Abstract
Background and Purpose
Previously, we demonstrated that glucosamine (GlcN) exerts a suppressive effect on LPS-induced inducible NOS (iNOS) through the inhibition of NF-κB activation in BV2 mouse microglial cells. The purpose of the present study was to examine the mechanisms by which GlcN inhibits NF-κB activation.
Experimental Approach
BV2 cells were stimulated with LPS with or without GlcN. NF-κB/c-Rel activities were studied by EMSA, nuclear translocation, reporter assay or chromatin immunoprecipitation. Wheat germ agglutinin precipitation or galactosyltransferase assay were used to measure O-linked N-acetylglucosamine (O-GlcNAc) modification (O-GlcNAcylation) of c-Rel. Protein-protein interactions were examined by co-immunoprecipitation.
Key Results
LPS stimulated the activation of c-Rel, increased the O-GlcNAcylation of c-Rel and enhanced the binding of c-Rel to the NF-κB site in the iNOS promoter; GlcN attenuated these effects of LPS. O-GlcNAcylation of both nuclear and cytosolic forms of c-Rel was increased by LPS and reduced by GlcN. LPS increased the interaction of c-Rel with O-GlcNAc transferase (OGT) and p50/p105, and GlcN suppressed these interactions. Knockdown of OGT reduced the c-Rel O-GlcNAcylation and c-Rel–p50 interaction in response to LPS, but did not affect either the binding of c-Rel to the iNOS promoter or the transcriptional activity of c-Rel.
Conclusions and Implications
In BV2 microglial cells, the anti-inflammatory effect of GlcN is mediated by prevention of the prolonged activation of transcription factors, c-Rel and NF-κB. Further clarification of the mechanism by which GlcN exerts this effect will facilitate the development of pharmacological strategies for preventing excessive NO formation when targeting inflammatory diseases of the periphery or CNS.
Keywords: O-GlcNAc, c-Rel, LPS, glucosamine, OGT
Introduction
NF-κB is the principal regulator of the transactivation of pro-inflammatory genes such as inducible NOS (iNOS) (Pahan et al., 1998; Dasgupta et al., 2004) (and see the review in Saha and Pahan, 2006). NF-κB/Rel proteins exist as homodimeric or heterodimeric complexes formed by various combinations of five distinct DNA-binding subunits: p65/RelA, RelB, c-Rel, p50 (a cleaved product of p105) and p52 (a cleaved product of p100). Under unstimulated conditions, these complexes are found in the cytosol, bound to IκB proteins (Karin and Ben-Neriah, 2000). The main mechanism of NF-κB activation is signal-induced proteolytic degradation of IκB. This, in turn, is followed by nuclear translocation of Rel proteins. In contrast to this simplistic model, recent research has indicated that NF-κB activation is much more sophisticated in nature. For example, post-translational modification of Rel proteins appears to be a component of an alternative regulatory mechanism. Phosphorylation of the regulatory protein p65/RelA at specific amino acids results in distinct functional changes (reviewed in Viatour et al., 2005). Additionally, O-linked N-acetylglucosamine (O-GlcNAc) modification (O-GlcNAcylation) of p65 has been suggested to play an important role in the regulation of NF-κB activity (Hwang et al., 2010).
A number of studies have examined the anti-inflammatory effects of GlcN. For example, GlcN is known to modulate the ability of IL-1β to activate NF-κB in chondrocytes (Gouze et al., 2002; Largo et al., 2003). GlcN also inhibits LPS-induced NO production in RAW264.7 macrophages and microglia (Meininger et al., 2000; Yi et al., 2005). Further, studies from our laboratory have demonstrated that GlcN exerts neuroprotective and anti-inflammatory effects under conditions of middle cerebral artery occlusion. Such effects are mediated by suppression of NF-κB activity in microglia (Hwang et al., 2010).
Infusion of GlcN into the cell elevates the level of uridine diphosphate (UDP) N-acetylglucosamine, which is an essential substrate for the transfer of O-GlcNAc to proteins induced by O-GlcNAc transferase (OGT) (Kreppel et al., 1997; Love and Hanover, 2005). This enzyme catalyses the addition of a single β-N-GlcNAc unit to serine or threonine residues of various nuclear and cytoplasmic proteins (Whisenhunt et al., 2006). This modulation of proteins by O-GlcNAc (O-GlcNAcylation) occurs ubiquitously and has been suggested to serve as a signalling mechanism analogous to dynamic protein phosphorylation (reviewed in Kamemura and Hart, 2003; Wells and Hart, 2003).
More recently, we found that c-Rel is modified by O-GlcNAc and that this effect is dynamically regulated by LPS with or without GlcN. We further investigated the possible functional implications of the O-GlcNAcylation-induced changes in c-Rel in the regulation of the transcriptional activity of c-Rel.
Methods
Reagents
Except where otherwise noted, all reagents were purchased from Sigma Chemical (St. Louis, MO, USA).
Cell cultures
A murine BV2 microglial cell line (obtained from Professor Tong H. Joh, Neurobiology Dept, Weill Cornell Medical College, New York, USA) which has been used as a suitable model for in vitro studies of activated microglia (Kim et al., 2004), and RAW264.7 murine macrophage cells (purchased from the Korean Cell Line Bank) were maintained at 37°C at 5% CO2 in DMEM supplemented with 10% FBS (Hyclone, Logan, Utah), streptomycin and penicillin.
Reverse transcription PCR
Total RNA from cells was extracted with TRIzol™ (Invitrogen, Carlsbad, CA, USA). PCR was performed using OGT specific primers of mouse F:TATGCCCGTTATTCCCATGA, R:ACTGCTGGAAAACGCAACAG. The final PCR products were electrophoresed in 1% agarose gel.
Immunoblotting and immunoprecipitation
Whole cell protein lysates was prepared in lysis buffer (10 mM Tris, 140 mM NaCl, 1% Triton, 0.5% SDS and protease inhibitors, pH 8.0). For immunoprecipitation, 500 μg of cell lysates were incubated with anti-OGT (Sigma Chemical) or -c-Rel (Santa Cruz Biotechnology, Santa Cruz, CA, USA) antibodies for 1 h. Antibody-protein complex was precipitated with protein G sepharose beads and analysed by Western blotting. Protein samples (20–40 μg for each) were separated by SDS-PAGE and transferred to Hybond™-Enhanced Chemiluminescence (ECL)™ nitrocellulose membrane (Amersham Biosciences, Piscataway, NJ, USA). The membrane was incubated with antibodies (Santa Cruz Biotechnology, otherwise noted) against c-Rel, p100/p52, p105/p50, RelB, OGT, IκBα, GAPDH, O-GlcNAc CTD110 (Covance, Berkeley, CA, USA), O-GlcNAc RL-2 (Han and Kudlow, 1997) or p65 antibodies. Next, HRP-conjugated (Amersham Biosciences, Piscataway, NJ, USA) (1:10 000 dilution in Tris-buffered saline with tween) or pre-adsorbed (abcam, Cambridge, MA, USA) secondary antibodies were applied and developed by the ECL detection system (Amersham Biosciences).
EMSA
Nuclear protein extracts were prepared as described previously (Kim et al., 2004). The double-stranded DNA oligonucleotide probe containing the consensus NF-κB binding site (Promega, Madison, WI, USA) was labelled by polynucleotide kinase (New England Biolabs, Beverly, MA, USA). Nuclear protein extracts (10 μg) were incubated with labelled oligonucleotides in binding buffer (50 mM KCl, 12.5 mM HEPES pH 7.6, 6.25 mM MgCl2, 0.05 mM EDTA, 0.5% Nonidet P-40, 0.5 mM DTT, 5% glycerol and 2 μg poly-[dI-dC]) for 30 min on ice. For supershift experiments, EMSA assay mixtures were pre-incubated with antibodies against p65, p50, c-Rel, RelB or p52 (Santa Cruz Biotechnology) for 15 min at room temperature.
Immunofluorescence microscopy
Cells were fixed in 4% paraformaldehyde and permeabilized with 0.1% Triton X-100 in PBS. Cells were then blocked with 5% BSA, incubated with anti-c-Rel antibody (Santa Cruz Biotechnology) overnight at 4°C, followed by 1 h incubation with FITC-conjugated secondary antibody (Molecular Probes, Eugene, OR, USA). Nuclei were counterstained with 2 μg·mL−1 of DAPI for 10 min. Slides were cover-slipped and viewed using a confocal laser scanning microscope (Zeiss LSM 510 META).
Galactosyltransferase labelling
Galactosyltransferase labelling assays were performed as described previously (Kelly et al., 1993). Protein lysates were immunoprecipitated with anti-c-Rel antibody and mixed with labelling buffer (5 mM MnCl2, 10 mM galactose, 50 mM HEPES pH 7.4). Galactosyltransferase labelling reactions were initiated by addition of 2 μCi UDP-[3H]-galactose (American Radiolabeled Chemicals, St Louis, MO, USA) in 5′-AMP solution (2.5 mM 5′-AMP) and 50 mU galactosyltransferase (Sigma Chemical), and then incubating at 37°C for 1 h. Reaction mixture proteins were separated by SDS-PAGE, intensified with EN3HANCE fluor (Perkin Elmer, Waltham, MA, USA), dried and exposed to X-ray film.
Streptavidin-agarose pull-down assay
Biotin pull-down assays were performed as described previously (Deng et al., 2003). The procedure allows for quantitative binding of transactivators or molecules of interest to a specific probe: a 20-nucleotide sequence containing the NF-κB binding site (5′-GCTAGGGGGATTTTCCCTCT-3′) at position −957/-977 of the iNOS promoter. Two complementary DNAs were synthesized and biotinylated by Bioneer Corporation (Bioneer Corporation, Daejeon, Korea) and annealed. Binding assays were performed by incubating 500 μg nuclear protein extracts with 2 μg biotinylated DNA probe and 25 μL streptavidin-conjugated agarose beads for 1 h. DNA-protein complexes were analysed by Western blotting using the indicated antibodies.
Transient transfection and luciferase assay
The NF-κB reporter contained three copies of the κB-binding sequence fused to the firefly luciferase gene (Clontech, Mountain View, CA, USA). An iNOS promoter containing a 973-bp region upstream from the transcription start site has been described previously (Kim et al., 2004). The plasmids encoding c-Rel, p50 and p65 were kindly provided by Dr C. Gélinas at Rutgers University. The pFR-luc vector was purchased from Stratagene (La Jolla, CA, USA). c-Rel (309–588) fused to GAL4 DNA-binding domain (DBD) corresponding to amino acids 1–147 (GAL4-c-Rel) was a generous gift from Dr Fresno (Martin et al., 2001). Cells were transfected by electroporation (Microporator, Digital Bio Technology, Daejeon, Korea). Luciferase activity was assayed 24 h after transfection and normalized to transfection efficiency using a cotransfected β-galactosidase plasmid. Bioluminescence was measured using a Turner Designs luminometer (TD-20/20).
Chromatin immunoprecipitation assay
Chromatin immunoprecipitation (ChIP) assays were performed as described previously (Boyd and Farnham, 1999; Hwang et al., 2010). Briefly, formaldehyde (1%) was added for cross-linking, and cell lysates were prepared. Samples were sonicated on ice in a Bioruptor (COSMO Bio Co., Ltd, Tokyo, Japan). Supernatant was precleared with 20 μg sheared salmon sperm DNA, 5 μg normal IgG and 50 μL protein G-sepharose for 2 h, and was immunoprecipitated by addition of c-Rel antibody (Santa Cruz Biotechnology, SC-71). After being washed, the immunoprecipitates were eluted with elution buffer. The eluted immunoprecipitates were treated with RNase A overnight at 65°C, and proteins were removed by treatment with EDTA, 1 M Tris Cl (pH 6.5) and proteinase K at 42°C for 1 h. The DNA was extracted using a DNA purification kit (QIAGEN, Hilden, Germany). The gene promoter sequences in the immunoprecipitates were amplified by PCR using the primers for iNOS promoter F (-904): GTGTACCTCAGACAAGGGC, R (-1058): CACACATGGCATGGAATTTT.
Quantitative real-time PCR
Precipitated DNAs were used as templates for PCR amplification, and products were quantitatively detected by measuring incorporation of fluorescent SYBR green into double-stranded DNA (iCycler iQ, Bio-Rad, Hercules, CA, USA). Relative DNA levels were calculated from the PCR profiles of each sample using the threshold cycle (Ct), corresponding to the cycle at which a statistically significant increase in fluorescence occurred. Ct is considered the amount of template present in the starting reaction. To correct for differences in the amount of total cDNA in the starting reaction, Ct values for an endogenous control (input DNA) were subtracted from those of the corresponding sample. Each experiment was repeated at least three times and GAPDH was used as a housekeeping gene for the endogenous control. The ChIP-quantitative PCR Negative Control Primers (QIAGEN) were used to measure the amount of non-specific genomic DNA that coprecipitates during a ChIP procedure.
OGT knockdown
RAW264.7 cells were transfected with a siRNA directed to a sequence in the COOH-terminal region of mouse OGT (Ref. NM-139144.2) corresponding to nucleotides 3420–3440 by electroporation (Microporator, Digital Bio Technology). The sequence of the siRNA against mouse OGT was sense 5′-AGGGAACUAGAUAACAUGCUU-3′. For the control, scrambled siRNA, the sequence was sense 5′-CGCAUUAAUCUAGUUCGCUUU-3′. This siRNA resulted in, on average, 50–60% suppression of OGT protein expression in RAW264.7 cells.
Statistical analysis
The data are expressed as the mean ± SEM and were analysed for statistical significance using anova, followed by Scheffe's test for multiple comparison. A P-value <0.05 was considered significant.
Results
GlcN suppresses the prolonged activation of NF-κB in response to LPS
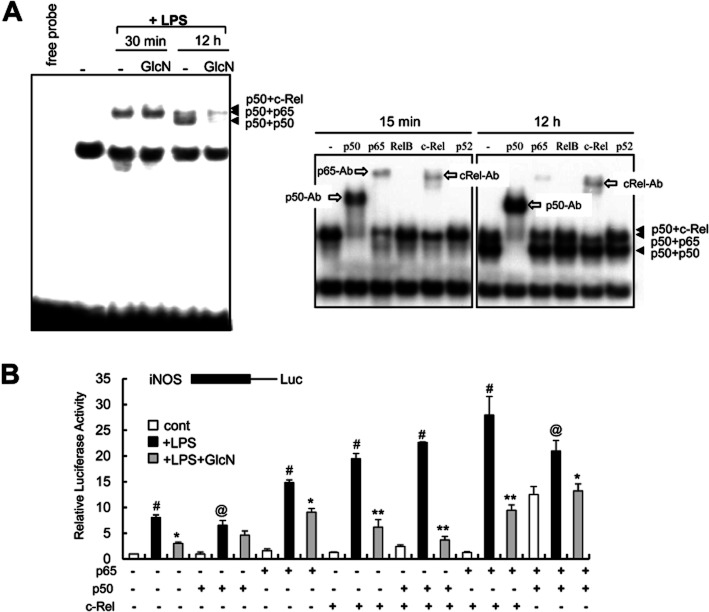
Previously, we demonstrated that GlcN has an inhibitory effect on LPS-induced activation of NF-κB in BV2 mouse microglial cells (Hwang et al., 2010). In the present study, GlcN inhibited the interaction of NF-κB with DNA-protein complexes in BV2 cells after 12 h of LPS stimulation. However, GlcN was ineffective in preventing the formation of DNA protein complexes after 30 min of LPS stimulation (Figure 1A). GlcN alone did not modify the position of the band in the NF-κB EMSA (data not shown). To characterize the proteins present in the DNA-protein complexes, supershift assays were performed in BV2 cells. The p50/p65 heterodimer and p50/c-Rel complexes were identified as the major forms of protein bound at the early time point (15 min) after LPS stimulation. In contrast, the p50/c-Rel heterodimer and the p50/p50 homodimer were the major forms observed at the 12 h time point (Figure 1A). The binding of p65 was less significant at 12 h after LPS treatment. Thus, GlcN appeared to inhibit the NF-κB complexes containing p50 or c-Rel. We verified this attenuation of LPS-induced NF-κB DNA binding and iNOS activation induced by GlcN using RAW264.7 cells (data not shown).
Figure 1.
Modulation of c-Rel containing DNA-binding complexes by LPS with or without GlcN. (A) BV2 cells were untreated or pretreated with 5 mM GlcN for 2 h and incubated with 0.1 μg·mL−1 LPS in the presence of or absence of 5 mM GlcN. Nuclear extracts were prepared at 30 min or 12 h and DNA binding to a 32P-labelled NF-κB probe was measured by EMSA (left panel). Supershift analysis using antibodies targeting p65, p50, c-Rel, RelB or p52 were performed at 15 min or 12 h after LPS stimulation (right panel). Data are representative of at least three independent experiments. (B) RAW264.7 cells were transfected with iNOS promoter-reporter constructs along with the indicated combinations of p65, p50 and c-Rel and stimulated with LPS (0.1 μg·mL−1) with or without 5 mM GlcN as indicated. Luciferase activity was measured at 24 h and shown as relative luciferase activity. #P < 0.01 control versus LPS-treated; @P < 0.05 control versus LPS-treated; *P < 0.05 LPS versus LPS+GlcN-treated; **P < 0.01 LPS versus LPS+GlcN-treated.
To gain an insight into the regulatory roles played by p50 and c-Rel proteins as iNOS promoters, we next examined the effects thereof using iNOS promoter- and NF-κB-reporter constructs in RAW264.7 macrophages overexpressing these Rel proteins. These transfection experiments were performed in RAW264.7 cells because we were only able to obtain extremely low (<2%) transfection efficiency with BV2 cells (with both the Lipofectamine and electroporation methods). The p65/p50 complex was a strong activator of NF-κB and iNOS under both basal and LPS-stimulated conditions (Figure 1B); LPS-stimulated induction of iNOS was potentiated by c-Rel. However, the effect of p50 on the expression of iNOS appeared to be more complicated. For example, overexpression of p50 inhibited the expression of NF-κB (Supporting Information Figure S1) but not iNOS (Figure 1B), whereas the combined overexpression of p50 and p65 stimulated the expression of both NF-κB and iNOS. The combined overexpression of p50 and c-Rel stimulated iNOS but not NF-κB (Figure 1B, Supporting Information Figure S1). These results suggest that c-Rel acts as a stimulator in all instances and plays an important role in the continuous induction of iNOS, whereas the effects of p50 on iNOS are not necessarily inhibitory. Instead, the effect of p50 may depend on the presence of other Rel family proteins and transcription factors. GlcN pretreatment inhibited the transcription of iNOS induced by p65, c-Rel and/or p50 in response to LPS.
We examined the time-dependent nuclear translocation of c-Rel in response to LPS with or without GlcN using an immunofluorescence approach (Supporting Information Figure S2). Nuclear translocation of c-Rel in BV2 cells was biphasic. The c-Rel protein showed an early response (at 30 min) to LPS treatment. Next, following an interval during which translocation to cytosol was evident at 2–6 h, c-Rel showed a secondary activation peak 12 h after LPS treatment, and this secondary activation continued to 24 h. GlcN inhibited this later activation of c-Rel, but had no effect on the immediate early response of c-Rel. Therefore, GlcN appears to modulate the LPS response by inhibiting the secondary activation of c-Rel.
GlcN suppresses LPS-stimulated binding of c-Rel to the NF-κB site in the iNOS promoter
Next, we assayed the binding of c-Rel to a biotinylated oligonucleotide corresponding to the distal NF-κB site (-957/-977). Stimulation with LPS for 24 h induced an increase in c-Rel binding, which was inhibited by GlcN pretreatment (Figure 2A).
Figure 2.
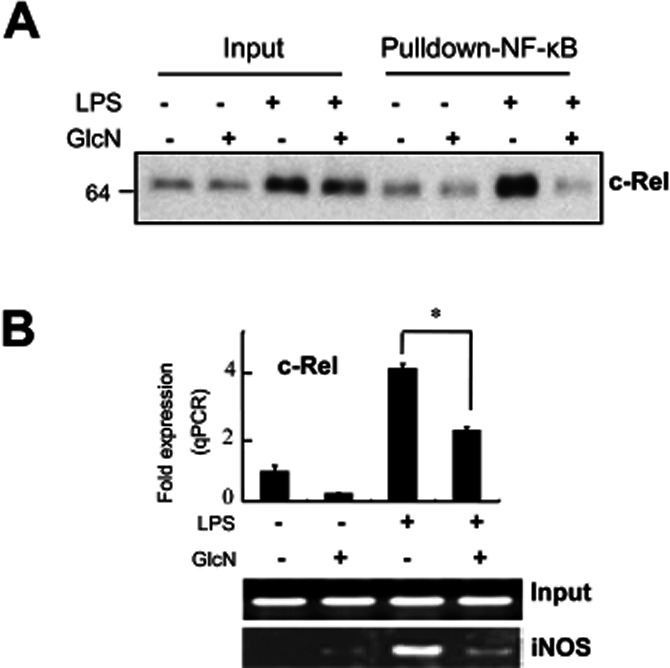
Inhibition of the binding of c-Rel to the NF-κB site of an iNOS promoter induced by GlcN. BV2 cells were untreated or pretreated with 5 mM GlcN for 2 h and stimulated with LPS (0.1 μg·mL−1) with or without 5 mM GlcN for 24 h. (A) Nuclear extracts were prepared and loaded (Input: 20 μg each). Binding of c-Rel to the iNOS promoter-derived, biotinylated NF-κB probe was measured by streptavidin-agarose pull-down assay (Pulldown-NF-κB) (as described in Methods) followed by Western blotting for c-Rel. (B) Chromatin DNA was immunoprecipitated with anti-c-Rel antibody and eluted DNA was quantified by real-time PCR or standard reverse transcriptase PCR (RT-PCR) for the iNOS promoter. Input represents each PCR product obtained from 2% of the pre-immunoprecipitated DNA. The real-time PCR data are expressed as mean ± SEM (error bars). The RT-PCR and Western blots shown are representative of three or more independent determinations. *Denotes significantly different from LPS-treated (P < 0.01). qPCR, quantitative PCR.
To confirm the physiological relevance of such oligonucleotide binding, we performed ChIP assays. LPS caused an increase in the binding of c-Rel to the endogenous iNOS promoter, which was suppressed by GlcN (Figure 2B). This demonstrates that c-Rel is recruited to the iNOS promoter in response to LPS stimulation. Furthermore, the data suggest an inhibitory role for GlcN in regulating the chromatin mobilization of c-Rel proteins.
LPS-dependent increases in c-Rel O-GlcNAcylation are reversed by GlcN
One potential mechanism by which LPS with or without GlcN may regulate the induction of iNOS is via post-translational O-GlcNAcylation of c-Rel. We performed wheat-germ agglutinin (WGA) pull-down assays using WGA-conjugated agarose beads that specifically precipitate proteins containing N-acetylglucosamine or N-acetylneuramic acid. The amount of c-Rel protein in the WGA precipitate was clearly increased by LPS, and this effect was reversed by GlcN (Figure 3A). These results strongly suggest that the c-Rel protein is post-translationally modified by O-GlcNAc. Interestingly, O-GlcNAcylation of total nucleocytoplasmic proteins was reduced by LPS treatment, although O-GlcNAcylation levels returned to basal values following treatment with GlcN. Using [3H]-galactose as a substrate, galactosyltransferase effectively radiolabelled immunoprecipitated c-Rel. Such radiolabelling was increased by LPS but reversed by GlcN (Figure 3B).
Figure 3.

Modulation of O-GlcNAc and the transcriptional activity of c-Rel induced by stimulation with LPS with or without GlcN. (A, B) BV2 cells were untreated or pretreated with GlcN (5 mM) for 2 h and stimulated with LPS (0.1 μg·mL−1) in the presence or absence of 5 mM GlcN for 24 h. Total cell lysates (Input) were prepared and subjected to precipitation (IP) with WGA-conjugated agarose beads. Precipitated proteins were immunoblotted by c-Rel, p50, O-GlcNAc (CTD110.6) or GAPDH antibodies (A). Cell lysates were immunoprecipitated with anti-c-Rel antibody. Immunoprecipitates were subjected to galactosyltransferase labelling using [3H]-UDP-galactose as a substrate (B). (C) RAW264.7 cells were transfected with pFR-luc promoter containing five GAL4 binding sites in its promoter with or without c-Rel protein fused to GAL4 DBD. Cells were then treated with 5 mM GlcN with or without LPS (0.1 μg·mL−1) for 24 h and luciferase activity, in relative light units, was measured. Data are representative of three independent experiments. *denotes P < 0.01 compared with LPS-treated samples.
The transcriptional activity of c-Rel in response to LPS with or without GlcN was assayed using chimeric protein in which c-Rel was fused to the GAL4 DBD 1–147. This allowed c-Rel to be artificially tethered to the luciferase reporter for transcriptional activation. In these experiments, the transcriptional activity of the GAL4-c-Rel chimeric protein was stimulated by LPS but inhibited by GlcN (Figure 3C).
Both cytosolic and nuclear forms of c-Rel are O-GlcNAcylated by LPS
To determine if O-GlcNAcylation of Rel proteins are affected by cellular location, nuclear and cytosolic fractions were isolated and precipitated from each fraction using WGA-agarose beads. Both cytosolic and nuclear c-Rel and p65 were O-GlcNAcylated (Figure 4). Furthermore, O-GlcNAcylation of c-Rel in both fractions was modified by LPS, with or without GlcN. Other Rel proteins, such as p52, p50 and RelB, were not precipitated by WGA. LPS treatment resulted in an overall reduction in O-GlcNAcylation of cytosolic proteins, which was reversed by GlcN pretreatment. In contrast, LPS treatment with or without GlcN did not significantly alter the O-GlcNAcylation of nuclear proteins. The OGT of both nuclear and cytosolic fractions was itself O-GlcNAcylated.
Figure 4.
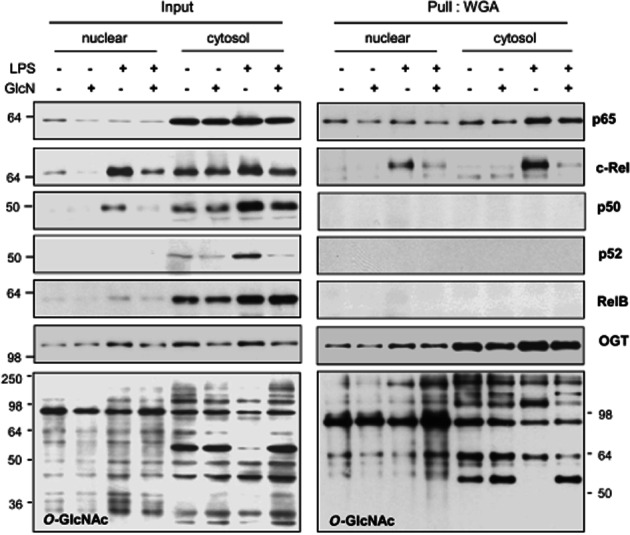
Changes in the O-GlcNAcylation of cytosolic and nuclear NF-κB/Rel proteins induced by LPS with or without GlcN. BV2 cells were pretreated with GlcN (5 mM) for 2 h and then treated with LPS (0.1 μg·mL−1) with or without 5 mM GlcN for 24 h. Cytosolic and nuclei lysates were prepared (Input) and each fraction was precipitated with WGA-conjugated agarose beads (Pull: WGA). The precipitates were immunoblotted by anti-p65, c-Rel, p50, p52, OGT or O-GlcNAc (CTD110.6) antibodies. The blots shown are representative of three independent experiments.
LPS increases the interaction of c-Rel with OGT, and p105/p50, but GlcN reduces these interactions
Next, we examined the interaction between OGT and c-Rel using co-immunoprecipitation assays. An antibody against OGT co-immunoprecipitated c-Rel in LPS-stimulated cell lysates; this interaction was inhibited by GlcN (Figure 5A). LPS and GlcN exerted similar effects on OGT and c-Rel interactions, as determined by reciprocal experiments that used an anti-c-Rel antibody to co-immunoprecipitate OGT (Figure 5B). Although LPS increased the amount of c-Rel associated with OGT, it did not affect the interaction of c-Rel with p65 or IκB-α. However, LPS treatment did increase the interaction of c-Rel with p50 and its precursor protein p105 (Figure 5B and Supporting Information Figure S3).
Figure 5.
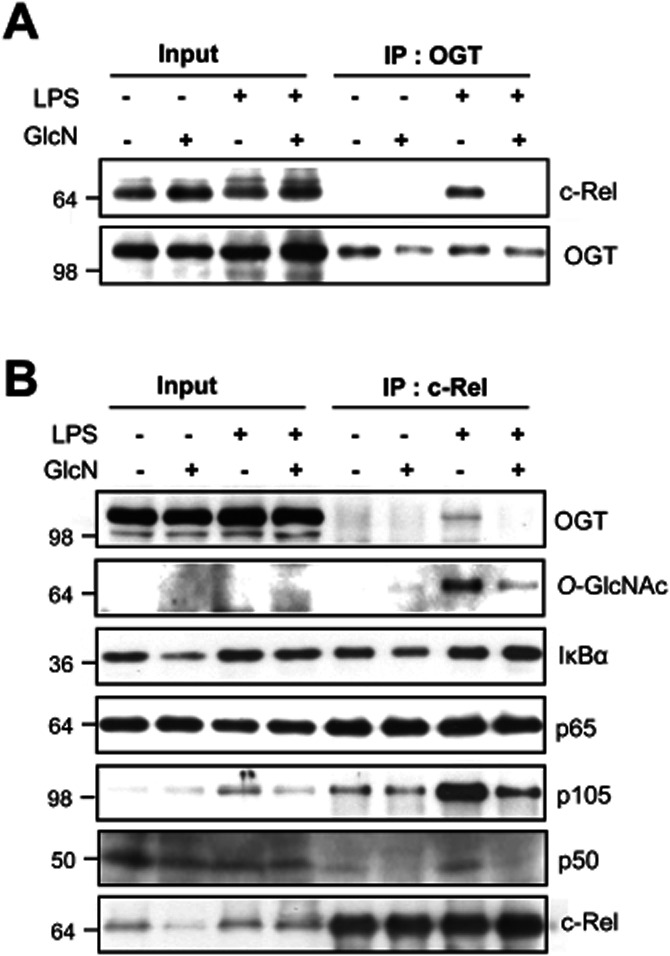
Modulation of the proteins associated with c-Rel in response to LPS with or without GlcN. BV2 cells were pretreated with GlcN (5 mM) and stimulated with LPS (0.1 μg·mL−1) in the presence or absence of 5 mM GlcN for 24 h, and total cell lysates were prepared. Lysates (Input) were immunoprecipitated (IP) with anti-OGT (A) or anti-c-Rel (B) antibodies. IP proteins were subjected to Western blotting with antibodies against OGT, IκBα, p65, p105, p50, O-GlcNAc or c-Rel. Data are representative of three independent experiments.
OGT knockdown reduces both c-Rel O-GlcNAcylation and the association of c-Rel with p50
We performed an OGT knockdown experiment to determine the effect of O-GlcNAcylation and transcriptional activity of c-Rel. At 72 h following si-OGT transfection, the level of OGT mRNA was significantly reduced compared to that in the cells transfected with control siRNA (Figure 6A). The level of c-Rel O-GlcNAcylation and its association with p50 were reduced following si-OGT transfection (Figure 6B). However, neither DNA binding to the iNOS promoter nor the transcriptional activity of c-Rel was significantly changed by OGT knockdown (Figure 6C and D). These data suggest that reduced levels of OGT increase p50 binding by a mechanism that is independent of the transcriptional activity of c-Rel. We determined the expression of iNOS and activity of c-Rel in the presence of the OGT inhibitor, alloxan, and compared the results with those obtained using OGT knockdown siRNA. Alloxan reduced the O-GlcNAcylation of all the nucleocytoplasmic proteins, but it did not reduce the O-GlcNAcylation of c-Rel (Supporting Information Figure S4).
Figure 6.
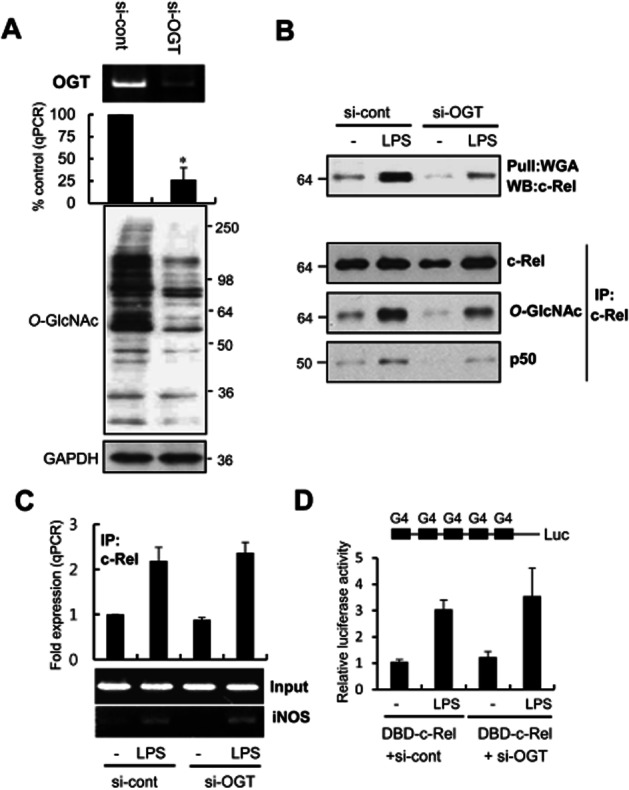
The functional effects of OGT knockdown. RAW264.7 cells were transfected with OGT-siRNA (si-OGT) or scrambled-siRNA (si-cont). (A) The effect of OGT knockdown on OGT mRNA was analysed after 72 h by PCR and real-time PCR (A, upper panel). The O-GlcNAcylation of all the nucleocytoplasmic proteins was determined by Western blotting using CTD110 or GAPDH antibodies (A, lower panel). (B) Control cells and OGT knockdown cells were stimulated with LPS (0.1 μg·mL−1) for 24 h. O-GlcNAcylation changes and interaction with p50 was determined by WGA-precipitation (upper panel) and c-Rel immunoprecipitation (lower panels). Precipitates were analysed by Western blotting with anti-c-Rel, CTD110.6 or anti-p50 antibodies. (C) ChIP analysis was performed to determine the binding of c-Rel to the iNOS promoter. Control cells and OGT knockdown cells (si-OGT) at 48 h were stimulated with LPS (0.1 μg·mL−1) for 24 h. The immunoprecipitated DNA was purified for real-time PCR or standard RT-PCR analyses. Input represents each PCR product obtained from 2% of the pre-immunoprecipitated DNA. (D) RAW264.7 Cells were transfected with si-control or si-OGT. pFR-luc reporter and/or DBD-c-Rel plasmids were transfected into the cell 36 h after the transfection of siRNAs. Cells were stimulated with LPS (0.1 μg·mL−1) for 24 h, and luciferase activity was compared. The real-time PCR data are expressed as mean ± SEM (error bars), and the RT-PCR and Western blots shown are representative of three or more independent determinations. *denotes P < 0.05 compared with siRNA control cells.
Discussion
This study demonstrates that O-GlcNAcylation of transcription factors, c-Rel and NF-κB, and the interaction of OGT with c-Rel are dynamically modified by LPS with or without GlcN in microglia. A number of reports have obtained results indicating that an increased concentration of GlcN augments the O-GlcNAcylation of proteins by upregulating substrate availability. However, we observed that the GlcN-induced changes in O-GlcNAcylation of individual proteins, as measured by Western blotting, were distinct and protein-specific manner; the O-GlcNAcylation of some proteins was decreased, whereas that of others was unchanged or increased by GlcN. Therefore, the modulation of O-GlcNAcylation levels of individual proteins is likely to be achieved through a number of distinct mechanisms and is not simply dependent on substrate availability. Such factors include OGT levels, OGT enzymatic activity, the availability and enzymatic activity of O-GlcNAcase, and differential substrate targeting. In this study, we initially demonstrated that c-Rel can be O-GlcNAcylated and that this process is dynamically modulated by LPS, with or without GlcN. Currently, the mechanism by which LPS specifically increases the O-GlcNAcylation of c-Rel remains to be elucidated.
The inhibition of LPS-stimulated DNA-binding and transcriptional activities of c-Rel by GlcN could be caused by simple inhibition of the nuclear translocation of c-Rel. However, the results from the present study suggest that O-GlcNAcylation of c-Rel does not seem to regulate its nuclear translocation directly, because the extent of O-GlcNAcylation of both nuclear and cytosolic forms of c-Rel was increased by LPS and reduced by GlcN (Figure 4). Nonetheless, it still remains to be elucidated if the O-GlcNAcylation of specific amino acid residues of c-Rel is important for c-Rel nuclear translocation and subsequent transcriptional activity. This question can be addressed in the future by conducting extended experiments using c-Rel mutated at O-GlcNAc sites.
As noted earlier, LPS can upregulate the interaction of OGT with c-Rel, whereas GlcN down-regulates this interaction, but OGT knockdown did not modify c-Rel transcriptional activity. Here, we present two scenarios that may explain the role(s) played by OGT in LPS-induced transcriptional regulation of iNOS. One possibility is that the association of OGT with c-Rel in response to LPS induces O-GlcNAcylation of these proteins. NF-κB, in turn, is activated by an increased interaction with p105/p50, thereby leading to an increase in iNOS transcription. Therefore, OGT appears to play a positive role in the regulation of the LPS-mediated transcriptional activity of NF-κB, at least at certain time points or at particular levels of stimulation. In an alternative scenario, O-GlcNAcylation of c-Rel may be independent of the transcriptional activities of the protein. Instead, OGT may regulate transcription through a mechanism independent of O-GlcNAcylation. In addition to performing an enzymatic function in O-GlcNAc regulation, the interaction of OGT with c-Rel may affect the association of c-Rel with other transcriptional complexes, resulting in either activation or inhibition of transcription. In fact, OGT has been shown to regulate transcription by recruiting transcriptional repressors such as mammalian Sin3A histone deacetylases to promoters (Yang et al., 2002; Toleman et al., 2004). Thus, OGT can potentially function as a regulator of both O-GlcNAcylation and the transcription of iNOS, but these two events are not necessarily related. Although O-GlcNAcylation of c-Rel by OGT plays an important role in the induction of iNOS through the increased c-Rel–p50 interaction, OGT also seems to regulate iNOS transcription by an as yet undefined mechanism(s).
One major goal of the research into neurodegenerative diseases is to define the mechanisms of inflammatory gene control, particularly in terms of resolving the pathophysiology of inflammation. Our discovery of a potential mechanism by which NF-κB is regulated by OGT will certainly add to the hypotheses pertaining to the regulation of NF-κB during the inflammatory response in the brain. By demonstrating the dynamic O-GlcNAcylation of c-Rel in response to an inflammatory stimulus (LPS), our results may provide a new insight into the regulation of NF-κB-dependent physiological processes.
Glossary
- BFA
brefeldin A
- CRE
cAMP-response element
- CREB
cAMP-response element binding protein
- GRP7
78-kDa glucose-regulated protein
- GlcN
glucosamine
- O-GlcNAc
O-linked N-acetylglucosamine
- LTA
lipoteichoic acid
- MTT
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrasolium bromide
- OGT
O-GlcNAc transferase
- PI-PC
polyinosinic:polycytidylic
- TG
thapsigargin
- TM
tunicamycin
- UPR
unfolded protein response
Conflict of interest
There is no conflict of interest in this paper.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
Figure S1 Regulation of NF-κB luciferase activities by p65, p50 and c-Rel in the presence or absence of LPS. RAW264.7 cells were transfected with NF-κB-responsive reporter constructs along with the indicated combinations of p65, p50 and c-Rel and incubated with or without LPS as indicated. Luciferase activity was measured at 24 h. *P < 0.05 control versus transfected; **P < 0.01 control versus transfected; #P < 0.05 LPS control versus LPS-transfected; **P < 0.01 LPS control versus LPS-transfected.
Figure S2 Regulation of nuclear translocation of c-Rel by LPS with or without GlcN. BV2 cells were pretreated with 5 mM GlcN and incubated with 0.1 μg·mL−1 LPS. Nuclear translocations of c-Rel at indicated times were measured by immunofluorescence staining using a FITC-conjugated secondary antibody (green). Nuclei were stained with DAPI (blue). The result shown is representative of three independent experiments.
Figure S3 Densitometric calculations for Western blot data shown Figure 5B. The intensities of bands in the Western blots were calculated using ImageJ, subtracting background. Values are expressed relative intensity of each band (compared with weakest band) and relative intensities of indicated proteins were graphed.
Figure S4 Changes in the O-GlcNAcylation of nucleocytoplasmic proteins and c-Rel induced by alloxan. BV2 cells were untreated or pretreated with alloxan (1 mM) for 1 h and stimulated with LPS (0.1 μg·mL−1) in the presence or absence of 5 mM GlcN for 24 h. O-GlcNAc levels of total cell lysates were measured by RL-2 antibody (left). Cell lysates were immunoprecipitated with anti-c-Rel antibody and precipitated proteins were immunoblotted by c-Rel or anti-O-GlcNAc (RL-2) antibodies (right).
References
- Boyd KE, Farnham PJ. Coexamination of site-specific transcription factor binding and promoter activity in living cells. Mol Cell Biol. 1999;19:8393–8399. doi: 10.1128/mcb.19.12.8393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta S, Jana M, Zhou Y, Fung YK, Ghosh S, Pahan K. Antineuroinflammatory effect of NF-kappaB essential modifier-binding domain peptides in the adoptive transfer model of experimental allergic encephalomyelitis. J Immunol. 2004;173:1344–1354. doi: 10.4049/jimmunol.173.2.1344. [DOI] [PubMed] [Google Scholar]
- Deng WG, Zhu Y, Montero A, Wu KK. Quantitative analysis of binding of transcription factor complex to biotinylated DNA probe by a streptavidin-agarose pulldown assay. Anal Biochem. 2003;323:12–18. doi: 10.1016/j.ab.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Gouze JN, Bianchi A, Becuwe P, Dauca M, Netter P, Magdalou J, et al. Glucosamine modulates IL-1-induced activation of rat chondrocytes at a receptor level, and by inhibiting the NF-kappa B pathway. FEBS Lett. 2002;510:166–170. doi: 10.1016/s0014-5793(01)03255-0. [DOI] [PubMed] [Google Scholar]
- Han I, Kudlow JE. Reduced O glycosylation of Sp1 is associated with increased proteasome susceptibility. Mol Cell Biol. 1997;17:2550–2558. doi: 10.1128/mcb.17.5.2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang SY, Shin JH, Hwang JS, Kim SY, Shin JA, Oh ES, et al. Glucosamine exerts a neuroprotective effect via suppression of inflammation in rat brain ischemia/reperfusion injury. Glia. 2010;58:1881–1892. doi: 10.1002/glia.21058. [DOI] [PubMed] [Google Scholar]
- Kamemura K, Hart GW. Dynamic interplay between O-glycosylation and O-phosphorylation of nucleocytoplasmic proteins: a new paradigm for metabolic control of signal transduction and transcription. Prog Nucleic Acid Res Mol Biol. 2003;73:107–136. doi: 10.1016/s0079-6603(03)01004-3. [DOI] [PubMed] [Google Scholar]
- Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu Rev Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- Kelly WG, Dahmus ME, Hart GW. RNA polymerase II is a glycoprotein. Modification of the COOH-terminal domain by O-GlcNAc. J Biol Chem. 1993;268:10416–10424. [PubMed] [Google Scholar]
- Kim WK, Hwang SY, Oh ES, Piao HZ, Kim KW, Han IO. TGF-beta1 represses activation and resultant death of microglia via inhibition of phosphatidylinositol 3-kinase activity. J Immunol. 2004;172:7015–7023. doi: 10.4049/jimmunol.172.11.7015. [DOI] [PubMed] [Google Scholar]
- Kreppel LK, Blomberg MA, Hart GW. Dynamic glycosylation of nuclear and cytosolic proteins. Cloning and characterization of a unique O-GlcNAc transferase with multiple tetratricopeptide repeats. J Biol Chem. 1997;272:9308–9315. doi: 10.1074/jbc.272.14.9308. [DOI] [PubMed] [Google Scholar]
- Largo R, Alvarez-Soria MA, Diez-Ortego I, Calvo E, Sanchez-Pernaute O, Egido J, et al. Glucosamine inhibits IL-1beta-induced NFkappaB activation in human osteoarthritic chondrocytes. Osteoarthritis Cartilage. 2003;11:290–298. doi: 10.1016/s1063-4584(03)00028-1. [DOI] [PubMed] [Google Scholar]
- Love DC, Hanover JA. The hexosamine signaling pathway: deciphering the ‘O-GlcNAc code’. Sci STKE. 2005;2005:re13. doi: 10.1126/stke.3122005re13. [DOI] [PubMed] [Google Scholar]
- Martin AG, San-Antonio B, Fresno M. Regulation of nuclear factor kappa B transactivation. Implication of phosphatidylinositol 3-kinase and protein kinase C zeta in c-Rel activation by tumor necrosis factor alpha. J Biol Chem. 2001;276:15840–15849. doi: 10.1074/jbc.M011313200. [DOI] [PubMed] [Google Scholar]
- Meininger CJ, Kelly KA, Li H, Haynes TE, Wu G. Glucosamine inhibits inducible nitric oxide synthesis. Biochem Biophys Res Commun. 2000;279:234–239. doi: 10.1006/bbrc.2000.3912. [DOI] [PubMed] [Google Scholar]
- Pahan K, Sheikh FG, Namboodiri AM, Singh I. N-acetyl cysteine inhibits induction of no production by endotoxin or cytokine stimulated rat peritoneal macrophages, C6 glial cells and astrocytes. Free Radic Biol Med. 1998;24:39–48. doi: 10.1016/s0891-5849(97)00137-8. [DOI] [PubMed] [Google Scholar]
- Saha RN, Pahan K. Regulation of inducible nitric oxide synthase gene in glial cells. Antioxid Redox Signal. 2006;8:929–947. doi: 10.1089/ars.2006.8.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toleman C, Paterson AJ, Whisenhunt TR, Kudlow JE. Characterization of the histone acetyltransferase (HAT) domain of a bifunctional protein with activable O-GlcNAcase and HAT activities. J Biol Chem. 2004;279:53665–53673. doi: 10.1074/jbc.M410406200. [DOI] [PubMed] [Google Scholar]
- Viatour P, Merville MP, Bours V, Chariot A. Phosphorylation of NF-kappaB and IkappaB proteins: implications in cancer and inflammation. Trends Biochem Sci. 2005;30:43–52. doi: 10.1016/j.tibs.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Wells L, Hart GW. O-GlcNAc turns twenty: functional implications for post-translational modification of nuclear and cytosolic proteins with a sugar. FEBS Lett. 2003;546:154–158. doi: 10.1016/s0014-5793(03)00641-0. [DOI] [PubMed] [Google Scholar]
- Whisenhunt TR, Yang X, Bowe DB, Paterson AJ, Van Tine BA, Kudlow JE. Disrupting the enzyme complex regulating O-GlcNAcylation blocks signaling and development. Glycobiology. 2006;16:551–563. doi: 10.1093/glycob/cwj096. [DOI] [PubMed] [Google Scholar]
- Yang X, Zhang F, Kudlow JE. Recruitment of O-GlcNAc transferase to promoters by corepressor mSin3A: coupling protein O-GlcNAcylation to transcriptional repression. Cell. 2002;110:69–80. doi: 10.1016/s0092-8674(02)00810-3. [DOI] [PubMed] [Google Scholar]
- Yi HA, Yi SD, Jang BC, Song DK, Shin DH, Mun KC, et al. Inhibitory effects of glucosamine on lipopolysaccharide-induced activation in microglial cells. Clin Exp Pharmacol Physiol. 2005;32:1097–1103. doi: 10.1111/j.1440-1681.2005.04305.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.