Abstract
Background
Leishmania major is an intracellular parasite transmitted through the bite of the female phlebotomine sand flies. Leishmania major is able to escape the host immune defense and survive within macrophages. Modulation of the NF-κB (Nuclear Factor-Kappa B) activation and suppression of the pro-inflammatory cytokines by L. major are the main evasion mechanisms that remain to be explored. This study aims to examine the expression level of the Monarch-1 in L. major-infected macrophages, as a negative regulator of the NF-κB activation.
Methods
Murine macrophage cell line (J774 A.1) was infected by metacyclic form of Leishmania promastigotes at macrophage/parasite ratio of 1:10. After harvesting infected cells at different times, total RNA was extracted and converted to cDNA. Semi-quantitative RT-PCR was performed for Monarch-1 by specific primers. Hypoxanthine Phospho-Ribosyl Transferase (HPRT) was used as an internal control to adjust the amount of mRNA in each sample.
Results
Semiquantitive analysis of Monarch-1 mRNA expression level showed a significant expression increase within 6 to 30 hours after L. major infection of macrophages when compared to the control macrophages.
Conclusion
Monarch-1 expression level reveals a significant increase in the early phase of macrophage infection with L. major, which in turn may suppress IL-12 production in Leishmania infected macrophages and deeply influence the relationship between host and parasite.
Keywords: Monarch-1, Leishmania major, J774 A.1, NALP12, Murine macrophage cell line
Introduction
Leishmania is an intracellular parasite lives inside the histiocytes of mammals. It exploits a numer of mechanisms to escape phagocytosis and survive within the macrophages. Leishmania suppresses different activities of macrophages including phagocytosis, nitric oxide synthesis, IL-12 production and MHC class II presentation (1, 2). Several parasites including L. major and Toxoplasma gondii, affect key components of NF-κB signaling pathway which participates in innate and acquired immune responses (3, 4). Since, the majority of these mechanisms is not well known, immunization and preparation of vaccine against leishmaniasis has not been successful.
Monarch-1 activation seems one of the possible mechanisms that could account for NF-κB inhibition and suppression of IL-12 production. The Monarch-1 molecule, also known as PYPAF7, NALP12,PAN6, is one of the Nucleotide-binding and leucine-rich repeat-containing receptors (NLRs) family which contains an N-terminal effector PYRIN domain and has anti-inflammatory or immunosuppressive functions (5). Monarch-1 is often expressed on the myeloid cells including monocytes and granulocytes and regulates non-canonical and canonical NF-κB activation (6). This regulatory effect is likely achieved by degradation of NF-κB inducing kinase (NIK) via proteasome-dependent and independent pathways. In this context, some researches imply that Monarch-1 suppresses production of pro-inflammatory cytokines and chemokines (7). It has been also reported that this molecule may interact with HSP90 and “adaptor-like” proteins, such as Fas-associated factor 1 (FAF-1) which links innate immunity and apoptosis signaling (8). Recently, it has been indicated that Monarch-1 also regulates migration of dendritic and myeloid cell into the cutaneous inflammation sites (9).
To examine the possible role of the Monarch-1 in Leishmania survival inside the macrophages, this study examines Monarch-1 (NALP12) mRNA expression in Leishmania-infected mouse macrophage cell line.
Material and Methods
Leishmania major, strain MRHO/IR/75/ER, obtained from Pasteur institute, Tehran. The parasites transferred into biphasic NNN medium containing 250 IU/ml penicillin and 250 µg/ml of streptomycin. After 3 days, a fresh smear from liquid phase of biphasic culture was obtained and examined under low power light microscope. The promastigotes were counted using the hemocytometer cell counting chamber. After parasite count reached to 2x106 /ml, the promastigotes were transferred to the culture tubes containing RPMI 1640 plus 10% Fetal calf Serum (FCS) and kept at 25 °C until their population increased to 7x107 promastigotes/ml. After cultivating of the L. major promastigotes for 3 to 4 days in liquid medium, L. major promastigotes transform from a less infective procyclic form to a highly infectious metacyclic promastigote during the stationary phase of growth. The mouse macrophage cell line, J774 A.1, was obtained from the cell bank of Pasteur institute of Iran. The macrophages were cultured into a flask containing RPMI 1640, 15% FCS, streptomycin 100 mg/ml and penicillin 100 IU/ml, and kept at 37 °C in an incubator containing 5% CO2. After growth of macrophages, they were trypsinized and seeded into six-well plates.
To infect macrophages by metacyclic form of promastigotes, 1 × 106 cells/well macrophages plated in six-well plates and kept at room temperature for 24 hrs. All wells with macrophage monolayers were infected with promastigotes in stationary phase using 10 parasites per cell and incubated at 37 °C for 2 h. To eliminate free swimming promastigotes from the cultures, the supernatant was discarded and the macrophages were washed 3 times gently by RPMI 1640. Then, 5 ml fresh RPMI 1640 was added to each culture chamber and incubated for 6, 18 and 30 hours (10–12). Each incubation time carried out in quadruplicate wells simultaneously. To the indicate macrophage infection, Intracellular parasites were enumerated by invert phase contrast microscopy. Non infected-macrophages also incubated over the same time in quadruplicate wells. After the incubation periods, the macrophages were trypsinized and harvested from the plates. RNA extraction was performed by TriPure isolation reagent (Boehringer Mannheim) according to the manufacturer's instruction. The extracted RNA was reverse transcribed to cDNA by cDNA synthesis kit (Fermentas Life Science, Lithuania). Then, semi-quantitative RT-PCR was performed for Monarch-1 by specific primers. PCR primer sequences were based on the Ensembl database (http://www.ensembl.org) and were designed by Gene Runner (Hastings Software Inc., Hastings, NY) and Primer Premier 5 (Premier Biosoft Inc). BLAST analysis against mouse mRNA was performed, using Ensembl database, to test the specificity of the primers (Table 1). To normalize the interest gene, Hypoxanthine Phospho-Ribosyl Transferase (HPRT) was used as an internal control to adjust the amount of mRNA in each sample.
Table 1.
Primers used for RT-PCR of Monarch 1 and HPRT
| Genes | Primers | Product size | Top | Accession No. |
|---|---|---|---|---|
| Monarch-1 (NALP12) | F: 5’ – GTACCAACTCCAACCTGATCG– 3’ R: 5’ –GAAGTAGAGGCCAGATTTGC– 3’ |
518 bp | 59 °C | CCDS51963.1 |
| HPRT | F:5’ – CGTCGTGATTAGCGATGATGAAC– 3’ R:5’ –TCACTAATGACACAAACGTGATTC– 3’ |
609 bp | 59 °C | CCDS40972.1 |
The intensity of obtained bands was determined by Kodak 1D software (Eastman Kodak Company, Rochester, NY). Graph Pad Prism-5 (Graph Pad Prism Software Inc) was used for the statistical analysis, Kruskal-Wallis test, and graph drawing.
Results
The result of PCR optimization experiments for HPRT gene showed that the best annealing temperature was 59 °C for 34 cycles to obtain a sharp band in the exponential phase of amplification. In this study, the expression level of Monarch-1 mRNA was evaluated by semi-quantitative RT-PCR in L. major treated and untreated J774 A.1 cell lines.
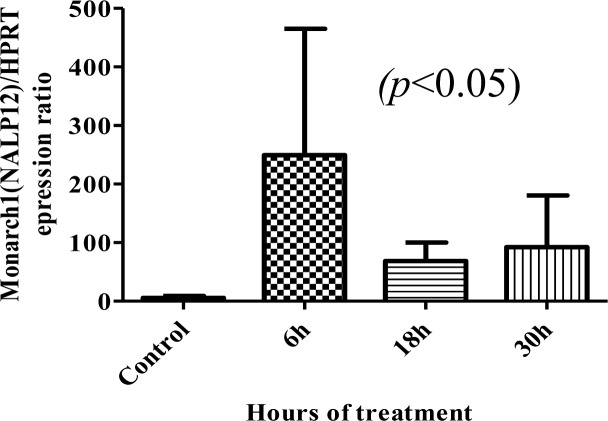
Considering the relative expression of Monarch-1 normalized to the HPRT expression level, Monarch-1 mRNA level showed a significant increase within 6 to 30 hrs after infection with promastigotes, while no expression were detected in the control samples (Figs. 1 and 2).
Fig. 1.
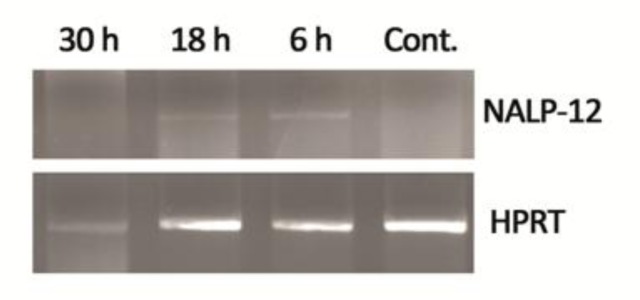
Semi-quantitative RT-PCR analysis of Monarch-1 expression in Leishmania major-infected and non-infected cells after 6, 18 and 30 hours
Fig. 2.
Comparison between the average of four separate experiments measuring mRNA Monarch-1 expression in Leishmania major-infected (6, 18 and 30 hours) and non-infected cells. Bar graph indicates the mean ± S.E.M
Discussion
In the mutual relationship between parasite and macrophage, there are various tricks for parasite survival in the macrophages. Leishmania evokes different strategies to subvert macrophage functions. One of them is modulation of the Rel/NF-κB transcription factor activity, which plays a pivotal role in several processes of immune and inflammatory responses (13, 14). In this study, findings indicate a significant expression of Monarch-1 in Leishmania infected macrophages. It seems one of the possible mechanisms that could account for NF-κB inhibition and suppression of IL-12 production.
Previous studies showed that Leishmania amastigotes prevent translocation of p50/p56 into the nucleus with an unknown mechanism (15). It has been also demonstrated that in the peritoneal macrophage of Leishmania-infected mouse, a few NF-κB proteins are bound on the NF-κB binding sites (16).
Previous studies indicated that macrophage suppression, which is induced by L. major, are initiated along with attachment of parasite on the macrophage surface. Leishmania entrance into the macrophages is mediated through molecules that induce translocation of new described NF-κB complex, p50/C-Re1, into the nucleus, instead of p50/p65. These receptors are not completely defined, but there are some evidences that imply on the role of TLRs in this mechanism (17–20).
In addition to the surface receptors, new family of intracellular receptors, so called NLR, has been found which could be a candidate for interaction with Leishmania and modulation of NF-κB activity. NALP12 or Monarch 1 is a regulatory protein of NLR family that has a proven suppressive effect on the NF-κB activity. This molecule exploits several mechanisms to modulate NF-κB signaling pathway, including hyperphosphorylation of the IRAK, degradation of NIK and interaction with other regulatory proteins (5, 21). To our knowledge, the present study is the first report on possible implications of this molecule in the relationship of L. major and host.
Conclusion
Although further analysis will be required to clarify the role of the Monarch-1 in immune evasion by L. major, our findings suggest that expression of this molecule in the early phase of macrophage infection with L. major, could be an explanation for suppression of IL-12 production in Leishmania infected macrophages. In turn, it might deeply influence on the future of the host and parasite relationship.
Acknowledgments
This paper is the result of a MS thesis No: A-248 and Research project holding Code No: 86420 which is approved and supported by the Deputy of Research, Mashhad University of Medical Sciences. The authors wish to thank the Deputy of Research for financial support. The authors declare that there is no conflict of interest.
References
- 1.Bourreau E, Gardon J, Pradinaud R, Pascalis H, Prevot-Linguet G, Kariminia A, Pascal L. Th2 responses predominate during the early phases of infection in patients with localized cutaneous leishmaniasis and precede the development of Th1 responses. Infect Immun. 2003;71(4):2244–2246. doi: 10.1128/IAI.71.4.2244-2246.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Souza Leao S, Lang T, Prina E, Hellio R, Antoine JC. Intracellular Leishmania amazonensis amastigotes internalize and degrade MHC class II molecules of their host cells. J Cell Sci. 1995;108(Pt 10):3219–3231. doi: 10.1242/jcs.108.10.3219. [DOI] [PubMed] [Google Scholar]
- 3.Orth K, Xu Z, Mudgett MB, Bao ZQ, Palmer LE, Bliska JB, et al. Disruption of signaling by Yersinia effector YopJ, a ubiquitin-like protein protease. Science. 2000;290(5496):1594–1597. doi: 10.1126/science.290.5496.1594. [DOI] [PubMed] [Google Scholar]
- 4.Shapira S, Speirs K, Gerstein A, Caamano J, Hunter CA. Suppression of NF-kappaB activation by infection with Toxoplasma gondii . J Infect Dis. 2002;185(Suppl 1):S66–72. doi: 10.1086/338000. [DOI] [PubMed] [Google Scholar]
- 5.Williams KL, Taxman DJ, Linhoff MW, Reed W, Ting JP. Cutting edge: Monarch-1: a pyrin/nucleotide-binding domain/leucine-rich repeat protein that controls classical and nonclassical MHC class I genes. J Immunol. 2003;170(11):5354–5358. doi: 10.4049/jimmunol.170.11.5354. [DOI] [PubMed] [Google Scholar]
- 6.Lich JD, Ting JP. Monarch-1/PYPAF7 and other CATERPILLER (CLR, NOD, NLR) proteins with negative regulatory functions. Microbes Infect. 2007;9(5):672–676. doi: 10.1016/j.micinf.2007.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lich JD, Williams KL, Moore CB, Arthur JC, Davis BK, Taxman DJ, Ting JP. Monarch-1 suppresses non-canonical NF-kappaB activation and p52-dependent chemokine expression in monocytes. J Immunol. 2007;178(3):1256–1260. doi: 10.4049/jimmunol.178.3.1256. [DOI] [PubMed] [Google Scholar]
- 8.Pinheiro AS, Eibl C, Ekman-Vural Z, Schwarzenbacher R, Peti W. The NLRP12 pyrin domain: structure, dynamics, and functional insights. J Mol Biol. 2011;413(4):790–803. doi: 10.1016/j.jmb.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arthur JC, Lich JD, Ye Z, Allen IC, Gris D, Wilson JE, et al. Cutting edge: NLRP12 controls dendritic and myeloid cell migration to affect contact hypersensitivity. J Immunol. 2012;185(8):4515–4519. doi: 10.4049/jimmunol.1002227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davies JQ, Gordon S. 3rd ed. Oxford, USA: Humana Press; 2004. Isolation and Culture of Human Macrophages Basic Cell Culture Protocols ; pp. 105–116. [DOI] [PubMed] [Google Scholar]
- 11.Madeira M, Barbosa-Santos E, Marzochi M. Experimental infection of canine peritoneal macrophages with visceral and dermotropic Leishmania strains. Mem Inst Oswaldo Cruz. 1999;94(5):645–648. doi: 10.1590/s0074-02761999000500015. [DOI] [PubMed] [Google Scholar]
- 12.Moreno ML, de Meirelles Mde N. In vitro method for isolation of amastigote forms of Leishmania amazonensis from J774 A.1 macrophage induced by temperature shifting. Mem Inst Oswaldo Cruz. 1998;93(1):99–102. doi: 10.1590/s0074-02761998000100017. [DOI] [PubMed] [Google Scholar]
- 13.Vallabhapurapu S, Karin M. Regulation and Function of NF-κB Transcription Factors in the Immune System. Annual Review of Immunology. 2009;27:693–733. doi: 10.1146/annurev.immunol.021908.132641. [DOI] [PubMed] [Google Scholar]
- 14.Guizani-Tabbane L, Ben-Aissa K, Belghith M, Sassi A, Dellagi K. Leishmania major Amastigotes Induce p50/c-Rel NF-κB Transcription Factor in Human Macrophages: Involvement in Cytokine Synthesis. Infection and Immunity. 2004;72(5):2582–2589. doi: 10.1128/IAI.72.5.2582-2589.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tato CM, Hunter CA. Host-pathogen interactions: subversion and utilization of the NF-kappa B pathway during infection. Infect Immun. 2002;70(7):3311–3317. doi: 10.1128/IAI.70.7.3311-3317.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reinhard K, Huber M, Lohoff M, Visekruna A. The role of NF-κB activation during protection against Leishmania infection. Int J Med Microbiol. 2012;302(4-5):230–235. doi: 10.1016/j.ijmm.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 17.Akira S, Hemmi H. Recognition of pathogen-associated molecular patterns by TLR family. Immunol Lett. 2003;85(2):85–95. doi: 10.1016/s0165-2478(02)00228-6. [DOI] [PubMed] [Google Scholar]
- 18.Alonso A, Bayon Y, Renedo M, Crespo MS. Stimulation of Fc gamma R receptors induces monocyte chemoattractant protein-1 in the human monocytic cell line THP-1 by a mechanism involving I kappa B-alpha degradation and formation of p50/p65 NF-kappa B/Rel complexes. Int Immunol. 2000;12(4):547–554. doi: 10.1093/intimm/12.4.547. [DOI] [PubMed] [Google Scholar]
- 19.Becker I, Salaiza N, Aguirre M, Delgado J, Carrillo-Carrasco N, Kobeh LG, et al. Leishmania lipophosphoglycan (LPG) activates NK cells through toll-like receptor-2. Mol Biochem Parasitol. 2003;130(2):65–74. doi: 10.1016/s0166-6851(03)00160-9. [DOI] [PubMed] [Google Scholar]
- 20.Thieblemont N, Haeffner-Cavaillon N, Haeffner A, Cholley B, Weiss L, Kazatchkine MD. Triggering of complement receptors CR1 (CD35) and CR3 (CD11b/CD18) induces nuclear translocation of NF-kappa B (p50/p65) in human monocytes and enhances viral replication in HIV-infected monocytic cells. J Immunol. 1995;155(10):4861–4867. [PubMed] [Google Scholar]
- 21.Williams KL, Lich JD, Duncan JA, Reed W, Rallabhandi P, Moore C, et al. The CATERPILLER protein Monarch-1 is an antagonist of toll-like receptor-, tumor necrosis factor alpha-, and Mycobacterium tuberculosis-induced pro-inflammatory signals. J Biol Chem. 2005;280(48):39914–39924. doi: 10.1074/jbc.M502820200. [DOI] [PMC free article] [PubMed] [Google Scholar]