Abstract
Background and Purpose
We investigated the effects of aging on the contributions of NO and endothelium-dependent hyperpolarization (EDH) to endothelium-dependent relaxation in saphenous arteries of male and female C57BL/6J mice aged 12, 34 and 64 weeks.
Experimental Approach
Vasomotor responses of saphenous arteries were analysed by wire myography in the absence and presence of stimuli of the endothelium, inhibitors of NOS, and inhibitors and stimulants of small (KCa2.3) and intermediate (KCa3.1) conductance calcium-activated potassium channels.
Key Results
Arterial relaxing responses to sodium nitroprusside and to ACh in the absence of pharmacological inhibitors (indomethacin and L-NAME), were similar in all age groups and sexes, but those mediated by endothelium-derived NO were slightly but significantly increased in 64-week-old male mice. In the presence of inhibitors, 12-week-old animals showed pronounced ACh-induced relaxation, which was significantly reduced in 34- and 64-week-old mice of both sexes. The EDH-related component of ACh-induced relaxations was abolished by TRAM-34 (KCa3.1 blocker) or UCL 1684 (KCa2.3 blocker). Although the maximal relaxation induced by NS309 (KCa activator) was not affected by aging, the sensitivity for NS309 significantly decreased with aging. The presence of SKA-31 (KCa modulator) potentiated relaxations induced by ACh in arteries of 12-week-old but not older mice.
Conclusion and Implications
In a small muscular artery of mice of either sex, total endothelium-dependent relaxation is not affected by age. However, possibly due to changes in KCa channel function, the contribution of EDH to endothelium-dependent relaxations decreased with age. The contribution of endothelium-derived NO increases in old male mice.
Keywords: EDH-related responses, KCa3.1, KCa2.3, L-NAME
Introduction
In humans, aging and male sex are associated with an increased risk for cardiovascular diseases (Castelli, 1984; Egashira et al., 1993; Farhat et al., 1996; Orshal and Khalil, 2004; Lloyd-Jones et al., 2010). These pathologies are characterized by an altered balance between endothelium-derived relaxing and contracting factors. Most investigations of this endothelial dysfunction address the reduced endothelium-dependent vasodilatation of large conduit arteries as a result of reduced bioavailability of endothelium-derived NO (EDNO) (Tschudi et al., 1996; Lesniewski et al., 2009). However, in small muscular resistance arteries, marked endothelium-dependent vasodilatation can persist during pharmacological inhibition of NOS or deficiency of NOS3 (Huang et al., 2000; Takaki et al., 2008b). This has been attributed to endothelium-derived hyperpolarizing factor (EDHF) (Feletou and Vanhoutte, 1999; Busse et al., 2002). This mechanism involves small and intermediate conductance calcium-activated potassium channels (KCa2.3 and KCa3.1, respectively) in the plasmalemma of the endothelial cells (Grgic et al., 2009; Edwards et al., 2010). Activation of these channels can cause endothelium-dependent vasodilatation by at least two mechanisms: (i) release of an alternative endothelium-derived relaxing factor such as K+ ions, H2O2 or epoxyeicosatrienoic acid; and (ii) conduction of the endothelial cell hyperpolarization to the underlying smooth muscle cells by heterocellular gap junctions (Feletou and Vanhoutte, 2009; de Wit and Griffith, 2010; Chadha et al., 2011). In contrast to NO, little is known about the effects of risk factors on endothelium-dependent hyperpolarization (EDH)-related arterial responses. Both impairment (Sunano et al., 1999; Wigg et al., 2001; Bussemaker et al., 2003; Haddock et al., 2011) and a compensatory up-regulation (Taddei et al., 2006; Goto et al., 2012) have been reported. Aging alters vascular functions at the level of both endothelium and smooth muscle cell. Age-related changes in EDH have been documented in normal and pathological conditions in rat mesenteric arteries (Fujii et al., 1993; Goto et al., 2012). In the current study, we tested the hypotheses that endothelium-dependent relaxation is impaired by aging in mice as a result of reduced EDH-related responses and that this is more pronounced in male mice as compared with female. For this purpose we used saphenous arteries (small muscular arteries) of young, adult and aging male and female mice, a species that is increasingly used for experimental cardiovascular research. We recorded vasomotor responses in the absence and presence of stimuli of the endothelium, inhibitors of NOS and inhibitors and stimulants of KCa2.3 and KCa3.1 channels.
Methods
Solutions and drugs
Krebs Ringer bicarbonate-buffered salt solution (KRB) contained (in mM): 118.5 NaCl, 4.7 KCl, 2.5 CaCl2, 1.2 MgSO4, 1.2 KH2PO4, 25.0 NaHCO3 and 5.5 glucose. The KRB solution was continuously aerated with 95% O2/5% CO2 and maintained at 37°C. In high K+-KRB solution was KRB in which all NaCl was replaced by KCl. Buffers containing intermediate K+ concentrations were prepared by mixing appropriate volumes of KRB and K+-KRB. Indomethacin (INDO) was purchased from Sigma Aldrich (Zwijndrecht, The Netherlands) and dissolved in ethanol. ACh, noradrenaline (NA), phenylephrine (PHE), Nω-nitro-L-arginine methyl ester (L-NAME) and sodium nitroprusside (SNP) were purchased from Sigma Aldrich and dissolved in KRB solution. 6, 7-dichloro-1H-indole-2,3-dione 3-oxime (NS309), naphtho[1,2-d]thiazol-2-ylamine (SKA-31) and 1-[(2-chlorophenyl)diphenylmethyl]-1H-pyrazole (TRAM-34) were purchased from Sigma Aldrich and dissolved in dimethyl sulfoxide (DMSO). 6,12,19,20,25,26-hexahydro-5,27:13, 18:21,24-trietheno-11,7-metheno-7H-dibenzo [b,n] [1,5,12,16]tetraazacyclotricosine-5,13-diium dibromide (UCL 1684) was purchased from Tocris Bioscience (Bristol, UK) and dissolved in DMSO.
Animals
Male and female C57BL/6J mice (Charles River, Wilmington, MA, USA) aged 12, 34 and 64 weeks were housed in standard cages (constant room temperature and humidity, 12 h light/12 h dark cycles) and had free access to standard chow diet (pellet) and tap water. All procedures were performed in accordance with the Committee for Animal Care and Use of Maastricht University.
Wire myography
Tissue preparation
Animals were killed by CO2/O2 inhalation. Saphenous arteries were dissected free from surrounding fat and connective tissue and directly mounted in a wire myograph (Danish Myo Technology, Aarhus, Denmark). The anatomical location of these vessels is illustrated in the supplement (Supporting Information Fig. S1). Arterial segments (2 mm) were distended to the diameter at which maximal contractile responses to 10 μM NA could be obtained (Hilgers et al., 2010). Optimal diameters (Dopt) and maximal contractile responses to NA are summarized in Supporting Information Table S1. The maximal relaxing response to ACh (10 μM) was recorded during contraction induced by 10 μM NA and arterial segments which showed less than 85% relaxation were discarded from the experiments. We performed several pharmacological protocols with the aim of discriminating between different pathways of endothelium-dependent relaxation. These were not intended to document normal physiological control of arterial function.
Contributions of NO, EDH and COX products to endothelium-dependent relaxation
Initially, a concentration response curve (CRC) for PHE (0.01–10 μM) was recorded. During the contraction induced by 10 μM PHE, an ACh CRC (0.01–10 μM) was generated. After 20 min recovery in drug-free solution, arteries were contracted using K+ (40 mM), and an ACh CRC (0.01–10 μM) was recorded during contraction induced by K+. These experiments were repeated in the presence of the COX inhibitor INDO (10 μM) and in the presence of both INDO (10 μM) and the NOS inhibitor L-NAME (100 μM).
Contribution of KCa2.3 and KCa3.1 to EDH-related relaxation
Arterial segments were exposed to INDO (10 μM) and L-NAME (100 μM). A PHE CRC (0.01–10 μM) was constructed and the effects of the KCa channel activator NS309 and of the positive allosteric modulator of KCa channels SKA 31 (1 μM) on this CRC were recorded. EDH-related relaxation in response to ACh (0.01–10 μM) was studied in the presence of INDO (10 μM) and L-NAME (100 μM) to rule out interference of NO and prostaglandins with KCa channels (Bolotina et al., 1994). To study the contribution of KCa channel subtypes, 1 μM UCL 1684 (Rosa et al., 1998) was used to block small conductance KCa channels, while 10 μM TRAM-34 was used to block intermediate conductance KCa channels (Hilgers et al., 2010; Hilgers and Webb, 2007).
Sensitivity of vascular smooth muscle to NO
Arteries were contracted with PHE (10 μM) in the presence of INDO (10 μM) and L-NAME (100 μM), and the relaxing effects of the NO donor SNP (0.01–10 μM) were recorded.
Statistical analysis
All CRCs for contractile stimuli were expressed as a percentage of the maximal response to 10 μM NA prior to the administration of any pharmacological inhibitor. Relaxing responses were expressed as percentage of the level of pre-contraction. Individual CRCs were fitted to a non-linear sigmoid regression curve (Graph Pad Prism 5.0, Graph Pad Software, La Jolla, CA, USA). Sensitivity (pEC50) and maximal effect (Emax) are shown as mean ± SEM. pEC50 and Emax were compared by unpaired t-test. Two-way analysis of variance followed by a Bonferroni post hoc test was used to compare multiple groups. A P value <0.05 was considered statistically significant and <0.1 as indicating a trend.
Results
Contractile reactivity
We characterized contractile responses in the saphenous arteries of different age groups of both sexes. The optimal diameters and the maximal contractile responses to 10 μM NA were comparable in all age groups of both sexes (Supporting Information Table S1). Furthermore, the sensitivity (pEC50; Supporting Information Table S1) and maximal contraction (Emax) to PHE (0.01–10 μM) or K+ (40 mM) in the absence or presence of NOS and COX inhibitors were comparable in all age groups of both sexes (Supporting Information Table S1).
Relaxing responses to ACh during PHE-induced contraction
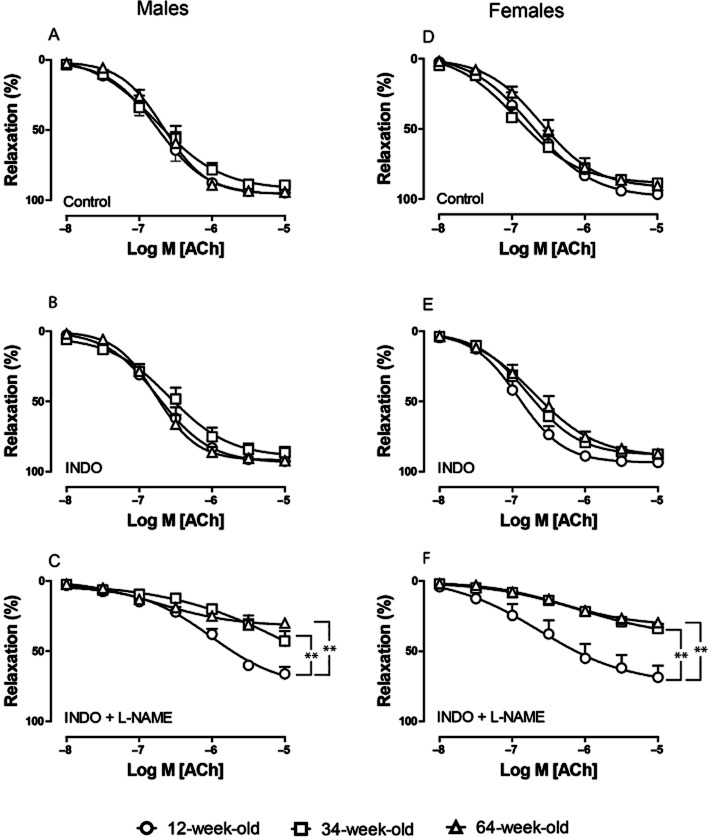
In the absence of inhibitors, the sensitivity of PHE-contracted saphenous arteries to ACh (0.01–10 μM)-induced relaxation and the maximal response were similar in 12-, 34- and 64-week-old male (Figure 1A) and female (Figure 1D) mice. The presence of INDO (10 μM) had no effect on the sensitivity or the maximal response to ACh in any age group or sex (Figure 1B, E; Table 1), demonstrating that, in our setting, COX products did not contribute to the relaxing responses.
Figure 1.
Effect of aging on relaxing responses to ACh (0.01–10 μM) during PHE-induced (10 μM) contraction in saphenous arteries of male (A–C) and female mice (D–F). Circles, 12-week; squares, 34-week; triangles, 64-week-old mice. (A, D) In the absence of pharmacological inhibitors. (B, E) In the presence of INDO (10 μM). (C, F) In the presence of both INDO (10 μM) and L-NAME (100 μM). Values are shown as means ± SEM (n = 7–14). **P < 0.001.
Table 1.
Effect of aging on endothelium-dependent and -independent relaxations
| 12 | 34 | 64 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Age (weeks) | pEC50 | Emax (%) | n | pEC50 | Emax (%) | n | pEC50 | Emax (%) | n |
| Males | |||||||||
| Relaxation responses to ACh | |||||||||
| Control | 6.6 ± 0.1 | 93 ± 1 | 9 | 6.7 ± 0.1 | 90 ± 3 | 6 | 6.6 ± 0.1 | 94 ± 1 | 8 |
| INDO | 6.7 ± 0.1 | 92 ± 1 | 9 | 6.6 ± 0.1 | 88 ± 3 | 6 | 6.7 ± 0.1 | 92 ± 2 | 8 |
| INDO + L-NAME | 6.1 ± 0.1 | 67 ± 4 | 8 | NA | 43 ± 7* | 6 | NA | 31 ± 2* | 7 |
| INDO + L-NAME + SKA-31 | 6.6 ± 0.1† | 76 ± 2 | 5 | NA | 32 ± 3* | 4 | NA | 34 ± 3* | 5 |
| INDO + L-NAME +TRAM-34 | 5.8 ± 0.3 | 6 ± 1† | 3 | ND | ND | ND | ND | ||
| INDO + L-NAME +UCL 1684 | 5.1 ± 2.8 | 9 ± 2† | 3 | ND | ND | ND | ND | ||
| INDO + L-NAME +TRAM-34 + UCL 1684 | 5.2 ± 1.2 | 7 ± 2† | 4 | NA | 12 ± 3 | 3 | NA | 12 ± 3 | 3 |
| Relaxation responses to SNP | |||||||||
| INDO + L-NAME | 7.2 ± 0.1 | 97 ± 1 | 8 | 7.2 ± 0.1 | 99 ± 1 | 5 | 7.1 ± 0.1 | 97 ± 1 | 7 |
| EDNO responses | |||||||||
| INDO | 6.1 ± 0.1 | 58 ± 3 | 8 | 6.1 ± 0.1 | 56 ± 6 | 7 | 6.2 ± 0.1 | 70 ± 3* | 5 |
| INDO + L-NAME | NA | 6 ± 2 | 3 | NA | 15 ± 1 | 3 | NA | 22 ± 2 | 8 |
| Relaxation responses to NS309 | |||||||||
| INDO + L-NAME | 5.8 ± 0.1 | 87 ± 2 | 6 | 5.3 ± 0.1* | 85 ± 2 | 3 | 5.2 ± 0.1* | 78 ± 2 | 5 |
| Females | |||||||||
| Relaxation responses to ACh | |||||||||
| Control | 6.8 ± 0.1 | 98 ± 1 | 11 | 6.8 ± 0.1 | 88 ± 3 | 10 | 6.6 ± 0.1 | 90 ± 5 | 9 |
| INDO | 6.9 ± 0.1 | 94 ± 1 | 9 | 6.8 ± 0.1 | 88 ± 3 | 14 | 6.7 ± 0.1 | 87 ± 2 | 8 |
| INDO + L-NAME | 5.9 ± 0.1 | 65 ± 7 | 12 | 6.0 ± 0.2 | 34 ± 4* | 12 | 6.2 ± 0.2 | 28 ± 3* | 11 |
| INDO + L-NAME + SKA-31 | 6.4 ± 0.1† | 66 ± 6 | 2 | ND | 24 ± 3 | 4 | ND | 32 ± 8 | 4 |
| INDO + L-NAME +TRAM-34 + UCL 1684 | NA | 19 ± 6† | 2 | NA | 8 ± 3 | 4 | NA | 18 ± 3 | 4 |
| Relaxation responses to SNP | |||||||||
| INDO + L-NAME | 7.6 ± 0.2 | 98 ± 1 | 7 | 7.5 ± 0.1 | 96 ± 1 | 8 | 7.4 ± 0.1 | 98 ± 1 | 11 |
| EDNO related responses | |||||||||
| INDO | 6.3 ± 0.1 | 60 ± 3 | 8 | 6.2 ± 0.1 | 48 ± 6 | 7 | 6.3 ± 0.2 | 57 ± 1 | 5 |
| INDO + L-NAME | NA | 16 ± 2 | 4 | NA | 18 ± 3 | 3 | NA | 19 ± 5 | 3 |
| Relaxation responses to NS309 | |||||||||
| INDO +L-NAME | 5.7 ± 0.1 | 82 ± 1 | 6 | 5.3 ± 0.1* | 77 ± 4 | 9 | 5.4 ± 0.1* | 86 ± 4 | 5 |
Emax expressed as % reduction of the maximal contractile response to 10 μM PHE except for EDNO responses (% reduction of maximal contractile response to 40 mM K). All values are shown as mean ± SEM.
P < 0.05 compared with arteries of 12-week-old animals of the same sex under the same condition.
P < 0.05 compared with arteries of 12-week-old animals of the same sex treated with INDO plus L-NAME.
ND, not determined; NA, not applicable.
EDNO responses
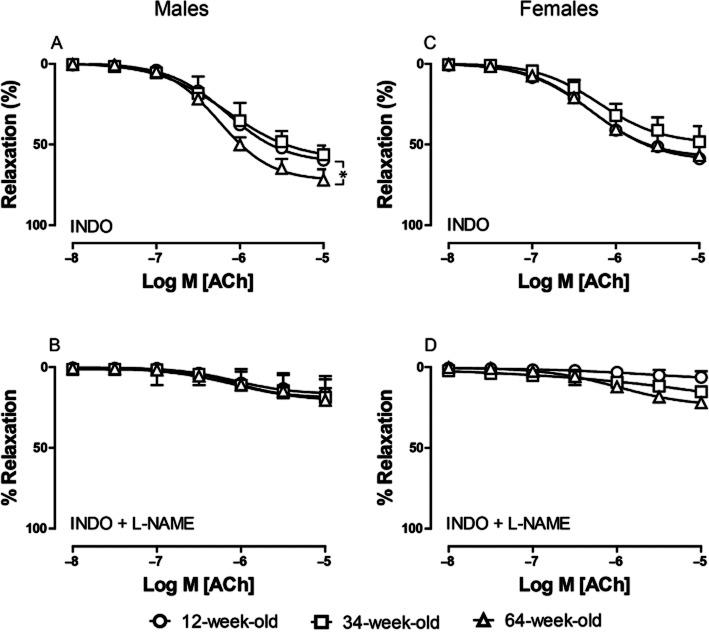
To evaluate the contribution of EDNO to arterial relaxation, we inhibited EDH-related relaxation by depolarizing the vessels with high potassium buffer [(K+) 40 mM] and COXs by INDO. In saphenous arteries of male mice, the sensitivity to ACh was not changed under these conditions in the three age groups (Figure 2A; Table 1), but the maximal relaxation to ACh was significantly greater in 64-week-old animals (70 ± 3%) than in 12-week-old mice (58 ± 3%, P = 0.022) and a trend was observed for 34-week-old animals (56 ± 6%, P = 0.0956). In contrast, arteries of female mice, showed similar sensitivity (Figure 2C; Table 1) and maximal relaxation to ACh in all age groups (60 ± 3, 48 ± 6 and 57 ± 1 % in 12-, 34- and 64-week-old mice, respectively).
Figure 2.
Effect of aging on relaxing responses to ACh (0.01–10 μM) during K+-induced (40 mM) contraction in saphenous arteries of male (A–B) and female (C–D) mice in the presence of INDO (A, C), INDO (10 μM) and L-NAME (100 μM) (B, D). Circles, 12-week; squares, 34-week; triangles, 64-week-old mice. Values shown as means ± SEM (n = 3–8). * = P < 0.05.
In both male and female mice, K+-contracted arteries, when treated with INDO (10 μM) plus L-NAME (100 μM), did not relax in response to ACh (Figure 2B, D). We, therefore, conclude that both NO and EDH (as will be described later) contribute to the ACh-induced vasorelaxation in murine saphenous arteries. In addition, we demonstrated that the contribution of NO to maximal relaxation increases in old male but not female mice.
Relaxing responses to SNP
To determine the endothelium-independent response to NO, PHE-contracted arteries were treated with INDO (10 μM) and L-NAME (100 μM) to block COXs and NOS, respectively. Subsequently, the relaxing response to the NO donor SNP (0.01–10 μM) was measured. Sensitivity and maximal relaxation to SNP were similar in all three age groups and in both sexes (Figure 3A, B; Table 1). Relaxing responses to the endothelium-independent NO donor SNP were not affected by aging and sex, indicating that the sensitivity of the vascular smooth muscle cells to NO is unchanged.
Figure 3.
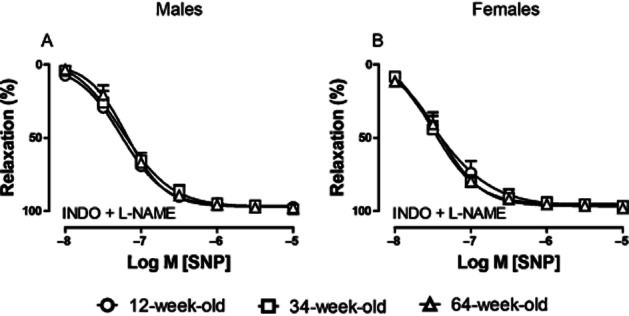
Effect of aging on relaxing responses to SNP (0.01–10 μM) during PHE-induced (10 μM) contraction in saphenous arteries of male (A) and female (B) mice in the presence of INDO (10 μM) and L-NAME (100 μM). Circles, 12-week; squares, 34-week; triangles, 64-week-old mice. Values are shown as means ± SEM; (n = 5–11).
ACh-induced EDH-related relaxations
To characterize the contribution of EDH to vascular relaxation, arteries were treated with L-NAME (100 μM) plus INDO (10 μM) and were contracted with 10 μM PHE. In the presence of NOS and COX inhibitors, the sensitivity to ACh was reduced, but did not differ between age groups (Figure 1C, F; Table 1). The maximal effect of ACh, however, did decrease with age under these conditions. In 12-week-old male and female mice, ACh induced a pronounced maximal relaxation (Emax 67 ± 4% and 65 ± 7% in males and females, respectively) that was significantly decreased in 34- and 64-week-old mice (Emax 43 ± 7 and 31 ± 2% in males, 34 ± 4 and 28 ± 3% in females, respectively; Figure 1C, F and Table 1). We, therefore, conclude that EDH-related relaxations are attenuated with age in both sexes.
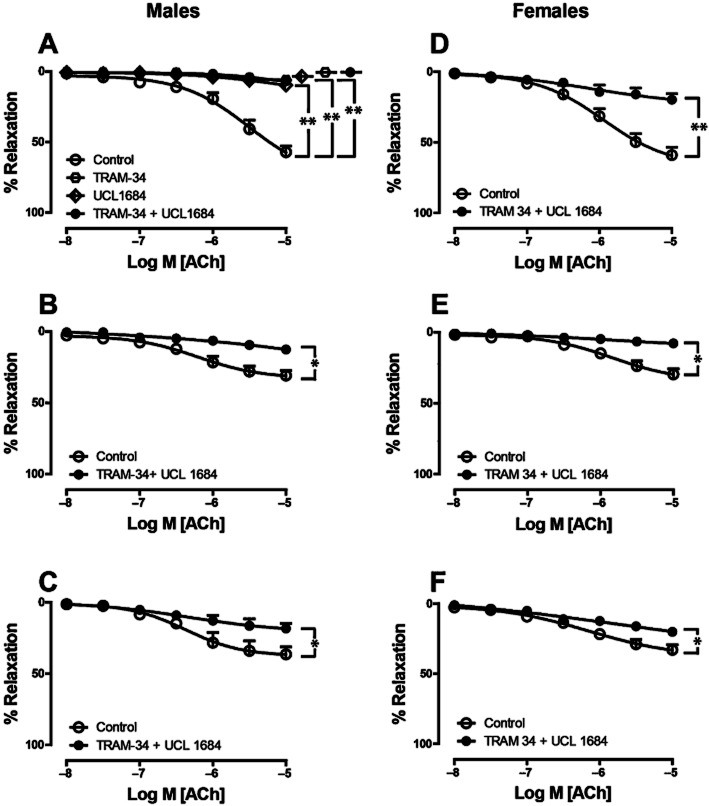
Contribution of KCa2.3 and KCa3.1 to EDH-related relaxation
To further characterize EDH-related relaxation and specifically to study the roles of KCa3.1 and KCa2.3 channels, we used TRAM-34 and UCL-1684 to block KCa3.1 and KCa2.3 channels, respectively. Saphenous arteries were incubated with a combination of INDO (10 μM), L-NAME (100 μM), TRAM-34 (10 μM) and UCL-1684 (1 μM), and were made to contract with PHE. In all age groups of both sexes, the relaxation response to ACh was completely blocked in the presence of the combination of these inhibitors [Figure 4A–C (males), 4D-F (females); Table 1]. This demonstrated that KCa3.1 and KCa2.3 channels account for the observed EDH-related effects. To evaluate the role of the individual channel subtypes in EDH-related relaxation in 12-week-old mice, saphenous arteries were treated with either TRAM-34 (10 μM) or UCL-1684 (1 μM) in combination with INDO and L-NAME. In young male mice, neither in the presence of TRAM-34 nor in the presence of UCL-1684 the arteries showed any residual relaxation (Figure 4A, Table 1), indicating that KCa3.1 and KCa2.3 can each mediate full and complete EDH-related relaxations in saphenous arteries of the mouse. Similar findings were obtained with 1 μM instead of 10 μM (Supporting Information Fig. S3).
Figure 4.
Effects of KCa3.1, KCa2.3 channel inhibitors on EDH-related relaxations during PHE-induced (10 μM) contraction in saphenous arteries of male (A–C) and female (D–F) mice aged 12 (A, D), 34 (B, E) and 64 (C, F) weeks. All experiments were performed in the presence of INDO (10 μM) and L-NAME (100 μM). In addition, arteries were treated with either TRAM-34 (10 μM) or UCL 1684 (1 μM) (12-week-old male mice only, A), or the combination of both (all age groups). Values are shown as means ± SEM (n = 3–4). **P < 0.001. *P < 0.05.
KCa channel activation by NS309
Furthermore, we characterized the relaxation response to NS309, a channel activator that does not discriminate between KCa3.1 and KCa2.3 channels (Strobaek et al., 2004) during PHE-induced contraction in the presence of INDO (10 μM) plus L-NAME (100 μM). In 34- and 64-week-old male and female mice, sensitivity to the relaxing effect of NS309 was significantly reduced in males (pEC50 5.3 ± 0.1, 5.2 ± 0.1) and females (pEC50 5.3 ± 0.1, 5.4 ± 0.1) compared with 12-week-old animals (pEC50 5.8 ± 0.1 in males and 5.7 ± 0.1 in females), but Emax was unchanged (Figure 5A, B; Table 1). This finding demonstrates that the sensitivity to KCa channel activation decreases with age. The relaxation responses induced by NS309 are largely endothelium dependent at lower concentrations (Supporting Information Fig. S4). Pharmacological inhibitors TRAM-34 and UCL-1684 alone or in combination completely block NS309-induced endothelium-dependent relaxation in young male mice (Supporting Information Fig. S4).
Figure 5.
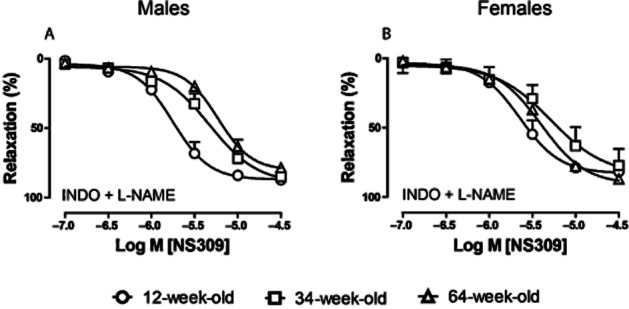
Effect of aging on relaxing responses to the KCa2.3 and KCa3.1 channel opener NS309 during PHE (10 μM)-induced contraction in saphenous arteries of male (A) and female (B) mice. All experiments were performed in the presence of INDO (10 μM) and L-NAME (100 μM). Circles, 12-week; squares, 34-week; triangles, 64-week-old mice. Values are shown as means ± SEM (n = 3–9).
KCa channel activation by SKA-31
Further we studied the potentiating effect of SKA-31, a newly developed allosteric modulator of KCa2.3 and KCa3.1 (Sankaranarayanan et al., 2009; Hasenau et al., 2011). The direct relaxing effect of SKA-31 was minor, and only observed at concentrations exceeding 3 μM (Supporting Information Fig. S2). The potentiating effects of this compound were measured in PHE-contracted arteries in the presence of INDO (10 μM) plus L-NAME (100 μM). In 12-week-old mice of both sexes, SKA-31 potentiated ACh-induced relaxation at a concentration of 1 μM [Figure 6A (males), 6D (females), Table 1], but this agent did not potentiate ACh-induced relaxations in 34- and 64-week-old males (Figure 6B, C) and females (Figure 6E, F). This underscores our hypothesis that KCa3.1 and KCa2.3 channel functions decrease with age in both sexes.
Figure 6.
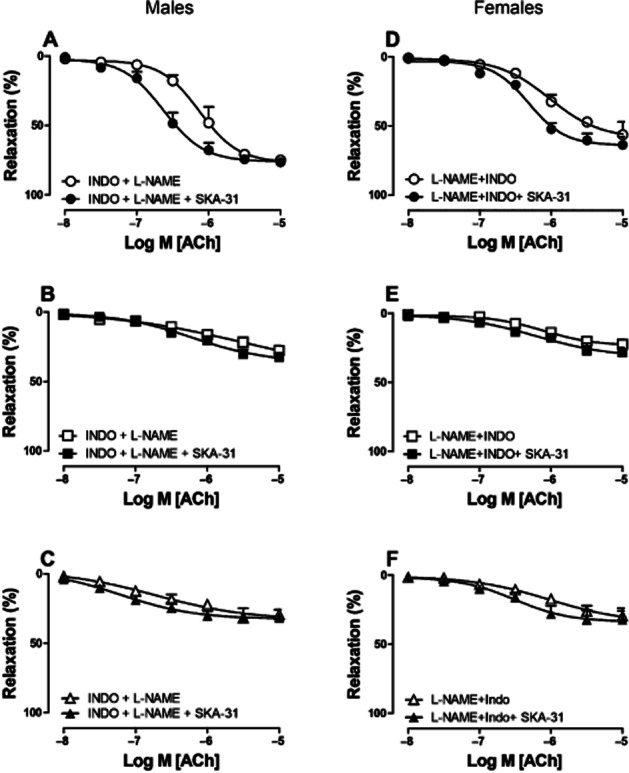
Potentiating effect of SKA-31 on relaxing responses in saphenous arteries of male (A–C) and female (D–F) mice of 12 (A, D), 34 (B, E) and 64 (C, F) weeks of age. Arteries were treated with either INDO (10 μM) and L-NAME (100 μM), or the combination of L-NAME (100 μM), INDO (10 μM) and SKA-31 (1 μM). Relaxing responses to ACh were recorded (0.01–10 μM) during PHE-induced (10 μM) contraction. Values are shown as means ± SEM (n = 4–5).
Discussion and conclusion
In the present study, the relative contribution of NO, EDH and prostaglandins to endothelium-dependent relaxation was studied in young, adult and aged mice of both sexes. We initially studied contractile responses induced by either PHE or K+. These contractile responses were not affected by age or sex. Interestingly, the contractile responses were also not affected by the presence of INDO (to block contributions of COX products) or the presence of both INDO and L-NAME.
Thereafter, we evaluated the responses mediated by EDNO in the presence of INDO in arteries that were depolarized with K+. In addition to activating arterial smooth muscle and peri-arterial nerves by depolarization, high K+ inhibits EDH. Responses mediated by EDNO were not affected by age in arteries isolated from female mice. Surprisingly, in arteries isolated from old male mice, EDNO-mediated responses were increased. This was not due to changes in the sensitivity of vascular smooth muscle for NO as these, in line with earlier findings in other labs (DeSouza et al., 2000; Taddei et al., 2001), were similar in all age groups. Our findings regarding EDNO are intriguing, since it is generally accepted that NO bioavailability decreases with age (Tschudi et al., 1996; Barton et al., 1997; Taddei et al., 2001). However, these studies on EDNO were performed on larger conduit arteries. In contrast, our study focused on the contribution of different vasoactive factors to endothelial function in saphenous arteries, which are muscular resistance arteries. The discrepancy between the previous studies and our findings may thus lie in the well-known and very profound regional vascular and interspecies heterogeneity.
The contribution of prostaglandins and EDH to endothelium-dependent relaxation was studied in PHE-contracted arteries. Total endothelium-dependent relaxation did not differ between age groups or sexes. Endothelium-dependent contractions, prominent in other types of murine arteries (e.g. mouse aorta; Zhou et al., 2005) were not observed and ACh-induced relaxations were not affected by the presence of INDO. Thus, in contrast to other murine arteries (aorta, carotid and femoral arteries) (Zhou et al., 2005; Liu et al., 2012), COX products do not contribute to endothelium-dependent relaxation in murine saphenous arteries.
In line with other studies in rats (Fujii et al., 1993; Goto et al., 2000; 2012), EDH-related relaxations decreased with age in murine saphenous arteries. Because the maximal ACh-induced relaxations did not change with age, we assume that the residual EDH-related responses together with the response mediated by EDNO are sufficient for full relaxation even in arteries isolated from older mice. It has been demonstrated that endothelial KCa channels are critically involved in the EDH-related phenomenon in various arteries in vitro and in vivo (Brahler et al., 2009; Grgic et al., 2009; Kohler and Ruth, 2010). Suppression of KCa2.3 expression by doxycycline administration in SK3T/T mice resulted in a pronounced elevation of blood pressure (∼30 mmHg) (Taylor et al., 2003). Deletion of KCa3.1 (KCa3.1−/− mice) decreased endothelial and smooth muscle hyperpolarizations in response to ACh and significantly increased arterial blood pressure (∼20 mmHg) (Si et al., 2006). Mice that were both KCa3.1-deficient and KCa2.3 (SK3T/T)-depleted showed impaired EDH-dependent relaxation as well as increased arterial blood pressure (Brahler et al., 2009). To establish whether KCa3.1 and KCa2.3 are also involved in the EDH-related responses of murine saphenous arteries, we evaluated their contribution to EDH-relatedrelaxations in arteries of young and old female and male mice. Blocking both channels simultaneously fully inhibited EDH-related responses in both sexes and all age groups. Similar results have been reported for murine mesenteric arteries in the presence of TRAM-34 and apamin (Harrington et al., 2007). Surprisingly, blocking only one of the two channel types, KCa2.3 or KCa3.1, had a similar effect as the blockade of both channels simultaneously. This is unlikely due to a lack of specificity of the inhibitors for either channel type (Rosa et al., 1998; Wulff et al., 2000). Interaction between KCa2.3 and KCa3.1 may, therefore, be crucial for EDH-related relaxation in murine saphenous arteries. In the future, co-immunoprecipitation experiments or in situ proximity ligation assays could establish the nature of the interaction between these channels. These experiments, however, were beyond the scope of our studies described here.
We relied on a classical pharmacological approach to study differences in channel expression and channel properties. In theory, decreased channel expression should result in a decreased Emax, but unchanged pEC50 when relaxations are induced by channel openers. In contrast, one would expect similar Emax, but altered pEC50 when the channel open probability is changed. Accordingly, we analysed relaxations induced by NS309 (an opener of KCa channels) to characterize receptor properties. We found that the Emax of NS309-induced relaxation was similar in all age groups and both sexes. However, arteries isolated from older mice were significantly less sensitive for NS309. It thus seems that aging alters the function of the KCa channels. To further address whether aging affects the characteristics of KCa channels in the endothelial cells of saphenous arteries, we used SKA-31, a recently developed positive allosteric modulator of KCa3.1 and KCa2.3 channels (Sankaranarayanan et al., 2009; Hasenau et al., 2011). In saphenous arteries of young mice, SKA-31 potentiated EDH-related relaxations, but not in those of 34- and 64-week-old mice. This, together with our findings with NS309, suggests that the functional properties of KCa channels are critically affected by age. Alternatively, the density of heterocellular gap junctions which have been shown to conduct endothelial hyperpolarization resulting from KCa channel activation to the underlying smooth muscle may decrease with age as recently documented (Sandow et al., 2004). Also, the mechanism by which smooth muscle cell hyperpolarization ultimately leads to relaxation and the role of intracellular calcium stores and plasmalemmal calcium channels merits further investigation. In addition to this, previous studies showed that NOS-derived H2O2, can be involved in EDH-related relaxations of both large and small arteries of mice and rats (Fujiki et al., 2005; Drouin et al., 2007; Takaki et al., 2008a). This has to be further addressed in the future studies.
We have attempted to quantify KCa3.1 and KCa2.3 expression using semi-quantitative immunohistochemistry. However, none of the presently available antibodies meets the criteria of specificity in paraffin sections of murine tissue (for details see online Supporting Information Figs. 6). This suggests that at least some published data using such antibodies should be treated with scepticism. It should, however, be pointed out that dedicated immunofluorescence analyses of whole mount preparations (Senadheera et al., 2012) would have been more suited.
The current study addressed age and sex differences in the vasomotor responses of murine saphenous arteries. We were able to demonstrate clear effects of aging on EDH-related relaxations. However, we did not observe major differences between the sexes, although a trend for increased sensitivity to exogenous NO was seen in female arteries and a modest increase in EDNO was observed in old male arteries. These mild sex differences regarding vasomotor function are surprising in view of the increased risk for cardiovascular disease associated with male gender. In addition, others have shown such gender differences previously, for example, the relative contribution of NO and EDH to endothelium-dependent relaxation differs in mesenteric arteries of male and female rats (McCulloch and Randall, 1998). In addition, studies on resistance arteries of endothelial NOS/COX-1 double-knockout mice (eNOS−/−/COX-1−/−) suggested that EDH is the main endothelium-dependent relaxing mechanism in female mice, whereas NO and PGI2 are the predominant mediators in male mice (Scotland et al., 2005). It is not easy to pinpoint the factors responsible for the discrepancy between our findings and earlier studies but clearly regional vascular heterogeneity and differences between species regarding the contribution of endothelium-derived relaxing factors to endothelium-dependent relaxation play a role (Hecker, 2000; Campbell and Gauthier, 2002).
Clinical implications
In vivo animal studies showed that treatment with SKA-31 lowers blood pressure (Sankaranarayanan et al., 2009; Damkjaer et al., 2012). In addition, KCa3.1- and KCa2.3-deficient mice are hypertensive (Taylor et al., 2003; Si et al., 2006). Thus, sensitizing KCa channels could be considered for the treatment of hypertension. However, in our current ex vivo study, arteries isolated from young mice were more sensitive to NS309 than those of older animals. In addition, SKA-31 was unable to potentiate EDH-related relaxations of saphenous arteries isolated from 34- or 64-week-old mice of both sexes. This implies that therapeutic interventions aimed at lowering blood pressure via KCa may be less efficacious in old than in young patients.
Summary
The present study shows that endothelium-dependent relaxations of murine saphenous arteries are maintained in aging mice of both sexes. However, the relative contribution of EDH to these relaxations diminishes with increasing age. The present study also shows that in murine saphenous arteries, both KCa3.1 and KCa2.3 are of crucial importance for EDH-related relaxation. Older mice display reduced sensitivity for KCa channel activators, possibly explaining the decreased contribution of EDH to endothelium-dependent relaxation in these mice. Only minor sex differences regarding endothelium-mediated relaxations were observed.
Acknowledgments
This work was supported by the grant from Dutch Heart Foundation (NHS) project 2008B107. The authors wish to thank to Dr R Köhler (University of Southern Denmark) for providing tissues for histology.
Glossary
- CRC
concentration response curve
- DMSO
dimethyl sulfoxide
- Dopt
optimal diameter
- EDH
endothelium-dependent hyperpolarization
- EDHF
endothelium-derived hyperpolarizing factor
- EDNO
endothelium-derived NO
- KCa2.3/SK3
small conductance calcium-activated potassium channel
- KCa3.1/IK1
intermediate conductance calcium-activated potassium channel
- KRB
Krebs Ringer bicarbonate-buffered salt solution
- INDO
indomethacin
- L-NAME
Nω-nitro-L-arginine methyl ester
- NA
noradrenaline
- NS309
6, 7-dichloro-1H-indole-2, 3-dione 3-oxime
- PHE
phenylephrine
- SKA-31
naphtho[1,2-d]thiazol-2-ylamine
- SNP
sodium nitroprusside
- TRAM-34
1-[(2-chlorophenyl) diphenylmethyl]-1H-pyrazole
- UCL 1684
6,12,19,20,25,26-hexahydro-5,27,13,18,21,24 -trietheno-11,7-metheno-7H-dibenzo [b,n] [1,5,12,16] tetraazacyclotricosine-5,13-diium dibromide
Conflicts of interest
The authors declare that there are no conflicts of interest.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
Figure S1 The blue line shows the anatomical location of saphenous artery used in the present study. The black line corresponds to the femoral artery.
Figure S2 Direct relaxing responses to SKA-31 (0.001–10μM) during PHE (10μM) – induced contraction in saphenous arteries of 12-week-old male mice (n = 4).
Figure S3 Relaxing responses to ACh (0.001–10μM) in PHE (10μM)-contracted saphenous arteries in the presence of L-NAME (100μM), INDO (10μM) (+L + I; diamonds), and the selective KCa3.1 channel blocker TRAM-34 at either 10μM (+L + I + T; squares) or 1μM (+L + I + T; squares) concentration. Values are expressed as mean ± SEM (n = 4–6).
Figure S4 Relaxing responses to the KCa2.3 and KCa3.1 channel opener NS309 in phenylephrine (10μM)-contracted saphenous arteries with (E) or without (−E; circles) endothelium. In the intact arteries, experiments were performed in the presence of LNAME (100μM), INDO (10μM), (+E + L + I; diamonds), the selective KCa3.1 channel blocker TRAM-34 (+E + L + I + T; 10μM; squares), the selective KCa2.3 channel blocker UCL-1684 (+E + L + I + U; 1μM; inverted triangles) and the combined incubation of TRAM-34 and UCL-1684 (+E + L + I + T + U; triangle). Values are expressed as mean ± SEM (n = 5–6).
Figure S5 Expression of KCa3.1 channels in saphenous arteries of 12- (A, E), 34- (B, F) and 64-week-old (C, G) male mice. All tissues were fixed in 4% formaldehyde and embedded in paraffin. Four millimetre sections were rehydrated and exposed for 20 min to 0.3% H2O2 at RT to block endogenous peroxidases. Subsequently, sections were incubated in a humidified chamber (overnight, 4°C) with successively sheep antibodies against IK1 [anti-KCa 3.1, 1:600 in normal goat serum (NGS); Alomone Labs (Jerusalem, Israel); product number: APC-064; Lot number: AN-02] and horseradish peroxidase-coupled rabbit antibodies against sheep IgG (1:400 in NGS, DAKO, Glostrup, Denmark). The localization of HRP was visualized with 3, 3,diaminobenzidine (Sigma Aldrich). All sections were counterstained with hematoxylin. Negative controls (A, B, C, D) were incubated with secondary antibody only. Mouse brain (12-week-old male) was used as positive control in absence (D) of and in the presence (H) of primary antibody. Corresponding magnified insets are marked with asterisk. Staining of endothelium (arrow) was observed in 12-week-old, but not in 34- and 64-week-old mice. However, we also observed staining in smooth muscle cells, which may be due to incomplete specificity of the antibodies. We also tried lower concentrations of the primary antibody, which only decreased the staining in the endothelium relative to the surrounding structures. Nevertheless, these observations are in line with reduced KCa3.1 protein expression in endothelium with aging.
Figure S6 Expression of KCa2.3 channels in saphenous arteries of 12- (A, E), 34- (B, F) and 64-week-old (C, G) male mice. Tissues were fixed and sections processed as described in the legend of Supporting Information Fig. S5, except that sections were incubated with sheep antibodies directed against SK3 (anti-KCa 2.3 N-terminal, 1:200 in NGS; Alomone Labs; Product number: APC-025; Lot number: AN-04) and horseradish peroxidase-coupled rabbit antibodies against sheep IgG (1:400 in NGS, DAKO, Glostrup, Denmark). The localization of HRP was visualized with 3, 3,diaminobenzidine (Sigma Aldrich). All sections were counterstained with hematoxylin. Negative controls (A, B, C, D) were incubated with secondary antibody only. Mouse brain (12-week-old male) was used as positive control in absence (D) of and in the presence (H) of primary antibody. Corresponding magnified insets are marked with asterisk. No staining was observed, indicating that the SK3 antiserum did not recognize KCa2.3 channels in the sections. Usage of whole mount arterial preparation merits further investigation.
Figure S7 Expression of KCa3.1 channels in saphenous arteries of 12- (A, E), 34- (B, F) and 64-week-old (C, G) male mice. All tissues were fixed in 4% formaldehyde and embedded in paraffin. Four millimetre sections were rehydrated and exposed for 20 min to 0.3% H2O2 at RT to block endogenous peroxidases. Subsequently, sections were incubated in a humidified chamber (overnight, 4°C) with successively sheep antibodies against IK1 (anti-KCa 3.1, 1:600 in normal goat serum (NGS); SIGMA; Product number: P 4997) and horseradish peroxidase-coupled rabbit antibodies against sheep IgG (1:400 in NGS, DAKO, Glostrup, Denmark). The localization of HRP was visualized with 3, 3,diaminobenzidine (Sigma Aldrich). All sections were counterstained with hematoxylin. Negative controls (A, B, C, D) were incubated with secondary antibody only. Mouse brain (12-weekold male) was used as positive control in absence (D) of and in the presence (H) of primary antibody. Corresponding magnified insets are marked with asterisk. Staining of endothelium (arrow) was observed in 12- week-old, but not in 34- and 64-week-old mice. However, we also observed staining in smooth muscle cells, which may be due to incomplete specificity of the antibodies. We also tried lower concentrations of the primary antibody, which only decreased the staining in the endothelium relative to the surrounding structures. Nevertheless, these observations are in line with reduced KCa3.1 protein expression in endothelium with aging.
Figure S8 Expression of KCa2.3 channels in saphenous arteries of 12- (A, E), 34- (B, F) and 64-week-old (C, G) male mice. Tissues were fixed and sections processed as described in the legend of Supporting Information Fig. S7, except that sections were incubated with sheep antibodies directed against SK3 (anti-KCa2.3 N-terminal, 1:200 in NGS; SIGMA; Product number: P 0608) and horseradish peroxidase-coupled rabbit antibodies against sheep IgG (1:400 in NGS, DAKO, Glostrup, Denmark). The localization of HRP was visualized with 3, 3,diaminobenzidine (Sigma Aldrich). All sections were counterstained with hematoxylin. Negative controls (A, B, C, D) were incubated with secondary antibody only. Mouse brain (12-week-old male) was used as positive control in absence (D) of and in the presence (H) of primary antibody. Corresponding magnified insets are marked with asterisk. No staining was observed, indicating that the SK3 antiserum did not recognize KCa2.3 channels in the sections.
Figure S9 Expression of KCa3.1(A,B,C) and KCa2.3 (D) channels in gut of WT (A, B, D) and knockout KCa3.1(C) mice. All tissues were fixed in 4% formaldehyde and embedded in paraffin. Four millimetre sections were rehydrated and exposed for 20 min to 0.3% H2O2 at RT to block endogenous peroxidases. Subsequently, sections were incubated in a humidified chamber (overnight, 4°C) with sheep antibodies against IK1 (anti-KCa3.1 1:600 in normal goat serum (NGS); SIGMA; Product number: P 4997) or SK3 (anti-KCa2.3 1:200 in normal goat serum (NGS); SIGMA; Product number: P 0608) and horseradish peroxidase-coupled rabbit antibodies against sheep IgG (1:400 in NGS, DAKO, Glostrup, Denmark). The localization of HRP was visualized with 3, 3,diaminobenzidine (Sigma Aldrich). All sections were counterstained with hematoxylin. Negative controls (A) were incubated with secondary antibody only. With SK3 antiserum no staining was observed in the gut (D). However, IK1 antiserum resulted intense staining in the gut of WT (B) compared with KCa2.3 knockout (C) mice. But KCa3.1 KO mice also showed some amount of staining in the gut (C, arrow), which may be due to incomplete specificity of the antibodies. Unfortunately due to the limited availability of tissues from KCa2.3 KO mice further investigation was not feasible in our lab.
Table S1 Effect of aging on optimal diameter and contractile responses.
References
- Barton M, Cosentino F, Brandes RP, Moreau P, Shaw S, Luscher TF. Anatomic heterogeneity of vascular aging: role of nitric oxide and endothelin. Hypertension. 1997;30:817–824. doi: 10.1161/01.hyp.30.4.817. [DOI] [PubMed] [Google Scholar]
- Bolotina VM, Najibi S, Palacino JJ, Pagano PJ, Cohen RA. Nitric oxide directly activates calcium-dependent potassium channels in vascular smooth muscle. Nature. 1994;368:850–853. doi: 10.1038/368850a0. [DOI] [PubMed] [Google Scholar]
- Brahler S, Kaistha A, Schmidt VJ, Wolfle SE, Busch C, Kaistha BP, et al. Genetic deficit of SK3 and IK1 channels disrupts the endothelium-derived hyperpolarizing factor vasodilator pathway and causes hypertension. Circulation. 2009;119:2323–2332. doi: 10.1161/CIRCULATIONAHA.108.846634. [DOI] [PubMed] [Google Scholar]
- Busse R, Edwards G, Feletou M, Fleming I, Vanhoutte PM, Weston AH. EDHF: bringing the concepts together. Trends Pharmacol Sci. 2002;23:374–380. doi: 10.1016/s0165-6147(02)02050-3. [DOI] [PubMed] [Google Scholar]
- Bussemaker E, Popp R, Fisslthaler B, Larson CM, Fleming I, Busse R, et al. Aged spontaneously hypertensive rats exhibit a selective loss of EDHF-mediated relaxation in the renal artery. Hypertension. 2003;42:562–568. doi: 10.1161/01.HYP.0000088852.28814.E2. [DOI] [PubMed] [Google Scholar]
- Campbell WB, Gauthier KM. What is new in endothelium-derived hyperpolarizing factors? Curr Opin Nephrol Hypertens. 2002;11:177–183. doi: 10.1097/00041552-200203000-00008. [DOI] [PubMed] [Google Scholar]
- Castelli WP. Epidemiology of coronary heart disease: the Framingham study. Am J Med. 1984;76(2A):4–12. doi: 10.1016/0002-9343(84)90952-5. [DOI] [PubMed] [Google Scholar]
- Chadha PS, Liu L, Rikard-Bell M, Senadheera S, Howitt L, Bertrand RL, et al. Endothelium-dependent vasodilation in human mesenteric artery is primarily mediated by myoendothelial gap junctions intermediate conductance calcium-activated K+ channel and nitric oxide. J Pharmacol Exp Ther. 2011;336:701–708. doi: 10.1124/jpet.110.165795. [DOI] [PubMed] [Google Scholar]
- Damkjaer M, Nielsen G, Bodendiek S, Staehr M, Gramsbergen JB, de Wit C, et al. Pharmacological activation of KCa3.1/KCa2.3 channels produces endothelial hyperpolarization and lowers blood pressure in conscious dogs. Br J Pharmacol. 2012;165:223–234. doi: 10.1111/j.1476-5381.2011.01546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, et al. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation. 2000;102:1351–1357. doi: 10.1161/01.cir.102.12.1351. [DOI] [PubMed] [Google Scholar]
- Drouin A, Thorin-Trescases N, Hamel E, Falck JR, Thorin E. Endothelial nitric oxide synthase activation leads to dilatory H2O2 production in mouse cerebral arteries. Cardiovasc Res. 2007;73:73–81. doi: 10.1016/j.cardiores.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Edwards G, Feletou M, Weston AH. Endothelium-derived hyperpolarising factors and associated pathways: a synopsis. Pflugers Arch. 2010;459:863–879. doi: 10.1007/s00424-010-0817-1. [DOI] [PubMed] [Google Scholar]
- Egashira K, Inou T, Hirooka Y, Kai H, Sugimachi M, Suzuki S, et al. Effects of age on endothelium-dependent vasodilation of resistance coronary artery by acetylcholine in humans. Circulation. 1993;88:77–81. doi: 10.1161/01.cir.88.1.77. [DOI] [PubMed] [Google Scholar]
- Farhat MY, Lavigne MC, Ramwell PW. The vascular protective effects of estrogen. FASEB J. 1996;10:615–624. [PubMed] [Google Scholar]
- Feletou M, Vanhoutte PM. The third pathway: endothelium-dependent hyperpolarization. J Physiol Pharmacol. 1999;50:525–534. [PubMed] [Google Scholar]
- Feletou M, Vanhoutte PM. EDHF: an update. Clin Sci (Lond) 2009;117:139–155. doi: 10.1042/CS20090096. [DOI] [PubMed] [Google Scholar]
- Fujii K, Ohmori S, Tominaga M, Abe I, Takata Y, Ohya Y, et al. Age-related changes in endothelium-dependent hyperpolarization in the rat mesenteric artery. Am J Physiol. 1993;265(2 Pt 2):H509–H516. doi: 10.1152/ajpheart.1993.265.2.H509. [DOI] [PubMed] [Google Scholar]
- Fujiki T, Shimokawa H, Morikawa K, Kubota H, Hatanaka M, Talukder MA, et al. Endothelium-derived hydrogen peroxide accounts for the enhancing effect of an angiotensin-converting enzyme inhibitor on endothelium-derived hyperpolarizing factor-mediated responses in mice. Arterioscler Thromb Vasc Biol. 2005;25:766–771. doi: 10.1161/01.ATV.0000158498.19027.75. [DOI] [PubMed] [Google Scholar]
- Goto K, Fujii K, Onaka U, Abe I, Fujishima M. Renin-angiotensin system blockade improves endothelial dysfunction in hypertension. Hypertension. 2000;36:575–580. doi: 10.1161/01.hyp.36.4.575. [DOI] [PubMed] [Google Scholar]
- Goto K, Kansui Y, Oniki H, Ohtsubo T, Matsumura K, Kitazono T. Upregulation of endothelium-derived hyperpolarizing factor compensates for the loss of nitric oxide in mesenteric arteries of dahl salt-sensitive hypertensive rats. Hypertens Res. 2012;35:849–854. doi: 10.1038/hr.2012.36. [DOI] [PubMed] [Google Scholar]
- Grgic I, Kaistha BP, Hoyer J, Kohler R. Endothelial Ca+-activated K+ channels in normal and impaired EDHF-dilator responses – relevance to cardiovascular pathologies and drug discovery. Br J Pharmacol. 2009;157:509–526. doi: 10.1111/j.1476-5381.2009.00132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddock RE, Grayson TH, Morris MJ, Howitt L, Chadha PS, Sandow SL. Diet-induced obesity impairs endothelium-derived hyperpolarization via altered potassium channel signaling mechanisms. PLoS ONE. 2011;6:e16423. doi: 10.1371/journal.pone.0016423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington LS, Carrier MJ, Gallagher N, Gilroy D, Garland CJ, Mitchell JA. Elucidation of the temporal relationship between endothelial-derived NO and EDHF in mesenteric vessels. Am J Physiol Heart Circ Physiol. 2007;293:H1682–H1688. doi: 10.1152/ajpheart.00389.2007. [DOI] [PubMed] [Google Scholar]
- Hasenau AL, Nielsen G, Morisseau C, Hammock BD, Wulff H, Kohler R. Improvement of endothelium-dependent vasodilations by SKA-31 and SKA-20, activators of small- and intermediate-conductance Ca2+ -activated K+ -channels. Acta Physiol (Oxf) 2011;203:117–126. doi: 10.1111/j.1748-1716.2010.02240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecker M. Endothelium-derived hyperpolarizing factor-fact or fiction? News Physiol Sci. 2000;15:1–5. [PubMed] [Google Scholar]
- Hilgers RH, Webb RC. Reduced expression of SKCa and IKCa channel proteins in rat small mesenteric arteries during angiotensin II-induced hypertension. Am J Physiol Heart Circ Physiol. 2007;292:H2275–H2284. doi: 10.1152/ajpheart.00949.2006. [DOI] [PubMed] [Google Scholar]
- Hilgers RH, Janssen GM, Fazzi GE, De Mey JG. Twenty-four-hour exposure to altered blood flow modifies endothelial Ca2+-activated K+ channels in rat mesenteric arteries. J Pharmacol Exp Ther. 2010;333:210–217. doi: 10.1124/jpet.109.161448. [DOI] [PubMed] [Google Scholar]
- Huang A, Sun D, Smith CJ, Connetta JA, Shesely EG, Koller A, et al. In eNOS knockout mice skeletal muscle arteriolar dilation to acetylcholine is mediated by EDHF. Am J Physiol Heart Circ Physiol. 2000;278:H762–H768. doi: 10.1152/ajpheart.2000.278.3.H762. [DOI] [PubMed] [Google Scholar]
- Kohler R, Ruth P. Endothelial dysfunction and blood pressure alterations in K+-channel transgenic mice. Pflugers Arch. 2010;459:969–976. doi: 10.1007/s00424-010-0819-z. [DOI] [PubMed] [Google Scholar]
- Lesniewski LA, Connell ML, Durrant JR, Folian BJ, Anderson MC, Donato AJ, et al. B6D2F1 mice are a suitable model of oxidative stress-mediated impaired endothelium-dependent dilation with aging. J Gerontol A Biol Sci Med Sci. 2009;64:9–20. doi: 10.1093/gerona/gln049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Luo W, Zhang Y, Li H, Zhu N, Huang D, et al. Involvement of cyclo-oxygenase-1-mediated prostacyclin synthesis in the vasoconstrictor activity evoked by ACh in mouse arteries. Exp Physiol. 2012;97:277–289. doi: 10.1113/expphysiol.2011.062034. [DOI] [PubMed] [Google Scholar]
- Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, et al. Heart disease and stroke statistics – 2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- McCulloch AI, Randall MD. Sex differences in the relative contributions of nitric oxide and EDHF to agonist-stimulated endothelium-dependent relaxations in the rat isolated mesenteric arterial bed. Br J Pharmacol. 1998;123:1700–1706. doi: 10.1038/sj.bjp.0701781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orshal JM, Khalil RA. Gender, sex hormones, and vascular tone. Am J Physiol Regul Integr Comp Physiol. 2004;286:R233–R249. doi: 10.1152/ajpregu.00338.2003. [DOI] [PubMed] [Google Scholar]
- Rosa JC, Galanakis D, Ganellin CR, Dunn PM, Jenkinson DH. Bis-quinolinium cyclophanes: 6,10-diaza-3(1,3),8(1,4)-dibenzena-1,5(1,4)- diquinolinacyclodecaphane (UCL 1684), the first nanomolar, non-peptidic blocker of the apamin-sensitive Ca(2+)-activated K+ channel. J Med Chem. 1998;41:2–5. doi: 10.1021/jm970571a. [DOI] [PubMed] [Google Scholar]
- Sandow SL, Goto K, Rummery NM, Hill CE. Developmental changes in myoendothelial gap junction mediated vasodilator activity in the rat saphenous artery. J Physiol. 2004;556(Pt 3):875–886. doi: 10.1113/jphysiol.2003.058669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaranarayanan A, Raman G, Busch C, Schultz T, Zimin PI, Hoyer J, et al. Naphtho[1,2-d]thiazol-2-ylamine (SKA-31), a new activator of KCa2 and KCa3.1 potassium channels, potentiates the endothelium-derived hyperpolarizing factor response and lowers blood pressure. Mol Pharmacol. 2009;75:281–295. doi: 10.1124/mol.108.051425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotland RS, Madhani M, Chauhan S, Moncada S, Andresen J, Nilsson H, et al. Investigation of vascular responses in endothelial nitric oxide synthase/cyclooxygenase-1 double-knockout mice: key role for endothelium-derived hyperpolarizing factor in the regulation of blood pressure in vivo. Circulation. 2005;111:796–803. doi: 10.1161/01.CIR.0000155238.70797.4E. [DOI] [PubMed] [Google Scholar]
- Senadheera S, Kim Y, Grayson TH, Toemoe S, Kochukov MY, Abramowitz J, et al. Transient receptor potential canonical type 3 channels facilitate endothelium-derived hyperpolarization-mediated resistance artery vasodilator activity. Cardiovasc Res. 2012;95:439–447. doi: 10.1093/cvr/cvs208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si H, Heyken WT, Wolfle SE, Tysiac M, Schubert R, Grgic I, et al. Impaired endothelium-derived hyperpolarizing factor-mediated dilations and increased blood pressure in mice deficient of the intermediate-conductance Ca2+-activated K+ channel. Circ Res. 2006;99:537–544. doi: 10.1161/01.RES.0000238377.08219.0c. [DOI] [PubMed] [Google Scholar]
- Strobaek D, Teuber L, Jorgensen TD, Ahring PK, Kjaer K, Hansen RS, et al. Activation of human IK and SK Ca2+ -activated K+ channels by NS309 (6,7-dichloro-1H-indole-2,3-dione 3-oxime) Biochim Biophys Acta. 2004;1665:1–5. doi: 10.1016/j.bbamem.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Sunano S, Watanabe H, Tanaka S, Sekiguchi F, Shimamura K. Endothelium-derived relaxing, contracting and hyperpolarizing factors of mesenteric arteries of hypertensive and normotensive rats. Br J Pharmacol. 1999;126:709–716. doi: 10.1038/sj.bjp.0702355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Ghiadoni L, Salvetti G, Bernini G, Magagna A, et al. Age-related reduction of NO availability and oxidative stress in humans. Hypertension. 2001;38:274–279. doi: 10.1161/01.hyp.38.2.274. [DOI] [PubMed] [Google Scholar]
- Taddei S, Versari D, Cipriano A, Ghiadoni L, Galetta F, Franzoni F, et al. Identification of a cytochrome P450 2C9-derived endothelium-derived hyperpolarizing factor in essential hypertensive patients. J Am Coll Cardiol. 2006;48:508–515. doi: 10.1016/j.jacc.2006.04.074. [DOI] [PubMed] [Google Scholar]
- Takaki A, Morikawa K, Murayama Y, Yamagishi H, Hosoya M, Ohashi J, et al. Roles of endothelial oxidases in endothelium-derived hyperpolarizing factor responses in mice. J Cardiovasc Pharmacol. 2008a;52:510–517. doi: 10.1097/FJC.0b013e318190358b. [DOI] [PubMed] [Google Scholar]
- Takaki A, Morikawa K, Tsutsui M, Murayama Y, Tekes E, Yamagishi H, et al. Crucial role of nitric oxide synthases system in endothelium-dependent hyperpolarization in mice. J Exp Med. 2008b;205:2053–2063. doi: 10.1084/jem.20080106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor MS, Bonev AD, Gross TP, Eckman DM, Brayden JE, Bond CT, et al. Altered expression of small-conductance Ca2+-activated K+ (SK3) channels modulates arterial tone and blood pressure. Circ Res. 2003;93:124–131. doi: 10.1161/01.RES.0000081980.63146.69. [DOI] [PubMed] [Google Scholar]
- Tschudi MR, Barton M, Bersinger NA, Moreau P, Cosentino F, Noll G, et al. Effect of age on kinetics of nitric oxide release in rat aorta and pulmonary artery. J Clin Invest. 1996;98:899–905. doi: 10.1172/JCI118872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigg SJ, Tare M, Tonta MA, O'Brien RC, Meredith IT, Parkington HC. Comparison of effects of diabetes mellitus on an EDHF-dependent and an EDHF-independent artery. Am J Physiol Heart Circ Physiol. 2001;281:H232–H240. doi: 10.1152/ajpheart.2001.281.1.H232. [DOI] [PubMed] [Google Scholar]
- de Wit C, Griffith TM. Connexins and gap junctions in the EDHF phenomenon and conducted vasomotor responses. Pflugers Arch. 2010;459:897–914. doi: 10.1007/s00424-010-0830-4. [DOI] [PubMed] [Google Scholar]
- Wulff H, Miller MJ, Hansel W, Grissmer S, Cahalan MD, Chandy KG. Design of a potent and selective inhibitor of the intermediate-conductance Ca2+-activated K+ channel, IKCa1: a potential immunosuppressant. Proc Natl Acad Sci U S A. 2000;97:8151–8156. doi: 10.1073/pnas.97.14.8151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Varadharaj S, Zhao X, Parinandi N, Flavahan NA, Zweier JL. Acetylcholine causes endothelium-dependent contraction of mouse arteries. Am J Physiol Heart Circ Physiol. 2005;289:H1027–H1032. doi: 10.1152/ajpheart.00226.2005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.