Abstract
Background and Purpose
Histamine and prostaglandin E2 (PGE2), directly and via their effects on other cells, regulate the behaviour of vascular smooth muscle (VSM), but their effects on human VSM are incompletely resolved.
Experimental Approach
The effects of PGE2 on histamine-evoked changes in intracellular free Ca2+ concentration ([Ca2+]i) and adenylyl cyclase activity were measured in populations of cultured human aortic smooth muscle cells (ASMCs). Selective ligands of histamine and EP receptors were used to identify the receptors that mediate the responses.
Key Results
Histamine, via H1 receptors, stimulates an increase in [Ca2+]i that is entirely mediated by activation of inositol 1,4,5-trisphosphate receptors. Selective stimulation of EP2 or EP4 receptors attenuates histamine-evoked Ca2+ signals, but the effects of PGE2 on both Ca2+ signals and AC activity are largely mediated by EP2 receptors.
Conclusions and Implications
Two important inflammatory mediators, histamine via H1 receptors and PGE2 acting largely via EP2 receptors, exert opposing effects on [Ca2+]i in human ASMCs.
Keywords: aortic smooth muscle, histamine, prostaglandin E2, cyclic AMP, Ca2+ signal, histamine H1 receptor, EP2 receptor, EP4 receptor
Introduction
Histamine released from mast cells and basophils initiates inflammatory responses, notably in the vasculature where its most immediate effects are dilatation of small vessels causing increased blood flow to sites of injury. These effects are mediated primarily by H1 histamine receptors on endothelial cells, which stimulate Gq and thereby phospholipase C (PLC) (Hill et al., 1997; Jones and Kearns, 2011). In larger vessels, including human arteries, H1 receptors directly stimulate contraction (Toda, 1987) and proliferation of vascular smooth muscle (VSM) cells (Satoh et al., 1994). There are, however, differences between species in the effects of histamine on the vasculature (Wang et al., 1993, and references therein).
Inflammation plays a key role in the development of atherosclerosis (Hansson, 2005; Swedenborg et al., 2011). Atherosclerotic lesions in human aorta are associated with enhanced local synthesis of histamine and increased expression of H1 receptors (Takagishi et al., 1995; Tanimoto et al., 2006). Furthermore, in animal models, prevention of histamine synthesis (Toda, 1987; Sasaguri et al., 2005) or competitive antagonists of H1 receptors (Miyazawa et al., 1998) reduce intimal thickening after vascular injury. These observations implicate H1 receptors of VSM in both inflammatory responses and the development of atherosclerosis and restenosis.
Prostaglandin E2 (PGE2), the most abundant PG in humans, is another inflammatory mediator with widespread effects that include regulation of blood vessels (Norel, 2007). Human VSM synthesizes PGE2 (Soler et al., 2000), and its synthesis is much increased in diseased human aorta (Bayston et al., 2003). PGE2 is associated with development of atherosclerosis (Gomez-Hernandez et al., 2006), and EP receptors are potential targets for its treatment (Yang et al., 2011). The effects of PGE2 are predominantly mediated by four classes of GPCRs. EP1 receptors stimulate PLC via Gq, EP2 and EP4 receptors stimulate adenylyl cyclase (AC) via Gs, and most splice variants of EP3 receptors regulate Gi (Norel, 2007; Sugimoto and Narumiya 2007). There is evidence, albeit rather sparse in humans, suggesting that all four EP receptor subtypes are expressed in various VSM (Coleman et al., 1994; see references in Breyer et al., 2001) including EP2 and EP3 receptors in human aortic smooth muscle (Bayston et al., 2003). Activation of EP1, or more often EP3, receptors causes contraction of various VSM (Jadhav et al., 2004; Jones and Woodward, 2011; Kobayashi et al., 2011) including human veins (Walch et al., 2001) and arteries (Qian et al., 1994; Jones et al., 1997; references in Walch et al., 2001). Stimulation of EP2 or EP4 receptors causes relaxation of many VSM (Jones et al., 1997; Yang et al., 2010), including human vessels (Qian et al., 1994; Davis et al., 2004; Foudi et al., 2008). These responses are consistent with the common observation that PLC-coupled receptors generally cause Ca2+-mediated contraction of smooth muscle, including VSM, while those that stimulate AC cause relaxation (references in Roscioni et al., 2010). The latter is widely thought to be mediated by cyclic AMP-dependent protein kinase, which via phosphorylation, can attenuate Ca2+ signalling and/or reduce the sensitivity of the contractile apparatus to Ca2+ (references in Roscioni et al., 2010). Collectively, these observations suggest that PGE2 and EP receptors play important roles in the normal physiology of blood vessels, and they are implicated in various pathological states including atherosclerosis (Yang et al., 2011).
The possibility that H1 and/or EP receptors might be targeted for treatment of vascular diseases is attractive because H1 receptor antagonists (Hill et al., 1997) and PG analogues (Abramovitz et al., 2000; Norel, 2007) are already established in clinical practice for other indications. Our choice of cultured VSM to investigate interactions between histamine and PGE2 is vindicated by the experimental opportunities that would not be accessible in studies of tissues, and by evidence that development of atherosclerosis is associated with a phenotypic change of VSM from a contractile to a proliferating (‘synthetic’) phenotype (Orr et al., 2010) similar to that of VSM in culture (House et al., 2008).
In the present study, we demonstrate that in human aortic smooth muscle cells (ASMCs), activation of H1 receptors by histamine stimulates an increase in intracellular free Ca2+ concentration ([Ca2+]i) entirely via activation of inositol 1,4,5-trisphosphate (IP3) receptors. Activation of EP2 or EP4 receptors substantially attenuates the Ca2+ signals evoked by histamine, but EP2 receptors are largely responsible for the inhibition of Ca2+ signals and stimulation of AC activity by PGE2.
Methods
Culture of human ASMCs
Human ASMCs were purchased from the American Tissue Culture Collection (ATCC, Manassas, VA, USA) or provided by Dr Trevor Littlewood (Division of Cardiovascular Medicine, University of Cambridge) (Boyle et al., 2002). Ethical committee approval for the latter was obtained from the Addenbrooke's NHS Trust. All cells were isolated from patients who died of causes unrelated to the cardiovascular system. Further details of the cells and the specific experiments for which they were used are provided in Supporting Information Table S1. Cells were cultured in DMEM supplemented with GlutaMAX-I, heat-inactivated FBS (10%), penicillin (100 units·mL−1) and streptomycin (0.1 mg·mL−1) at 37°C in humidified air containing 5% CO2. Cells from ATCC were first cultured according to the supplier's instructions (in ATCC vascular cell basal medium supplemented with ATCC VSM growth kit), and then as described earlier before use in experiments. Cells were used between passages 2 and 6, during which they retained an elongated shape and immunostained for α-smooth muscle actin (not shown).
Measurement of [Ca2+]i in cell populations
Confluent cultures of ASMC grown in 96-well plates were loaded in HEPES-buffered saline (HBS) with fluo-4 by incubation (1 h, 20°C) with fluo-4-acetoxymethyl ester (4 μM, Invitrogen, Paisley, UK), probenecid (2.5 mM) and pluronic F127 (0.02%, v/v) (Govindan et al., 2010). After a further incubation (30 min) in HBS supplemented with only probenecid, the medium was replaced with HBS and the cells were used for experiments. HBS had the following composition (mM): NaCl 135, KCl 5.9, MgCl2 1.2, CaCl2 1.5, glucose 11.5 and HEPES 11.6 (pH 7.3). Fluorescence (excitation at 485 nm, emission at 525 nm) was recorded during appropriate additions using a FlexStation 3 fluorescence spectrometer (MDS Analytical Technologies, Wokingham, UK). Fluorescence signals (F) were calibrated to [Ca2+]i from [Ca2+]i = KD(F − Fmin)/(Fmax − F), where KD is the equilibrium dissociation constant of fluo-4 for Ca2+ (345 nM) (Gee et al., 2000), Fmin is the fluorescence of Ca2+-free indicator [recorded from cells treated with 0.05% Triton X-100 and 10 mM 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA) in Ca2+-free HBS] and Fmax is the fluorescence of Ca2+-saturated indicator (cells treated with 0.05% Triton X-100 and 10 mM CaCl2 in HBS). Ca2+ signals were recorded after addition of histamine alone or in the presence of additional drugs added 5 min before histamine. Further details are provided in the figure legends.
Measurement of AC activity
Two assays were used to determine AC activity in confluent cultures of ASMC. A HitHunter cAMP HS+ (DiscoveRx, Birmingham, UK) enzyme complementation assay was used for most analyses. Briefly, confluent cultures of ASMC in 96-well plates were washed twice with HBS and incubated with appropriate stimuli (5 min). Reactions were stopped by aspirating the medium followed by addition of ice-cold ethanol (100 μL). After 10 min, the ethanol was evaporated (60°C, 30 min). This heating step also inactivates an endogenous β-galactosidase-like activity that otherwise contributes to the final luminescence measurement. The cAMP contents of the extracts were then determined according to the manufacturer's instructions. Because the AC inhibitors used [1 mM SQ 22536 with 200 μM DDA (SQ/DDA)] interfere with the HitHunter assay, analysis of their effects used a 3H-adenine-labelling assay in which cultures of ASMC in 24-well plates were incubated with [2,8-3H] adenine (1 μCi·mL−1, 1 mL per well) in DMEM for 2 h at 37°C in humidified air containing 5% CO2. After washing twice with HBS, cells in HBS at 20°C were used for experiments. Incubations were terminated by aspirating the medium and then adding ice-cold trichloroacetic acid (5% v/v, 1 mL). After 30 min at 4°C, 3H-labelled adenine nucleotides were separated using sequential Dowex 50WX4-400 (Bio-Rad, Hemel Hampstead, UK) and alumina columns (Sigma-Aldrich, Poole, UK) (Salomon et al., 1974).
Analyses of EP receptor expression by quantitative PCR (QPCR)
Confluent cultures of human ASMC in 24-well plates were lysed with Fastlane cDNA kit (500 μL per well, Qiagen, Crawley, UK). QPCR reactions were conducted according to the manufacturer's instructions using Rotorgene SYBR green PCR kit (Qiagen) with an initial denaturation (95°C, 5 min) followed by 40 cycles of amplification (95°C for 5 s and 60°C for 10 s). Fluorescence was measured at the end of each cycle (Govindan et al., 2010). Primers specific for EP2 (Qiagen Quantitect Primer Assay, code: Hs_PTGER2_1_SG; Qiagen) or EP4 receptors (Hs_PTGER4_2_SG) or, for calibration, primers for GAPDH (forward, ACCACAGTCCATGCCATCAC; reverse, TCCACCACCCTGTTGCTGTA) were used. The authenticity of each PCR product was confirmed by melting-curve analysis. Amplification efficiency (E) was calculated as 10m, where m is the average increase in fluorescence for four cycles after the cycle threshold (CT). Expression levels relative to the housekeeping product (GAPDH) were calculated from
Reactions were performed in duplicate with extracts from at least three different wells for each patient.
Statistical analysis
Concentration–effect relationships were individually fitted by non-linear curve fitting to Hill equations (GraphPad Prism version 5; GraphPad Software, La Jolla, CA, USA). Antagonist affinities (pKD) were calculated from dose ratios. Two-tailed paired Student's t-test or one-way anova with Bonferroni's post hoc test was used as appropriate, with P < 0.05 considered significant.
Materials
Cell culture materials, except FBS (Sigma), were from Invitrogen. Ionomycin, U73122 (1-[6-[[(17β)-3-methoxyestra-1,3,5(10)-trien-17-yl]amino]hexyl]-1H-pyrrole-2,5-dione), U73343 (1-[6-[[(17β)-3-methoxyestra-1,3,5(10)-trien-17-yl]amino]hexyl]-2,5-pyrrolidinedione), SQ 22536 (9-(tetrahydro-2-furanyl)-9H-purin-6-amine) and DDA (2′,5′-dideoxyadenosine) were from Merck Chemicals Ltd. (Nottingham, UK). trans-Ned-19 [(1R,3S)-1-[3-[[4-(2-fluorophenyl)piperazin-1-yl]methyl]-4-methoxyphenyl]-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylic acid] was from Enzo Life Sciences (Exeter, UK). Thapsigargin was from Alomone Labs Ltd. (Jerusalem, Israel). Histamine dihydrochloride, cimetidine hydrochloride, mepyramine maleate, AH6809 (6-isopropoxy-9-oxoxanthene-2-carboxylic acid), BW 245C [(4S)-(3-[(3R,S)-3-cyclohexyl-3-hydroxypropyl]-2,5-dioxo)-4-imidazolidineheptanoic acid], 2-APB (2-aminoethoxydiphenyl borate) and PGE2 were from Sigma. Butaprost (free acid), GW627368X (2-[4-(4,9-diethoxy-3-oxo-1H-benzo[f]isoindol-2-yl)phenyl]-N-phenylsulfonylacetamide), latanoprost (free acid) and L902,688 (5-[(1E,3R)-4,4-difluoro-3-hydroxy-4-phenyl-1-buten-1-yl]-1-[6-(2H-tetrazol-5R-yl)hexyl]-2-pyrrolidinone) were from Cayman Chemicals (Ann Arbor, MI, USA). Indomethacin and sulprostone were from R&D Systems (Minneapolis, MN, USA). Ryanodine was from AbCam (Cambridge, UK). [2,8-3H] adenine (36 Ci·mmol−1) was from PerkinElmer (Seer Green, Bucks, UK). All other reagents were from Sigma or sources specified in the relevant Methods section. Where dimethyl sulphoxide was used as a solvent (usually 0.01% v/v; 0.1% and 0.5% v/v in Figure 1F,E respectively), it was also included in controls. Key properties of the drugs used are provided in Supporting Information Table S2. The nomenclature of receptors and ligands follows Alexander et al. (2011).
Figure 1.
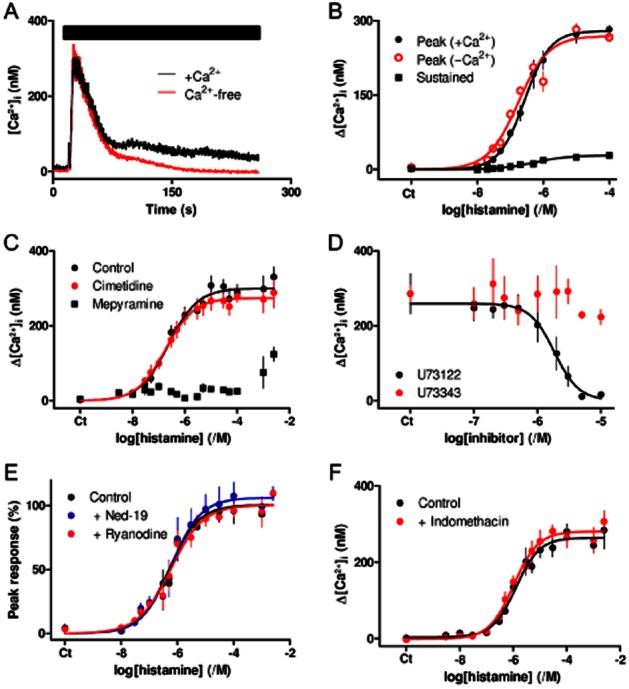
Histamine H1 receptors stimulate an increase in [Ca2+]i via IP3. (A) Histamine (100 μM, bar) stimulates an increase in [Ca2+]i in populations of ASMC incubated in HBS or Ca2+-free HBS. Results show means ± SEM from three wells on a single 96-well plate and are typical of results from four independent plates. (B) Concentration-dependent effects of histamine on the peak [Ca2+]i in the presence or absence of extracellular Ca2+, and on the sustained Ca2+ entry (measured ∼230 s after histamine addition). Results are means ± SEM from four independent plates, with one to three wells on each plate. (C) Effect of mepyramine (0.5 μM) and cimetidine (50 μM), each added 5 min before histamine, on histamine-evoked Ca2+ signals. Results are means ± SEM from nine independent plates, with one to three wells on each plate. (D) Effects of the indicated concentrations of U73122 and U73343 (added 5 min before histamine) on the peak increase in [Ca2+]i evoked by histamine (100 μM). Results are means ± SEM from three independent plates, with two wells on each plate. (E) Effects of trans-Ned-19 (1 μM) and ryanodine (100 μM), each added 5 min before histamine, on the peak increase in [Ca2+]i evoked by histamine. Results (percentage of the maximal response) are means ± SEM from three independent plates with one to three wells on each plate. (F) Effect of indomethacin (10 μM, added 5 min before histamine) on the peak Ca2+ signals evoked by histamine. Results are means ± SEM from four independent plates, each with three wells. (B–F) Ct denotes control.
Results
Histamine H1 receptors evoke Ca2+ signals via IP3 receptors in human ASMCs
Histamine evoked a concentration-dependent increase in [Ca2+]i in populations of human ASMC. Removal of extracellular Ca2+ had no effect on the initial peak response to histamine, but it abolished the very small sustained increase in [Ca2+]i (Figure 1A,B). The two phases of the Ca2+ signal, Ca2+ release from intracellular stores (pEC50 = 6.56 ± 0.09, n = 4) and Ca2+ entry (pEC50 = 6.12 ± 0.30, n = 4), were similarly sensitive to histamine. Responses to histamine were unaffected by cimetidine (50 μM, 5 min), a selective antagonist of H2 histamine receptors, but mepyramine (0.5 μM, 5 min), a competitive antagonist of H1 histamine receptors (Alexander et al., 2011), shifted the concentration–effect relationship to ∼26 000-fold higher concentrations of histamine, suggesting a pKD for mepyramine of ∼10.7 (Figure 1C). Pre-equilibration with mepyramine, which dissociates slowly from H1 histamine receptors, and the need to measure acute responses to histamine probably exaggerate the effect the antagonist would have at equilibrium, but the results are consistent with histamine, evoking Ca2+ signals via a receptor with high affinity for mepyramine and undetectable affinity for cimetidine. U73122, an inhibitor of PLC (Bleasdale et al., 1990), caused a concentration-dependent inhibition (pIC50 = 5.76 ± 0.09, n = 3) of the Ca2+ signals evoked by histamine, while its inactive analogue, U73343 (≤10 μM), had no effect (Figure 1D).
Similar results, although with slightly different sensitivities to histamine and peak Ca2+ signals, were obtained with ASMC isolated from different patients (Supporting Information Figure S1). These differences, presumably arising from different levels of expression of H1 receptors or downstream signalling proteins, highlight the need for paired comparisons of experimental manipulations in subsequent analyses of human ASMC. It also justifies our presentation of some results as percentages of matched control responses.
There are no membrane-permeant selective antagonists of IP3 receptors, although 2-APB has often been used (Taylor and Tovey, 2010). 2-APB caused a concentration-dependent inhibition of the Ca2+ signals evoked by either maximally or submaximally effective concentrations of histamine (Supporting Information Figure S2A–D). However, in keeping with published results (Peppiatt et al., 2003), similar concentrations of 2-APB also caused a substantial loss of Ca2+ from the intracellular stores (Supporting Information Figure S2E,F). While the slightly greater sensitivity of the histamine responses to 2-APB is consistent with the expected involvement of IP3 receptors, the results highlight the limitations of 2-APB as a useful antagonist of IP3 receptors.
Ryanodine receptors and the intracellular Ca2+ channels that are activated by nicotinic acid adenine dinucleotide phosphate (NAADP), probably two-pore channels (Calcraft et al., 2009), can also mediate Ca2+ release from intracellular stores, including those of VSM (Boittin et al., 2002; Tugba Durlu-Kandilci et al., 2010). However, caffeine (10 mM), which activates ryanodine receptors, had no effect on [Ca2+]i (Supporting Information Figure S3A), and the responses to histamine were unaffected by concentrations of either trans-Ned-19 (1 μM, 5 min) that block responses to NAADP (Naylor et al., 2009; Brailoiu et al., 2010) or of ryanodine (100 μM, 5 min) that block ryanodine receptors (Zheng et al., 2005) (Figure 1E).
These results demonstrate that activation of H1 receptors by histamine stimulates Ca2+ release via IP3 receptors in human ASMC, and that neither ryanodine receptors nor two-pore channels contribute to the response. Our results are consistent with widespread expression of H1 histamine receptors in most smooth muscles (Hill et al., 1997) and with evidence that histamine, via H1 receptors, stimulates PLC and an increase in [Ca2+]i in human ASMC (Satoh et al., 1994). In light of subsequent evidence demonstrating effects of PGE2 and cAMP on Ca2+ signals, we considered the possibility that histamine might influence Ca2+ signals via activation of endogenous H2 receptors or stimulate production of endogenous prostanoids. However, neither cimetidine, a selective antagonist of H2 receptors (Figure 1C), nor indomethacin, an inhibitor of cyclooxygenases (Figure 1F), had any effect on histamine-evoked Ca2+ signals in human ASMC.
PGE2 inhibits histamine-evoked Ca2+ signals
PGE2 alone had no effect on [Ca2+]i (Supporting Information Figure S3B), but the Ca2+ signals evoked by histamine (100 μM) were attenuated by PGE2 (10 μM, 5 min) (Figure 2A). PGE2 decreased the sensitivity of ASMC to histamine (pEC50 = 6.32 ± 0.10 and 5.74 ± 0.12, n = 7, in control and PGE2-treated cells respectively) and it reduced the maximal response by 51 ± 2% (Figure 2B). PGE2 had similar effects on both phases of the Ca2+ signal: Ca2+ release and the small Ca2+ entry (Figure 2C). The effects of PGE2 on the peak increase in [Ca2+]i evoked by histamine (3 μM) were concentration dependent (pIC50 = 8.99 ± 0.10, n = 15; Figure 2D). Similar results, although again with some variation in absolute sensitivities, were obtained from different patients (Supporting Information Figure S1 and Table S3). PGE2 (10 μM) did not affect the Ca2+ content of the intracellular stores whether assessed by addition, in Ca2+-free HBS, of thapsigargin or cyclopiazonic acid to inhibit the Ca2+ pump (SERCA) of the sarcoplasmic reticulum (SR), or of ionomycin to release Ca2+ directly (Figure 2E). These results demonstrate that PGE2 causes a concentration-dependent inhibition of the Ca2+ signals evoked by activation of H1 histamine receptors without affecting the Ca2+ content of the SR.
Figure 2.
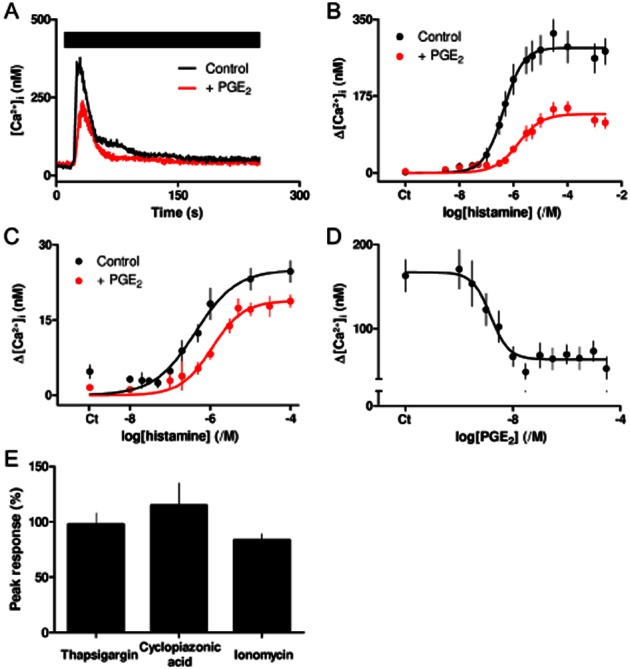
PGE2 inhibits histamine-evoked Ca2+ release. (A) Ca2+ signals evoked by histamine (100 μM, bar) alone or with PGE2 (10 μM, added 5 min before and then with histamine). Results, means ± SEM from three wells on a single plate, are typical of results from four independent plates. (B) Effect of PGE2 (10 μM) on the peak Ca2+ signals evoked by the indicated concentrations of histamine. Results are means ± SEM from seven independent plates, each with one to three wells. (C) Effect of PGE2 on the sustained Ca2+ signals evoked by histamine. Results are means ± SEM from 11 independent plates, each with one to three wells. (D) Effect of the indicated concentrations of PGE2 (added 5 min before histamine) on the peak increase in [Ca2+]i evoked by histamine (3 μM). Results are means ± SEM from 15 independent plates, with one to three wells analysed from each. (B–D) Ct denotes control. Similar results from ASMC isolated from different patients are shown in Supporting Information Figure S1. (E) Effects of pretreatment with PGE2 (10 μM, 5 min) on the peak Ca2+ signals evoked by subsequent addition of thapsigargin (1 μM), cyclopiazonic acid (10 μM) or ionomycin (1 μM) to ASMC in Ca2+-free HBS. Results (as percentages of the responses obtained without PGE2) are means ± SEM from three independent plates, with seven wells analysed on each.
Activation of EP2 or EP4 receptors inhibits histamine-evoked Ca2+ signals
PGE2 activates many prostanoid receptors, with EP1–4, DP1 and FP receptors being the most sensitive (Abramovitz et al., 2000; Alexander et al., 2011). EP1 receptors cannot mediate inhibition of histamine-evoked Ca2+ signals because their coupling to Gq would be expected to increase [Ca2+]i. Sulprostone binds selectively to EP3 receptors, with an affinity similar to that of PGE2. BW 245C and latanoprost are selective high-affinity agonists of DP1 and FP receptors respectively (Abramovitz et al., 2000) (Supporting Information Table S2). Sulprostone (1 μM), BW 245C (10 nM) and latanoprost (100 nM) had no effect on histamine-evoked Ca2+ signals (Supporting Information Figure S3C), suggesting that neither EP3, DP1 nor FP receptors contribute to the inhibition of Ca2+ signals by PGE2. Subsequent experiments therefore assess the contributions of EP2 and EP4 receptors, both of which are expressed in human VSM (Qian et al., 1994; Bayston et al., 2003; Davis et al., 2004; Foudi et al., 2008).
QPCR analysis of ASMC from two patients established that transcripts for EP2 and EP4 receptors were expressed at similar levels. In patients a and d (Supporting Information Table S1), 48 ± 2% and 59 ± 1% of the transcripts were for the EP2 receptor. Although we have not directly assessed protein expression, the results are consistent with comparable levels of expression of EP2 and EP4 receptors in human ASMC.
Butaprost, a selective agonist of EP2 receptors (Sugimoto and Narumiya, 2007; Alexander et al., 2011), caused a concentration-dependent inhibition of the Ca2+ signals evoked by 3 μM histamine (pIC50 = 8.22 ± 0.05, n = 3) (Figure 3A, Table 1). AH6809 is a poorly selective low-affinity antagonist of EP2 receptors (pKD = 5.9), but it does not interact with EP4 receptors (Supporting Information Table S4). The response to butaprost was competitively antagonized by AH6809 (30 μM, ΔpIC50 = 1.12 ± 0.15, where ΔpIC50 = pIC50control − pIC50+antagonist), but insensitive to GW627368X (1 μM, ΔpIC50 = −0.21 ± 0.14), an antagonist of EP4 receptors (Wilson et al., 2006) (Figure 3A, Table 1). These results show that butaprost inhibits histamine-evoked Ca2+ signals via a receptor with an affinity (pKD) for AH6809 of 5.6. This confirms that the inhibition is mediated by EP2 receptors (Supporting Information Table S4).
Figure 3.
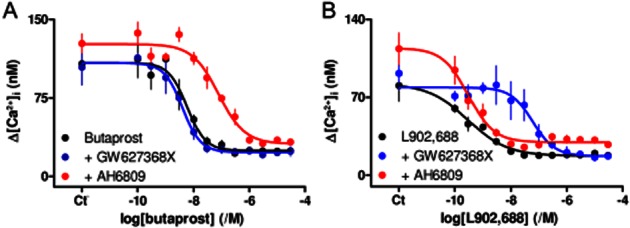
Activation of EP2 or EP4 receptors inhibits histamine-evoked Ca2+ signals. (A, B) Effect of butaprost (A, 5 min) or L902,688 (B, 5 min) on the Ca2+ signals evoked by histamine (3 μM) alone or in the presence of AH6809 (30 μM, 5 min) or GW627368X (1 μM, 5 min). Results (means ± SEM from three independent plates, with two wells analysed from each) show amplitudes of the peak changes in [Ca2+]i evoked by histamine. Ct denotes control. Summary results are shown in Table 1.
Table 1.
Effects of PGE2 and selective ligands of EP2 and EP4 receptors on histamine-evoked Ca2+ signals and cAMP accumulation
| [Ca2+]i, pIC50 (/M) | cAMP, pEC50 (/M) | |||
|---|---|---|---|---|
| Control | AH6809 | GW627368X | Control | |
| PGE2 | 9.27 ± 0.12 (4) | 8.65 ± 0.07* (4) | 9.03 ± 0.13 (4) | 6.62 ± 0.10 (4) |
| Butaprost | 8.22 ± 0.05 (3) | 7.10 ± 0.19* (3) | 8.43 ± 0.17 (3) | 5.68 ± 0.09 (4) |
| L902,688 | 9.52 ± 0.25 (3) | 9.53 ± 0.05 (3) | 7.31 ± 0.21* (3) | 7.76 ± 0.11 (3) |
Concentration-dependent effects of the indicated agonists of EP receptors on the increase in [Ca2+]i evoked by histamine (3 μM) (pIC50) or cAMP accumulation (pEC50) are shown alone or in the presence of antagonists of EP2 (AH6809, 30 μM) or EP4 (GW627368X, 1 μM) receptors. Measurements of cAMP accumulation were performed using the HitHunter HS+ assay. Results are means ± SEM, with the number of independent experiments shown in parentheses.
P < 0.05 relative to control.
L902,688 is a selective agonist of EP4 receptors with slightly greater affinity than PGE2 (Young et al., 2004) (Supporting Information Table S4). L902,688 also caused a concentration-dependent inhibition of histamine-evoked Ca2+ signals (pIC50 = 9.52 ± 0.25, n = 3) (Figure 3B, Table 1). This inhibition was insensitive to AH6809 (30 μM, ΔpIC50 = −0.01 ± 0.27), but competitively inhibited by GW627368X (1 μM, ΔpIC50 = 2.20 ± 0.28). The latter suggests that L902,688 acts via a receptor with an affinity (pKD) for GW627368X of 8.2, consistent with it being an EP4 receptor (Supporting Information Table S4). We conclude that selective activation of either EP2 or EP4 receptors in human ASMC inhibits histamine-evoked Ca2+ release. For butaprost, the sensitivity of the functional response (pIC50 = 8.22) and the published binding affinity (pKD = 7.04) differ by more than 10-fold, whereas they are more similar for L902,688 (9.5 and 9.4 respectively). This suggests that for inhibition of histamine-evoked Ca2+ signals, there is a greater receptor reserve for EP2, than for EP4, receptors.
PGE2 inhibits histamine-evoked Ca2+ signals largely via EP2 receptors
Maximally effective concentrations of PGE2, butaprost or L902,688, caused similar inhibition of histamine-evoked Ca2+ signals, and no combination of the three stimuli caused any greater inhibition than a single stimulus (Figure 4A,B). This suggests that the three stimuli converge to cause inhibition via a common pathway. Subsequent experiments resolve which of the two receptors (EP2 or EP4) mediates the response to the endogenous stimulus, PGE2.
Figure 4.
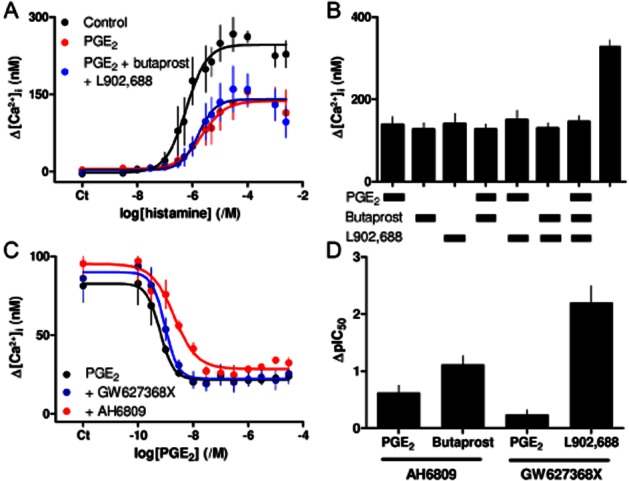
PGE2 inhibits histamine-evoked Ca2+ signals largely via EP2 receptors. (A) Effect of PGE2 (10 μM) alone or in combination with butaprost and L902,688 (10 μM each) on the peak Ca2+ signals evoked by the indicated concentrations of histamine. Results are means ± SEM from three independent plates, each with one to two wells. (B) Peak Ca2+ signals evoked by histamine (3 μM) alone or with (5 min) PGE2 (10 μM), butaprost (100 μM), L902,688 (10 μM) or the indicated combinations. Results are means ± SEM from 11 to 14 wells. (C) Effect of PGE2 (5 min) on the peak Ca2+ signals evoked by histamine (3 μM) alone or in the presence of AH6809 (30 μM, 5 min) or GW627368X (1 μM, 5 min). Results are means ± SEM from four independent plates, each with one to two wells. (A, C) Ct denotes control. (D) ΔpIC50 values for PGE2, butaprost and L902,688 and the antagonists of EP2 (AH6809, 30 μM) or EP4 (GW627368X, 1 μM) receptors. Results are means ± SEM from three to four independent experiments.
Inhibition of histamine-evoked Ca2+ signals by PGE2 (pIC50 = 9.27 ± 0.12, n = 4) was antagonized by AH6809 (30 μM, ΔpIC50 = 0.62 ± 0.13), but to a lesser extent than the response to butaprost (ΔpIC50 = 1.12 ± 0.15). GW627368X (1 μM) also inhibited the responses to PGE2 (ΔpIC50 = 0.24 ± 0.08), but to a much lesser degree than the response to L902,688 (ΔpIC50 = 2.20 ± 0.28) (Figure 4C, Table 1). These results suggest that both EP2 and EP4 receptors contribute to the PGE2-mediated inhibition of histamine-evoked Ca2+ signals, but the magnitudes of the disparities between the effects of the competitive antagonists on responses to PGE2 and those evoked by the subtype-selective agonists (Figure 4D) suggest that EP2 receptors mediate most responses to PGE2. EP4 receptors bind PGE2 with approximately sixfold greater affinity than EP2 receptors (Abramovitz et al., 2000). The predominant role of the latter must, therefore, be due to their greater expression or more effective coupling to downstream signalling, consistent with our suggestion that only EP2 receptors have a receptor reserve. Our evidence that transcripts for EP2 and EP4 receptors are expressed at similar levels would be consistent with more effective coupling of EP2 receptors.
EP2 and EP4 receptors differ in their abilities to stimulate AC
Because EP2 and EP4 receptors share an ability to activate AC via the G protein Gs (Alexander et al., 2011), we examined the effects of their activation on cAMP accumulation in human ASMC. PGE2 evoked a concentration-dependent accumulation of cAMP whether assessed using 3H-adenine labelling (pEC50 = 6.67 ± 0.06, n = 3) or the HitHunter assay (pEC50 = 6.62 ± 0.10, n = 4) (Figure 5). This stimulation of cAMP accumulation was reduced by 83 ± 1% (n = 3) in the presence of inhibitors of AC, SQ 22536 (1 mM) and DDA (200 μM) (hereafter described as SQ/DDA) (Figure 5B). We note that as with histamine-evoked Ca2+ signals, there was a variability between patients and cell passages in the absolute amounts of cAMP produced in response to PGE2, again dictating the need for paired comparisons of treatments (Table 1). These results are consistent with an earlier report in which PGE2, but not histamine, evoked an increase in cAMP in human ASMC (Satoh et al., 1994). Butaprost also stimulated AC activity (pEC50 = 5.68 ± 0.09, n = 4; Figure 5A, Table 1). The similar maximal effects of PGE2 and butaprost, and the ∼10-fold higher EC50 of the latter, are consistent with evidence that butaprost is a full agonist of human EP2 receptors with ∼20-fold lower affinity than PGE2 (Narumiya et al., 1999; Abramovitz et al., 2000) (Supporting Information Table S4).
Figure 5.
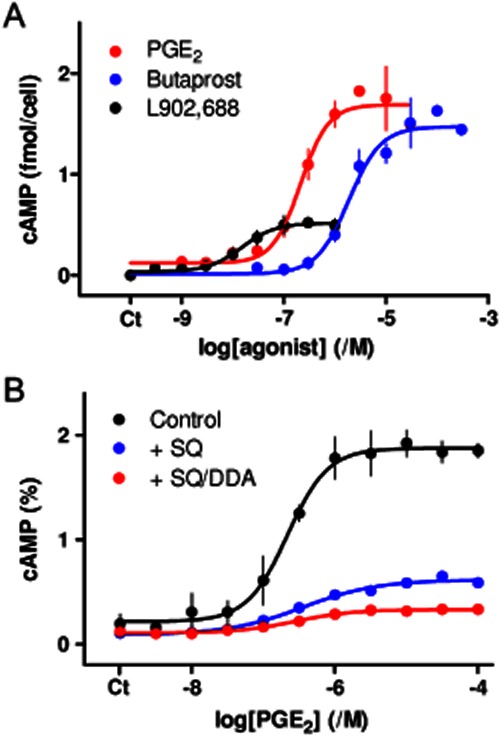
PGE2 stimulates AC predominantly via EP2 receptors. (A) Effects of the indicated concentrations of PGE2, butaprost and L902,688 (5 min) on cAMP formation measured using a HitHunter assay. (B) Effect of PGE2 (5 min) on cAMP accumulation (determined using 3H-adenine labelling, and expressed as percentages of 3H-ATP + 3H-ADP) alone or after treatment with SQ 22536 (1 mM SQ) alone (n = 1) or SQ 22536 with DDA (200 μM) (n = 3). Both inhibitors were added 20 min before and then during stimulation with PGE2. Results (except for SQ alone in panel B) are means ± SEM from three to four independent plates. Ct denotes control.
L902,688, which has approximately twofold greater affinity than PGE2 for EP4 receptors (Supporting Information Table S4), stimulated a concentration-dependent (pEC50 = 7.76 ± 0.11) accumulation of cAMP that was only 32 ± 2% (n = 3) of that evoked by a maximally effective concentration of PGE2 (Figure 5A). The lesser maximal response to L902,688 and greater sensitivity relative to PGE2 (Figure 5A) suggest that EP4 receptors probably mediate the effect of L902,688 on AC activity (Supporting Information Table S4). We suggest that both EP2 and EP4 receptors stimulate AC in human ASMC, but the former is either more abundant or more effectively coupled to AC, and largely mediates the effects of PGE2. More effective coupling of EP2 receptors would be consistent with our analysis of transcripts for EP2 and EP4 receptors, and with analyses showing that EP2 receptors evoke greater stimulation of AC than do EP4 receptors when each is heterologously expressed at a similar level (Fujino et al., 2002).
Discussion
Activation of histamine H1 receptors evokes an increase in [Ca2+]i in human ASMC that results from Ca2+ release via IP3 receptors, followed by a small sustained response mediated by Ca2+ entry, most likely via a store-operated Ca2+ entry pathway (Figure 1). Such biphasic Ca2+ signals are typical of those evoked by PLC-coupled receptors in VSM (Berridge, 2008) and other tissues (Putney, 1997). PGE2 is another inflammatory mediator with widespread actions in the vasculature (Norel, 2007). The receptors through which PGE2 regulates human ASMC have not hitherto been defined, although both EP2 and EP4 receptors mediate relaxation and/or attenuation of Ca2+ signalling in other human smooth muscles (Baxter et al., 1995; Jones et al., 1997; Benyahia et al., 2012).
We have shown that in human ASMC, PGE2 attenuates histamine-evoked Ca2+ signals without affecting the Ca2+ content of the intracellular stores (Figure 2). This inhibition is mediated by cAMP (Pantazaka et al., unpubl. obs.). Inhibition of histamine-evoked Ca2+ signals is mimicked by selective activation of either EP2 or EP4 receptors (Figures 3 and 4A–C), but results with selective antagonists suggest that EP2 receptors predominantly mediate the effect of PGE2 on [Ca2+]i (Figure 4D). This is consistent with the relationships between pIC50 and published KD values for the subtype-selective agonists suggesting a greater receptor reserve for EP2 receptors. It also aligns with evidence that both EP2 and EP4 receptors stimulate AC activity, but the maximal response is much larger for EP2 receptors (Figure 5A). The predominant role of EP2, relative to EP4, receptors, despite the lower KD of the latter (Supporting Information Table S4), could arise from higher levels of expression of EP2 receptors in human ASMC or their more effective coupling to stimulation of AC (Fujino et al., 2002). The latter seems more likely because transcripts for EP2 and EP4 receptors are expressed at similar levels in human ASMC. There is some evidence that functional EP receptors, including EP2 and EP4 receptors, are expressed in both the plasma membrane and the nuclear envelope of some cells (Zhu et al., 2006). We have not assessed whether this contributes to the ability of PGE2 to inhibit histamine-evoked Ca2+ signals.
Our results demonstrate that two important inflammatory mediators, histamine and PGE2, acting via receptors that are targets of existing clinically approved drugs, interact to control [Ca2+]i in human ASMC. EP2, EP4 and histamine H1 receptors might thereby provide effective therapeutic targets for treatment of atherosclerosis.
Acknowledgments
We thank Trevor Littlewood (Division of Cardiovascular Medicine) for provision of ASMC and advice on their culture. We thank Stephen Tovey, Taufiq Rahman and Nadia Shah for discussions; Sriram Govindan for preliminary analyses of ASMC; and Kathryn Shelley for contributions to QPCR analyses. This work was supported by the Medical Research Council (G0900049), the Wellcome Trust (085295) and a research studentship (to W. G. B.) from the British Heart Foundation (FS/11/77/29327).
Glossary
- 2-APB
2-aminoethoxydiphenyl borate
- AC
adenylyl cyclase
- AH6809
6-isopropoxy-9-oxoxanthene-2-carboxylic acid
- ASMC
aortic smooth muscle cell
- BW 245C
(4S)-(3-[(3R,S)-3-cyclohexyl-3-hydroxypropyl]-2,5-dioxo)-4-imidazolidineheptanoic acid
- [Ca2+]i
intracellular free Ca2+ concentration
- DDA
2′,5′-dideoxyadenosine
- GW627368X
2-[4-(4,9-diethoxy-3-oxo-1H-benzo[f]isoindol-2-yl)phenyl]-N-phenylsulfonylacetamide
- HBS
HEPES-buffered saline
- IP3
inositol 1,4,5-trisphosphate
- L902,688
5-[(1E,3R)-4,4-difluoro-3-hydroxy-4-phenyl-1-buten-1-yl]-1-[6-(2H-tetrazol-5R-yl)hexyl]-2-pyrrolidinone
- NAADP
nicotinic acid adenine dinucleotide phosphate
- pEC50 (pIC50)
negative logarithm of the half-maximally effective (inhibitory) concentration
- PLC
phospholipase C
- SQ 22536
9-(tetrahydro-2-furanyl)-9H-purin-6-amine
- SQ/DDA
1 mM SQ 22536 with 200 μM DDA (used to inhibit AC)
- SR
sarcoplasmic reticulum
- trans-Ned-19
(1R,3S)-1-[3-[[4-(2-fluorophenyl)piperazin-1-yl]methyl]-4-methoxyphenyl]-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylic acid
- U73122
1-[6-[[(17β)-3-methoxyestra-1,3,5(10)-trien-17-yl]amino]hexyl]-1H-pyrrole-2,5-dione
- U73343
1-[6-[[(17β)-3-methoxyestra-1,3,5(10)-trien-17 yl]amino]hexyl]-2,5-pyrrolidinedione
- VSM
vascular smooth muscle
Conflict of interest
None.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
Figure S1 Inhibition of histamine-evoked Ca2+ signals by PGE2 in ASMC from different patients. (A–C) Peak Ca2+ signals evoked by the indicated concentrations of histamine alone or with PGE2 (10 μM added 5 min before histamine). Results are from three different patients (codes in Supporting Information Table S1) and show means ± SEM from five (A), 15 (B) and 11 (C) independent experiments. (D). Effect of the indicated concentrations of PGE2 (added 5 min before histamine) on the pEC50 for histamine-evoked peak Ca2+ signals. Ct denotes control.
Figure S2 Inhibition of histamine-evoked Ca2+ signals and depletion of intracellular Ca2+ stores by 2-APB. (A, C) Effect of 2-APB (added 5 min before histamine) on the Ca2+ signals evoked by submaximal (3 μM) (A) or maximal (1 mM) (C) concentrations of histamine in Ca2+-free HBS. (E) Similar analyses of the effects of 2-APB on the Ca2+ content of the intracellular stores assessed by addition of ionomycin (1 μM). The code shown in panel E applies to all three panels (B, D, F). Summary results show peak Ca2+ signals evoked by histamine (B, D) or ionomycin (F) after the indicated treatments with 2-APB. Results (A–F) are means ± SEM from three independent plates, with two wells analysed from each.
Figure S3 Caffeine and PGE2 do not directly affect [Ca2+]i, and neither EP3, DP1 nor FP receptors contribute to inhibition of Ca2+ signals by PGE2. (A) Populations of ASMC in HBS were stimulated with caffeine (A, 10 mM) and then histamine (3 μM). (B) In similar experiments, ASMCs were stimulated with histamine alone (3 μM) or after pretreatment with PGE2 (10 μM) as shown. The trace shows that although PGE2 inhibits histamine-evoked Ca2+ signals, it does not itself affect [Ca2+]i. Results (A and B) are means ± SEM from four wells on a single 96-well plate and are typical of results from three independent experiments. (C) The peak increase in [Ca2+]i evoked by histamine (3 μM) is shown for histamine alone or in the presence of PGE2 (100 nM) or agonists selective for EP3 receptors (sulprostone, 1 μM), DP1 receptors (BW 245C, 10 nM) or FP receptors (latanoprost, 100 nM) (each added 5 min before histamine). Results are means ± SEM from 8–29 wells.
Table S1 Sources of the human ASMC used.
Table S2 Key properties of the drugs used.
Table S3 Attenuation of histamine-evoked Ca2+ signals in human ASMC from different patients.
Table S4 Reported affinities (pKD) of drugs used for analysis of EP2 and EP4 receptors.
References
- Abramovitz M, Adam M, Boie Y, Carriere M, Denis D, Godbout C, et al. The utilization of recombinant prostanoid receptors to determine the affinities and selectivities of prostaglandins and related analogs. Biochim Biophys Acta. 2000;1483:285–293. doi: 10.1016/s1388-1981(99)00164-x. [DOI] [PubMed] [Google Scholar]
- Alexander SP, Mathie A, Peters JA. Guide to receptors and channels (GRAC), 5th edition. Br J Pharmacol. 2011;164(Suppl. 1):S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter GS, Clayton JK, Coleman RA, Marshall K, Sangha R, Senior J. Characterization of the prostanoid receptors mediating constriction and relaxation of human isolated uterine artery. Br J Pharmacol. 1995;116:1692–1696. doi: 10.1111/j.1476-5381.1995.tb16393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayston T, Ramessur S, Reise J, Jones KG, Powell JT. Prostaglandin E2 receptors in abdominal aortic aneurysm and human aortic smooth muscle cells. J Vasc Surg. 2003;38:354–359. doi: 10.1016/s0741-5214(03)00339-2. [DOI] [PubMed] [Google Scholar]
- Benyahia C, Gomez I, Kanyinda L, Boukais K, Danel C, Leseche G, et al. PGE2 receptor (EP4) agonists: potent dilators of human bronchi and future asthma therapy. Pulm Pharmacol Ther. 2012;25:115–118. doi: 10.1016/j.pupt.2011.12.012. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Smooth muscle cell calcium activation mechanisms. J Physiol. 2008;586:5047–5061. doi: 10.1113/jphysiol.2008.160440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleasdale JE, Thakur NR, Gremban RS, Bundy GL, Fitzpatrick FA, Smith RJ, et al. Selective inhibition of receptor-coupled phospholipase C-dependent processes in human platelets and polymorphonuclear neutrophils. J Pharmacol Exp Ther. 1990;255:756–768. [PubMed] [Google Scholar]
- Boittin FX, Galione A, Evans AM. Nicotinic acid adenine dinucleotide phosphate mediates Ca2+ signals and contraction in arterial smooth muscle via a two-pool mechanism. Circ Res. 2002;91:1168–1175. doi: 10.1161/01.res.0000047507.22487.85. [DOI] [PubMed] [Google Scholar]
- Boyle JJ, Weissberg PL, Bennett MR. Human macrophage-induced vascular smooth muscle cell apoptosis requires NO enhancement of Fas/Fas-L interactions. Arterioscler Thromb Vasc Biol. 2002;22:1624–1630. doi: 10.1161/01.atv.0000033517.48444.1a. [DOI] [PubMed] [Google Scholar]
- Brailoiu E, Rahman T, Churamani D, Prole DL, Brailoiu GC, Taylor CW, et al. An NAADP-gated two-pore channel targeted to the plasma membrane uncouples triggering from amplifying Ca2+ signals. J Biol Chem. 2010;285:38611–38616. doi: 10.1074/jbc.M110.162073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breyer RM, Bagdassarian CK, Myers SA, Breyer MD. Prostanoid receptors: subtypes and signaling. Annu Rev Pharmacol Toxicol. 2001;41:661–690. doi: 10.1146/annurev.pharmtox.41.1.661. [DOI] [PubMed] [Google Scholar]
- Calcraft PJ, Ruas M, Pan Z, Cheng X, Arredouani A, Hao X, et al. NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature. 2009;459:596–600. doi: 10.1038/nature08030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman RA, Smith WL, Narumiya S. International Union of Pharmacology classification of prostanoid receptors: properties, distribution, and structure of the receptors and their subtypes. Pharmacol Rev. 1994;46:205–229. [PubMed] [Google Scholar]
- Davis RJ, Murdoch CE, Ali M, Purbrick S, Ravid R, Baxter GS, et al. EP4 prostanoid receptor-mediated vasodilatation of human middle cerebral arteries. Br J Pharmacol. 2004;141:580–585. doi: 10.1038/sj.bjp.0705645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foudi N, Kotelevets L, Louedec L, Leseche G, Henin D, Chastre E, et al. Vasorelaxation induced by prostaglandin E2 in human pulmonary vein: role of the EP4 receptor subtype. Br J Pharmacol. 2008;154:1631–1639. doi: 10.1038/bjp.2008.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujino H, West KA, Regan JW. Phosphorylation of glycogen synthase kinase-3 and stimulation of T-cell factor signaling following activation of EP2 and EP4 prostanoid receptors by prostaglandin E2. J Biol Chem. 2002;277:2614–2619. doi: 10.1074/jbc.M109440200. [DOI] [PubMed] [Google Scholar]
- Gee KR, Brown KA, Chen WN, Bishop-Stewart J, Gray D, Johnson I. Chemical and physiological characterization of fluo-4 Ca2+-indicator dyes. Cell Calcium. 2000;27:97–106. doi: 10.1054/ceca.1999.0095. [DOI] [PubMed] [Google Scholar]
- Gomez-Hernandez A, Martin-Ventura JL, Sanchez-Galan E, Vidal C, Ortego M, Blanco-Colio LM, et al. Overexpression of COX-2, prostaglandin E synthase-1 and prostaglandin E receptors in blood mononuclear cells and plaque of patients with carotid atherosclerosis: regulation by nuclear factor-κ B. Atherosclerosis. 2006;187:139–149. doi: 10.1016/j.atherosclerosis.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Govindan S, Taylor EJA, Taylor CW. Ca2+ signalling by P2Y receptors in cultured rat aortic smooth muscle cells. Br J Pharmacol. 2010;160:1953–1962. doi: 10.1111/j.1476-5381.2010.00763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- Hill SJ, Ganellin CR, Timmerman H, Schwartz JC, Shankley NP, Young JM, et al. International Union of Pharmacology. XIII. Classification of histamine receptors. Pharmacol Rev. 1997;49:253–278. [PubMed] [Google Scholar]
- House SJ, Potier M, Bisaillon J, Singer HA, Trebak M. The non-excitable smooth muscle: calcium signaling and phenotypic switching during vascular disease. Pflugers Arch. 2008;456:769–785. doi: 10.1007/s00424-008-0491-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadhav V, Jabre A, Lin SZ, Lee TJ. EP1- and EP3-receptors mediate prostaglandin E2-induced constriction of porcine large cerebral arteries. J Cereb Blood Flow Metab. 2004;24:1305–1316. doi: 10.1097/01.WCB.0000139446.61789.14. [DOI] [PubMed] [Google Scholar]
- Jones BL, Kearns GL. Histamine: new thoughts about a familiar mediator. Clin Pharmacol Ther. 2011;89:189–197. doi: 10.1038/clpt.2010.256. [DOI] [PubMed] [Google Scholar]
- Jones RL, Woodward DF. Interaction of prostanoid EP and TP receptors in guinea-pig isolated aorta: contractile self-synergism of 11-deoxy-16,16-dimethyl PGE. Br J Pharmacol. 2011;162:521–531. doi: 10.1111/j.1476-5381.2010.01039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RL, Qian Y, Wong HN, Chan H, Yim AP. Prostanoid action on the human pulmonary vascular system. Clin Exp Pharmacol Physiol. 1997;24:969–972. doi: 10.1111/j.1440-1681.1997.tb02730.x. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Murata T, Hori M, Ozaki H. Prostaglandin E2-prostanoid EP3 signal induces vascular contraction via nPKC and ROCK activation in rat mesenteric artery. Eur J Pharmacol. 2011;660:375–380. doi: 10.1016/j.ejphar.2011.03.032. [DOI] [PubMed] [Google Scholar]
- Miyazawa N, Watanabe S, Matsuda A, Kondo K, Hashimoto H, Umemura K, et al. Role of histamine H1 and H2 receptor antagonists in the prevention of intimal thickening. Eur J Pharmacol. 1998;362:53–59. doi: 10.1016/s0014-2999(98)00716-x. [DOI] [PubMed] [Google Scholar]
- Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties, and functions. Physiol Rev. 1999;79:1193–1226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- Naylor E, Arredouani A, Vasudevan SR, Lewis AM, Parkesh R, Mizote A, et al. Identification of a chemical probe for NAADP by virtual screening. Nat Chem Biol. 2009;5:220–226. doi: 10.1038/nchembio.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norel X. Prostanoid receptors in the human vascular wall. ScientificWorldJournal. 2007;7:1359–1374. doi: 10.1100/tsw.2007.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr AW, Hastings NE, Blackman BR, Wamhoff BR. Complex regulation and function of the inflammatory smooth muscle cell phenotype in atherosclerosis. J Vasc Res. 2010;47:168–180. doi: 10.1159/000250095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppiatt CM, Collins TJ, Mackenzie L, Conway SJ, Holmes AB, Bootman MD, et al. 2-Aminoethoxydiphenyl borate (2-APB) antagonises inositol 1,4,5-trisphosphate-induced calcium release, inhibits calcium pumps and has a use-dependent and slowly reversible action on store-operated calcium entry channels. Cell Calcium. 2003;34:97–108. doi: 10.1016/s0143-4160(03)00026-5. [DOI] [PubMed] [Google Scholar]
- Putney JW., Jr . Capacitative Calcium Entry. Austin, TX: R.G. Landes Company; 1997. [Google Scholar]
- Qian YM, Jones RL, Chan KM, Stock AI, Ho JK. Potent contractile actions of prostanoid EP3-receptor agonists on human isolated pulmonary artery. Br J Pharmacol. 1994;113:369–374. doi: 10.1111/j.1476-5381.1994.tb16997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roscioni SS, Maarsingh H, Elzinga CR, Schuur J, Menzen M, Halayko AJ, et al. Epac as a novel effector of airway smooth muscle relaxation. J Cell Mol Med. 2010;15:1551–1563. doi: 10.1111/j.1582-4934.2010.01150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon Y, Londos C, Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974;58:541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Sasaguri Y, Wang KY, Tanimoto A, Tsutsui M, Ueno H, Murata Y, et al. Role of histamine produced by bone marrow-derived vascular cells in pathogenesis of atherosclerosis. Circ Res. 2005;96:974–981. doi: 10.1161/01.RES.0000166325.00383.ed. [DOI] [PubMed] [Google Scholar]
- Satoh T, Sugama K, Matsuo A, Kato S, Ito S, Hatanaka M, et al. Histamine as an activator of cell growth and extracellular matrix reconstruction for human vascular smooth muscle cells. Atherosclerosis. 1994;110:53–61. doi: 10.1016/0021-9150(94)90067-1. [DOI] [PubMed] [Google Scholar]
- Soler M, Camacho M, Escudero JR, Iniguez MA, Vila L. Human vascular smooth muscle cells but not endothelial cells express prostaglandin E synthase. Circ Res. 2000;87:504–507. doi: 10.1161/01.res.87.6.504. [DOI] [PubMed] [Google Scholar]
- Sugimoto Y, Narumiya S. Prostaglandin E receptors. J Biol Chem. 2007;282:11613–11617. doi: 10.1074/jbc.R600038200. [DOI] [PubMed] [Google Scholar]
- Swedenborg J, Mayranpaa MI, Kovanen PT. Mast cells: important players in the orchestrated pathogenesis of abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2011;31:734–740. doi: 10.1161/ATVBAHA.110.213157. [DOI] [PubMed] [Google Scholar]
- Takagishi T, Sasaguri Y, Nakano R, Arima N, Tanimoto A, Fukui H, et al. Expression of the histamine H1 receptor gene in relation to atherosclerosis. Am J Pathol. 1995;146:981–988. [PMC free article] [PubMed] [Google Scholar]
- Tanimoto A, Sasaguri Y, Ohtsu H. Histamine network in atherosclerosis. Trends Cardiovasc Med. 2006;16:280–284. doi: 10.1016/j.tcm.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Taylor CW, Tovey SC. IP3 receptors: towards understanding their activation. Cold Spring Harb Perspect Biol. 2010;2:a004010. doi: 10.1101/cshperspect.a004010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda N. Mechanism of histamine actions in human coronary arteries. Circ Res. 1987;61:280–286. doi: 10.1161/01.res.61.2.280. [DOI] [PubMed] [Google Scholar]
- Tugba Durlu-Kandilci N, Ruas M, Chuang KT, Brading A, Parrington J, Galione A. TPC2 proteins mediate nicotinic acid adenine dinucleotide phosphate (NAADP)- and agonist-evoked contractions of smooth muscle. J Biol Chem. 2010;285:24925–24932. doi: 10.1074/jbc.M110.129833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walch L, de Montpreville V, Brink C, Norel X. Prostanoid EP1- and TP-receptors involved in the contraction of human pulmonary veins. Br J Pharmacol. 2001;134:1671–1678. doi: 10.1038/sj.bjp.0704423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Okamura T, Toda N. Mechanisms of histamine-induced relaxation in external and internal ophthalmic arteries. Invest Ophthalmol Vis Sci. 1993;34:41–48. [PubMed] [Google Scholar]
- Wilson RJ, Giblin GM, Roomans S, Rhodes SA, Cartwright KA, Shield VJ, et al. GW627368X ((N-{2-[4-(4,9-diethoxy-1-oxo-1,3-dihydro-2H-benzo[f]isoindol-2-yl)phenyl]acetyl} benzene sulphonamide): a novel, potent and selective prostanoid EP4 receptor antagonist. Br J Pharmacol. 2006;148:326–339. doi: 10.1038/sj.bjp.0706726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Kwan YW, Au AL, Poon CC, Zhang Q, Chan SW, et al. 14,15-Epoxyeicosatrienoic acid induces vasorelaxation through the prostaglandin EP2 receptors in rat mesenteric artery. Prostaglandins Other Lipid Mediat. 2010;93:44–51. doi: 10.1016/j.prostaglandins.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Yang C, Liu X, Cao Q, Liang Q, Qiu X. Prostaglandin E receptors as inflammatory therapeutic targets for atherosclerosis. Life Sci. 2011;88:201–205. doi: 10.1016/j.lfs.2010.11.015. [DOI] [PubMed] [Google Scholar]
- Young RN, Billot X, Han Y, Slipetz DA, Chauret N, Belley M, et al. Discovery and synthesis of a potent, selective and orally bioavailble EP4 receptor agonist. Heterocycles. 2004;64:437–446. [Google Scholar]
- Zheng YM, Wang QS, Rathore R, Zhang WH, Mazurkiewicz JE, Sorrentino V, et al. Type-3 ryanodine receptors mediate hypoxia-, but not neurotransmitter-induced calcium release and contraction in pulmonary artery smooth muscle cells. J Gen Physiol. 2005;125:427–440. doi: 10.1085/jgp.200409232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu T, Gobeil F, Vazquez-Tello A, Leduc M, Rihakova L, Bossolasco M, et al. Intracrine signaling through lipid mediators and their cognate nuclear G-protein-coupled receptors: a paradigm based on PGE2, PAF, and LPA1 receptors. Can J Physiol Pharmacol. 2006;84:377–391. doi: 10.1139/y05-147. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


