Abstract
Background and Purpose
3′,5′-Cyclic nucleotide PDE4 is expressed in several inflammatory and immune cells, and PDE4 catalyses the hydrolysis of cAMP to 5′AMP, down-regulating cAMP signalling in cells. MAPK phosphatase-1 (MKP-1) is an endogenous p38 MAPK signalling suppressor and limits inflammatory gene expression and inflammation. In the present study, we investigated the effect of a PDE4 inhibitor rolipram on MKP-1 expression and whether MKP-1 is involved in the anti-inflammatory effects of rolipram.
Experimental Approach
The effect of rolipram on TNF production was investigated in J774 mouse macrophage cell line and in primary mouse peritoneal macrophages (PM) from wild-type (WT) and MKP-1(–/–) mice. We also investigated the effect of rolipram on carrageenan-induced paw inflammation in WT and MKP-1(–/–) mice.
Key Results
MKP-1 expression was enhanced by rolipram, by a non-selective PDE inhibitor IBMX and by a cAMP analogue 8-Br-cAMP in J774 cells and in PM. Enhanced MKP-1 mRNA expression by rolipram was reversed by a PKA inhibitor. Rolipram, IBMX and 8-Br-cAMP also inhibited TNF production in activated macrophages. Accordingly, rolipram inhibited TNF production in PMs from WT mice but, interestingly, not in PMs from MKP-1(–/–) mice. Furthermore, rolipram attenuated carrageenan-induced paw inflammation in WT but not in MKP-1(–/–) mice.
Conclusions and Implications
PDE4 inhibitor rolipram was found to enhance the expression of MKP-1, and MKP-1 mediated, at least partly, the anti-inflammatory effects of PDE4 inhibition. The results suggest that compounds that enhance MKP-1 expression and/or MKP-1 activity hold potential as novel anti-inflammatory drugs.
Keywords: MKP-1, PDE4, TNF, MAPK, DUSP1, PDE inhibitors
Introduction
cAMP is a fundamental second-messenger molecule produced in essentially all mammalian cells. 3′5′-Cyclic nucleotide PDEs are the enzymatic machinery that is responsible for the degradation of cAMP in cells by catalysing the hydrolysis of cAMP to 5′AMP. There are 11 known families (PDE1–11) in PDE superfamily, and, currently, at least 24 PDE isoforms have been characterized. Compounds that selectively inhibit PDE4 have prominent anti-inflammatory effects, and selective PDE4 inhibitors have recently entered to the clinics for the treatment of chronic obstructive pulmonary disease, and they have been proven effective in the treatment of plaque psoriasis and psoriatic arthritis as well (Chong et al., 2011; Page and Spina, 2012; Papp et al., 2012; Schett et al., 2012).
PDE4 family members specifically degrade cAMP, and they are expressed essentially in all immune cells including macrophages, neutrophils, eosinophils and lymphocytes, and in many other cell types, including epithelial cells, endothelium and fibroblasts. PDE4 isoforms differ from one another in structure, substrate specificity, tissue and cell distribution, intracellular localization, and regulation by other proteins. PDE4 family contains four genes, namely PDE4A, PDE4B, PDE4C and PDE4D, and they give rise to more than 20 alternative splice variants (Bender and Beavo, 2006; Spina, 2008; Keravis and Lugnier, 2012; Page and Spina, 2012). PDE4 isoforms interact with various scaffold proteins, such as β-arrestin, disrupted in schizophrenia 1, receptor for activated C-kinase 1, A-kinase-anchoring proteins, p75 neurotrophin receptor and aryl-hydrocarbon receptor interacting protein. Interactions with specific scaffold proteins evoke sequestered localization of specific PDE4 isoforms in cells. By virtue of such sequestered intracellular localization, PDE4 isoforms create cAMP gradients in cells that elicit temporally and spatially coordinated, also called a compartmentalized, cAMP signalling. PDE4 activity is regulated also by PKA and ERK1/2, and the regulatory outcome on the PDE4 activity by these kinases varies between isoforms and splice variant (Houslay and Adams, 2003; Houslay et al., 2005; Houslay, 2010).
PDE4 plays an important role in immune cell functions. PDE4B(–/–) mice displayed improved tolerance to the development of shock in endotoxemia, and it was associated with the reduced TNF production by macrophages (Jin et al., 2005). Moreover, in ovalbumin-sensitized lung inflammation model, PDE4B-deficient mice had markedly attenuated airway constriction, reduced lung eosinophilia and suppressed production of T helper 2 cytokines after ovalbumin inhalation challenge (Jin et al., 2010). Rolipram, a classic PDE4-specific inhibitor that elevates cAMP levels, inhibited TNF production in monocytes and macrophages (Kelly et al., 1996; Gantner et al., 1997; Wang et al., 1997). Oral or intratracheal administration of PDE4-specific inhibitors have been shown to reduce lung inflammation and airway hyper-responsiveness, attenuate leukocyte infiltration and inhibit cytokine production in the lung (Dastidar et al., 2009; Kobayashi et al., 2011; Nials et al., 2011).
MAPKs include ERK1/2 and ERK5, and stress-activated protein kinases p38 MAPK and JNK. They regulate many cellular responses such as mitosis, differentiation, cellular growth and inflammatory response. MAPKs are activated by phosphorylation of regulatory tyrosine and threonine residues within the activation loop by upstream kinases in response to extracellular stimuli such as cellular stress, bacterial products, growth factors, cytokines or chemokines through activation of GPCRs or kinase-linked receptors (Plotnikov et al., 2011).
MAPK phosphatases (MKPs) are a subgroup of a larger family of dual specificity phosphatases that dephosphorylate phosphotyrosine and phosphoserine/phosphothreonine residues in regulatory and signalling proteins. MKPs have a distinct MAPK binding domain, which enables them to interact with MAPK, and hence, they function as endogenous inhibitors of MAPK pathways (Patterson et al., 2009). MKPs include more than 10 phosphatases and they have differences in expressional pattern, tissue distribution, intracellular location and substrate specificity. MKP-1 is a nuclear phosphatase present in most cell types and tissues. The expression of MKP-1 is up-regulated by factors such as cellular stress, cytokines and LPS (Boutros et al., 2008). MKP-1 mainly inhibits p38 MAPK and, in some cell types, JNK signalling, and it suppresses the expression of inflammatory genes, including TNF (Chi et al., 2006; Zhao et al., 2006; Turpeinen et al., 2010; Korhonen et al., 2011). MKP-1(–/–) mice display more severe inflammatory response and increased mortality to LPS in vivo, and they have reduced IL-12 production and impaired antimicrobial clearance (Chi et al., 2006; Zhao et al., 2006; Huang et al., 2011; Korhonen et al., 2012).
Glucocorticoids and anti-rheumatic gold compounds increase the expression of MKP-1, and their anti-inflammatory effects have been shown to be mediated, at least in part, by MKP-1 (Abraham et al., 2006; Nieminen et al., 2010). The promoter of MKP-1 contains two cAMP-responsive elements (CRE) (Kwak et al., 1994), which bind a transcription factor cAMP-responsive element binding protein (CREB), and MKP-1 expression has been reported to be regulated by cAMP-PKA-CREB pathway (Zhang et al., 2008; Brion et al., 2011; Lee et al., 2012). Therefore, we hypothesized that MKP-1 expression may be regulated by PDE4 inhibitors. In the present study, we investigated the effects of PDE4 inhibitor rolipram on MKP-1 expression and on TNF production in activated macrophages and on the development of an acute carrageenan-induced oedema response in vivo. Interestingly, rolipram increased MKP-1 expression, inhibited TNF production in macrophages and suppressed carrageenan-induced inflammation in mice, and these effects were mediated by MKP-1.
Methods
Materials
Reagents were obtained as follows: LPS from Escherichia coli strain 0111:B4 (Sigma-Aldrich Inc., St. Louis, MO, USA), rolipram [4-(3-cyclopentyloxy-4-methoxy-phenyl)-pyrrolidin-2-one; Axon MedChem, Groningen, The Netherlands], PKA inhibitor 6–22 amide (PKAi; Millipore, Merck Chemicals Ltd., Nottingham, UK) and BIRB 796 [1-(5-tert-butyl-2-p-tolyl-2H-pyrazol-3-yl)-3(4-(2-morpholin-4-yl-ethoxy)naphthalen-1-yl)urea; Axon MedChem] were purchased as indicated. IBMX and 8-Br-cAMP (8-bromoadenosine 3′,5′-cyclic monophosphate) were obtained from Sigma-Aldrich Inc. Antibodies for phospho-CREB (#9196), CREB (#9197), phospho-p38 MAPK (#9211), MAPK-activated protein kinase 2 (MK2) (#3042) and phospho-MK2 (27B7, #3007) were purchased from Cell Signaling Technology Inc. (Beverly, MA, USA). p38 MAPK antibody (ab27986) and PDE4A antibody (ab14607) were purchased from Abcam plc. (Cambridge, UK) and MKP-1 antibody (SAB2500331) was obtained from Sigma-Aldrich Inc. Actin antibody (sc-1615), PDE4B antibody (sc-25812), PDE4D antibody (sc-25814), polyclonal anti-rabbit antibody (sc-2004) and polyclonal anti-goat antibody (sc-2020) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Lipofectamine 2000 (Life Technologies Europe BV, Espoo, Finland) and Luciferase Assay System (Promega, Madison, WI, USA) were obtained as indicated. CRE reporter [pCRE(luc)neo] was a kind gift from Professor Hartmut Kleinert (Johannes Gutenberg University, Mainz, Germany). All other reagents were from Sigma-Aldrich Inc., unless otherwise stated.
Cell culture
J774 murine macrophages (ATCC, Rockville Pike, MD, USA) were cultured at 37°C in 5% CO2 atmosphere in DMEM supplemented with glutamax-1 containing 10% heat-inactivated FBS, 100 U·mL−1 penicillin, 100 μg·mL−1 streptomycin and 250 ng·mL−1 amphotericin B (all from Invitrogen, Paisley, UK). For experiments, cells were seeded on 24-well plates at a density of 2 × 105 cells per well. Cell monolayers were grown for 72 h before the experiments were started. Rolipram, IBMX and BIRB 796 were dissolved in dimethyl sulfoxide (DMSO), and 8-Br-cAMP in HBSS. LPS (10 ng·mL−1) or the compounds of interest at concentrations indicated or the solvent (DMSO, 0.1% v/v) were added to the cells in fresh culture medium containing 10% FBS and the supplements. Cells were further incubated for the time indicated.
The effect of LPS and the tested chemicals on cell viability was evaluated by Cell Proliferation Kit II (XTT) (Roche Diagnostics, Mannheim, Germany) (Korhonen et al., 2001; Hämäläinen et al., 2009). Neither LPS nor the other chemicals used in the experiments were observed to evoke cytotoxicity.
Animals
Inbred C57BL/6 MKP-1(–/–) mice were originally generated by the R. Bravo laboratory at Bristol-Myers Squibb Pharmaceutical Research Institute (Dorfman et al., 1996) and bred at the University of Tampere School of Medicine animal facilities. MKP-1(–/–) mice and their wild-type (WT) littermates were housed under conditions of optimum light, temperature and humidity (12:12 h light–dark cycle, 22 ± 1°C, 50–60%) with food and water provided ad libitum.
Isolation of peritoneal macrophages and cell culture
For the collection of peritoneal macrophages (PMs), mice were killed according to the regulations of the University of Tampere School of Medicine Animal Unit. Mice were killed by suffocation with CO2, followed by an immediate cervical dislocation. Primary mouse PMs were obtained by i.p. lavage with sterile PBS supplemented with 0.2 mM EDTA. Cells were washed, resuspended in RPMI 1640 medium supplemented with 2% heat-inactivated FBS, 100 U·mL−1 penicillin, 100 μg·mL−1 streptomycin, and seeded on 24-well plates (5 × 105 cells per well). The cells were incubated overnight and washed with PBS to remove non-adherent cells before the experiments. Pharmacological compounds or the solvent (DMSO, 0.1% v/v) were added to the cells in fresh culture medium containing 2% heat-inactivated FBS and the antibiotics (see previous discussion), and cells were stimulated with LPS (100 ng·mL−1). Cells were further incubated for the time indicated. The results are reported in accordance with the ARRIVE guidelines (McGrath et al., 2010).
Carrageenan-induced paw oedema
The study was approved by the National Animal Experiment Board. C57BL/6 mice (20–25 g) were divided into groups of six mice and treated with 200 μL of PBS or rolipram (100 mg·kg−1 in PBS) by an i.p. injection 2 h before applying carrageenan. Before the administration of carrageenan, the mice were anaesthetized by i.p. injection of 0.5 mg·kg−1 of medetomidine (Domitor® 1 mg·mL−1; Orion Oyj, Espoo, Finland) and 75 mg·kg−1 of ketamine (Ketalar® 10 mg·mL−1; Pfizer Oy Animal Health, Helsinki, Finland). The mice received a 30 μL i.d. injection of carrageenan (1.5%, dissolved in normal saline) in one hind paw. The contralateral paw received 30 μL of saline and it was used as a control. Paw volume was measured before and 3 h after the carrageenan injection with a plethysmometer (Ugo Basile, Comerio, Italy). Oedema is expressed as a change in paw volume over time. After the experiments, the anaesthetized animals were killed by cervical dislocation. The results are reported in accordance with the ARRIVE guidelines (McGrath et al., 2010).
Preparation of cell lysates and Western blot analysis
At the indicated time points, the culture medium was removed from the cells. Cells were rapidly washed with ice-cold PBS and solubilized in cold lysis buffer containing 10 mM Tris–HCl, 5 mM EDTA, 50 mM NaCl, 1% Triton X-100, 0.5 mM phenylmethylsulfonyl fluoride, 1 mM sodium orthovanadate, 20 μg·mL−1 leupeptin, 50 μg·mL−1 aprotinin, 5 mM sodium fluoride, 2 mM sodium pyrophosphate and 10 μM n-octyl-β-D-glucopyranoside. After incubation for 15 min on ice, lysates were centrifuged, and the supernatants were collected and mixed in a ratio of 1:4, with SDS loading buffer (62.5 mM Tris–HCl, pH 6.8, 10% glycerol, 2% SDS, 0.025% bromophenol blue and 5% β-mercaptoethanol), and stored at −20°C until analysed.
Prior to Western blot analysis, the samples were boiled for 10 min. Equal aliquots of protein (20 μg) were loaded on a 10% SDS-polyacrylamide gel and separated by electrophoresis. Proteins were transferred to Hybond-enhanced chemiluminescence nitrocellulose membrane (Amersham, Buckinghamshire, UK) by semi-dry electroblotting. After transfer, the membrane was blocked in TBS/T [20 mM Tris-base (pH 7.6), 150 mM NaCl, 0.1% Tween-20] containing 5% non-fat milk for 1 h at room temperature. For detection of phosphorylated proteins, membranes were blocked in TBS/T containing 5% BSA. Membranes were incubated overnight at 4°C with primary antibody and for 1 h with secondary antibody, and the chemiluminescent signal was detected by ImageQuant™ LAS 4000 mini (GE Healthcare Bio-Sciences AB, Uppsala, Sweden). The chemiluminescent signal was quantified with ImageQuant TL 7.0 Image Analysis Software (GE Healthcare Bio-Sciences AB).
RNA extraction and real-time RT-PCR
At the indicated time points, the culture medium was removed, and cell homogenization and RNA extraction were carried out using GenElute™ Mammalian Total RNA Miniprep Kit (Sigma-Aldrich Inc.) according to the manufacturer's instruction. Reverse transcription of RNA to cDNA was performed by TaqMan® reverse transcription reagents (Applied Biosystems, Foster City, CA, USA), according to the supplier's instructions. The primer and probe sequences and concentrations were optimized according to the manufacturer's guidelines in TaqMan Universal PCR Master Mix Protocol part number 4304449 revision C (Applied Biosystems) and were as follows: 5′-CAAGGATGCTGGAGGGAGAGT-3′ (forward, 300 nM), 5′-TGAGGTAAGCAAGGCAGATGGT-3′ (reverse, 300 nM), 5′-TTTGTTCATTGCCAGGCCGGCAT-3′ (probe containing 6-FAM as 5′-reporter dye and TAMRA as 3′-quencher, 150 nM) for mouse MKP-1, 5′-AATGGCCTCCCTCTCATCAGTT-3′ (forward, 300 nM), 5′-TCCTCCACTTGGTGGTTTGC-3′ (reverse, 300 nM), 5′-CTCAAAATTCGAGTGACAAGCCTGTAGCCC-3′ (probe containing 6-FAM as 5′-reporter dye and TAMRA as 3′-quencher, 150 nM) for mouse TNF, 5′-GCATGGCCGGCCGTGTTC-3′ (forward, 300 nM), 5′-GATGTCATCATACTTGGCAGGTTT-3′ (reverse, 300 nM) and 5′-TCGTGGATCTGACGTGCCGCC-3′ (probe containing 6-FAM as 5′-reporter dye and TAMRA as 3′-quencher, 150 nM) for mouse GAPDH. Primers and probes were obtained from Metabion (Martinsried, Germany). TaqMan Gene Expression assays for mouse PDE4A (Mm01147149_m1), mouse PDE4B (Mm00456879_m1), mouse PDE4C (Mm01333237_m1) and mouse PDE4D (Mm01253862_m1) were purchased from Life Technologies (Life Technologies Europe BV). The PCR parameters were as follows: incubation at 50°C for 2 min, incubation at 95°C for 10 min, and thereafter, 40 cycles of denaturation at 95°C for 15 s and annealing and extension at 60°C for 1 min. Each sample was determined in duplicate. A standard curve method was used to estimate the relative mRNA levels. When calculating the results, MKP-1 and TNF mRNA levels were first normalized against GAPDH.
ELISA
Culture medium samples were collected and stored at −20°C until assayed. The concentrations of mouse TNF (DuoSet® ELISA, R&D Systems Europe Ltd., Abingdon, UK) were determined by elisa according to the manufacturer's instructions. The concentrations of acetylated cAMP in J774 cell lysates were determined using cAMP elisa Kit (Cell Biolabs Inc., San Diego, CA, USA) according to the manufacturer's instructions.
Statistics
Results are expressed as mean ± SEM. When appropriate, one-way anova with Dunnett's or Bonferroni's post-test, two-way anova with Bonferroni's post-test or two-way repeated measures anova with Bonferroni's post-test was performed using GraphPad Prism 5 for Windows version 5.04 (GraphPad Software Inc., La Jolla, CA, USA). Differences were considered significant at *P < 0.05, **P < 0.01 or ***P < 0.001.
Results
PDE4 inhibitor rolipram enhanced MKP-1 expression in activated macrophages
PDE4 isoforms are widely expressed in different cell types, including inflammatory cells (Bender and Beavo, 2006). First, we investigated the expression of PDE4 in J774 macrophages and PM. PDE4 isoforms A, B and D have been reported to be present in monocytes and PDE4B is expressed in macrophages (Shepherd et al., 2004). Both mRNA and protein of PDE4A, PDE4B and PDE4D were found to be present in J774 cells. LPS stimulation for 1 h did not change PDE4A, PDE4B or PDE4D levels in J774 cells (Supporting Information Figure S1). PDE4A mRNA was the most abundant isoform detected by qRT-PCR in J774 cells. Only PDE4B mRNA was detected in mouse primary PM from WT and MKP-1(–/–) mice. Three hour stimulation with LPS increased PDE4B mRNA in PM (Supporting Information Figure S1). mRNA levels of PDE4A and PDE4D were very low, and PDE4C was not detected in PMs from WT or MKP-1(–/–) mice (Supporting Information Figure S1).
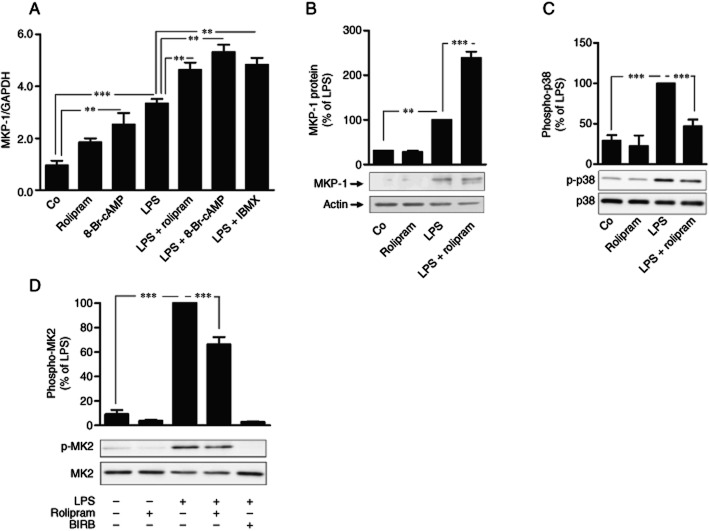
Rolipram is a selective PDE4 inhibitor that inhibits all PDE4 isoforms A, B, C and D (Wang et al., 1997). Rolipram increased cAMP levels in J774 macrophages, as expected (Supporting Information Table S1). MKP-1 promoter contains two copies of cAMP responsive (Kwak et al., 1994), and CREB has been reported to regulate the transcription of MKP-1 gene (Zhang et al., 2008). Therefore, we investigated the effect of a PDE4 inhibitor rolipram on MKP-1 expression in J774 mouse macrophages. MKP-1 mRNA and protein expression was increased by LPS and it was further enhanced in the presence of rolipram in J774 cells (Figure 1, B). MKP-1 mRNA expression was increased also by a cAMP analogue 8-Br-cAMP and a non-selective PDE inhibitor IBMX in J774 cells (Figure 1A).
Figure 1.
Effects of rolipram, IBMX and 8-Br-cAMP on MKP-1 expression and p38 MAPK phosphorylation in activated J774 macrophages. (A) J774 cells were stimulated with LPS (10 ng·mL−1) in the presence or absence of rolipram (2 μM), IBMX (100 μM) or 8-Br-cAMP (100 μM) for 1 h. MKP-1 mRNA was detected by quantitative RT-PCR, and MKP-1 mRNA expression levels were normalized against GAPDH mRNA levels. The results are expressed as mean ± SEM, number of repeats = 6. J774 cells were stimulated with LPS (10 ng·mL−1) in the presence or absence of rolipram (2 μM) or BIRB 796 (100 nM) and (B) MKP-1 protein (at 1 h), (C) phosphorylated p38 MAPK (at 30 min) and (D) phosphorylated MK2 (at 30 min) were detected by Western blot. The chemiluminescent signal was quantified and the amounts of MKP-1 and phosphorylated p38 MAPK or MK2 were normalized against actin and total p38 MAPK or MK2 respectively. Phosphorylated p38 MAPK and MK2 levels are expressed in arbitrary units, LPS-simulated cells were set as 100%, and the other values were related to that value. The results are expressed as mean ± SEM; number of repeats = 4 for MKP-1 and MK2 and number of repeats = 6 for p38 MAPK. One-way anova with Bonferroni's post-test was performed and statistical significance is indicated as **P < 0.01 and ***P < 0.001.
Because MKP-1 is an endogenous inhibitor of p38 MAPK in macrophages (Chi et al., 2006; Zhao et al., 2006; Korhonen et al., 2011), we investigated the effect of rolipram on p38 MAPK pathway. Phosphorylation of p38 MAPK and its direct substrate MK2 was increased in response to LPS and they were reduced by rolipram (Figure 1, D). p38 MAPK inhibitor BIRB 796, used as a control compound, inhibited MK2 phosphorylation, as expected (Figure 1D). LPS-stimulated MKP-1 expression was increased by rolipram in PM also (Figure 2).
Figure 2.
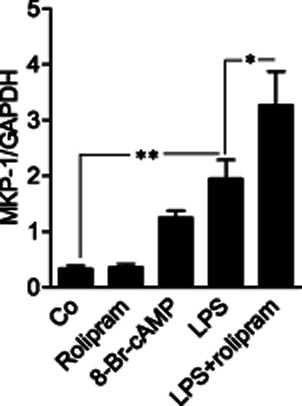
Effect of rolipram on MKP-1 mRNA levels in primary mouse peritoneal macrophages. Peritoneal macrophages were treated with LPS (100 ng·mL−1) in the presence or in the absence of rolipram (2 μM) or with 8-Br-cAMP (100 μM) alone for 1 h. MKP-1 mRNA levels were measured by quantitative RT-PCR and normalized against GAPDH mRNA levels. The results are expressed as mean ± SEM, number of repeats = 4. One-way anova with Bonferroni's post-test was performed and statistical significance is indicated as *P < 0.05 and **P < 0.01.
Increased MKP-1 expression by rolipram was inhibited by a PKA inhibitor
MKP-1 expression has been shown to be increased in response to cAMP (Brion et al., 2011), and PDE4B has recently been reported to inhibit MKP-1 expression in mucosa by a mechanism dependent on cAMP and PKA (Lee et al., 2012). Therefore, we continued by investigating the effect of PKA inhibitor on MKP-1 expression in J774 macrophages. PKA inhibitor 6–22 amide (PKAi), alone or in combination with LPS, did not affect MKP-1 levels in J774 cells. Rolipram increased LPS-induced MKP-1 mRNA expression, but that effect was not seen in the presence of PKAi (Figure 3A). Rolipram also increased CREB phosphorylation in LPS-treated cells, and this effect was also reverted by PKAi in J774 cells (Figure 3B). Furthermore, rolipram increased luciferase activity in A549 cells transfected with a CRE reporter plasmid, and the increased luciferase activity was reversed by the PKAi (Supporting Information Figure S2). These results suggest that rolipram increased MKP-1 expression by a mechanism dependent on cAMP-PKA-CREB pathway. In addition, reduction in LPS-induced p38 MAPK phosphorylation by rolipram was also reversed in the presence of PKAi (Figure 3C), supporting the hypothesis that PDE4 inhibition leads to the increased MKP-1 expression and this has functional significance to the activity of p38 MAPK.
Figure 3.
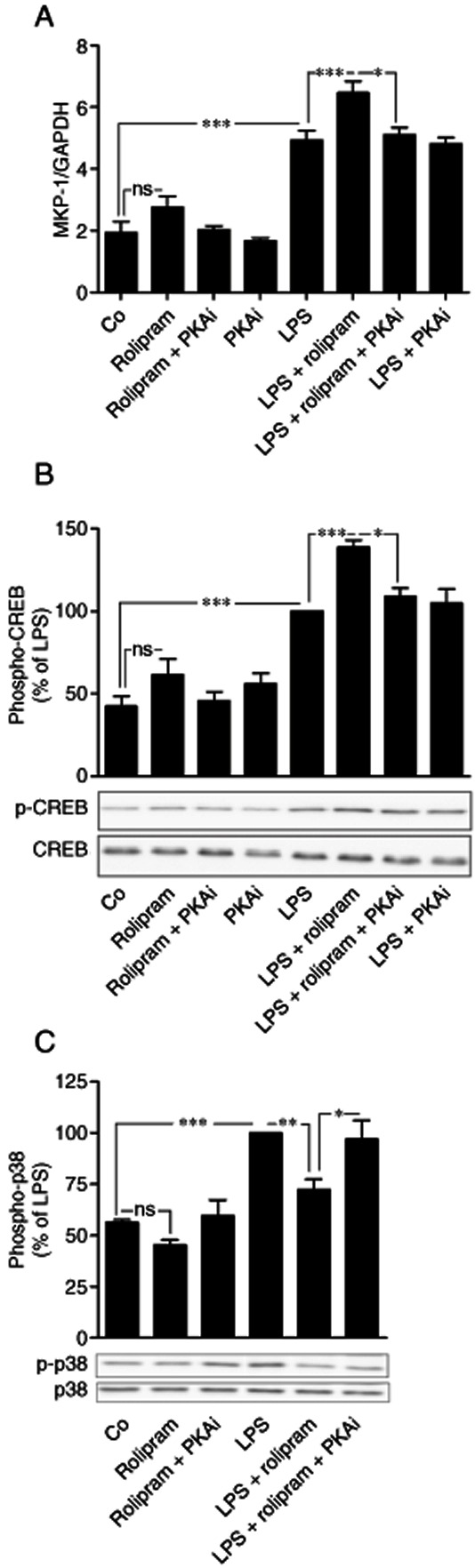
Effects of rolipram and PKA inhibitor on the expression of MKP-1 mRNA and phosphorylation of CREB and p38 MAPK in activated J774 macrophages. (A) J774 cells were pre-incubated with rolipram (2 μM) or PKA inhibitor 6–22 amide (PKAi, 5 μM) for 1 h and stimulated then with LPS (10 ng·mL−1) for 1 h. MKP-1 mRNA was detected by quantitative RT-PCR and normalized against GAPDH mRNA levels. The results are expressed as mean ± SEM, number of repeats = 8. (B, C) J774 cells were pre-incubated with rolipram (2 μM) or PKA inhibitor 6–22 amide (PKAi, 5 μM) for 1 h and stimulated with LPS (10 ng·mL−1) for 30 min and phophorylated CREB and phosphorylated p38 MAPK were detected by Western blot. The chemiluminescent signal was quantified, and the phosphorylated CREB and p38 MAPK were normalized against total CREB and total p38 MAPK respectively. Phosphorylated CREB and p38 MAPK levels are expressed in arbitrary units, LPS-simulated cells were set as 100%, and the other values were related to that value. The results are expressed as mean ± SEM, number of repeats = 8. One-way anova with Bonferroni's post-test was performed and statistical significance is indicated as *P < 0.05, **P < 0.01 and ***P < 0.001.
PDE4 inhibitor rolipram inhibited TNF production in activated mouse macrophages
TNF is a cytokine whose expression is known to be regulated by MKP-1 and p38 MAPK. Therefore, we continued by investigating the effect of rolipram on TNF production in macrophages. Rolipram inhibited LPS-induced TNF production in a dose-dependent manner (IC50 25.9 nM), and maximal/submaximal inhibition was observed with 2 μM drug concentration in J774 cells (Figure 4A). Rolipram also reduced LPS-induced TNF mRNA levels in J774 macrophages (Figure 4C). TNF production was also inhibited by a non-selective PDE inhibitor IBMX and by a cAMP analogue 8-Br-cAMP (Figure 4B).
Figure 4.
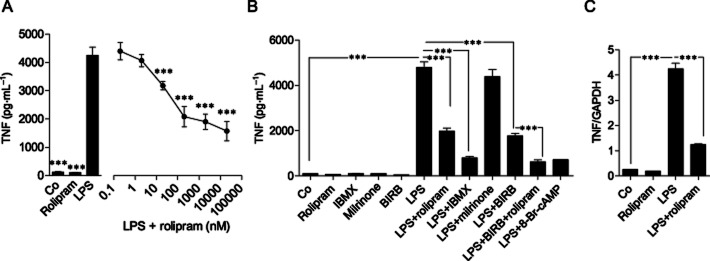
Effects of rolipram, IBMX and 8-Br-cAMP on TNF expression in J774 mouse macrophages. (A) J774 cells were incubated with rolipram alone (2 μM) or in the presence of increasing concentrations of rolipram for 1 h and stimulated then with LPS (10 ng·mL−1) for 24 h, and TNF accumulated into the culture medium was measured by elisa. (B, C) J774 cells were pre-incubated by rolipram (2 μM), IBMX (100 μM), milrinone (10 μM), BIRB 796 (100 nM) and stimulated with LPS (10 ng·mL−1) or with the combination of LPS and 8-Br-cAMP (100 μM) for 24 h (TNF protein) or 3 h (TNF mRNA). TNF accumulated into the culture medium was measured by elisa. TNF mRNA levels were measured by quantitative RT-PCR and normalized against GAPDH mRNA levels. The results are expressed as mean ± SEM, number of repeats = 6 for TNF protein and number of repeats = 3 for TNF mRNA. One-way anova with Dunnett's post-test (A) or Bonferroni's post-test (B, C) was performed and statistical significance is indicated as ***P < 0.001.
In addition to PDE4, monocytes and macrophages have been reported to express PDE3B, which degrades both cAMP and cGMP in a manner that cGMP inhibits the degradation of cAMP (Bender and Beavo, 2006). A PDE3 inhibitor milrinone at 10 μM concentration (Sudo et al., 2000) did not inhibit TNF production in J774 cells (Figure 4B), suggesting that the PDE4 activity is selectively involved in the regulation of TNF production in J774 macrophages. A p38 inhibitor BIRB 796 also inhibited TNF production as expected and it further enhanced the effect of rolipram (Figure 4B). TNF production was inhibited by the p38 MAPK inhibitor BIRB 796 in PMs also (Figure 5).
Figure 5.
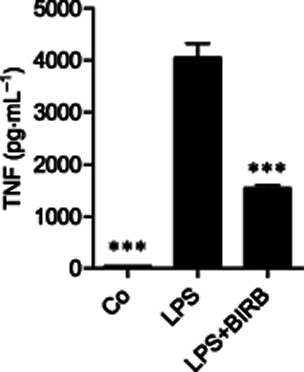
Effect of p38 MAPK inhibitor BIRB 796 on TNF production in primary mouse peritoneal macrophages. Peritoneal macrophages were pre-incubated with a p38 MAPK inhibitor BIRB 796 (100 nM) for 30 min and stimulated then with LPS (100 ng·mL−1) for 24 h. TNF accumulated into the culture medium was measured by elisa. The results are expressed as mean ± SEM, number of repeats = 4. One-way anova with Bonferroni's post-test was performed and statistical significance is indicated as ***P < 0.001.
The inhibition of TNF expression and carrageenan-induced paw inflammation by a PDE4 inhibitor rolipram was mediated by MKP-1
Because rolipram increased MKP-1 expression and inhibited TNF production, we hypothesized that MKP-1 could mediate the anti-inflammatory effects of rolipram. To test the hypothesis, we first investigated the effect of rolipram on LPS-induced TNF production in PM from WT and MKP-1(–/–) mice. TNF mRNA and protein expression was induced by LPS in PM from WT mice, and that was clearly (by 74 and 63% for TNF mRNA and TNF protein, respectively) inhibited by rolipram. LPS-induced TNF production was enhanced in PM from MKP-1(–/–) mice as compared to that in PM from WT mice, which is in line with the published results (Chi et al., 2006; Zhao et al., 2006; Korhonen et al., 2011). Interestingly, the inhibition of TNF mRNA and protein expression by rolipram was markedly attenuated in PM from MKP-1(–/–) mice and did not reach statistical significance (Figure 6).
Figure 6.
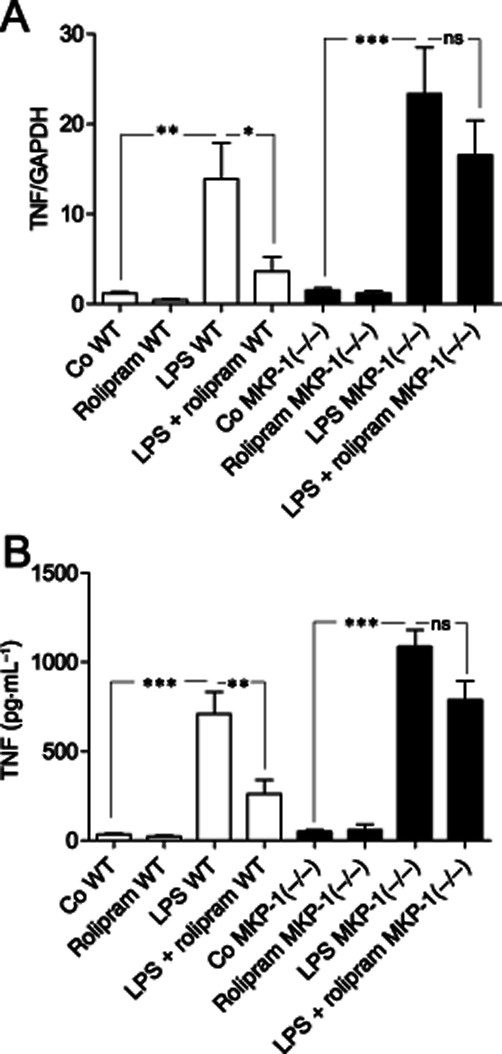
Effect of MKP-1 on the inhibition of TNF production by rolipram in primary mouse peritoneal macrophages. Peritoneal macrophages from wild-type (WT, open bars) and MKP-1(–/–) (black bars) mice were incubated with LPS (100 ng·mL−1) for (A) 3 h and (B) 24 h for TNF mRNA and protein determinations respectively. TNF mRNA levels were measured by quantitative RT-PCR and normalized against GAPDH mRNA levels. TNF protein accumulated into the culture medium was measured by elisa. The results are expressed as mean ± SEM, number of repeats = 6 for TNF mRNA and number of repeats = 8 for TNF protein. Two-way anova with Bonferroni's post-test was performed and statistical significance is indicated as ns, non-significant; *P < 0.05, **P < 0.01 and ***P < 0.001.
To investigate whether MKP-1 could mediate the anti-inflammatory effects of rolipram also in vivo, we tested the effect of rolipram on the severity of carrageenan-induced paw inflammation in WT and MKP-1(–/–) mice. Carrageenan-induced paw oedema was markedly attenuated by rolipram in WT mice, while rolipram did not have any effect on paw oedema in MKP-1(–/–) mice (Figure 7).
Figure 7.
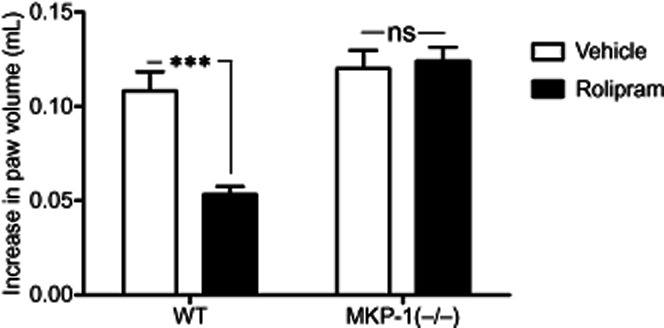
Effect of rolipram and MKP-1 on carrageenan-induced acute paw inflammation. Rolipram (100 mg·kg−1, i.p.) was administered 2 h prior to the experiment. In the beginning of the experiment (0 h), hind paw volumes were measured with a plethysmometer. Carrageenan or vehicle (control) was i.d. injected, and paw volumes were measured after 3 h with a plethysmometer. Oedema is expressed as the difference between the volume changes of the carrageenan treated paw and the control paw, mean ± SEM, number of repeats = 6. Two-way repeated measures anova with Bonferroni's post-test was performed and statistical significance is indicated as ns (non-significant) and ***P < 0.001.
Discussion and conclusions
In the present study, we investigated the effects of a PDE4 inhibitor rolipram on TNF expression in macrophages and on carrageenan-induced acute paw inflammation in vivo, and the role of MKP-1 in mediating those effects of rolipram. The results show that rolipram increased the expression of MKP-1, inhibited the production of TNF in activated macrophages and reduced the severity of carrageenan-induced paw inflammation. Furthermore, using MKP-1(–/–) mice and macrophages from these animals, we showed that those effects of rolipram were mediated by MKP-1.
Studies with different PDE4 isoform-deficient mice and with selective PDE4 inhibitors have shown that PDE4 is a potential target for anti-inflammatory drug treatments. PDE4A, PDE4B and PDE4D are present in human and mouse monocytes and macrophages, and the expression of PDE4B is increased after in vivo LPS challenge (Verghese et al., 1995; Jin and Conti, 2002). Earlier studies have shown that in U937 human monocytes, certain splice variants of PDE4A and PDE4D predominates and when these cells are differentiated to macrophages, PDE4B2 activity becomes predominating (Shepherd et al., 2004). In this study, PDE4A, PDE4B and PDE4D were found to be present in J774 cells, while in PM, which differentiate from monocytes to macrophages in vivo, only PDE4B expression was observed.
Of the PDE4 isoforms present in inflammatory cells, PDE4B, and PDE4D to some extent, seems to be an important regulator of inflammation and immune response. PDE4B-deficient mice display suppressed innate immune response and increased survival after in vivo LPS administration, and macrophages from PDE4B(–/–) mice, but not from PDE4A(–/–) or PDE4D(–/–) mice, produce less TNF in response to LPS as compared to WT macrophages (Jin and Conti, 2002; Jin et al., 2005). Furthermore, PDE4B(–/–) mice have attenuated ovalbumin-specific T-cell responses, and this was associated with reduced lung inflammation evoked by inhaled ovalbumin and ameliorated airway hyper-responsiveness induced by methacholine (Jin et al., 2010). Adhesion of neutrophils to endothelium and their chemotaxis is impaired in PDE4B(–/–) and PDE4D(–/–) mice, showing that both PDE4B and PDE4D are intrinsic and non-redundant regulators of neutrophil functions (Ariga et al., 2004). PDE4 selective inhibitor rolipram has been shown to inhibit airway hyper-responsiveness, leukocytes accumulation and cytokine release in the lung in ovalbumin-asthma model in mice (Ikemura et al., 2000; Kanehiro et al., 2001). In humans, selective PDE4 inhibitor roflumilast has been approved for the treatment of chronic obstructive pulmonary disease, and it improves lung functions and reduces the incidence of disease exacerbations (Chong et al., 2011). Furthermore, roflumilast has been reported to attenuate lung inflammation in patients with mild asthma after allergen challenge (Gauvreau et al., 2011). Another selective PDE4 inhibitor apremilast has been recently proven effective in the treatment of plaque psoriasis and psoriatic arthritis (Papp et al., 2012; Schett et al., 2012).
MKP-1 is a nuclear tyrosine/threonine phosphatase that primarily regulates the phosphorylation of p38 MAPK and JNK, and MKP-1(–/–) mice display excessive inflammatory response and increased mortality to LPS challenge (Chi et al., 2006; Zhao et al., 2006; Korhonen et al., 2011) as well as exacerbated chronic inflammation in arthritis models (Salojin et al., 2006; Vattakuzhi et al., 2012). MKP-1 promoter contains two CRE (Kwak et al., 1994), and cAMP-PKA-CREB pathways have been reported to regulate MKP-1 expression (Zhang et al., 2008; Brion et al., 2011; Lee et al., 2012). In this study, we found that rolipram increased cAMP levels and enhanced CREB phosphorylation and MKP-1 expression in J774 cells and CREB-driven transcription in A549 cells, and those effects were decreased by a PKA inhibitor. In addition, cAMP analogue 8-Br-cAMP increased MKP-1 levels in J774 cells. Moreover, rolipram inhibited p38 MAPK phosphorylation and that effect was removed by the PKA inhibitor. These results suggest that the increased MKP-1 expression by rolipram was due to activation of cAMP-PKA-CREB pathway. Our results also show that the increased MKP-1 expression by rolipram via cAMP-PKA-CREB pathway had functional effects on p38 MAPK pathway, as evidenced by reduced phosphorylation of MK2, which is a direct down-stream target of p38 MAPK. This was further supported by the findings that rolipram did not inhibit TNF expression in PMs from MKP-1(–/–) mice, and that carrageenan-induced paw inflammation was inhibited by rolipram in WT mice, but not in MKP-1(–/–) mice. These findings strongly suggest that the anti-inflammatory effects of rolipram on TNF expression and on carrageenan-induced inflammation in vivo are mediated by MKP-1, which is a novel finding and a new anti-inflammatory mechanism of action of PDE4 inhibitors.
PDE3B is expressed in macrophages, and it degrades both cAMP and cGMP. PDE3 has been suggested to participate in insulin, insulin-like growth factor 1 and leptin signalling (Barber et al., 2004; Bender and Beavo, 2006). In our experiments, selective PDE3 inhibitor milrinone (Sudo et al., 2000) did not inhibit TNF production, which is in line with a previous study reporting that pharmacological inhibition of PDE3 does not affect TNF production in macrophages (Gantner et al., 1997).
MKP-1 has also been linked to the pathogenesis of chronic inflammatory diseases and anti-inflammatory drug effects. MKP-1(–/–) mice display increased disease penetrance and exacerbated inflammation and osteolysis in experimental arthritis models (Salojin et al., 2006; Vattakuzhi et al., 2012). This is interesting because PDE4 inhibitors may hold therapeutic potential in the treatment of chronic inflammation other than inflammatory lung diseases. PDE4 inhibitors ameliorate the development of arthritis in murine models, and roflumilast and another selective PDE4 inhibitor apremilast reduce inflammatory cytokine release from human rheumatoid synovial cells (Ross et al., 1997; Yamaki et al., 2007; McCann et al., 2010; Crilly et al., 2011). Recently, apremilast has been reported to be effective in the treatment of plaque psoriasis and psoriatic arthritis (Papp et al., 2012; Schett et al., 2012). Apremilast also reduced the epidermal thickening, keratinocyte proliferation and the development of psoriform-like histopathological features in human skin xenograft/severe combined immunodeficiency mouse model of psoriasis (Schafer et al., 2010). It is possible that enhanced MKP-1 expression, at least in part, mediates the anti-inflammatory effects of PDE4 inhibitors in chronic inflammation.
There is evidence showing that MKP-1 is associated with effects of other anti-inflammatory drugs. MKP-1 expression is increased by glucocorticoids (Kassel et al., 2001; Shipp et al., 2010), and the macrophages from MKP-1(–/–) mice are less sensitive to the inhibition of inflammatory gene expression by glucocorticoids (Abraham et al., 2006). Furthermore, the attenuation of the acetylcholine-induced bronchial contraction by glucocorticoids was reduced in MKP-1(–/–) mice (Li et al., 2011). We have recently reported that anti-rheumatic gold compounds increase the expression of MKP-1 in chondrocytes and cartilage from rheumatoid arthritis patients; and using siRNA and MKP-1(–/–) mice, we showed that the inhibition of inflammatory gene expression by gold compounds was mediated by MKP-1 (Nieminen et al., 2010). In addition, MKP-1 gene transfer suppressed osteoclastogenesis in macrophages, reduced the accumulation of inflammatory cells into the bone and attenuated the inflammatory bone loss evoked by LPS (Yu et al., 2010). Based on these findings, it is possible that some anti-inflammatory drug effects are mediated by MKP-1 also in patients. Importantly, MKP-1 expression in dendritic cells has been shown to be important for development of effective antimicrobial responses (Huang et al., 2011). Also, MKP-1 enhances the expression of interferon regulatory factor 1 and IL-12 (Korhonen et al., 2012), both important factors for the development of cell-mediated immune response and effective anti-microbial defence. Hence, the present study and the published reports support the idea that MKP-1 holds potential as a novel anti-inflammatory drug target. MKP-1 is an endogenous factor that suppresses inflammatory response and the development of pathological changes in tissues without compromising anti-microbial defence.
In conclusion, the present study shows that a PDE4 inhibitor rolipram enhanced MKP-1 expression through cAMP-PKA-CERB pathway and inhibited TNF production in activated macrophages and attenuated carrageenan-induced paw inflammation in mice. Importantly, rolipram failed to inhibit TNF production in macrophages from MKP-1(–/–) mice and to attenuate carrageenan-induced paw inflammation in MKP-1-deficient animals. We propose that the anti-inflammatory effects of PDE4 inhibitors are, at least partly, mediated by enhanced MKP-1 expression, which is a novel finding and a new mechanism of action for the anti-inflammatory effects of PDE4 inhibitors. The results also support the idea that compounds that enhance MKP-1 expression or its function hold potential as novel anti-inflammatory drugs.
Acknowledgments
We would like to thank Bristol–Myers Squibb Pharmaceutical Research Institute for providing MKP-1 knockout mice. cAMP responsive element (CRE) reporter [pCRE(luc)neo] was a kind gift from Professor Hartmut Kleinert (Johannes Gutenberg University, Mainz, Germany). Mrs Salla Hietakangas, Ms Meiju Kukkonen, Mr Jan Koski and Ms Mirva Järvelä-Stölting are warmly thanked for their excellent technical assistance and Mrs Heli Määttä for skilful secretarial help. This work was financially supported by grants from The Academy of Finland, The Competitive Research Funding of the Pirkanmaa Hospital District, Finland, Tampere Tuberculosis Foundation, Finland and Scandinavian Rheumatology Research Foundation. Funding sources were not involved in the study design, in the collection, analysis or interpretation of the data, or in the writing of the manuscript.
Glossary
- 8-Br-cAMP
8-bromoadenosine 3′,5′-cyclic monophosphate
- CRE
cAMP-responsive element
- CREB
cAMP-responsive element binding protein
- DMSO
dimethyl sulfoxide
- MK2
MAPK-activated protein kinase 2
- MKP-1
MAPK phosphatase-1
- PKAi
PKA inhibitor 6–22 amide
- PM
peritoneal macrophages
Conflicts of interest
None.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
Figure S1 The expression of PDE4A, PDE4B, PDE4C and PDE4D in J774 macrophages and mouse primary peritoneal macrophages (PM) from WT and MKP-1(–/–) mice.
Figure S2 cAMP-responsive element-driven transcription was increased by rolipram and inhibited PKA inhibitor 6-22 amide (PKAi) in A549 human bronchial epithelial cells.
Table S1 The effect of rolipram on cAMP levels in J774 macrophages.
References
- Abraham SM, Lawrence T, Kleiman A, Warden P, Medghalchi M, Tuckermann J, et al. Antiinflammatory effects of dexamethasone are partly dependent on induction of dual specificity phosphatase 1. J Exp Med. 2006;203:1883–1889. doi: 10.1084/jem.20060336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariga M, Neitzert B, Nakae S, Mottin G, Bertrand C, Pruniaux MP, et al. Nonredundant function of phosphodiesterases 4D and 4B in neutrophil recruitment to the site of inflammation. J Immunol. 2004;173:7531–7538. doi: 10.4049/jimmunol.173.12.7531. [DOI] [PubMed] [Google Scholar]
- Barber R, Baillie GS, Bergmann R, Shepherd MC, Sepper R, Houslay MD, et al. Differential expression of PDE4 cAMP phosphodiesterase isoforms in inflammatory cells of smokers with COPD, smokers without COPD, and nonsmokers. Am J Physiol Lung Cell Mol Physiol. 2004;287:L332–L343. doi: 10.1152/ajplung.00384.2003. [DOI] [PubMed] [Google Scholar]
- Bender AT, Beavo JA. Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol Rev. 2006;58:488–520. doi: 10.1124/pr.58.3.5. [DOI] [PubMed] [Google Scholar]
- Boutros T, Chevet E, Metrakos P. Mitogen-activated protein (MAP) kinase/MAP kinase phosphatase regulation: roles in cell growth, death, and cancer. Pharmacol Rev. 2008;60:261–310. doi: 10.1124/pr.107.00106. [DOI] [PubMed] [Google Scholar]
- Brion L, Maloberti PM, Gomez NV, Poderoso C, Gorostizaga AB, Mori Sequeiros Garcia MM, et al. MAPK phosphatase-1 (MKP-1) expression is up-regulated by hCG/cAMP and modulates steroidogenesis in MA-10 Leydig cells. Endocrinology. 2011;152:2665–2677. doi: 10.1210/en.2011-0021. [DOI] [PubMed] [Google Scholar]
- Chi H, Barry SP, Roth RJ, Wu JJ, Jones EA, Bennett AM, et al. Dynamic regulation of pro- and anti-inflammatory cytokines by MAPK phosphatase 1 (MKP-1) in innate immune responses. Proc Natl Acad Sci U S A. 2006;103:2274–2279. doi: 10.1073/pnas.0510965103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong J, Poole P, Leung B, Black PN. Phosphodiesterase 4 inhibitors for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2011;(5) doi: 10.1002/14651858.CD002309.pub3. CD002309. [DOI] [PubMed] [Google Scholar]
- Crilly A, Robertson SE, Reilly JH, Gracie JA, Lai WQ, Leung BP, et al. Phosphodiesterase 4 (PDE4) regulation of proinflammatory cytokine and chemokine release from rheumatoid synovial membrane. Ann Rheum Dis. 2011;70:1130–1137. doi: 10.1136/ard.2010.134825. [DOI] [PubMed] [Google Scholar]
- Dastidar SG, Ray A, Shirumalla R, Rajagopal D, Chaudhary S, Nanda K, et al. Pharmacology of a novel, orally active PDE4 inhibitor. Pharmacology. 2009;83:275–286. doi: 10.1159/000209608. [DOI] [PubMed] [Google Scholar]
- Dorfman K, Carrasco D, Gruda M, Ryan C, Lira SA, Bravo R. Disruption of the erp/mkp-1 gene does not affect mouse development: normal MAP kinase activity in ERP/MKP-1-deficient fibroblasts. Oncogene. 1996;13:925–931. [PubMed] [Google Scholar]
- Gantner F, Kupferschmidt R, Schudt C, Wendel A, Hatzelmann A. In vitro differentiation of human monocytes to macrophages: change of PDE profile and its relationship to suppression of tumour necrosis factor-alpha release by PDE inhibitors. Br J Pharmacol. 1997;121:221–231. doi: 10.1038/sj.bjp.0701124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauvreau GM, Boulet LP, Schmid-Wirlitsch C, Cote J, Duong M, Killian KJ, et al. Roflumilast attenuates allergen-induced inflammation in mild asthmatic subjects. Respir Res. 2011;12:140. doi: 10.1186/1465-9921-12-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hämäläinen M, Korhonen R, Moilanen E. Calcineurin inhibitors down-regulate iNOS expression by destabilizing mRNA. Int Immunopharmacol. 2009;9:159–167. doi: 10.1016/j.intimp.2008.07.012. [DOI] [PubMed] [Google Scholar]
- Houslay MD. Underpinning compartmentalised cAMP signalling through targeted cAMP breakdown. Trends Biochem Sci. 2010;35:91–100. doi: 10.1016/j.tibs.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Houslay MD, Adams DR. PDE4 cAMP phosphodiesterases: modular enzymes that orchestrate signalling cross-talk, desensitization and compartmentalization. Biochem J. 2003;370:1–18. doi: 10.1042/BJ20021698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houslay MD, Schafer P, Zhang KY. Keynote review: phosphodiesterase-4 as a therapeutic target. Drug Discov Today. 2005;10:1503–1519. doi: 10.1016/S1359-6446(05)03622-6. [DOI] [PubMed] [Google Scholar]
- Huang G, Wang Y, Shi L, Kanneganti T, Chi H. Signaling by the phosphatase MKP-1 in dendritic cells imprints distinct effector and regulatory T cell fates. Immunity. 2011;35:45–58. doi: 10.1016/j.immuni.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemura T, Schwarze J, Makela M, Kanehiro A, Joetham A, Ohmori K, et al. Type 4 phosphodiesterase inhibitors attenuate respiratory syncytial virus-induced airway hyper-responsiveness and lung eosinophilia. J Pharmacol Exp Ther. 2000;294:701–706. [PubMed] [Google Scholar]
- Jin SL, Conti M. Induction of the cyclic nucleotide phosphodiesterase PDE4B is essential for LPS-activated TNF-alpha responses. Proc Natl Acad Sci U S A. 2002;99:7628–7633. doi: 10.1073/pnas.122041599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin SL, Lan L, Zoudilova M, Conti M. Specific role of phosphodiesterase 4B in lipopolysaccharide-induced signaling in mouse macrophages. J Immunol. 2005;175:1523–1531. doi: 10.4049/jimmunol.175.3.1523. [DOI] [PubMed] [Google Scholar]
- Jin SL, Goya S, Nakae S, Wang D, Bruss M, Hou C, et al. Phosphodiesterase 4B is essential for T(H)2-cell function and development of airway hyperresponsiveness in allergic asthma. J Allergy Clin Immunol. 2010;126:1252–1259. doi: 10.1016/j.jaci.2010.08.014. .e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanehiro A, Ikemura T, Makela MJ, Lahn M, Joetham A, Dakhama A, et al. Inhibition of phosphodiesterase 4 attenuates airway hyperresponsiveness and airway inflammation in a model of secondary allergen challenge. Am J Respir Crit Care Med. 2001;163:173–184. doi: 10.1164/ajrccm.163.1.2001118. [DOI] [PubMed] [Google Scholar]
- Kassel O, Sancono A, Kratzschmar J, Kreft B, Stassen M, Cato AC. Glucocorticoids inhibit MAP kinase via increased expression and decreased degradation of MKP-1. EMBO J. 2001;20:7108–7116. doi: 10.1093/emboj/20.24.7108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly JJ, Barnes PJ, Giembycz MA. Phosphodiesterase 4 in macrophages: relationship between cAMP accumulation, suppression of cAMP hydrolysis and inhibition of [3H]R-(-)-rolipram binding by selective inhibitors. Biochem J. 1996;318:425–436. doi: 10.1042/bj3180425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keravis T, Lugnier C. Cyclic nucleotide phosphodiesterase (PDE) isozymes as targets of the intracellular signalling network: benefits of PDE inhibitors in various diseases and perspectives for future therapeutic developments. Br J Pharmacol. 2012;165:1288–1305. doi: 10.1111/j.1476-5381.2011.01729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Kubo S, Iwata M, Ohtsu Y, Takahashi K, Shimizu Y. ASP3258, an orally active potent phosphodiesterase 4 inhibitor with low emetic activity. Int Immunopharmacol. 2011;11:732–739. doi: 10.1016/j.intimp.2011.01.023. [DOI] [PubMed] [Google Scholar]
- Korhonen R, Kankaanranta H, Lahti A, Lähde M, Knowles RG, Moilanen E. Bi-directional effects of the elevation of intracellular calcium on the expression of inducible nitric oxide synthase in J774 macrophages exposed to low and to high concentrations of endotoxin. Biochem J. 2001;354:351–358. doi: 10.1042/0264-6021:3540351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen R, Turpeinen T, Taimi V, Nieminen R, Goulas A, Moilanen E. Attenuation of the acute inflammatory response by dual specificity phosphatase 1 by inhibition of p38 MAP kinase. Mol Immunol. 2011;48:2059–2068. doi: 10.1016/j.molimm.2011.06.439. [DOI] [PubMed] [Google Scholar]
- Korhonen R, Huotari N, Hömmö T, Leppänen T, Moilanen E. The expression of interleukin-12 is increased by MAP kinase phosphatase-1 through a mechanism related to interferon regulatory factor 1. Mol Immunol. 2012;51:219–226. doi: 10.1016/j.molimm.2012.03.019. [DOI] [PubMed] [Google Scholar]
- Kwak SP, Hakes DJ, Martell KJ, Dixon JE. Isolation and characterization of a human dual specificity protein-tyrosine phosphatase gene. J Biol Chem. 1994;269:3596–3604. [PubMed] [Google Scholar]
- Lee J, Komatsu K, Lee BC, Lim JH, Jono H, Xu H, et al. Phosphodiesterase 4B mediates extracellular signal-regulated kinase-dependent up-regulation of mucin MUC5AC protein by streptococcus pneumoniae by inhibiting cAMP-protein kinase A-dependent MKP-1 phosphatase pathway. J Biol Chem. 2012;287:22799–22811. doi: 10.1074/jbc.M111.337378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Zhang M, Hussain F, Triantaphyllopoulos K, Clark AR, Bhavsar PK, et al. Inhibition of p38 MAPK-dependent bronchial contraction after ozone by corticosteroids. Eur Respir J. 2011;37:933–942. doi: 10.1183/09031936.00021110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann FE, Palfreeman AC, Andrews M, Perocheau DP, Inglis JJ, Schafer P, et al. Apremilast, a novel PDE4 inhibitor, inhibits spontaneous production of tumour necrosis factor-alpha from human rheumatoid synovial cells and ameliorates experimental arthritis. Arthritis Res Ther. 2010;12:R107. doi: 10.1186/ar3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nials AT, Tralau-Stewart CJ, Gascoigne MH, Ball DI, Ranshaw LE, Knowles RG. In vivo characterization of GSK256066, a high-affinity inhaled phosphodiesterase 4 inhibitor. J Pharmacol Exp Ther. 2011;337:137–144. doi: 10.1124/jpet.110.173641. [DOI] [PubMed] [Google Scholar]
- Nieminen R, Korhonen R, Moilanen T, Clark AR, Moilanen E. Aurothiomalate inhibits cyclooxygenase 2, matrix metalloproteinase 3, and interleukin-6 expression in chondrocytes by increasing MAPK phosphatase 1 expression and decreasing p38 phosphorylation: MAPK phosphatase 1 as a novel target for antirheumatic drugs. Arthritis Rheum. 2010;62:1650–1659. doi: 10.1002/art.27409. [DOI] [PubMed] [Google Scholar]
- Page C, Spina D. Selective PDE inhibitors as novel treatments for respiratory diseases. Curr Opin Pharmacol. 2012;12:275–286. doi: 10.1016/j.coph.2012.02.016. [DOI] [PubMed] [Google Scholar]
- Papp K, Cather JC, Rosoph L, Sofen H, Langley RG, Matheson RT, et al. Efficacy of apremilast in the treatment of moderate to severe psoriasis: a randomised controlled trial. Lancet. 2012;380:738–746. doi: 10.1016/S0140-6736(12)60642-4. [DOI] [PubMed] [Google Scholar]
- Patterson KI, Brummer T, O'Brien PM, Daly RJ. Dual-specificity phosphatases: critical regulators with diverse cellular targets. Biochem J. 2009;418:475–489. doi: 10.1042/bj20082234. [DOI] [PubMed] [Google Scholar]
- Plotnikov A, Zehorai E, Procaccia S, Seger R. The MAPK cascades: signaling components, nuclear roles and mechanisms of nuclear translocation. Biochim Biophys Acta. 2011;1813:1619–1633. doi: 10.1016/j.bbamcr.2010.12.012. [DOI] [PubMed] [Google Scholar]
- Ross SE, Williams RO, Mason LJ, Mauri C, Marinova-Mutafchieva L, Malfait AM, et al. Suppression of TNF-alpha expression, inhibition of Th1 activity, and amelioration of collagen-induced arthritis by rolipram. J Immunol. 1997;159:6253–6259. [PubMed] [Google Scholar]
- Salojin KV, Owusu IB, Millerchip KA, Potter M, Platt KA, Oravecz T. Essential role of MAPK phosphatase-1 in the negative control of innate immune responses. J Immunol. 2006;176:1899–1907. doi: 10.4049/jimmunol.176.3.1899. [DOI] [PubMed] [Google Scholar]
- Schafer PH, Parton A, Gandhi AK, Capone L, Adams M, Wu L, et al. Apremilast, a cAMP phosphodiesterase-4 inhibitor, demonstrates anti-inflammatory activity in vitro and in a model of psoriasis. Br J Pharmacol. 2010;159:842–855. doi: 10.1111/j.1476-5381.2009.00559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schett G, Wollenhaupt J, Papp K, Joos R, Rodrigues JF, Vessey AR, et al. Oral apremilast in the treatment of active psoriatic arthritis: results of a multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum. 2012;64:3156–3167. doi: 10.1002/art.34627. [DOI] [PubMed] [Google Scholar]
- Shepherd MC, Baillie GS, Stirling DI, Houslay MD. Remodelling of the PDE4 cAMP phosphodiesterase isoform profile upon monocyte-macrophage differentiation of human U937 cells. Br J Pharmacol. 2004;142:339–351. doi: 10.1038/sj.bjp.0705770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipp LE, Lee JV, Yu C, Pufall M, Zhang P, Scott DK, et al. Transcriptional regulation of human dual specificity protein phosphatase 1 (DUSP1) gene by glucocorticoids. PLoS ONE. 2010;5:e13754. doi: 10.1371/journal.pone.0013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spina D. PDE4 inhibitors: current status. Br J Pharmacol. 2008;155:308–315. doi: 10.1038/bjp.2008.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudo T, Tachibana K, Toga K, Tochizawa S, Inoue Y, Kimura Y, et al. Potent effects of novel anti-platelet aggregatory cilostamide analogues on recombinant cyclic nucleotide phosphodiesterase isozyme activity. Biochem Pharmacol. 2000;59:347–356. doi: 10.1016/s0006-2952(99)00346-9. [DOI] [PubMed] [Google Scholar]
- Turpeinen T, Nieminen R, Moilanen E, Korhonen R. Mitogen-activated protein kinase phosphatase-1 negatively regulates the expression of interleukin-6, interleukin-8, and cyclooxygenase-2 in A549 human lung epithelial cells. J Pharmacol Exp Ther. 2010;333:310–318. doi: 10.1124/jpet.109.157438. [DOI] [PubMed] [Google Scholar]
- Vattakuzhi Y, Abraham SM, Freidin A, Clark AR, Horwood NJ. Dual-specificity phosphatase-1 null mice exhibit spontaneous osteolytic disease and enhanced inflammatory osteolysis in experimental arthritis. Arthritis Rheum. 2012;62:2201–2210. doi: 10.1002/art.34403. [DOI] [PubMed] [Google Scholar]
- Verghese MW, McConnell RT, Lenhard JM, Hamacher L, Jin SL. Regulation of distinct cyclic AMP-specific phosphodiesterase (phosphodiesterase type 4) isozymes in human monocytic cells. Mol Pharmacol. 1995;47:1164–1171. [PubMed] [Google Scholar]
- Wang P, Myers JG, Wu P, Cheewatrakoolpong B, Egan RW, Billah MM. Expression, purification, and characterization of human cAMP-specific phosphodiesterase (PDE4) subtypes A, B, C, and D. Biochem Biophys Res Commun. 1997;234:320–324. doi: 10.1006/bbrc.1997.6636. [DOI] [PubMed] [Google Scholar]
- Yamaki K, Li X, Hossain MA, Alam AH, Taneda S, Yanagisawa R, et al. Difference in preventive effects between the phosphodiesterase iv inhibitor rolipram and anti-arthritic drugs on antigen-induced arthritis in mice. Immunol Invest. 2007;36:131–145. doi: 10.1080/08820130600746008. [DOI] [PubMed] [Google Scholar]
- Yu H, Li Q, Herbert B, Zinna R, Martin K, Junior CR, et al. Anti-inflammatory effect of MAPK phosphatase-1 local gene transfer in inflammatory bone loss. Gene Ther. 2010;18:344–353. doi: 10.1038/gt.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Wang Q, Zhu N, Yu M, Shen B, Xiang J, et al. Cyclic AMP inhibits JNK activation by CREB-mediated induction of c-FLIP(L) and MKP-1, thereby antagonizing UV-induced apoptosis. Cell Death Differ. 2008;15:1654–1662. doi: 10.1038/cdd.2008.87. [DOI] [PubMed] [Google Scholar]
- Zhao Q, Wang X, Nelin LD, Yao Y, Matta R, Manson ME, et al. MAP kinase phosphatase 1 controls innate immune responses and suppresses endotoxic shock. J Exp Med. 2006;203:131–140. doi: 10.1084/jem.20051794. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.