Abstract
Background
The free-living amoebae Acanthamoeba spp., have been recognized as etiologic agents of amoebic encephalitis, keratitis, otitis, lung lesions and other skin infections mainly in immuno-compromised individuals. In this study, morpho-physiological and biochemical characterization of Acanthamoeba strains isolated from the Egyptian aquatic environment were surveyed.
Methods
Some Acanthamoeba species were cultivated on non-nutrient agar. Isolated strains of Acanthamoeba were identification based on the morphology of trophic and cyst forms in addition to temperature and osmo-tolerance assays. Biochemical characterization of the isolated amoeba strains was performed using quantitative assay as well as qualitative determination of proteolytic activity in zymograph analysis.
Results
Potentially pathogenic Acanthamoeba species were isolated from all of the examined water sources. Colorimetric assays showed protease activity in the heat-tolerant isolates of Acanthamoeba. All pathogenic isolates of Acanthamoeba exhibited higher protease activity than did the non-pathogenic ones. The zymographic protease assays showed various banding patterns for different strains of Acanthamoeba.
Conclusion
The incidence and prevalence of the pathogenic Acanthamoeba species in the aquatic environment using parasitological and biochemical diagnostic tools will provide baseline data against which the risk factors associated with waterborne transmission can be identified.
Keywords: Acanthamoeba, Heat-tolerance, Proteases, Water, Swimming pools, Egypt
Introduction
There is an increasing interest and awareness of the free-living amoeba, Acanthamoeba, over recent years as an opportunistic pathogen of medical importance. Acanthamoeba is one of the more abundant protozoa on earth, which can be isolated from soil, dust, air, treated and untreated tap water, swimming pools, air-conditioning units and numerous other domestic and outdoor environments (1). The protozoa's life cycle consists of an active feeding trophozoite phase and dormant cyst phase, which is activated by unfavorable conditions, such as exposure to extreme temperature, high or low pH, dryness or starvation. They are the causative agents of granulomatous amebic encephalitis and amebic keratitis and have been associated with cutaneous lesions and sinusitis. Human diseases caused by Acanthamoeba continue to rise worldwide, which is probably due to increasing populations of contact lens wearers and HIV+ patients (2). Moreover, Acanthamoeba serves as a carrier for different pathogens viruses, bacteria such as Legionella, Pseudomonas and Helicobacter (3). These bacterial pathogens can lead to severe human disease or manifest as complications of amoebic keratitis. Indeed, these amebas can transfer different microorganisms to humans and to date, Acanthamoeba is introduced as a vehicle for circulation of pathogens between human and environment (4).
The first human infection by Acanthamoeba was described causing granulomatous amoebic encephalitis (GAE) (5). In Egypt, Acanthamoeba spp. were isolated from the majority of environmental samples (70.27%).They were isolated from swimming pools, surface water and canals, River Nile, rain water, soil and air in different Egyptian governorates (6–9). The author reported the growth of the isolated Acanthamoeba spp. at 37 °C but not at 43 °C. Water samples from aquatic sites were examined for the presence of free living amoeba. In Lower Egypt, A. culbertsoni, A. rhysodes and A. glebae were isolated. Five Acanthamoeba spp. were isolated from Mahmoudia and Nubaria canals, which are the main water supply in Alexandria. These were A. rhysodes, A. glebae, A. culbertsoni, A. astronyxis and A. palastiniensis (10). Acanthamoeba polyphaga was isolated from the nasal swab of healthy children (11, 12).
The aim of this study was to determine the presence of Acanthamoeba spp. in different aquatic environment of Egypt. This finding lead to additional researches for investigating presence of pathogenic Acanthamoeba strains in water sources using morpho-physiological, and biochemical characterization methods which can be a risk factor for people especially contact lens wearers and immuno-compromised patients.
Materials and Methods
Samples and sampling sites
Water samples (3 liters each) were collected from different localities in Delta region, Egypt (Table 1) for the detection and isolation of freshwater amoebae. Samples were collected from the Nile River, tap water and swimming pool water in clean, dry autoclavable polypropylene containers and sent to the laboratory in icebox and processed at the same day of collection.
Table 1.
Samples and sampling sites
| Governorate | Type of water samples |
|---|---|
| Cairo | Nile, swimming pools and tap |
| Giza | Nile and tap |
| Qalubeya | Nile and tap |
| Behera | Nile and tap |
| Gharbeya | Nile and tap |
| Dakahleya | Nile and tap |
| Helwan | Nile, swimming pools and tap |
| Kafr-Elshikh | Tap |
| Sharkeya | Tap |
| Minofeya | Tap |
Isolation and morphologic identification of Acanthamoeba spp. from water samples
Collected water samples (1 liter each) were concentrated by using the membrane filtration technique. One liter of each water sample was filtered through a nitrocellulose membrane filters (0.45 µm pore size and 47 mm in diameter) (Whatman, WCN type, Cat No. 7141-104) (13). After filtration the membranes were separately inverted face to face on the surface of a non-nutrient (NN) agar plates previously seeded with 100 µl Escherichia coli suspension. All the inoculated plates were incubated at 40°C for one week with daily microscopic examination for the presence of any amoebic growth (14). Identification of the obtained Acanthamoeba spp. were achieved according to the morphological characteristics of both trophic and cyst stages (6, 15, 16).
Osmo-tolerance assay for pathogenicity of isolated Acanthamoeba strains
The isolated free-living amoebae that proved morphologically to be Acanthamoeba were cultivated on non-nutrient agar plates containing one molar (1 M) manitol. Approximately 5 ml of late log phase cultures of Escherichia coli were poured on non-nutrient agar plates containing 1 M manitol and left for 5 min, after which the excess bacterial culture fluid was removed and plates were left to dry for 10 min. After that, 50 µl of each Acanthamoeba strain were separately inoculated at the center of the plate. Inoculated plates were incubated at 30°C for up to 72 hours with daily observation. Growth and persistence of pathogenic Acanthamoeba spp. was observed by measuring the increase in diameter of clearance zone in the bacterial lawn. The increase in zone diameter was an indication of the increase in pathogenicity of the inoculated Acanthamoeba spp. (17).
Biochemical characterization of isolated Acanthamoeba spp.
Grown amoebae were harvested from cultured NN agar plates by scraping of the agar surface in eppendorf tubes containing 0.5 ml sterile Page's amoebae saline. The harvested amoebae were centrifuged at 1500 rpm for 10 min. The supernatant was discarded and the final pellet was re-suspended in 100 µl Page's amoebae saline and homogenized for 5 minutes in a tissue grinder. After that, the homogenate was transferred to a fresh eppendorf tube and centrifuged at 14000 rpm for 10 min. The supernatant was aspirated, divided into aliquots and stored at -80°C till being used. These steps were repeated for each sample (18).
Quantitative assays for proteinase activity using chromomeric substrates
An aliquot was taken from samples prepared and stored at -80°C as mentioned above. The protease activities in different Acanthamoeba were quantitatively measured using the trypsin-like proteases specific substrate (Boc-Val-Leu-Gly-Arg-PNA L-1195, Bachem Biochemica, Heidelberg, Germany) at λmax 405 nm using Sun Rise reader (TECAN, Austria) according to (19–21). The intensity of the yellow colour was directly proportional to the enzyme activity.
Qualitative determination of proteolytic activity in zymograph analysis (gelatin sodium dodecyl sulphate-polyacrylamide gel electrophoresis, SDS-PAGE gels)
The proteolytic activity of Acanthamoeba isolates were characterized by zymography on SDS-polyacrylamide gels copolymerized with gelatin (21).
Statistical analysis
All obtained data were analyzed by the student's t-test using the Graph Pad InStat Soft ware.
Results
Prevalence of heat-tolerant Acanthamoeba spp. in different types of water
Heat-tolerant Acanthamoeba species were isolated from 56.0, 58.6 and 49.2% of the examined Nile water, tap water and swimming pools water samples, respectively.
Nile water samples collected from Helwan Governorate showed the highest incidence of heat-tolerant Acanthamoeba species (66.7%). The least incidence of Acanthamoeba species in Nile water samples occurred in Qalubeya (52.8%) and Gharbeya (38.9%) Governorates (Table 2). Tap water samples collected from Qalubeya Governorate showed the highest incidence of heat-tolerant Acanthamoeba species (88.9%). On the contrary, tap water of Gharbeya Governorate showed the lowest incidence of heat-tolerant Acanthamoeba species (41.7%) (Table 2). Water samples collected from swimming pools number 6 and 10 showed the highest incidence of heat-tolerant Acanthamoeba species (83.3%). Swimming pool number 2 recorded the least incidence of heat-tolerant Acanthamoeba species (25.0%). In addition, the heat-tolerant Acanthamoeba species were not recorded in water samples collected from swimming pool number 4 (Table 3).
Table 2.
Prevalence of Acanthamoeba spp. in Nile and tap water samples
| Sampling sites (Governorate) | Heat-tolerant Acanthamoeba grown at 40 °C | |||||
|---|---|---|---|---|---|---|
| Nile water | Tap water | |||||
| Examined samples (n) | +Ve samples | % | Examined samples (n) | +Ve samples | % | |
| Cairo | 36 | 21 | 58.3 | 36 | 18 | 50.0 |
| Giza | 36 | 20 | 55.6 | 36 | 17 | 47.2 |
| Qalubeya | 36 | 19 | 52.8 | 36 | 32 | 88.9 |
| Behera | 36 | 23 | 63.9 | 36 | 20 | 55.6 |
| Gharbeya | 36 | 14 | 38.9 | 36 | 15 | 41.7 |
| Dakahleya | 36 | 24 | 66.7 | 36 | 27 | 75.0 |
| Helwan | 36 | 20 | 55.6 | 36 | 22 | 61.1 |
| Kafr-Elshikh | - | - | - | 36 | 16 | 44.4 |
| Sharkeya | - | - | - | 36 | 28 | 77.8 |
| Minofeya | - | - | - | 36 | 16 | 44.4 |
| Total | 252 | 141 | 56.0 | 360 | 211 | 58.6 |
Table 3.
Prevalence of Acanthamoeba spp. in swimming pool samples
| Swimming pools | Examined samples (n) | Heat-tolerant free-living amoebae at 40°C Acanthamoeba spp. | |
|---|---|---|---|
| +Ve | % | ||
| 1 | 12 | 7 | 58.3 |
| 2 | 12 | 3 | 25.0 |
| 3 | 12 | 6 | 50.0 |
| 4 | 12 | - | - |
| 5 | 12 | 5 | 41.7 |
| 6 | 12 | 10 | 83.3 |
| 7 | 12 | 3 | 25.0 |
| 8 | 12 | 8 | 66.7 |
| 9 | 12 | 7 | 58.3 |
| 10 | 12 | 10 | 83.3 |
| Total | 120 | 59 | 49.2 |
Osmo-tolerance differentiation between pathogenic and non-pathogenic Acanthamoeba
Results showed that pathogenic Acanthamoeba exhibit growth at increased osmolarity and this physiological determinant can be used to differentiate between pathogenic and non-pathogenic Acanthamoeba.
It was observed that 73 (51.8%) of 141 Acanthamoeba strains isolated from Nile water demonstrated pathogenic potential. Moreover, 23 of 211 (10.9%) Acanthamoeba strains isolated from tap water sources demonstrated pathogenic potential, but only 5 of 59 (8.5%) samples detected from swimming pools were considered as potentially pathogenic amoebae (Tables 4 and 5).
Table 4.
Distribution of osmo-tolerant Acanthamoeba spp. in Nile and tap water samples
| Sampling site (Governorate) | Tap water | Nile water | ||
|---|---|---|---|---|
| Osmo-tolerant +Ve samples/ Acanthamoeba +Ve samples | ||||
| +Ve/ No. | % | +Ve/ No. | % | |
| Cairo | 4/18 | 22.2 | 12/21 | 57.1 |
| Giza | 2/17 | 11.8 | 12/20 | 60.0 |
| Qalubeya | 12/32 | 37.5 | 16/19 | 84.2 |
| Behera | 2/20 | 10.0 | 18/23 | 78.3 |
| Gharbeya | 0/15 | 0.0 | 3/14 | 21.4 |
| Dakahleya | 1/27 | 3.7 | 5/24 | 20.8 |
| Helwan | 2/22 | 9.1 | 7/20 | 35.0 |
| Kafr-Elshikh | 0/16 | 0.0 | - | - |
| Sharkeya | 4/28 | 14.3 | - | - |
| Minofeya | 5/16 | 31.3 | - | - |
| Total | 23/211 | 10.9 | 73/141 | 51.8 |
Table 5.
Distribution of osmo-tolerant Acanthamoeba spp. in swimming pool samples
| Swimming pool number | Acanthamoeba +Ve samples | Osmo-tolerant Acanthamoeba +Ve samples | |
|---|---|---|---|
| No. | % | ||
| 1 | 7 | 1 | 14.3 |
| 2 | 3 | 0 | 0.0 |
| 3 | 6 | 0 | 0.0 |
| 4 | 0 | 0 | 0.0 |
| 5 | 5 | 0 | 0.0 |
| 6 | 10 | 2 | 20.0 |
| 7 | 3 | 0 | 0.0 |
| 8 | 8 | 0 | 0.0 |
| 9 | 7 | 1 | 14.3 |
| 10 | 10 | 1 | 10.0 |
| Total | 59 | 5 | 8.5 |
Morpho-physiological characteristics of isolated Acanthamoeba species
Identification of the different species of Acanthamoeba was performed according to the shape and size of cysts in addition to the number, shape, size and arrangement of the cyst pores. Six species of Acanthamoeba could be morphologically recognized, namely Acanthamoeba castellanii, A. polyphaga, A. rhysodes, A. mauritaniensis, A. triangularis and A. royreba.
Quantitatively absolute enzyme activity in Acanthamoeba isolates
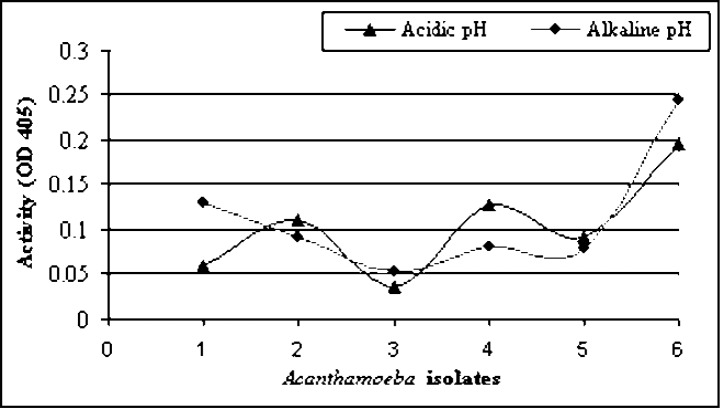
The examined Acanthamoeba isolates were classified according to absolute trypsin-like proteolytic activities into relatively pathogenic and non-pathogenic at both acidic and alkaline pH values (Fig. 1). Acanthamoeba isolates number 6 and 3 (isolated from swimming pools) showed the highest and lowest absolute trypsin-like activities at both acidic and alkaline pH values, respectively. These two Acanthamoeba isolates were morphologically identified as A. polyphaga and A. triangularis, respectively. Both of them exhibited thermo-tolerant activity. Concerning osmo-tolerance activity, the isolate number 6 exhibited growth at high osmolarity and is considered as potentially pathogenic while isolate number 3 exhibited no growth at high osmolarity and is considered as potentially non-pathogenic. Both Acanthamoeba isolates number 4, 5 and isolate number 2 (isolated from Nile water and tap water, respectively) showed moderately high proteolytic activities lying in between isolate number 6 and isolate number 3 at both acidic and alkaline pH values. They were morphologically identified as A. rhysodes, A. royreba,and A. castellanii respectively. Finally, isolate number 1 (isolated from tap water) showed moderately high proteolytic activity at alkaline pH value but low proteolytic activity at acidic pH value. This Acanthamoeba isolate, morphologically identified as A. mauritaniensis, exhibited both thermo- and osmo-tolerant properties and is considered as potentially pathogenic (Fig. 1).
Fig. 1.
Tryptase activity in individual Acanthamoeba isolates at both acidic and alkaline pH
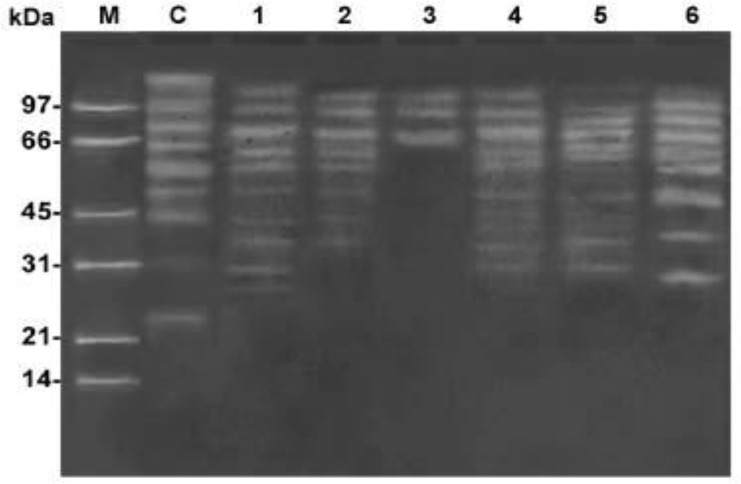
Qualitatively proteolytic activity in lysates of different Acanthamoeba isolates visualized by gelatin SDS-PAGE
The proteolytic profile of prepared lysates from bacterial control sample containing no Acanthamoeba was totally different from that of different Acanthamoeba isolates. Bacterial control sample had molecular weights 147, 110, 87, 70, 59, 53, 46, 31 and 23 kDa. The proteolytic profile of prepared lysates from Acanthamoeba isolates showed activity only at alkaline pH value 10, while at the acidic pH values no proteolytic activities appeared. Isolates number 1 and 2 (isolated from tap water and morphologically identified as A. mauritaniensis and A. castellanii, respectively) showed different proteolytic activities with common bands at molecular weights 63, 51, 43 and 36 kDa. The proteolytic activities of A. mauritaniensis were visualized at 118, 94, 75, 63, 57, 51, 43, 36, 30 and 27 kDa, while those of A. castellanii were visualized at 115, 93, 73, 63, 58, 51, 43 and 36 kDa (Fig. 2). Acanthamoeba isolates number 3 and 6 (isolated from swimming pools and morphologically identified as A. triangularis and A. polyphaga, respectively) showed completely different proteolytic activities. Only three proteolytic bands were seen at 115, 94 and 70 kDa in isolate number 3 (A. triangularis), while isolate number 6 (A. polyphaga) the gelatin digestion bands were evidenced at 104, 87, 71, 61, 57, 48, 34 and 29 kDa (Fig. 2).
Fig. 2.
Serine-like protease activity in Acanthamoeba isolates was visualized in gelatin SDS-PAGE, where C: negative control bacteria; 1: A. mauritaniensis; 2: A. castellanii; 3: A. triangularis; 4: A. rhysodes; 5: A. royreba; 6: A. polyphaga; M: marker
The proteolytic activity from isolates number 4 and 5 (isolated from Nile water, morphologically identified as A. rhysodes and A. royreba, respectively) showed different proteolytic activities with a common band at molecular weight 30 kDa. The proteolytic activities of A. rhysodes were visualized at 113, 90, 75, 62, 49, 45, 39, 36 and 30 kDa, while in A. royreba the gelatin digestion bands were evidenced at 124, 97, 83, 73, 65, 61, 57, 50, 37 and 30 KDa (Fig. 2). The intensity of the bands varied among species. The pH profile revealed that these proteases were active in all strains at pH 10 only. Interestingly, these proteases showed over expression only in potentially pathogenic species of Acanthamoeba.
Discussion
The present study deals with the natural distribution of pathogenic Acanthamoeba in the aquatic environment at different localities in the Nile Delta region, Egypt. Of special interest were the isolates capable of proliferating at temperatures of 37°C and above, as well as the biochemical characterization of these organisms. Previous studies on this subject in Egypt are non-existent. Therefore this study represents the first field investigation initiated in Egypt to estimate the presence of potentially pathogenic Acanthamoeba in the Nile, tap and swimming pools waters by using culture and biochemical techniques.
Prevalence of heat tolerant free-living amoebae in different types of water
In the present study, free-living amoebae grown at 40°C were isolated from 78.9% of the total examined freshwater samples. In Oklahoma, USA, (22) recorded a relatively lower incidence of free-living amoebae (63.0%) isolated at 42°C from pond filled mainly by runoff water. A year later, the same authors recorded free-living amoebae in a much lower incidence (28.5%) when water samples from Lake Tenkiller were cultured at 42°C (23).
In the present work, free-living amoebae were isolated at 37°C from 90.5% of the examined tap water samples. In Egypt Hamadto et al. (7) and Hilali et al. (24) recorded lower incidence of free-living amoebae in tap water (4 and 23.6%, respectively). This difference might be due to the difference in the number of the samples examined: 50 and 72 tap water samples respectively, compared to 360 tap water samples in the present investigation. Certain workers in UK also detected free-living amoebae in 89% of the examined household tap-water samples (25). However, in Korea, Jeong and Yu (26) recorded freshwater amoebae in a percentage of domestic tap water samples (46.9%) lower than that recorded in our results. In the present study, free-living amoebae grown at 40°C were isolated from 60% of the swimming pool samples. In Poland, researchers (27) recorded a lower incidence of free-living amoebae (37.2%) isolated at 42°C from swimming pools.
Prevalence of Acanthamoeba in different types of water
In the present study, it was found that the incidence of Acanthamoeba spp. reached 56.0% in the examined Nile water samples. In Egypt also Acanthamoeba spp. were detected in 66.67, 40 and 43.1% of the examined Nile water samples (6, 7, 24), respectively. Lorenzo-Morales et al. (8) in Spain detected Acanthamoeba in 43.3% of fresh water samples examined. Other workers in Thailand, USA and Saudi Arabia recorded a lower incidence of Acanthamoeba (35, 38.89 and 36.7%, respectively) in freshwater samples (22, 28, 29). In USA, Ettinger et al. (30) recorded Acanthamoeba spp. from James River in a percentage (7%) lower than that recorded in our results.
In the present work, it was found that the incidence of Acanthamoeba spp. reached 58.6% in the examined tap water samples. However, in Egypt Hamadto et al. (7) detected Acanthamoeba in tap water in a much lower incidence (4%). This difference might be due to the difference in the number of the examined samples as the mentioned authors examined 50 tap water samples; while in the present work a much higher number of samples (360) were examined. Other workers in Egypt detected Acanthamoeba in a lower percentage (11.1%) in the examined tap water (24) than that of our results. Other workers in other countries, in Spain for example, the incidence of Acanthamoeba in tap water recorded 59.5% (31). Other workers in other countries also recorded lower incidence of Acanthamoeba (26.9 and 5.8%) in tap water samples in UK and Korea, respectively (25, 26), for example.
In the present work, it was found that the incidence of Acanthamoeba spp. reached 49.2% in the examined swimming pool samples. However, in Egypt Acanthamoeba spp. were detected in a lower incidence (25%) of the examined swimming pool samples (7). Other workers in NewZeland, Mexico, Thailand, and Taiwan recorded lower incidences of Acanthamoeba (20, 32, 8.1, 13.0 and 5.9%, respectively) in swimming pools (32–36). These findings may indicate that the used disinfection procedures were not adequate for the elimination of amoebae from the swimming pools examined.
Osmo-tolerance differentiation between pathogenic and non-pathogenic Acanthamoeba
Acanthamoeba spp. exhibiting growths at 1M manitol are considered potentially pathogenic. This physiological assay can be used to differentiate between pathogenic and non-pathogenic Acanthamoeba spp. (17, 37, 31). In the present work it was found that 92.9% of the isolated Acanthamoeba strains from Nile water demonstrated pathogenic potential using osmo-tolerance assay. Also in the present work, the percentage of Acanthamoeba spp. exhibiting osmo-tolerance reached 15.2 and 8.5% in tap water and swimming pools, respectively. Other workers in Egypt, recorded a lower incidence of osmo-tolerant Acanthamoeba spp. (69%) isolated from freshwater samples of the Nile Delta (8). Other workers in Turkey from fresh water samples reported a lower percentage of Acanthamoeba spp. exhibited osmo-tolerance (66.6%) (38).
Biochemical Characterization of isolated free-living amoebae
Proteases are enzymes that catalyze the hydrolysis of peptide bonds in a broad spectrum of important biological reactions including the pathogenesis of parasitic disease (39). Proteases secreted by Acanthamoeba are regarded as an important factor in the pathogenesis (39).
In the present work, the isolated Acanthamoeba species were classified according both osmo-tolerant and enzymatic activity tests to 5 potentially pathogenic (morphologically identified as Acanthamoeba rhysodes, A. polyphaga, A. mauritaniensis, A. royreba and A. castellanii) and 1 non-pathogenic (morphologically identified as A. triangularis) species. Our result agreed with other workers in UK (18) in that A. castellanii exhibited significantly higher protease activity, while our result disagreed in that A. polyphaga and A. royreba (that were potentially pathogenic in our result) exhibited a lower protease activity. In the present work, A. polyphaga showed proteolytic activity ranging from 29 to 104 kDa by zymography. Other workers in Mexico (40) found that A. polyphaga gave proteolytic activity ranging from 34 to 144 kDa and they agreed with our results in 2 bands (34 and 71 kDa). Other workers reported that A. polyphaga zymography gave 43, 59, 70 and 100 to 130 kDa cysteine protease, (41) and our results also showed a dense band of 104 kDa. Mitro et al. (42) clearly showed only three protease activities of 36, 49 and 66 kDa, accompanied by additional faint bands that were discernible between 22 and 24 kDa and between 49 and 66 kDa.
In the present work, A. castellanii showed proteolytic activity ranging from 36 to 115 kDa by zymography. In Egypt, Nashed et al. (43) found complete agreement among the A. culbertsonii strains with respect to the banding patterns of phosphoglucomutase using disc electrophoresis of seven different enzymes to compare A. culbertsonii from Egypt with other A. culbertsonii from New York and India and A. castellanii from London. Other workers in Mexico (40) stated that A. castellanii gave a characteristic zymography bands ranging from 30 to 178 kDa but they reported a band giving 73 kDa which we recorded in the present work. On the other hand, A. castellanii was described as having different bands by zymography (44–47).
Other workers described that a characteristic band at 33 kDa was considered as an indicator of pathogenicity due to the presence of serine proteases in different Acanthamoeba spp. (44, 47–49). In the present study a 34 kDa band was only observed in A. polyphaga and 36 kDa was only in A. castellanii.
Conclusion
The incidence and prevalence of the pathogenic Acanthamoeba species in the aquatic environment using parasitological and biochemical diagnostic tools will provide baseline data against which the risk factors associated with waterborne transmission can be identified.
Acknowledgements
The authors declare that there is no conflict of interest.
References
- 1.Marciano-Cabral F, Cabral G. Acanthamoeba spp. as agents of disease in humans. Clin Microbiol Rev. 2003;16:273–307. doi: 10.1128/CMR.16.2.273-307.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khan NA. Pathogenesis of Acanthamoeba infections. Microb Pathog. 2003;34:277–28. doi: 10.1016/s0882-4010(03)00061-5. [DOI] [PubMed] [Google Scholar]
- 3.Krusnell J, Linder E. Bacterial infections of free-living amoebae. Res Microbiol. 2001;152:613–619. doi: 10.1016/s0923-2508(01)01240-2. [DOI] [PubMed] [Google Scholar]
- 4.Lorenzo-Morales J, Lopez-Darias M, Martinez-Carretero E, Valladares B. Isolation of potentially pathogenic strains of Acanthamoeba in wild squirrels from Canary Islands and Morocco. Exp Parasitol. 2007;117:74–79. doi: 10.1016/j.exppara.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 5.Jager B, Stamm W. Brain abscesses caused by free-living amoebae probably of the genus Hartmannella in a patient with Hodgkin's disease. Lancet. 1972;ii:1343–1345. doi: 10.1016/s0140-6736(72)92781-x. [DOI] [PubMed] [Google Scholar]
- 6.Al-Herrawy A. In vitro cultivation of agents of amoebic meningo-encephalitis isolated from water and sewage; Egypt: Alexandria Univ; 1992. Ph. D. thesis, Fac. Vet. Med. [Google Scholar]
- 7.Hamadto H, Aufy S, El-Hayawan I, Saleh M, Nagaty I. Study of free-living amoebae in Egypt. J Egypt Soci Parasitol. 1993;23:631–637. [PubMed] [Google Scholar]
- 8.Lorenzo-Morals J, Ortiga-Rivas A, Martinez E, Khoubbane M, Artigas P, Periago M, Foronda P, Abreu-Acosta N, Valladares B, Mas-Coma S. Acanthamoeba isolates belonging to T1, T2, T3, T4 and T7 genotypes from environmental freshwater samples in the Nile Delta region, Egypt. Acta Trop. 2006;100:63–69. doi: 10.1016/j.actatropica.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 9.Ismail M. General studies on free living amoeba isolated from the environment; Cairo University; 1995. MD. Thesis. [Google Scholar]
- 10.Sadaka H, El-Nassery S, Abou-Samra L, Awadalla H. Isolation and identification of free-living Amoebae from some water sources in Alexandria. J Egypt Soc Parasitol. 1994;24(2):247–57. [PubMed] [Google Scholar]
- 11.El-Marhoumy S, Antonics S, Warhurst D. Isolation of soil amoebae from the nasal mucosa of children in Tanta-Egypt. J Egypt Soc Parasitol. 1988;18(2):665. [PubMed] [Google Scholar]
- 12.El-Deeb MAB. Preliminary studies on free-living amoebae; Cairo University; 1990. M.Sc. Thesis. [Google Scholar]
- 13.Gradus M, Koenig S, Hyndiuk R, De Carlo J. Filter-culture technique using amoebae saline transport medium for the noninvasive diagnosis of Acanthamoeba keratitis. Am J Clin Pathol. 1989;92:682–685. doi: 10.1093/ajcp/92.5.682. [DOI] [PubMed] [Google Scholar]
- 14.De Jonckheere J, Van Dijk P, De Voord H. Evaluation of the indirect fluorescent antibody technique for identification of Naegleria species. Appl. Microbiol. 1974;28:159–164. doi: 10.1128/am.28.2.159-164.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pussard M, Pons R. Morphologie de la paroi kystique et taxonomie du genre Acanthamoeba (Protozoa, Amoebida) Protistol. 1977;TXIII:557–598. [Google Scholar]
- 16.Page F. Freshwater Biol. Ass: Ambleside; 1988. A New Key to Freshwater and Soil Gymnamoebae. [Google Scholar]
- 17.Khan NA, Jarroll E, Paget T. Molecular and physiological differentiation between pathogenic and non-pathogenic Acanthamoeba. Curr Microbiol. 2002;45:197–202. doi: 10.1007/s00284-001-0108-3. [DOI] [PubMed] [Google Scholar]
- 18.Khan NA, Jarroll E, Panjwani N, Cao Z, Pager T. Proteases as markers of differentiation of pathogenic and non-pathogenic Acanthamoeba. J Clin Microbiol. 2000;38:2858–2861. doi: 10.1128/jcm.38.8.2858-2861.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iwanaga Y. Studies on host-parasite relationship between the Puerto Rican strain of Schistosoma mansoni and Biomphalaria snails. Southeast Asian J Trop Med Public Health. 1994;25:509–15. [PubMed] [Google Scholar]
- 20.Bahgat M, Ruppel A. Biochemical comparison of the serine protease (elastase) activities in cercarial secretions from Trichobilharzia ocellata and Schistosoma mansoni. Parasitol Res. 2002;88:495–500. doi: 10.1007/s00436-002-0597-4. [DOI] [PubMed] [Google Scholar]
- 21.Bahgat M, Sorgho H, Ouédraogo J. Enzyme-linked immunosorbent assay with worm vomit and cercarial secretions of Schistosoma mansoni to detect infections in an endemic focus of Burkina Faso. J Helminthol. 2006;80:19–23. doi: 10.1079/joh2005312. [DOI] [PubMed] [Google Scholar]
- 22.John D, Howard M. Seasonal distribution of pathogenic free-living amoebae in Oklahoma water. Parasitol Res. 1995;81:193–201. doi: 10.1007/BF00937109. [DOI] [PubMed] [Google Scholar]
- 23.John D, Howard M. Isolation of thermo-tolerant free-living amoebae from Lake Tenkiller. Oklahoma Proc Okla Acad Sci. 1996;76:1–4. [Google Scholar]
- 24.Hilali M, Ashmawy K, Samaha H, Draz A, Abu El-Waf S, Salem A. Preliminary studies on amoebic pathogens isolated from water and sewage with respect to Naegleria and Acanthamoeba. J Egypt Vet Med Ass. 1994;53:215–224. [Google Scholar]
- 25.Kilvington S, Gray T, Dart J, Morlet N, Beeching J, Frazer D, Matheson M. Acanthamoeba keratitis: the role of domestic tap water contamination in the United Kingdom. Invest. Ophthalmol Vis Sci. 2004;45:165–169. doi: 10.1167/iovs.03-0559. [DOI] [PubMed] [Google Scholar]
- 26.Jeong H, Yu H. The role of domestic water in Acanthamoeba contamination in contact lens storage cases in Korea. Korean J Parasitol. 2005;43:47–50. doi: 10.3347/kjp.2005.43.2.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gronik K, Kuzna-Grygiel W. Presence of virulent strains of amphizoic amoebae in swimming pools of the city of Szczecin. Ann Agric Environ Med. 2004;11:233–236. [PubMed] [Google Scholar]
- 28.Al-Herrawy A, Al-Rasheid K. Identification of Acanthamoeba strains isolated from a freshwater course in Saudi Arabia. J Egypt Pub Healt Ass. 1998;LXXIII:621–633. [PubMed] [Google Scholar]
- 29.Nacapunchai D, Kino H, Ruangsitticha C, Sriwichai P, Ishih A, Terada M. A brief survey of free-living amoebae in Thailand and Hamamatsu district, Japan. Southeast Asian J Trop Med Public Health. 2001;32:179–82. [PubMed] [Google Scholar]
- 30.Ettinger M, Webb S, Harris S, McIninch S, Garman G, Brown B. Distribution of free-living amoebae in James River, Virginia, USA. Parasitol Res. 2003;89:6–15. doi: 10.1007/s00436-002-0707-3. [DOI] [PubMed] [Google Scholar]
- 31.Lorenzo-Morals J, Ortiga-Rivas A, Martinez E, Foronda P, Valladares B. Isolation and identification of pathogenisis Acanthamoeba strains in Tenerife, Canary Island, Spain from water sources. Parasitol Res. 2005;95:273–277. doi: 10.1007/s00436-005-1301-2. [DOI] [PubMed] [Google Scholar]
- 32.Brown T. The occurrence and distribution of pathogenic free-living amoebae in thermal areas of the North Island of New Zealand. New Zealand Journal of Marine and Freshwater Research. 1983;17:59–69. [Google Scholar]
- 33.Rivera F, Ramirez P, Vilaclara G, Robles E, Medina F. A survey of pathogenic and free-living amoebae inhabiting swimming pool water in Mexico City. Environ Res. 1983;32:205–211. doi: 10.1016/0013-9351(83)90207-4. [DOI] [PubMed] [Google Scholar]
- 34.Rivera F, Ramirez E, Bonilla P, Calderon A, Gallegos E, Rodríguez S, Ortiz R, Zaldivar B, Ramirez P, Duran A. Pathogenic and free-living amoebae isolated from swimming and physiotherapy tubs in Mexico. Environ Res. 1993;62:43–52. doi: 10.1006/enrs.1993.1087. [DOI] [PubMed] [Google Scholar]
- 35.Lekkla A, Sutthikornchai C, Bovornkitti S, Sukthana Y. Free-living amoebae contamination in natural hot springs in Thailand. Southeast Asian J Trop Med Public Health. 2005;36:5–9. [PubMed] [Google Scholar]
- 36.Hsu B, Ma P, Liou T, Chen J, Shine F. Identification of 18S ribosomal DNA genotype of Acanthamoeba from hot spring recreation areas in the central range, Taiwan. Journal of Hydrobiology. 2009;367:249–254. [Google Scholar]
- 37.Khan N, Tareen N. Genotypic, phenotypic, biochemical, physiological and pathogenicity-based categorization of Acanthamoeba strain. Folia Parasitologica. 2003;50:97–104. doi: 10.14411/fp.2003.017. [DOI] [PubMed] [Google Scholar]
- 38.Kilic A, Tanyuksel M, Sissons J, Jayasekera S, Khan N. Isolation of Acanthamoeba isolates belonging to T2, T3, T4, and T7 genotypes from environmental samples in Ankara, Turkey. Acta Parasitol. 2004;49:246–252. [Google Scholar]
- 39.Mckerrow J, Sun E, Rosenthal P, Bouvier J.N. Isolation of Acanthamoeba The proteases and pathogenicity of parasitic protozoa. Annu Rev Microbiol. 1993;47:821–853. doi: 10.1146/annurev.mi.47.100193.004133. [DOI] [PubMed] [Google Scholar]
- 40.Serrano-Luna J, Cervantes-Sandoval I, Calderon J, Navarro-Garcia F, Tsutsumi V, Shibayama M.N. Protease activities of Acanthamoeba polyphaga and Acanthamoeba castellanii. Can J Microbiol. 2006;52:16–23. doi: 10.1139/w05-114. [DOI] [PubMed] [Google Scholar]
- 41.Alfieri S, Correia C, Motegi S, Pral E. Proteinase activities in total extracts and in medium conditiond by Acanthamoeba polyphaga trophozoites. J Parasitol. 2000;86:220–227. doi: 10.1645/0022-3395(2000)086[0220:PAITEA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 42.Mitro K, Bhagavathiammai A, Zhou O, Bobbett G, Mckerrow J, Chokshi R, Chokshi B, James E. Partial characterization of the proteolytic secretions of Acanthamoeba polyphaga. Exp Parasitol. 1994;78:377–385. doi: 10.1006/expr.1994.1041. [DOI] [PubMed] [Google Scholar]
- 43.Nashed N, Youssef F, Mansour N. Isoenzyme characterization of Acanthamoeba from water sites in Egypt as compared to other geographical Acanthamoeba species and strains. Invertebrate Zoology and Parasitology. 1993;11:43–54. [Google Scholar]
- 44.Cho J, Na B, Kim T, Song C. Purification and characterization of an extracellular serine proteinase from Acanthamoeba castellanii. IUBMB Life. 2000;50:209–214. doi: 10.1080/152165400300001534. [DOI] [PubMed] [Google Scholar]
- 45.Alsam S, Sisson J, Jayasekera S, Khan N. Extracellular proteases of Acanthamoeba castellanii (encephalitis isolate belonging to T1 genotype) contribute to increased permeability in an in vitro model of the human blood-brain barrier. J Inf. 2005;51:150–156. doi: 10.1016/j.jinf.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 46.Sissons J, Alsam S, Goldsworthy G, Lightfoot M, Jarroll E, Khan N. Identification and properties of proteases from an Acanthamoeba isolate capable of producing granulomatous encephalitis. BMC Microbiology. 2006;6:42–49. doi: 10.1186/1471-2180-6-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim W, Kong H, Ha Y, Hong Y, Jeong H, Yu H, Chung D. Comparison of specific activity and cytopathic effects of purified 33 kDa serine proteinase from Acanthamoeba strains with different degree of virulence. Korean J Parasitol. 2006;4:321–330. doi: 10.3347/kjp.2006.44.4.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kong H, Kim T, Chung D. Purification and characterization of secretory serine proteinase of Acanthamoeba healyi isolated from gave. J Parasitol. 2000;43:391–395. doi: 10.1645/0022-3395(2000)086[0012:PACOAS]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 49.Blaschitz M, Kohsler M, Aspock H, Walochnik J. Detection of a serine proteinase gene in Acanthamoeba genotype T6 (Amoebozoa: Lobosea) Exp Parasitol. 2006;114:26–33. doi: 10.1016/j.exppara.2006.02.004. [DOI] [PubMed] [Google Scholar]