Abstract
The purified lymphocytosis promoting factor (LPF) from Bordetella pertussis was found to be a potent mitogen for peripheral blood lymphocytes (PBL) from normal adults as well as for cord blood lymphocytes. Proliferation occurred in autologous plasma or fetal calf serum, regardless of previous exposure to pertussis infection or immunization. Only one adult human serum, from a physician constantly working with B. pertussis, inhibited the mitogenic response to LPF and this serum was shown to contain precipitating antibody against LPF. The proliferative effect of LPF was characteristic of a “nonspecific” mitogen and not of antigen stimulation of sensitized cells.
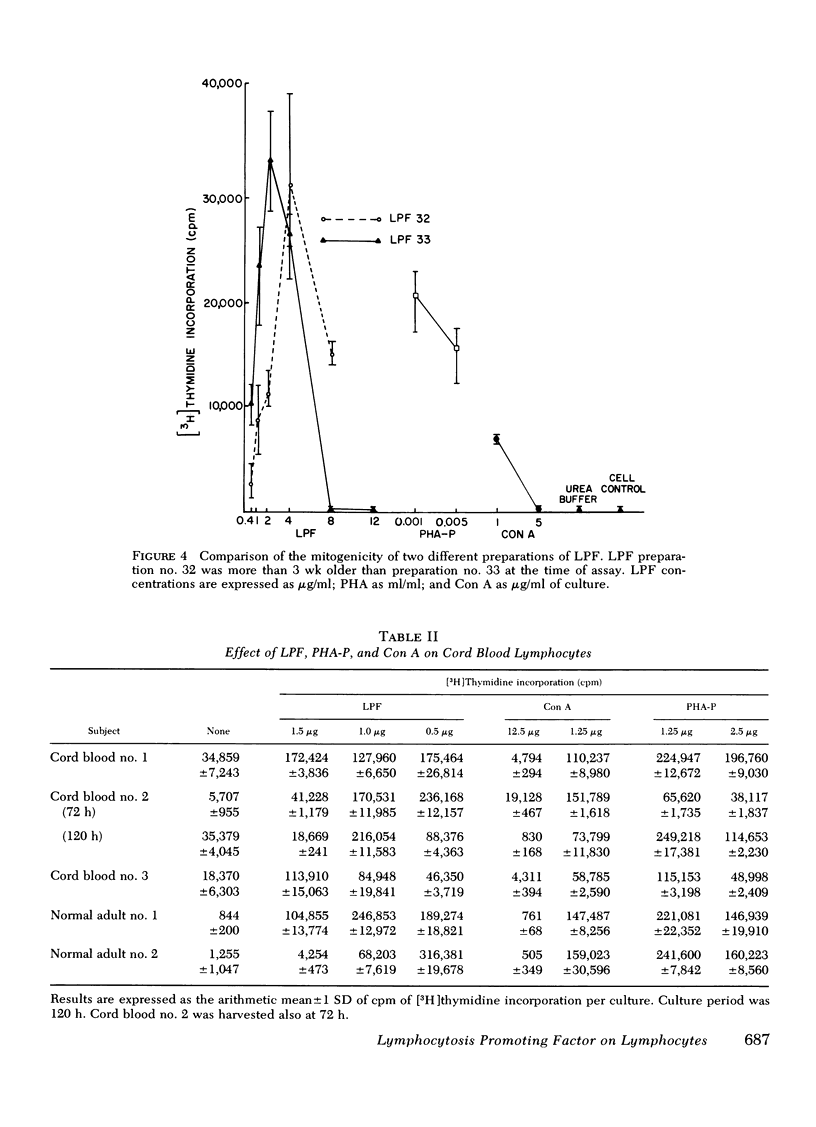
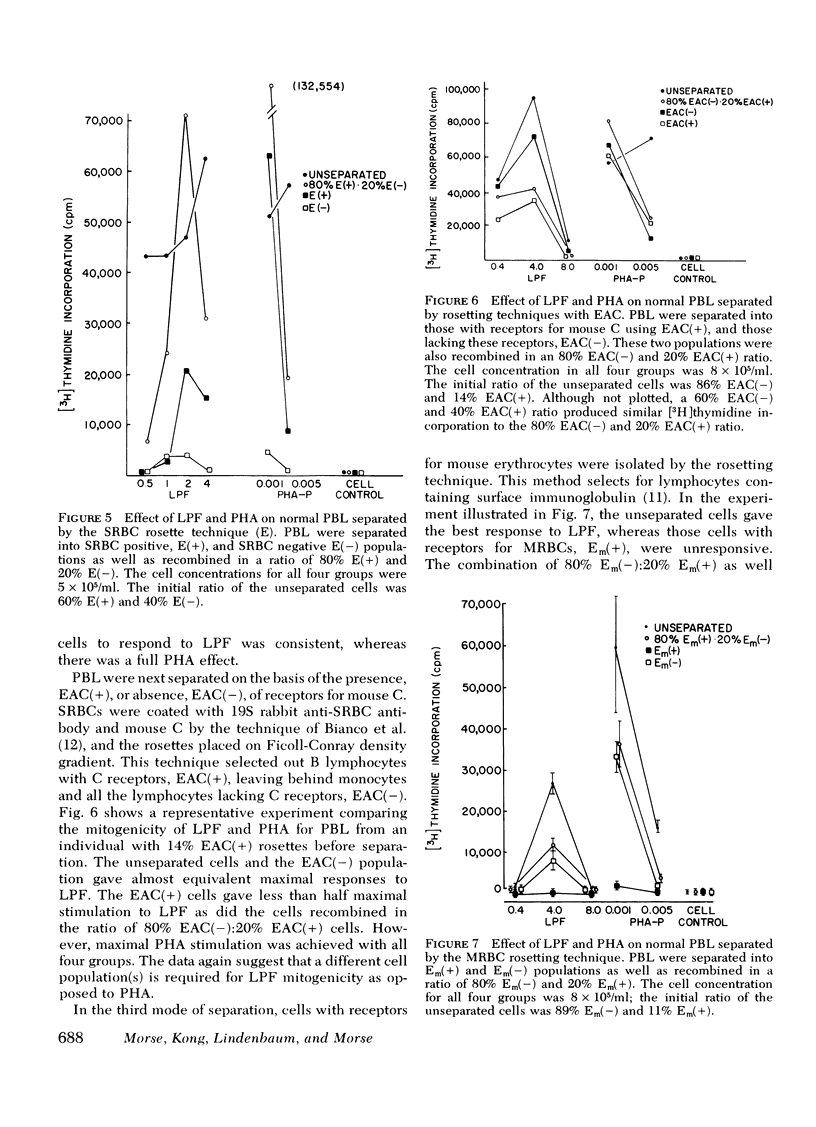
LPF, phytohemagglutinin, and concanavalin A were approximately equal in potency although variation occurred depending upon the cell donor. Experiments with lymphocyte subpopulations obtained by rosetting techniques employing sheep erythrocytes, mouse erythrocytes, and sheep erythrocytes coated with antibody and complement suggested the requirement of a multicellular system for LPF mitogencity.
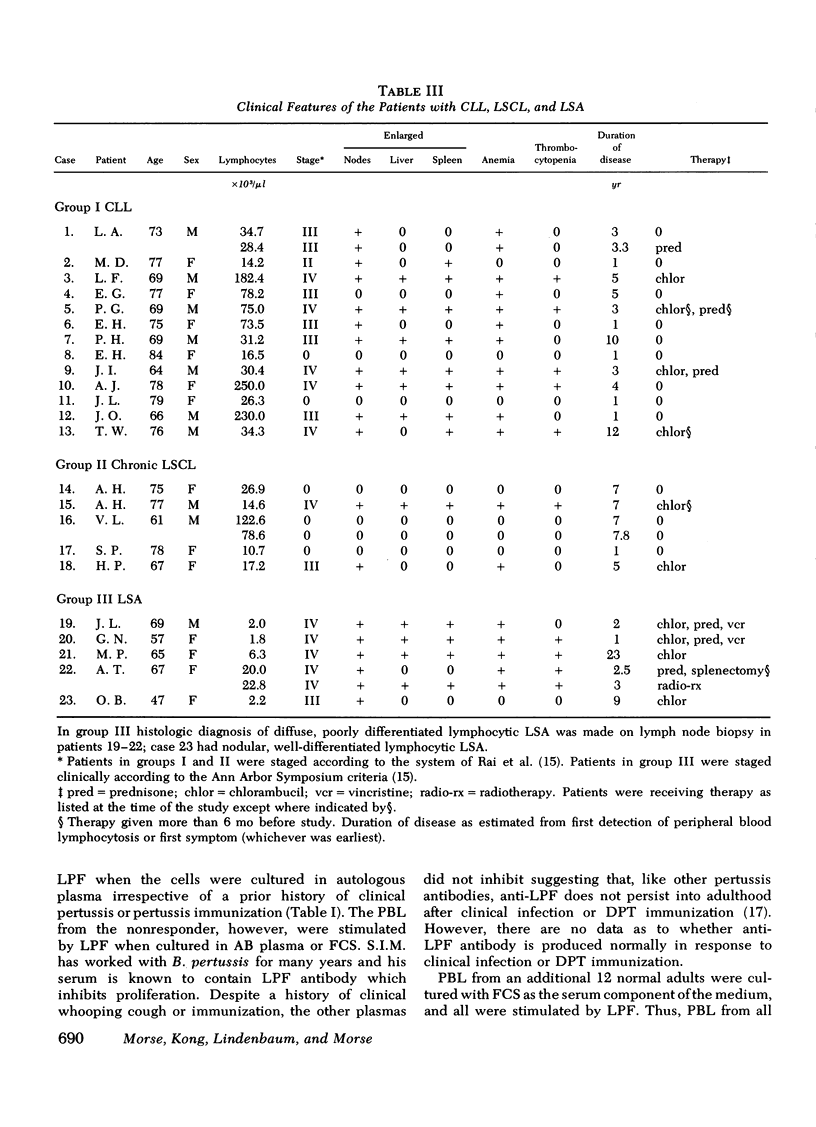
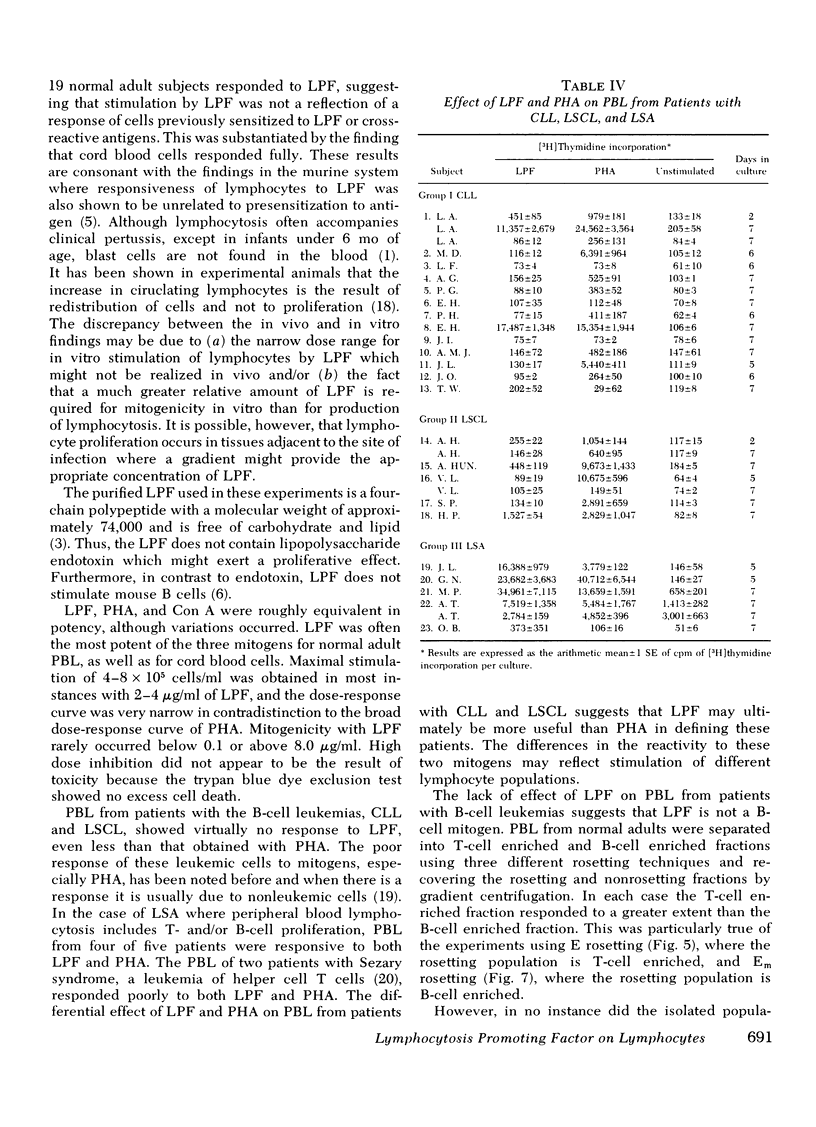
PBL from most patients with chronic lymphatic leukemia and lymphosarcoma cell leukemia were even less responsive to LPF than to phytohemagglutinin, whereas PBL from patients with lymphosarcoma usually responded to both mitogens. It can be inferred from the results of experiments with both normal and leukemic cells that LPF, which is a murine thymus-derived (T)-cell mitogen, is also a T-cell mitogen for human PBL. The exact cell requirement and mode of action, however, are as yet unknown.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen V., Hertz J. B., Sorensen S. F., Baekgaard P., Christensen P. E., Ramhoj W., Hansen G. A., Wardlaw A. C., Sato Y. In vitro stimulation of human lymphocytes by Bordetella Pertussis. Acta Pathol Microbiol Scand C. 1977 Feb;85(1):65–72. doi: 10.1111/j.1699-0463.1977.tb03612.x. [DOI] [PubMed] [Google Scholar]
- Bianco C., Patrick R., Nussenzweig V. A population of lymphocytes bearing a membrane receptor for antigen-antibody-complement complexes. I. Separation and characterization. J Exp Med. 1970 Oct 1;132(4):702–720. doi: 10.1084/jem.132.4.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broder S., Edelson R. L., Lutzner M. A., Nelson D. L., MacDermott R. P., Durm M. E., Goldman C. K., Meade B. D., Waldmann T. A. The Sézary syndrome: a malignant proliferation of helper T cells. J Clin Invest. 1976 Dec;58(6):1297–1306. doi: 10.1172/JCI108585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouet J. C., Flandrin G., Seligmann M. Indications of the thymus-derived nature of the proliferating cells in six patients with Sézary's syndrome. N Engl J Med. 1973 Aug 16;289(7):341–344. doi: 10.1056/NEJM197308162890703. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Carbone P. P., Kaplan H. S., Musshoff K., Smithers D. W., Tubiana M. Report of the Committee on Hodgkin's Disease Staging Classification. Cancer Res. 1971 Nov;31(11):1860–1861. [PubMed] [Google Scholar]
- Gupta S., Grieco M. H. Rosette formation with mouse erythrocytes: probable marker for human B lymphocytes. Int Arch Allergy Appl Immunol. 1975;49(6):734–742. doi: 10.1159/000231457. [DOI] [PubMed] [Google Scholar]
- Harrison M. R., Thurman G. B., Thomas G. M. A simple and versatile harvesting device for processing radioactive label incorporated into and-or released from cells in microculture. J Immunol Methods. 1974 Jan;3(1):11–16. doi: 10.1016/0022-1759(74)90027-1. [DOI] [PubMed] [Google Scholar]
- Kong A. S., Morse S. I. The in vitro effects of Bordetella pertussis lymphocytosis-promoting factor on murine lymphocytes. I. Proliferative response. J Exp Med. 1977 Jan 1;145(1):151–162. doi: 10.1084/jem.145.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong A. S., Morse S. I. The in vitro effects of Bordetella pertussis lymphocytosis-promoting factor on murine lymphocytes: II. Nature of the responding cells. J Exp Med. 1977 Jan 1;145(1):163–174. doi: 10.1084/jem.145.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAGERGREN J. The white blood cell count and the erythrocyte sedimentation rate in pertussis. Acta Paediatr. 1963 Jul;52:405–409. doi: 10.1111/j.1651-2227.1963.tb03798.x. [DOI] [PubMed] [Google Scholar]
- LAMBERT H. J. EPIDEMIOLOGY OF A SMALL PERTUSSIS OUTBREAK IN KENT COUNTY, MICHIGAN. Public Health Rep. 1965 Apr;80:365–369. [PMC free article] [PubMed] [Google Scholar]
- Morse S. I., Bray K. K. The occurrence and properties of leukocytosis and lymphocytosis-stimulating material in the supernatant fluids of Bordetella pertussis cultures. J Exp Med. 1969 Mar 1;129(3):523–550. doi: 10.1084/jem.129.3.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse S. I., Morse J. H. Isolation and properties of the leukocytosis- and lymphocytosis-promoting factor of Bordetella pertussis. J Exp Med. 1976 Jun 1;143(6):1483–1502. doi: 10.1084/jem.143.6.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse S. I., Riester S. K. Studies on the leukocytosis and lymphocytosis induced by Bordetella pertussis. II. The effect of pertussis vaccine on the thoracic duct lymph and lymphocytes of mice. J Exp Med. 1967 Apr 1;125(4):619–628. doi: 10.1084/jem.125.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai K. R., Sawitsky A., Cronkite E. P., Chanana A. D., Levy R. N., Pasternack B. S. Clinical staging of chronic lymphocytic leukemia. Blood. 1975 Aug;46(2):219–234. [PubMed] [Google Scholar]
- Salem N. B., Morse J. H. Lymphocyte response to mitogens in progressive systemic sclerosis. Arthritis Rheum. 1976 Sep-Oct;19(5):875–882. doi: 10.1002/art.1780190507. [DOI] [PubMed] [Google Scholar]
- Shohat B., Joshua H. Formation of mouse red cell rosettes by lymphocytes from patients with chronic lymphatic leukemia and assessment of T cell function. Clin Immunol Immunopathol. 1976 Nov;6(3):389–393. doi: 10.1016/0090-1229(76)90092-1. [DOI] [PubMed] [Google Scholar]
- Wybran J., Carr M. C., Fudenberg H. H. The human rosette-forming cell as a marker of a population of thymus-derived cells. J Clin Invest. 1972 Oct;51(10):2537–2543. doi: 10.1172/JCI107069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharski L. R., Linman J. W. Chronic lymphocytic leukemia versus chronic lymphosarcoma cell leukemia. Analysis of 496 cases. Am J Med. 1969 Jul;47(1):75–81. doi: 10.1016/0002-9343(69)90242-3. [DOI] [PubMed] [Google Scholar]