Abstract
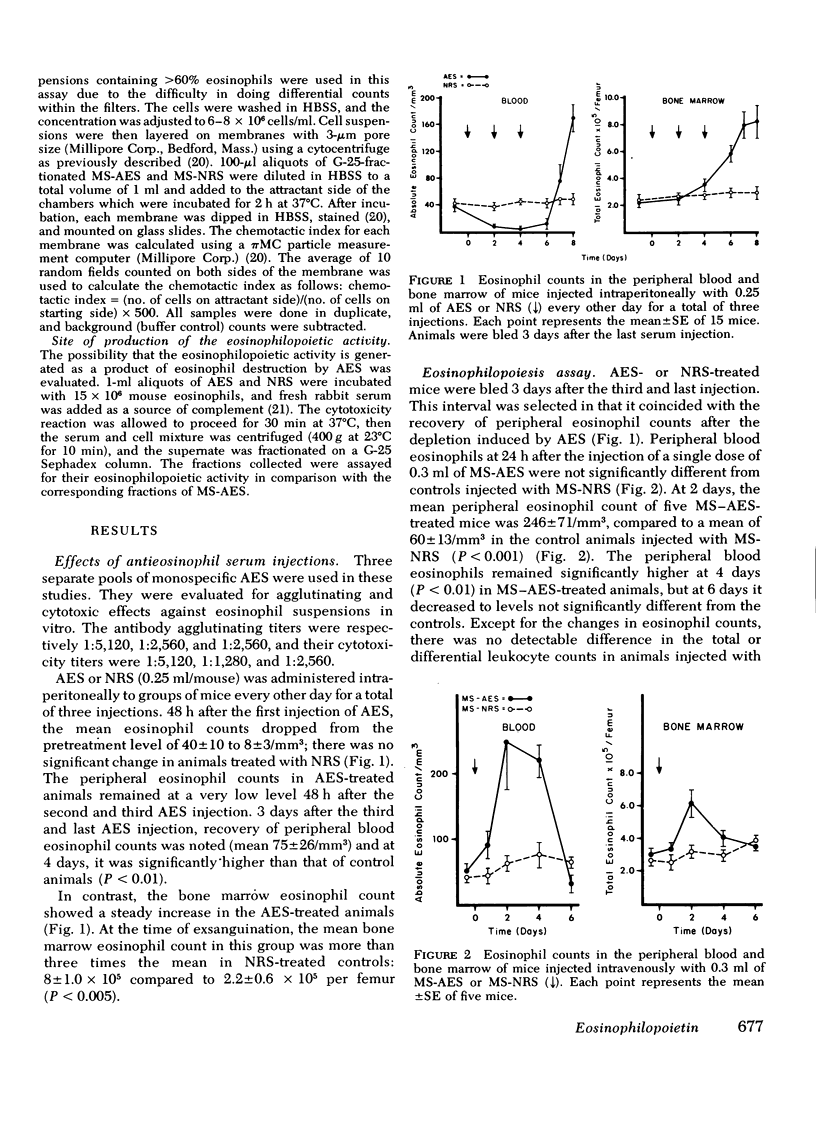
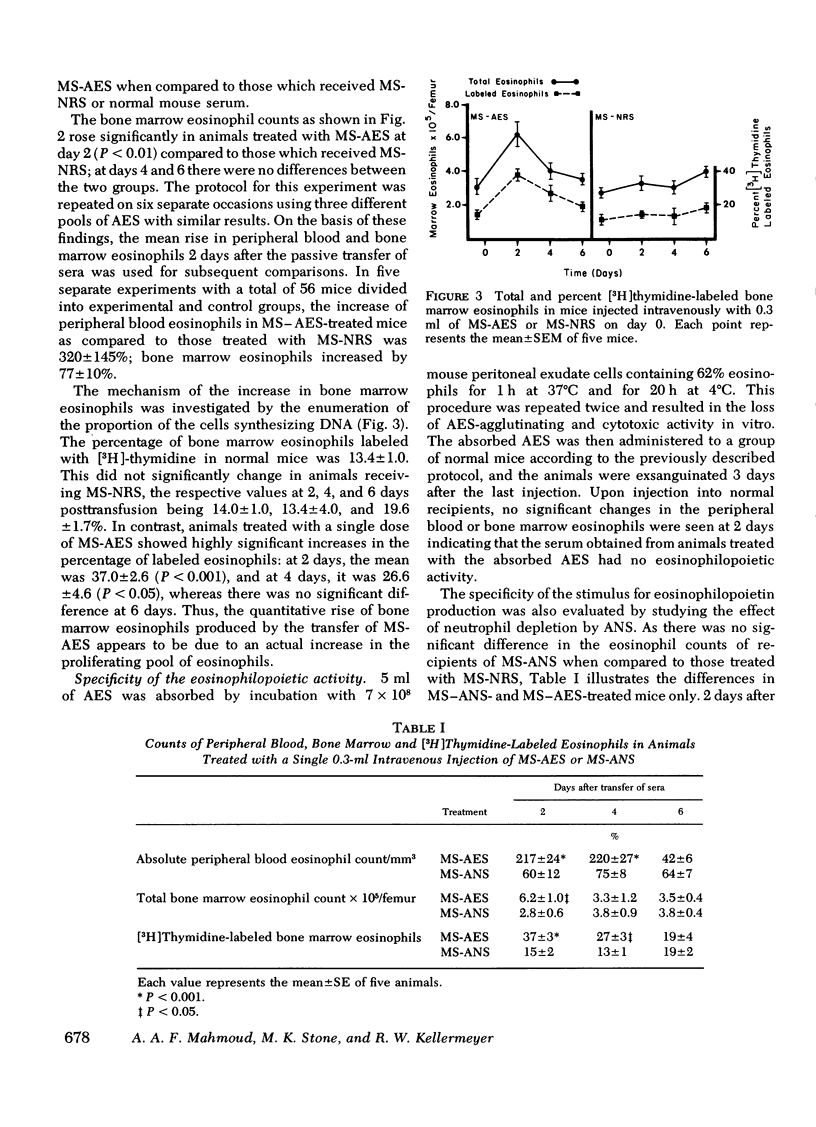
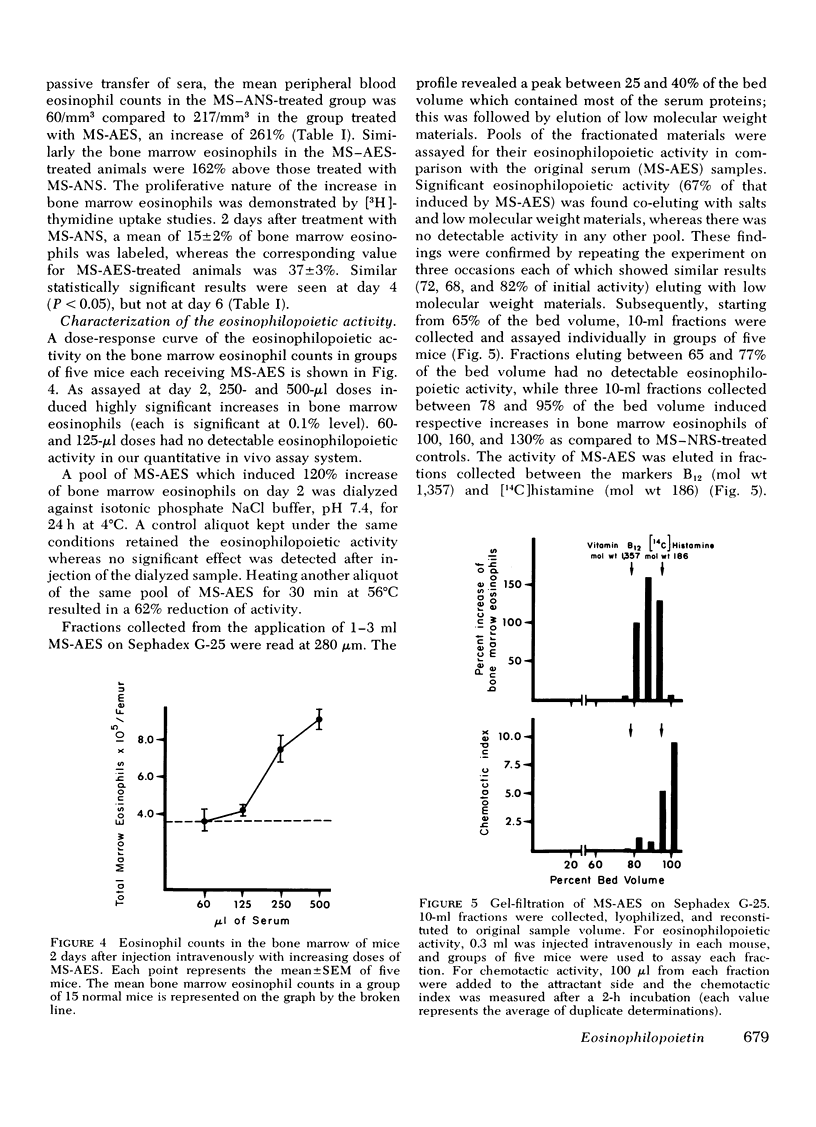
In earlier studies, methods were developed to raise specific antibodies in rabbits against purified suspensions of mouse or human eosinophils. On administration of antieosinophil serum (AES) to mice, the mature eosinophils in tissues, peripheral blood, and bone marrow were depleted, while the immature eosinophil pool in the bone marrow was observed to proliferate. The current investigations explore the generation of eosinophilopoietic factors during AES-induced eosinophilopenia. Mice received three injections of AES, one every other day. As the peripheral eosinophil counts started to recover after the last AES injection, the serum was collected and transferred to normal animals. Within 2 days the recipients showed an increase in peripheral blood as well as in bone marrow eosinophils. The rise in bone marrow eosinophils was due to newly formed cells as evidenced by increased uptake of [3H]thymidine. The generation of eosinophilopoietic activity was specifically related to depletion of eosinophils but not neutrophils. The eosinophilopoietic activity was: (a) dependent on the volume of serum transferred, (b) lost on dialysis, and (c) largely heat labile. The activity eluted as a low molecular weight substance on G-25 Sephadex and was digested by pronase but not by trypsin. Active fractions collected from G-25 columns were not chemotactic for the eosinophils in vitro. Thus, specific depletion of mature eosinophils generates a low molecular weight peptide which stimulates eosinophilopoiesis in vivo. It is suggested that this substance be named eosinophilopoietin.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bass D. A. Behavior of eosinophil leukocytes in acute inflammation. II. Eosinophil dynamics during acute inflammation. J Clin Invest. 1975 Oct;56(4):870–879. doi: 10.1172/JCI108166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basten A., Beeson P. B. Mechanism of eosinophilia. II. Role of the lymphocyte. J Exp Med. 1970 Jun 1;131(6):1288–1305. doi: 10.1084/jem.131.6.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggs D. R., Chervenick P. A., Marsh J. C., Cartwright G. E., Wintrobe M. M. Neutrophil-releasing activity in plasma of dogs injected with endotoxin. J Lab Clin Med. 1968 Aug;72(2):177–191. [PubMed] [Google Scholar]
- Butterworth A. E., David J. R., Franks D., Mahmoud A. A., David P. H., Sturrock R. F., Houba V. Antibody-dependent eosinophil-mediated damage to 51Cr-labeled schistosomula of Schistosoma mansoni: damage by purieid eosinophils. J Exp Med. 1977 Jan 1;145(1):136–150. doi: 10.1084/jem.145.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth A. E., Sturrock R. F., Houba V., Mahmoud A. A., Sher A., Rees P. H. Eosinophils as mediators of antibody-dependent damage to schistosomula. Nature. 1975 Aug 28;256(5520):727–729. doi: 10.1038/256727a0. [DOI] [PubMed] [Google Scholar]
- Butterworth A. E., Sturrock R. F., Houba V., Taylor R. Schistosoma mansoni in baboons. Antibody-dependent cell-mediated damage to 51Cr-labelled schistosomula. Clin Exp Immunol. 1976 Jul;25(1):95–102. [PMC free article] [PubMed] [Google Scholar]
- Chusid M. J., Dale D. C., West B. C., Wolff S. M. The hypereosinophilic syndrome: analysis of fourteen cases with review of the literature. Medicine (Baltimore) 1975 Jan;54(1):1–27. [PubMed] [Google Scholar]
- Clark R. A., Gallin J. I., Kaplan A. P. The selective eosinophil chemotactic activity of histamine. J Exp Med. 1975 Dec 1;142(6):1462–1476. doi: 10.1084/jem.142.6.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S., Ward P. A. In vitro and in vivo activity of a lymphocyte and immune complex-dependent chemotactic factor for eosinophils. J Exp Med. 1971 Jan 1;133(1):133–146. doi: 10.1084/jem.133.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colley D. G. Eosinophils and immune mechanisms. Eosinophil stimulation promoter (ESP): a lymphokine induced by specific antigen or phytohemagglutinin. J Immunol. 1973 May;110(5):1419–1423. [PubMed] [Google Scholar]
- Engelfriet C. P., Britten A. Cytotoxic antibodies against leucocytes. Vox Sang. 1966 May-Jun;11(3):334–344. [PubMed] [Google Scholar]
- GORDON A. S., HANDLER E. S., SIEGEL C. D., DORNFEST B. S., LOBUE J. PLASMA FACTORS INFLUENCING LEUKOCYTE RELEASE IN RATS. Ann N Y Acad Sci. 1964 Feb 28;113:766–789. doi: 10.1111/j.1749-6632.1964.tb40703.x. [DOI] [PubMed] [Google Scholar]
- Greene B. M., Colley D. G. Eosinophils and immune mechanisms. II. Partial characterization of the lymphokine eosinophil stimulation promoter. J Immunol. 1974 Sep;113(3):910–917. [PubMed] [Google Scholar]
- Grove D. I., Mahmoud A. A., Warren K. S. Eosinophils and resistance to Trichinella spiralis. J Exp Med. 1977 Mar 1;145(3):755–759. doi: 10.1084/jem.145.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay A. B., Stechschulte D. J., Austen K. F. An eosinophil leukocyte chemotactic factor of anaphylaxis. J Exp Med. 1971 Mar 1;133(3):602–619. doi: 10.1084/jem.133.3.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazura J. W., Mahmoud A. A., Karb K. S., Warren K. S. The lymphokine eosinophil stimulation promoter and human schistosomiasis mansoni. J Infect Dis. 1975 Dec;132(6):702–706. doi: 10.1093/infdis/132.6.702. [DOI] [PubMed] [Google Scholar]
- Mahmoud A. A., Kellermeyer R. W., Warren K. S. Monospecific antigranulocyte sera against human neutrophils, eosinophils, basophils, and myeloblasts. Lancet. 1974 Nov 16;2(7890):1163–1166. doi: 10.1016/s0140-6736(74)90809-5. [DOI] [PubMed] [Google Scholar]
- Mahmoud A. A., Kellermeyer R. W., Warren K. S. Production of monospecific rabbit antihuman eosinophil serums and demonstration of a blocking phenomenon. N Engl J Med. 1974 Feb 21;290(8):417–420. doi: 10.1056/NEJM197402212900801. [DOI] [PubMed] [Google Scholar]
- Mahmoud A. A., Warren K. S., Boros D. I. Production of a rabbit antimouse eosinophil serum with no cross-reactivity to neutrophils. J Exp Med. 1973 Jun 1;137(6):1526–1531. doi: 10.1084/jem.137.6.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud A. A., Warren K. S., Graham R. C., Jr Antieosinophil serum and the kinetics of eosinophilia in Schistosomiasis mansoni. J Exp Med. 1975 Sep 1;142(3):560–574. doi: 10.1084/jem.142.3.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud A. A., Warren K. S., Peters P. A. A role for the eosinophil in acquired resistance to Schistosoma mansoni infection as determined by antieosinophil serum. J Exp Med. 1975 Oct 1;142(4):805–813. doi: 10.1084/jem.142.4.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. M., Colley D. G., McGarry M. P. Spleen cells from Schistosoma mansoni-infected mice produce diffusible stimulator of eosinophilopoiesis in vivo. Nature. 1976 Aug 12;262(5569):586–587. doi: 10.1038/262586a0. [DOI] [PubMed] [Google Scholar]
- Robinson W. A., Mangalik A. The kinetics and regulation of granulopoiesis. Semin Hematol. 1975 Jan;12(1):7–25. [PubMed] [Google Scholar]
- Rothstein G., Hügl E. H., Chervenick P. A., Athens J. W., Macfarlane J. Humoral stimulators of granulocyte production. Blood. 1973 Jan;41(1):73–78. [PubMed] [Google Scholar]
- Spry C. J. Mechanism of eosinophilia. V. Kinetics of normal and accelerated eosinopoiesis. Cell Tissue Kinet. 1971 Jul;4(4):351–364. [PubMed] [Google Scholar]
- Spry C. J. Mechanism of eosinophilia. VI. Eosinophil mobilization. Cell Tissue Kinet. 1971 Jul;4(4):365–374. [PubMed] [Google Scholar]
- Stohlman F., Quesenberry P. J., Tyler W. S. The regulation of myelopoiesis as approached with in vivo and in vitro techniques. Prog Hematol. 1973;8:259–297. [PubMed] [Google Scholar]
- Tyler W. S., Niskanen E., Stohlman F., Jr, Keane J., Howard D. The effect of neutropenia on myeloid growth and the stem cell in an in vivo culture system. Blood. 1972 Nov;40(5):634–645. [PubMed] [Google Scholar]
- Warren K. S., Karp R., Pelley R. P., Mahmoud A. A. The eosinophil stimulation promoter test in murine and human Trichinella spiralis infection. J Infect Dis. 1976 Sep;134(3):277–280. doi: 10.1093/infdis/134.3.277. [DOI] [PubMed] [Google Scholar]
- Wasserman S. I., Goetzl E. J., Ellman L., Austen K. F. Tumor-associated eosinophilotactic factor. N Engl J Med. 1974 Feb 21;290(8):420–424. doi: 10.1056/NEJM197402212900802. [DOI] [PubMed] [Google Scholar]
- Wine A. C., Kellermeyer R. A computer-based method for the quantification of leukocyte chemotaxis. J Lab Clin Med. 1976 Sep;88(3):487–490. [PubMed] [Google Scholar]
- Zucker-Franklin D. Eosinophil function and disorders. Adv Intern Med. 1974;19:1–25. [PubMed] [Google Scholar]


