DPAT
| GSK557296 i.t. T12-T13 (μg per rat) | |||
|---|---|---|---|
| Vehicle | 3.5 | 35 | |
| Ejaculation | |||
| Number | 0.3 ± 0.2 (7) | 0 ± 0 (5) | 0 ± 0 (7) |
| Latency first ejaculation (s) | 178–205 (2) | – | – |
| SVP responses | |||
| Number | 2.3 ± 1.0 (7) | 1.6 ± 1.0 (5) | 0 ± 0 (7) |
| Latency first response (s) | 99 ± 12 (4) | 82–112 (2) | – |
| Mean duration (s) | 12.9 ± 1.4 (4) | 12.9–16.5 (2) | – |
| Mean amplitude (mmHg) | 3.4 ± 1.3 (4) | 0.7–1.0 (2) | – |
| BS responses | |||
| Number | 1.9 ± 0.7 (7) | 1.8 ± 1.0 (5) | 0 ± 0 (7) |
| Latency first response (s) | 101 ± 13 (4) | 126 ± 29 (3) | – |
| Mean duration (s) | 16.2 ± 0.6 (4) | 15.1 ± 2.4 (3) | – |
| Mean frequency (s−1) | 0.94 ± 0.03 (4) | 0.88 ± 0.06 (3) | – |
The oxytocin antagonist (GSK557296) was injected i.t. at level of T12-T13 spinal segments 10 min prior to 7-OH-DPAT (i.v., 1 mg·kg−1). Ejaculation, seminal vesicle pressure (SVP) and bulbospongiosus muscle (BS) EMG were recorded and quantified over 20 min following 7-OH-DPAT delivery. The values are the means ± SEM of (n) rats.
Treatment delivery at the L5-L6 segment
GSK557296, whatever the dose, abolished ejaculation (Table 4). A dose-dependent decrease in the mean number of SVP responses was observed in GSK557296-treated rats (one-way anova, F(2,19) = 3.7, P = 0.046; Table 4). A posteriori SNK test yielded a significantly diminished mean number of SVP responses in GSK557296 35 μg-treated group as compared to control (P < 0.05). A dose-dependent decrease in the mean number of BS responses was found in rats treated with GSK557296, although one-way anova test yielded a P-value > 0.05 (F(2,19) = 3.4, P = 0.057; Table 4). Nevertheless, post hoc SNK test yielded a significant difference between L5-L6 GSK557296 35 μg and vehicle groups (P < 0.05).
Table 4.
Effects of GSK557296 i.t. L5-L6 levels on ejaculation, and SVP and BS responses induced by 7-OH-DPAT
| GSK557296 i.t. L5-L6 (μg per rat) | |||
|---|---|---|---|
| Vehicle | 3.5 | 35 | |
| Ejaculation | |||
| Number | 0.3 ± 0.2 (8) | 0 ± 0 (5) | 0 ± 0 (7) |
| Latency first ejaculation (s) | 191–247 (2) | – | – |
| SVP responses | |||
| Number | 2.8 ± 0.7 (8) | 1.2 ± 0.8 (5) | 0.4 ± 0.4 (7) |
| Latency first response (s) | 179 ± 98 (6) | 88–362 (2) | 94 (1) |
| Mean duration (s) | 13.1 ± 0.8 (4) | 10.6–11.5 (2) | 10.2 (1) |
| Mean amplitude (mmHg) | 5.0 ± 1.7 (6) | 1.6–2.4 (2) | 6.3 (1) |
| BS responses | |||
| Number | 2.6 ± 0.7 (8) | 1.2 ± 0.7 (5) | 0.4 ± 0.4 (7) |
| Latency first response (s) | 193 ± 100 (6) | 294 ± 141 (3) | 88 (1) |
| Mean duration (s) | 14.6 ± 1.2 (6) | 15.2 ± 2.4 (3) | 13.8 (1) |
| Mean frequency (s−1) | 1.07 ± 0.07 (6) | 1.02 ± 0.06 (3) | 1.06 (1) |
The oxytocin antagonist (GSK557296) was injected i.t. at level of L5-L6 spinal segments 10 min prior to 7-OH-DPAT (i.v., 1 mg·kg−1). Ejaculation, seminal vesicle pressure (SVP) and bulbospongiosus muscle (BS) EMG were recorded and quantified over 20 min following 7-OH-DPAT delivery. The values are the means ± SEM of (n) rats. Statistics: one-way anova + Student-Newman-Keuls' post hoc test for comparisons. Bold figures indicate significant difference as compared to vehicle.
Because of the low number of values (Tables 3 and 4), statistical comparison of the quantitative parameters of SVP and BS responses could not be performed in i.t.-treated animals.
Discussion and conclusions
The present study investigating the effects of the selective OT receptor antagonist GSK557296 injected via different routes in a model of pharmacologically induced ejaculation suggests a multi-level inhibitory action of the compound on ejaculation.
Decrease in 7-OH-DPAT-induced SVP responses with no change in BS responses in rats treated with the highest i.v. dose of GSK557296 (Table 1) leads to postulate a specific action for this compound on seminal vesicle contractions. Modulation of contractile activity of epididymis by OT has been evidenced in vitro and in vivo (Filippi et al., 2002; 2003). In addition, OT receptors have been found throughout the seminal tract, including seminal vesicle, in various species thereby supporting a peripheral role for OT on smooth muscle tone (Maggi et al., 1987; Filippi et al., 2003). Further investigation indicates an important role for peripheral OT in steroidogenesis (Frayne and Nicholson, 1998). However, peripheral action of OT in the seminal tract has recently been reported to be mediated by arginine vasopressin receptors (Gupta et al., 2008). At last, in an experimental model similar to the present one, i.v. injection of a peptide OT receptor antagonist had no action on SVP (Clément et al., 2008). The reasons for such a discrepancy between previous and present results are unclear. Differences in the effects of OT receptor antagonists may reside in distinct pharmacodynamic properties including pharmacological profile and intratissular diffusion. The inhibitory action of i.v. GSK557296 on 7-OH-DPAT-induced ejaculation resulted in a trend for reduced number of ejaculation but without marked lengthening of ejaculation latency. Whether or not such a specific effect of i.v. GSK557296 in an experimental context could translate in delayed ejaculation in a natural context remains to be addressed.
Regarding CNS delivery experiments, we first noticed that the occurrence of ejaculation and SVP and BS responses was reduced in rats delivered with vehicle via i.c.v. cannula or i.t. catheter as compared to i.v. catheter. A likely factor explaining this observation is the solution (acetate buffer, pH∼4) used for dissolving GSK557296. We suggest that direct CNS delivery of 10 μL vehicle acidic solution resulted in decrease in cerebrospinal fluid pH, which has interfered with centrally driven 7-OH-DPAT-induced ejaculation. We however consider that it does not alter the interpretation of the results and the conclusions of the study. Action of i.v. delivery of GSK557296 on 7-OH-DPAT-induced ejaculation is to be distinguished from that elicited by central delivery (i.t. or i.c.v.) of the same compound where inhibition of physiological markers of emission (SVP) and expulsion (BS EMG) phases was observed. A dose-dependent inhibitory effect of i.c.v. GSK557296 on the occurrence of ejaculation was found (Table 2). These results, confirming previous ones (Clément et al., 2008), support the role of brain OT receptors in mediating the pro-ejaculatory activity of 7-OH-DPAT. This preferential DA D3 agonist triggers ejaculation by stimulating brain receptors in anaesthetized animals, that is, outside a sexual context (Clément et al., 2007). More notably, activation of DA D3 receptors located in the medial preoptic area of the hypothalamus (MPOA) is sufficient to trigger ejaculation (Kitrey et al., 2007). Projections have been identified from the MPOA to brain structures known to be involved in the control of ejaculation and containing OT binding sites such as the bed nucleus of the stria terminalis and the amygdala (Freund-Mercier et al., 1987; Elands et al., 1988). We therefore can hypothesize that blockade of OT receptors in these areas may interfere with the pro-ejaculatory activity of 7-OH-DPAT.
GSK557296 delivered in the vicinity of the T12-T13 or L5-L6 spinal segments prevented 7-OH-DPAT-induced ejaculation. Examination of GSK effects on SVP and BS responses led to suggest that this compound does not exert the same action at the T12-T13 and L5-L6 levels (Tables 3 and 4). Axons originating in the MPOA have been described to terminate in the paraventricular nucleus of the hypothalamus (PVN; Simerly and Swanson, 1988). Within the parvocellular division of the PVN, a group of OT neurons has been shown to project to thoracolumbar and lumbosacral preganglionic autonomic neurons innervating pelvi-perineal viscera (Luiten et al., 1985; Tang et al., 1998). Abolition by the highest GSK557296 dose (35 μg) at the T12-T13 segment of SVP and BS responses (Table 3) suggests an inhibitory effect for this compound on the command of ejaculation by acting on thoracolumbar (T12-L1) sympathetic preganglionic neurons innervating the anatomical structures involved in ejaculation (Nadelhaft and McKenna, 1987). Blockade of the thoracolumbar sympathetic output to the periphery cannot explain inhibition of BS muscles contractions, which are commanded by lumbosacral motoneurons. However, the inhibitory activity of T12-T13 GSK557296 on BS contractions was not found to be dose dependent since the lowest dose (3.5 μg) had no effect on the number of BS responses (Table 3). It thus can be proposed that the action on the expulsion phase of ejaculation at the T12-T13 level of GSK557296 resulted from the spreading of the compound to lumbosacral and/or brain levels in sufficient quantities to efficiently block OT receptors in these areas. In contrast, SVP and BS responses were found to be dose dependently inhibited by L5-L6 GSK557296 (Table 4). Notably, the amplitude of the decrease in SVP number caused by 3.5 μg dose at L5-L6 level was higher than that of the same dose at T12-T13 level (−58 vs. −30% as compared to corresponding vehicle respectively; Tables 3 and 4). This is in favour of an inhibitory action of this compound on 7-OH-DPAT-induced ejaculation by acting on lumbosacral parasympathetic preganglionic neurons and motoneurons involved in the command of the physiological peripheral events leading to ejaculation. Blockade of OT PVN descending outputs to L6-S1 parasympathetic neurons that control secretion of seminal fluid may result in altered emission phase. Although OT receptors have never been detected in the vicinity of lumbosacral motoneurons nucleus (Onuf's nucleus), OT binding sites have been reported in the rat in neurons of the dorsal grey commissure of L6-S1 segments (Veronneau-Longueville et al., 1999), which project to motoneurons of the same segment (Sasek et al., 1984).
The results yielded by the present i.t. experiments come into an apparent contradiction with previous ones using d(CH2)51,Tyr(Me)2,Orn8-Oxytocin, a selective peptide OT receptor antagonist (Clément et al., 2008). It was shown that the former studied peptide antagonist (i) had no noticeable effect on ejaculation as well as on SVP and BS responses when administrated at T12-T13 levels, and (ii) had a moderate inhibitory effect on ejaculation and SVP responses when delivered at L5-L6 levels. As already discussed for the i.v. study, distinct pharmacodynamic properties between GSK557296 and peptide antagonist, and notably higher capacity of intratissular diffusion for the former, may explain these conflicting findings.
In conclusion, the non-peptide selective OT receptor antagonist GSK557296 interferes with ejaculation induced by stimulation of DA D3 receptors in anaesthetized rats by acting at multiple levels with different modalities. While blockade of peripheral OT receptors and supposedly at lower thoracic spinal levels specifically impairs the emission phase of ejaculation, the blockade of brain and spinal lower lumbar segments OT receptors alters both emission and expulsion phases of ejaculation. Whether GSK557296 effects in the present experimental setting translate in effective lengthening of ejaculation in a more physiological paradigm is to be assessed. These findings led to the suggestion that the development of OT receptor antagonists capable of diffusing into the CNS might represent a promising therapeutical avenue for future research for the treatment of premature ejaculation.
Acknowledgments
This study was sponsored by an unrestricted grant from GlaxoSmithKline.
Glossary
- 7-OH-DPAT
7-hydroxy-2-(di-N-propylamino)tetralin
- BS
bulbospongiosus muscle
- DA
dopamine
- MPOA
medial preoptic area
- OT
oxytocin
- PVN
paraventricular nucleus of the hypothalamus
- SGE
spinal generator of ejaculation
- SVP
seminal vesicle pressure
Conflict of interest
S. McCallum is employed as clinical director at GlaxoSmithKline, King of Prussia, USA.
References
- Argiolas A, Collu M, Gessa GL, Melis MR, Serra G. The oxytocin antagonist d(CH2)5Tyr(Me)-Orn8-vasotocin inhibits male copulatory behaviour in rats. Eur J Pharmacol. 1988;149:389–392. doi: 10.1016/0014-2999(88)90675-9. [DOI] [PubMed] [Google Scholar]
- Arletti R, Bazzani C, Castelli M, Bertolini A. Oxytocin improves male copulatory performance in rats. Horm Behav. 1985;19:14–20. doi: 10.1016/0018-506x(85)90002-9. [DOI] [PubMed] [Google Scholar]
- Borgdorff AJ, Bernabé J, Denys P, Alexandre L, Giuliano F. Ejaculation elicited by microstimulation of lumbar spinothalamic neurons. Eur Urol. 2008;54:449–456. doi: 10.1016/j.eururo.2008.03.043. [DOI] [PubMed] [Google Scholar]
- Borthwick AD, Liddle J, Davies DE, Exall AM, Hamlett C, Hickey DM, et al. Pyridil-2,5-diketopiperazines as potent, selective, and orally bioavailable oxytocin antagonists: synthesis, pharmacokinetics, and in vivo potency. J Med Chem. 2012;55:783–796. doi: 10.1021/jm201287w. [DOI] [PubMed] [Google Scholar]
- Carmichael MS, Humbert R, Dixen J, Palmisano G, Greenleaf W, Davidson JM. Plasma oxytocin increases in the human sexual response. J Clin Endocrinol Metab. 1987;64:27–31. doi: 10.1210/jcem-64-1-27. [DOI] [PubMed] [Google Scholar]
- Clément P, Bernabé J, Denys P, Alexandre L, Giuliano F. Ejaculation induced by i.c.v. injection of the preferring dopamine D3 receptor agonist 7-hydroxy-2-(di-N-propylamino)tetralin in anesthetized rats. Neuroscience. 2007;145:605–610. doi: 10.1016/j.neuroscience.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Clément P, Peeters M, Bernabé J, Denys P, Alexandre L, Giuliano F. Brain oxytocin receptors mediate ejaculation elicited by 7-hydroxy-2-(di-N-propylamino) tetralin (7-OH-DPAT) in anaesthetized rats. Br J Pharmacol. 2008;154:1150–1159. doi: 10.1038/bjp.2008.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coolen LM, Peters HJ, Veening JG. Anatomical interrelationships of the medial preoptic area and other brain regions activated following male sexual behavior: a combined fos and tract-tracing study. J Comp Neurol. 1998;397:421–435. doi: 10.1002/(sici)1096-9861(19980803)397:3<421::aid-cne8>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Elands J, Beetsma A, Barberis C, de Kloet ER. Topography of the oxytocin receptor system in rat brain: an autoradiographical study with a selective radioiodinated oxytocin antagonist. J Chem Neuroanat. 1988;1:293–302. [PubMed] [Google Scholar]
- Filippi S, Vannelli GB, Granchi S, Luconi M, Crescioli C, Mancina R, et al. Identification, localization and functional activity of oxytocin receptors in epididymis. Mol Cell Endocrinol. 2002;193:89–100. doi: 10.1016/s0303-7207(02)00101-6. [DOI] [PubMed] [Google Scholar]
- Filippi S, Vignozzi L, Vannelli GB, Ledda F, Forti G, Maggi M. Role of oxytocin in the ejaculatory process. J Endocrinol Invest. 2003;26:82–86. [PubMed] [Google Scholar]
- Frayne J, Nicholson HD. Localization of oxytocin receptors in the human and macaque monkey male reproductive tracts: evidence for a physiological role of oxytocin in the male. Mol Hum Reprod. 1998;4:527–532. doi: 10.1093/molehr/4.6.527. [DOI] [PubMed] [Google Scholar]
- Freund-Mercier MM, Stoeckel ME, Palacios JM, Pazos A, Reichart JM, Porte A, et al. Pharmacological characteristics and anatomical distribution of [3H]oxytocin-binding sites in the Wistar rat brain studied by autoradiography. Neuroscience. 1987;20:599–614. doi: 10.1016/0306-4522(87)90113-8. [DOI] [PubMed] [Google Scholar]
- Giuliano F, Clément P. Neuroanatomy and physiology of ejaculation. Annu Rev Sex Res. 2005;16:190–216. [PubMed] [Google Scholar]
- Giuliano F, Clément P. Pharmacology for the treatment of premature ejaculation. Pharmacol Rev. 2012;64:621–644. doi: 10.1124/pr.111.004952. [DOI] [PubMed] [Google Scholar]
- Gupta J, Russell R, Wayman C, Hurley D, Jackson V. Oxytocin-induced contractions within rat and rabbit ejaculatory tissues are mediated by vasopressin V1A receptors and not oxytocin receptors. Br J Pharmacol. 2008;155:118–126. doi: 10.1038/bjp.2008.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamson DK, Watson NV. Regional brainstem expression of Fos associated with sexual behavior in male rats. Brain Res. 2004;1006:233–240. doi: 10.1016/j.brainres.2004.01.072. [DOI] [PubMed] [Google Scholar]
- Heeb MM, Yahr P. Anatomical and functional connections among cell groups in the gerbil brain that are activated with ejaculation. J Comp Neurol. 2001;439:248–258. doi: 10.1002/cne.1346. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. NC3Rs Reporting Guidelines Working Group. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitrey ND, Clément P, Bernabé J, Alexandre L, Giuliano F. Microinjection of the preferential dopamine receptor D3 agonist 7-hydroxy-N,N-DI-n-propylaminotetralin hydrobromide into the hypothalamic medial preoptic area induced ejaculation in anesthetized rats. Neuroscience. 2007;149:636–641. doi: 10.1016/j.neuroscience.2007.06.051. [DOI] [PubMed] [Google Scholar]
- Luiten PG, Ter Horst GJ, Karst H, Steffens AB. The course of paraventricular hypothalamic efferents to autonomic structures in medulla and spinal cord. Brain Res. 1985;329:374–378. doi: 10.1016/0006-8993(85)90554-2. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi M, Malozowski S, Kassis S, Guardabasso V, Rodbard D. Identification and characterization of two classes of receptors for oxytocin and vasopressin in porcine tunica albuginea, epididymis, and vas deferens. Endocrinology. 1987;120:986–994. doi: 10.1210/endo-120-3-986. [DOI] [PubMed] [Google Scholar]
- Murphy MR, Seckl JR, Burton S, Checkley SA, Lightman SL. Changes in oxytocin and vasopressin secretion during sexual activity in men. J Clin Endocrinol Metab. 1987;65:738–741. doi: 10.1210/jcem-65-4-738. [DOI] [PubMed] [Google Scholar]
- Nadelhaft I, McKenna KE. Sexual dimorphism in sympathetic preganglionic neurons of the rat hypogastric nerve. J Comp Neurol. 1987;256:308–315. doi: 10.1002/cne.902560210. [DOI] [PubMed] [Google Scholar]
- Pattij T, de Jong TR, Uitterdijk A, Waldinger MD, Veening JG, Cools AR, et al. Individual differences in male rat ejaculatory behaviour: searching for models to study ejaculation disorders. Eur J Neurosci. 2005;22:724–734. doi: 10.1111/j.1460-9568.2005.04252.x. [DOI] [PubMed] [Google Scholar]
- Sasek CA, Seybold VS, Elde RP. The immunohistochemical localization of nine peptides in the sacral parasympathetic nucleus and the dorsal grey commissure in rat spinal cord. Neuroscience. 1984;12:855–873. doi: 10.1016/0306-4522(84)90175-1. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Swanson LW. Projections of the medial preoptic nucleus: a Phaseolus vulgaris leucoagglutinin anterograde tract-tracing study in the rat. J Comp Neurol. 1988;270:209–242. doi: 10.1002/cne.902700205. [DOI] [PubMed] [Google Scholar]
- Stoneham MD, Everitt BJ, Hansen S, Lightman SL, Todd K. Oxytocin and sexual behaviour in the male rat and rabbit. J Endocrinol. 1985;107:97–106. doi: 10.1677/joe.0.1070097. [DOI] [PubMed] [Google Scholar]
- Tang Y, Rampin O, Calas A, Facchinetti P, Giuliano F. Oxytocinergic and serotonergic innervation of identified lumbosacral nuclei controlling penile erection in the male rat. Neuroscience. 1998;82:241–254. doi: 10.1016/s0306-4522(97)00290-x. [DOI] [PubMed] [Google Scholar]
- Truitt WA, Coolen LM. Identification of a potential ejaculation generator in the spinal cord. Science. 2002;297:1566–1569. doi: 10.1126/science.1073885. [DOI] [PubMed] [Google Scholar]
- Veronneau-Longueville F, Rampin O, Freund-Mercier M-J, Tang Y, Calas A, Marson L, et al. Oxitocinergic innervation of autonomic nuclei controlling penile erection in the rat. Neuroscience. 1999;93:1437–1447. doi: 10.1016/s0306-4522(99)00262-6. [DOI] [PubMed] [Google Scholar]