Abstract
While an excess of metals such as zinc, cadmium or nickel (Ni) is toxic for most plants, about 500 plant species called hyperaccumulators are able to accumulate high amounts of these metals. These plants and the underlying mechanisms are receiving an increasing interest because of their potential use in sustainable biotechnologies such as biofortification, phytoremediation, and phytomining. Among hyperaccumulators, about 400 species scattered in 40 families accumulate Ni. Despite this wide diversity, our current knowledge of the mechanisms involved in Ni accumulation is still limited and mostly restricted to temperate herbaceous Brassicaceae. New Caledonia is an archipelago of the tropical southwest pacific with a third of its surface (5500 km2) covered by Ni-rich soils originating from ultramafic rocks. The rich New Caledonia flora contains 2145 species adapted to these soils, among which 65 are Ni hyperaccumulators, including lianas, shrubs or trees, mostly belonging to the orders Celastrales, Oxalidales, Malpighiales, and Gentianales. We present here our current knowledge on Ni hyperaccumulators from New Caledonia and the latest molecular studies developed to better understand the mechanisms of Ni accumulation in these plants.
Keywords: nickel hyperaccumulator, New Caledonia, ultramafic soils, adaptation, comparative transcriptomic
Introduction
New Caledonia is an archipelago located in the southwest Pacific off the east coast of Australia (20–23 S, 164–167 E). New Caledonia separated from Gondwana in the Cretaceous, and after a submersion during Paleocene and Eocene, the main island emerged 37 million years ago (Pelletier, 2006) and was likely exclusively re-colonized by plants through long-distance dispersal (Grandcolas et al., 2008; Cruaud et al., 2012; Pillon, 2012). At the time of its emersion it was probably entirely covered with ultramafic rocks that still occupy 34% (5500 km2) of the surface of the main island. The weathering of these rocks produced a variety of soils that have a very low level of phosphorus, potassium, calcium and high level of magnesium and heavy metals including nickel (Ni), cobalt and chromium (Jaffré, 1980; Brooks, 1987). New Caledonia harbors a rich and original vegetation likely resulting from the adaptation of plants over a long period of time to these challenging edaphic conditions. New Caledonia is renowned for its rich, endemic and threatened vascular flora (Morat et al., 2012) and is therefore considered as one of the world biodiversity hotspots (Myers et al., 2000). In particular, 2145 species, among which 80% are endemic, are developing on these metal rich soils (Jaffré et al., 2009). As a consequence, New Caledonia has also been listed among the main hotspots for metallophytes (Whiting et al., 2004).
Metal hyperaccumulators from new Caledonia: history and current knowledge
Unusually high concentration of nickel (Ni) in plants were first reported in the Italian Brassicaceae Alyssum bertolonii (Minguzzi and Vegnano, 1948) and later in Hybanthus floribundus (Violaceae) from Australia (Severne and Brooks, 1972). Ni concentration exceeding 1% (dry weight) in New Caledonian plants growing on ultramafic soils was first reported in Psychotria gabriellae (previously known as P. douarrei, Rubiaceae), Hybanthus austrocaledonicus, H. caledonicus (Violaceae), Geissois pruinosa (Cunoniaceae), and Homalium guillainii (Salicaceae) (Brooks et al., 1974; Jaffré and Schmid, 1974). These plants accumulating more than 1% Ni were then qualified as “hypernickelophore” (Jaffré and Schmid, 1974). The more widely used term Ni “hyperaccumulator” was first coined by Jaffré et al. (1976) for the description of Pycnandra acuminata (previously known as Sebertia acuminata, Sapotaceae) in which Ni represents up to 25% of the latex dry weight. The term ‘hyperaccumulator’ was later defined more precisely by Brooks et al. (1977) to qualify plants with a concentration of Ni above 0.1% (1000 ppm) in dried leaves and extended to other metal accumulators, although with different thresholds (Baker and Brooks, 1989; van der Ent et al., 2013a). Following these pioneering studies, a large number of species that hyperaccumulate Ni were described from the New Caledonian flora (Jaffré, 1977a, 1980; Jaffré et al., 1979a,b; Kersten et al., 1979; Amir et al., 2007). Today, we consider that 65 Ni hyperaccumulator taxa scattered in 12 plant families have been identified (see Supplementary Table), placing New Caledonia, along with Cuba that contains about 140 hyperaccumulators (Reeves et al., 1996, 1999), as one of the most important reservoirs of Ni hyperaccumulators. We can predict that the number of Ni hyperaccumulators in New Caledonia will slightly increase in the coming years considering that the hyperaccumulator status of known taxa (e.g., Pancheria ferruginea, Cunoniaceae; Capparis artensis, Capparaceae) was revealed by recent analyses and that new hyperaccumulator taxa, such as Pycnandra caeruleilatex (Swenson and Munzinger, 2010), are still being described. The New Caledonian flora also contains manganese hyperaccumulators (above 1% of dry weight) such as Denhamia fournieri (previously known as Maytenus fournieri, Celastraceae), Alyxia poyaensis (previously a subspecies of Alyxia rubricaulis, Apocynaceae), Virotia neurophylla (previously known as Macadamia neurophylla, Proteaceae), and Garcinia amplexicaulis from the Clusiaceae family (Jaffré, 1977b, 1979; Fernando et al., 2008, 2010). Interestingly, Denhamia fournieri is also able to hyperaccumulate Ni when growing on ultramafic soil.
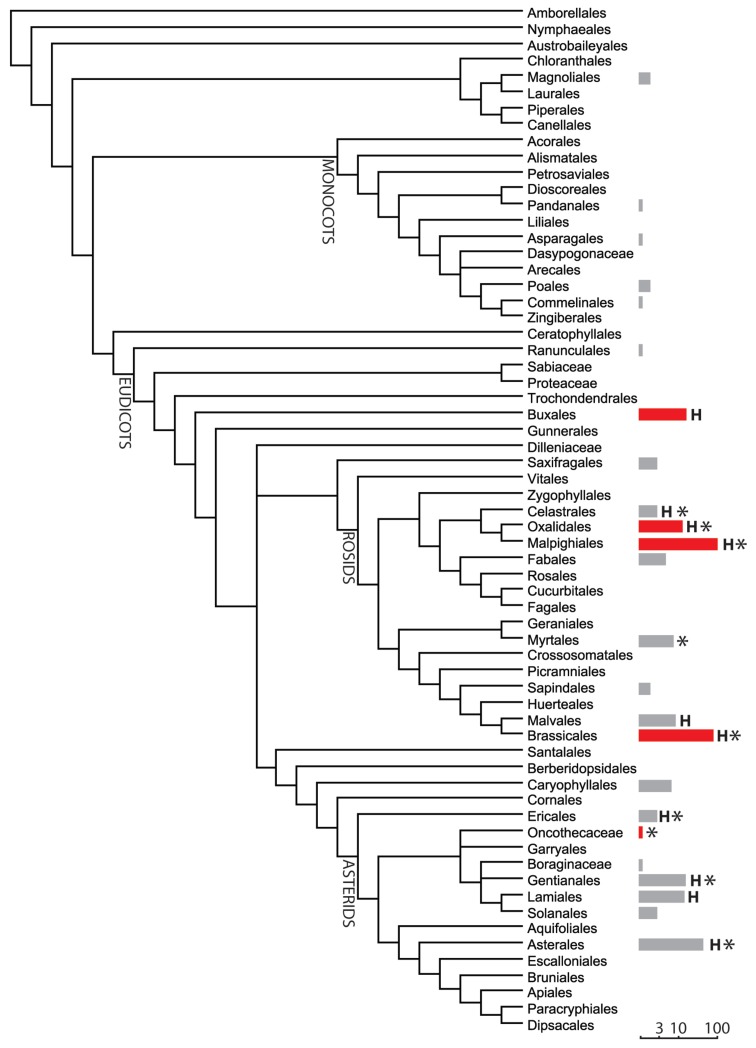
Taxonomic distribution of nickel hyperaccumulators and evolution of the hyperaccumulator trait
Worldwide about 400 nickel (Ni) hyperaccumulators scattered in more than 40 families have been reported almost exclusively in angiosperms (Verbruggen et al., 2009; Krämer, 2010), however at least one fern of the Adiantum genus (Brooks et al., 1990), and several epiphytic bryophytes and liverworts growing on hyperaccumulating shrubs in New Caledonia can be considered as Ni hyperaccumulators (Boyd et al., 2009), suggesting that Ni hyperaccumulation could have appeared early in the evolution of terrestrial plants. Within angiosperms, Ni hyperaccumulators are mainly found in Eudicots, although few examples in Magnolianae and Monocots have been described (Figure 1). Ni hyperaccumulators are taxonomically scattered, nevertheless phylogenetic trends can be observed (Pillon et al., 2010). A third of the Ni hyperaccumulators belongs to the COM clade (Celastrales, Oxalidales, and Malpighiales) which form part of Rosids that account for two third of the Ni hyperaccumulating species. Although this character appeared many times in the course of evolution, it tends to come out more often in certain lineages. In New Caledonia, 83% of the Ni hyperaccumulators are from the COM clade while only one hyperaccumulator from each of the orders Brassicales and Asterales have been identified (see Supplementary Table). The explanation for this bias remains not fully understood.
Figure 1.
Phylogenetic distribution of nickel hyperaccumulators using APG III classification. The number of Ni hyperaccumulator in each order is presented as a bar (logarithmic scale). The red bar indicates that the observed number of hyperaccumulators significantly exceed the theoretical number (hyperaccumulating species randomly distributed using a binomial law with a Bonferroni correction for multiple tests). H, indicates the presence of hypernickelophore species accumulating more than 10,000 ppm nickel. *, indicates the presence of hyperaccumulators from New Caledonia.
There are still only few phylogenetic studies that have investigated how nickel hyperaccumulating species evolved from non-accumulating ancestors, and whether the ability to grow on ultramafic soils is a prerequisite for this character to appear. In some genera, such as Australian Stackhousia (Burge and Barker, 2010) or Californian Streptanthus (Mayer and Soltis, 1994), Ni hyperaccumulation is a rare character found only in one species, although several other related species are growing on Ni-rich soils. This is also the case in New Caledonian Psychotria, in which P. gabriellae is the only Ni hyperaccumulator while 60 Psychotria species, including P. semperflorens, are present on ultramafic soils (Jaffré, 1980; Baker et al., 1985). In contrast, in New Caledonian Phyllanthus (Euphorbiaceae; Kersten et al., 1979), and in Cuban Buxus (Buxaceae; Reeves et al., 1996), a significant numbers of species growing on ultramafic soils hyperaccumulates Ni. In Meditterranean Alyssum sect. Odontarrhena (Cecchi et al., 2010) and in New Caledonian Geissois (Cunoniaceae; Jaffré et al., 1979a), almost all species occurring on ultramafic soils hyperaccumulate nickel, but they are phylogenetically entangled with non-accumulating species restricted to non-ultramafic soils (Cecchi et al., 2010; Pillon et al., unpublished). In the Carribean genus Leucocroton (Euphorbiaceae), mostly diversified on Cuba, all 26 species are restricted to ultramafic soils and hyperaccumulate nickel (Jestrow et al., 2012). Therefore, the link between the adaptation to ultramafic soils and Ni hyperaccumulation shows some striking variations that are also found within New Caledonian hyperaccumulators. Both characters seem to appear recurrently in some genera in distant areas, e.g., in Psychotria and Phyllanthus from New Caledonia, Cuba and other regions (Reeves, 2003). Although no specific study have addressed this question, molecular phylogenetic analyses indicated that Phyllanthus and Psychotria species, including nickel hyperaccumulators, from New Caledonia and the Caribbean region belong to distant clades within these genera (Andersson, 2002; Kathriarachchi et al., 2006; Barrabé, 2013).
Parallel evolution of the characters at broad taxonomical and geographical scales may be facilitated by an ancestral fortuitous pre-adaptation, that can be called exaptation, to ultramafic soils (Pillon et al., 2010). At a smaller scale, it may have evolved only once and then have been transmitted horizontally through hybridization and introgression between closely related species, as observed for the adaptation to ultramafic soils (Pillon et al., 2009a,b), in a process that could be qualified as collective evolution (Morjan and Rieseberg, 2004). The great diversity of plants adapted to ultramafic soils and that can hyperaccumulate Ni in New Caledonia, combined with its geographic isolation, makes this archipelago an ideal system to study the evolution of these two characters.
Molecular studies on new Caledonian nickel hyperaccumulators
Our current understanding of the hyperaccumulation of metals in plants has been recently recapitulated in excellent review articles (Verbruggen et al., 2009; Krämer, 2010; Rascio and Navari-Izzo, 2011). It now clearly appears that molecular mechanisms involved in metal chelation and transport play key roles in this remarkable adaptation. However, only few mechanisms involved in nickel (Ni) hyperaccumulation have been identified at the molecular level, and this knowledge only originates from studies of hyperaccumulators from the Brassicaceae family. The study of Ni hyperaccumulators from other plant families would therefore likely bring new and original information on the mechanisms involved in Ni hyperaccumulation in plants.
After the identification of Ni hyperaccumulators in New Caledonia in the early 70's, one of the first questions was to identify molecules that are able to chelate Ni and therefore play a role in detoxification. Early analyses of New Caledonian hyperaccumulators revealed that Ni was essentially associated with organic acids. Citrate was identified as a main ligand for Ni in the latex of Pycnandra acuminata and in leaves of several hyperaccumulators in the Homalium and Hybanthus genera (Lee et al., 1977, 1978), while 63% of Ni was bound to malate in leaves of Psychotria gabriellae (Kersten et al., 1980). The existence of Ni-citrate complexes in several hyperaccumulators was largely confirmed since then in several studies that use state-of-the art techniques of chromatography and mass spectrometry (Sagner et al., 1998; Schaumlöffel et al., 2003; Callahan et al., 2008, 2012). These recent studies also revealed the presence of Ni-nicotianamine (NA) complexes in most of the New Caledonian species that were studied, including P. acuminata and P. gabriellae (Schaumlöffel et al., 2003; Callahan et al., 2012). Callahan et al. (2008, 2012) developed a metabolic profiling approach based on GC-MS to identify putative ligands whose abundance in New Caledonian hyperaccumulators correlates with Ni concentration. Putative Ni-metabolite complexes were then directly identified using LC-MS. These analyses identified 2,4,5-trihydroxy-3-methoxy-1,6-hexan-dioic acid as a new ligand for Ni in the latex of P. acuminata. In contrast to Alyssum and Noccaea hyperaccumulators (Krämer et al., 1996; Persans et al., 1999), no correlation between the amino acid histidine and Ni was observed so far in the New Caledonian hyperaccumulators. Rather, a significant correlation was observed with aspartic acid, which has a relatively high association constant for Ni (lg K = 7.2), but LC-MS analyses failed to directly detect Ni-aspartic acid complexes in these species. Analyses of P. gabriellae samples revealed several Ni-organic acid complexes, including malonic and maleic acids, but did not confirm the existence of a complex with malic acid. In addition, the authors pointed out that several intense Ni-containing ions could not be identified by LC-MS analysis from P. gabriellae samples (Callahan et al., 2012). These studies confirmed that Ni forms complexes with a plethora of organic acids, which could be of biological significance for the storage of Ni in vacuoles. NA that is able to bind Ni with a high affinity (lg K = 16), seems to be an ubiquitous Ni ligand in dicotyledon hyperaccumulators and may play an important role in cytoplasmic Ni detoxification and long range transport (Kim et al., 2005; Pianelli et al., 2005; Gendre et al., 2007). In contrast, histidine might not play a significant role in Ni homeostasis in New Caledonian hyperaccumulators and the presence of Ni-complexes with other amino acids still needs to be confirmed. Additional metabolomic studies using alternative extraction and separation methods will be required to validate and identify new Ni-complexes from New Caledonian hyperaccumulators. Ultimately, the speciation of Ni in New Caledonian hyperaccumulators should be also confirmed in vivo, for example using X-ray spectrometric methods (Montarges-Pelletier et al., 2008).
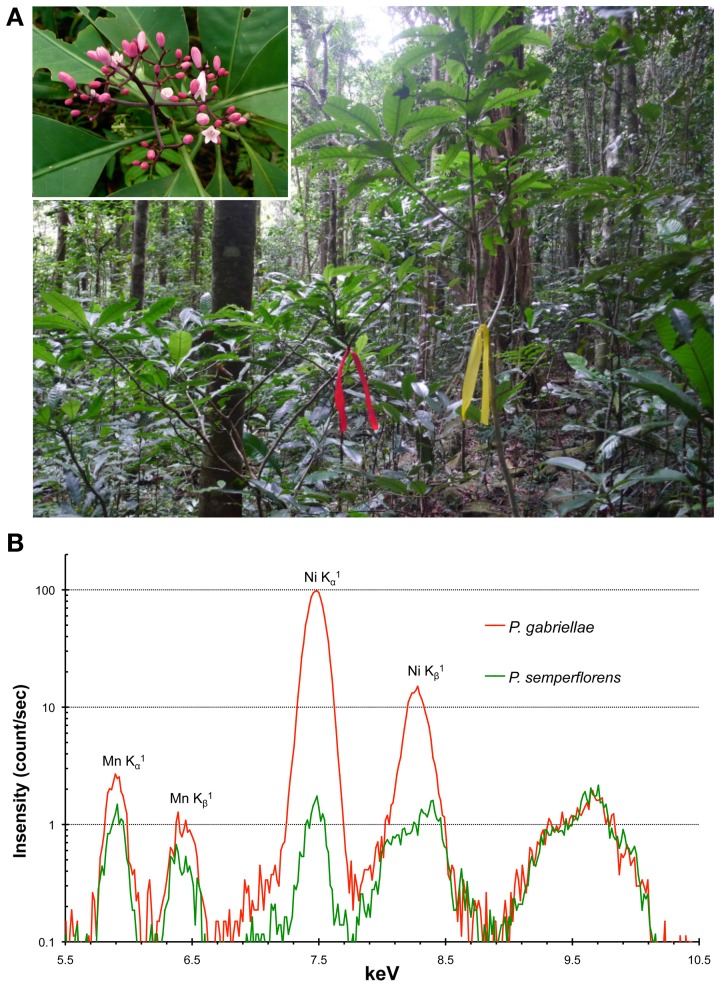
In parallel to these metabolomic studies, it is also important to identify genes coding for key enzymes in the biosynthesis of Ni ligands and for Ni membrane transporters. However, no nucleotide sequences were available until recently for any of the New Caledonian hyperaccumulators. The recent development of Next Generation Sequencing (NGS) technologies and the creation of a molecular biology platform in New Caledonia (Majorel-Loulergue et al., 2009), allowed us to initiate the sequencing of the P. gabriellae leaf transcriptome using Roche GS-FLX titanium technology. We obtained more than 500,000 reads that are publicly available at the European Nucleotide Archive under accession number ERP001334. The de novo assembly of the sequence reads generated approximately 30,000 contigs that represented the first EST data set for P. gabriellae. We are currently using these original sequences to identify putative Ni transporters from the NRAMP, IREG/FPN, YSL, ZIP/IRT families (Verbruggen et al., 2009; Krämer, 2010; Rascio and Navari-Izzo, 2011), that might be involved in nickel tolerance and hyperaccumulation in leaves (Merlot et al., submitted). To identify additional genes important for Ni accumulation, it will be necessary to sequence the root transcriptome of P. gabriellae. In parallel, the seed transcriptome of P. gabriellae was sequenced by the 1KP project in collaboration with the University of New Caledonia (www.onekp.com/index.html). Also, because it is not presently possible to genetically manipulate this species, one strategy to target genes of interest is to compare its transcriptome with that of a phylogenetically related, non-accumulating species and thereby identify genes whose expression is linked to the hyperaccumulation trait. Psychotria semperflorens, which lives in sympatry with P. gabriellae in rain forest on ultramafic soil but does not accumulate Ni (Figure 2), represents an excellent candidate to conduct comparative transcriptomic studies. Interestingly, the flora of Cuba contains at least five Ni hyperaccumulators of the Psychotria genus including P. costivenia and P. clementis (Reeves et al., 1999). The comparison of the molecular mechanisms involved in Ni adaptation and hyperaccumulation in Psychotria species from the flora of Cuba and New Caledonia that have different origins, will help to understand the evolution of these traits and possibly reveal the genetic basis of the exaptation to ultramafic soils. A similar approach using New Caledonian species could be used to study the evolution of Ni hyperaccumulation mechanisms in the Phyllanthaceae genus Phyllanthus (including Glochidion) that is also present in Cuba and Indonesia (Kersten et al., 1979).
Figure 2.
Psychotria gabriellae and Psychotria semperflorens as a model system to study nickel hyperaccumulation. The Ni hyperaccumulator P. gabriellae (red tag) and the closely related non-accumulator P. semperflorens (yellow tag) are living in sympatry in rain forest on ultramafic soil in New Caledonia (A). Both species are 1–3 meters shrub with very similar pink flowers (top right insert: P. semperflorens inflorescence). In natural growth condition, P. gabriellae accumulates up to 4% Ni in leaves while P. semperflorens accumulates approximately 100 times less Ni. These differences can be semi-quantitatively visualized on XRF spectra (B).
Concluding remarks
The hyperaccumulation of nickel (Ni) is a complex trait that appeared several times during evolution of plants. New Caledonia hosts a rich and phylogenetically diverse set of Ni hyperaccumulators and offers significant opportunities to broaden our knowledge of the molecular mechanisms involved in Ni hyperaccumulation that was mostly studied so far in European Brassicaceae. Today, such studies are realistic considering the rapid development of NGS technologies that give access to virtually all plant genomes and also because of the relatively good knowledge of the diversity of New Caledonian hyperaccumulators. The identification of genes involved in Ni accumulation in these species will be important to identify conserved and divergent mechanisms involved in Ni hyperaccumulation and also adaptation to ultramafic soils. We think these results will support the development of phytoremediation and phytomining technologies that are particularly important in regions such as New Caledonia (Losfeld et al., 2012) and Indonesia (van der Ent et al., 2013b), where the nickel mining industry and the economical development are often opposed to the protection of environment and endangered flora. In every respect, future studies on New Caledonian hypernickelophore accumulating more than 1% Ni and producing a high biomass such as species of Geissois (Fogliani et al., 2009) or Homalium, being related to Populus, will be of particular interest (Chaney et al., 2007).
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Because of space limitation, we were not able to cite all studies that would have been relevant to this topic, but we would like to collectively acknowledge researchers from the University of New Caledonia (UNC), the neo-Caledonian Agronomic Institute (IAC), IRD, CNRS, or from other institutions and also volunteers that are acting in New Caledonia to better describe, understand, valorize and protect its peculiar flora. We would like to especially thank Laurent L'Huillier (IAC) for funding some recent metal analyses, Laure Barrabé and Nadia Robert for unpublished information and the picture presented in Figure 2, and Fondis Bioritech (Guyancourt, France) for the use of the hand-held XRF instrument. The costs for this publication were supported by funding from CNRS.
Supplementary material
The Supplementary Material for this article can be found online at: http://www.frontiersin.org/Plant_Physiology/10.3389/fpls.2013.00279/abstract
References
- Amir H., Perrier N., Rigault F., Jaffré T. (2007). Relationships between Ni-hyperaccumulation and mycorrhizal status of different endemic plant species from New Caledonian ultramafic soils. Plant Soil 293, 23–35 10.1007/s11104-007-9238-0 [DOI] [Google Scholar]
- Andersson L. (2002). Relationships and generic circumscriptions in the Psychotria complex (Rubiaceae, Psychotrieae). Syst. Geogr. Pl. 72, 167–202 [Google Scholar]
- Baker A. J. M., Brooks R. R. (1989). Terrestrial higher plants which hyperaccumulate metallic elements. a review of their distribution, ecology and phytochemistry. Biorecovery 1, 81–126 [Google Scholar]
- Baker A. J. M., Brooks R. R., Kersten W. J. (1985). Accumulation of nickel by Psychotria species from the Pacific Basin. Taxon 34, 89–95 10.2307/1221569 [DOI] [Google Scholar]
- Barrabé L. (2013). Phylogénie, Systématique et évolution du genre Psychotria (Rubiaceae) en Nouvelle-Calédonie, Adaptation Aux Terrains Ultramafiques. Ph.D. thesis, University of New Caledonia, 381.
- Boyd R. S., Wall M. A., Jaffré T. (2009). Do tropical nickel hyperaccumulator mobilize metals into epiphytes? A test using Bryophytes from New Caledonia. Northeast Nat. 16, 139–154 10.1656/045.016.0512 [DOI] [Google Scholar]
- Brooks R. R. (1987). Serpentine and Its Vegetation: a Multidisciplinary Approach. Kent: Croom Helm, Limited [Google Scholar]
- Brooks R. R., Lee J., Jaffré T. (1974). Some New Zealand and New Caledonian plant accumulators of nickel. J. Ecol. 62, 493–499 10.2307/2258995 [DOI] [Google Scholar]
- Brooks R. R., Lee J., Reeves R. D., Jaffré T. (1977). Detection of nickeliferous rocks by analysis of herbarium specimens of indicator plants. J. Geochem. Explor. 7, 49–57 10.1016/0375-6742(77)90074-7 [DOI] [Google Scholar]
- Brooks R. R., Reeves R. D., Baker A. J. M., Rizzo J. A., Diaz Ferreira H. (1990). The Brazilian serpentine plant expedition (BRASPEX). Natl. Geogr. Res. 6, 205–219 [Google Scholar]
- Burge D. O., Barker W. R. (2010). Evolution of nickel hyperaccumulation by Stackhousia tryonii (Celastraceae), a serpentine-endemic plant from Queensland, Australia. Aust. Syst. Bot. 23, 415–430 10.1071/SB10029 [DOI] [Google Scholar]
- Callahan D. L., Roessner U., Dumontet V., De Livera A. M., Doronila A., Baker A. J., et al. (2012). Elemental and metabolite profiling of nickel hyperaccumulators from New Caledonia. Phytochemistry 81, 80–89 10.1016/j.phytochem.2012.06.010 [DOI] [PubMed] [Google Scholar]
- Callahan D. L., Roessner U., Dumontet V., Perrier N., Wedd A. G., O'Hair R. A., et al. (2008). LC-MS and GC-MS metabolite profiling of nickel(II) complexes in the latex of the nickel-hyperaccumulating tree Sebertia acuminata and identification of methylated aldaric acid as a new nickel(II) ligand. Phytochemistry 69, 240–251 10.1016/j.phytochem.2007.07.001 [DOI] [PubMed] [Google Scholar]
- Cecchi L., Gabbrielli R., Arnetoli M., Gonnelli C., Hasko A., Selvi F. (2010). Evolutionary lineages of nickel hyperaccumulation and systematics in European Alysseae (Brassicaceae): evidence from nrDNA sequence data. Ann. Bot. 106, 751–767 10.1093/aob/mcq162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaney R. L., Angle J. S., Broadhurst C. L., Peters C. A., Tappero R. V., Sparks D. L. (2007). Improved understanding of hyperaccumulation yields commercial phytoextraction and phytomining technologies. J. Environ. Qual. 36, 1429–1443 10.2134/jeq2006.0514 [DOI] [PubMed] [Google Scholar]
- Cruaud A., Jabbou-Zahab R., Genson G., Ungricht S., Rasplus J. Y. (2012). Testing the emergence of New Caledonia: fig wasp mutualism as a case study and a review of evidence. PLoS ONE 7:e30941 10.1371/journal.pone.0030941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernando D. R., Mizuno T., Woodrow I. E., Baker A. J. M., Collins R. N. (2010). Characterization of foliar manganese (Mn) in Mn (hyper)accumulators using X-ray absorption spectroscopy. New Phytol. 188, 1014–1027 10.1111/j.1469-8137.2010.03431.x [DOI] [PubMed] [Google Scholar]
- Fernando D. R., Woodrow I. E., Jaffré T., Dumontet V., Marshall A. T., Baker A. J. M. (2008). Foliar manganese accumulation by Maytenus founieri (Celastraceae) in its native New Caledonian habitats: populational variation and localization by X-ray microanalysis. New Phytol. 177, 178–185 [DOI] [PubMed] [Google Scholar]
- Fogliani B., Hopkins H. C. F., Bouraima-Madjebi S., Medevielle V. (2009). Morphological development of Geissois pruinosa (Cunoniaceae) from seed to adult, and the expression of plesiomorphic characters in seedlings. Flora 204, 7–16 10.1016/j.flora.2007.11.009 [DOI] [Google Scholar]
- Gendre D., Czernic P., Conejero G., Pianelli K., Briat J. F., Lebrun M., et al. (2007). TcYSL3, a member of the YSL gene family from the hyper-accumulator Thlaspi caerulescens, encodes a nicotianamine-Ni/Fe transporter. Plant J. 49, 1–15 10.1111/j.1365-313X.2006.02937.x [DOI] [PubMed] [Google Scholar]
- Grandcolas P., Murienne J., Robillard T., Desutter-Grandcolas L., Jourdan H., Guilbert E., et al. (2008). New Caledonia: a very old Darwinian island? Philos. Trans. R. Soc. B Biol. Sci. 363, 3309–3317 10.1098/rstb.2008.0122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffré T. (1977a). Composition chimique élémentaire des tissus foliaires des espèces végétales colonisatrices des anciennes mines de nickel en Nouvelle-Calédonie. Cahiers ORSTOM. Série Biologie Ecologie et Biologie Végétale 12, 323–330 [Google Scholar]
- Jaffré T. (1977b). Manganese accumulation by plants on ultrabasic rocks in New Caledonia. C.R. Acad. Sci. D Nat. 284, 1573–1575 [Google Scholar]
- Jaffré T. (1979). Manganese accumulation by New Caledonian Proteaceae. C.R. Acad Sci. D Nat. 289, 425–428 [Google Scholar]
- Jaffré T. (1980). Etude écologique du Peuplement Végétal Des Sols Dérivés de Roches Ultrabasiques en Nouvelle-Calédonie. Paris: ORSTOM [Google Scholar]
- Jaffré T., Brooks R. R., Lee J., Reeves R. D. (1976). Sebertia acuminata: a hyperaccumulator of nickel from New Caledonia. Science 193, 579–580 10.1126/science.193.4253.579 [DOI] [PubMed] [Google Scholar]
- Jaffré T., Brooks R. R., Trow J. M. (1979a). Hyperaccumulation of nickel by Geissois species. Plant Soil 51, 157–162 10.1007/BF0220593722795763 [DOI] [Google Scholar]
- Jaffré T., Kersten W. J., Brooks R. R., Reeves R. D. (1979b). Nickel uptake by Flacourtiaceae of New Caledonia. Proc. R. Soc. Lond. B Biol. Sci. 205, 385–394 10.1098/rspb.1979.0072 [DOI] [PubMed] [Google Scholar]
- Jaffré T., Rigault F., Dagostini G., Tinel-Fambart J., Wulff A., Munzinger J. (2009). Input of the different vegetation units to the richness and endemicity of New Caledonia flora, in 11th Pacific Science Inter-Congress (Tahiti: ). ISBN: 978-2-11-098964-2. [Google Scholar]
- Jaffré T., Schmid M. (1974). Accumulation du nickel par une Rubiacée de Nouvelle-Calédonie, Psychotria douarrei (G. Beauvisage) Däniker. C.R. Acad. Sci. D Nat. 278, 1727–1730 [Google Scholar]
- Jestrow B., Gutièrez Amaro J., Francisco-Ortega J. (2012). Islands within islands: a molecular phylogenetic study of the Leucocroton alliance (Euphorbiaceae) across the Carribean Islands and within the serpentinite archipelago of Cuba. J. Biogeogr. 39, 452–464 10.1111/j.1365-2699.2011.02607.x [DOI] [Google Scholar]
- Kathriarachchi H., Samuel R., Hoffmann P., Mlinarec J., Wurdack K. J., Ralimanana H. N., et al. (2006). Phylogenetics of tribe Phyllantheae (Phyllanthaceae; Euphorbiaceae sensu lato) based on nrITS and plastid matK DNA sequence data. Am. J. Bot. 93, 637–655 10.3732/ajb.93.4.637 [DOI] [PubMed] [Google Scholar]
- Kersten W. J., Brooks R. R., Reeves R. D., Jaffré T. (1979). Nickel uptake by New Caledonian species of Phyllanthus. Taxon 38, 529–534 10.2307/1219791 [DOI] [Google Scholar]
- Kersten W. J., Brooks R. R., Reeves R. D., Jaffré T. (1980). Nature of nickel-complexes in Psychotria douarrei and other nickel-accumulating plants. Phytochemistry 19, 1963–1965 10.1016/0031-9422(80)83013-5 [DOI] [Google Scholar]
- Kim S., Takahashi M., Higuchi K., Tsunoda K., Nakanishi H., Yoshimura E., et al. (2005). Increased nicotianamine biosynthesis confers enhanced tolerance of high levels of metals, in particular nickel, to plants. Plant Cell Physiol. 46, 1809–1818 10.1093/pcp/pci196 [DOI] [PubMed] [Google Scholar]
- Krämer U. (2010). Metal hyperaccumulation in plants. Annu. Rev. Plant. Biol. 61, 517–534 10.1146/annurev-arplant-042809-112156 [DOI] [PubMed] [Google Scholar]
- Krämer U., Cotterhowells J. D., Charnock J. M., Baker A. J. M., Smith J. A. C. (1996). Free histidine as a metal chelator in plants that accumulate nickel. Nature 379, 635–638 10.1038/379635a0 [DOI] [Google Scholar]
- Lee J., Reeves R. D., Brooks R. P., Jaffré T. (1977). Isolation and identification of a citrato-complex of nickel from nickel-accumulating plants. Phytochemistry 16, 1503–1505 10.1016/0031-9422(77)84010-7 [DOI] [Google Scholar]
- Lee J., Reeves R. D., Brooks R. R., Jaffré T. (1978). Relation between nickel and citric-acid in some nickel-accumulating plants. Phytochemistry 17, 1033–1035 10.1016/S0031-9422(00)94274-2 [DOI] [Google Scholar]
- Losfeld G., Escande V., Jaffré T., L'Huillier L., Grison C. (2012). The chemical exploitation of nickel phytoextraction: an environmental, ecologic and economic opportunity for New Caledonia. Chemosphere 89, 907–910 10.1016/j.chemosphere.2012.05.004 [DOI] [PubMed] [Google Scholar]
- Majorel-Loulergue C., Maggia L., Wabete N., Goarant C., Lebrun M., Amir H., et al. (2009). The regional genomic core research facilities for life science in New-Caledonia, in 11th Pacific Science Inter-Congress (Tahiti: ). ISBN: 978-2-11-098964-2. [Google Scholar]
- Mayer M. S., Soltis P. S. (1994). The evolution of serpentine endemics: a chloroplast DNA phylogeny of the Streptanthus glandulosus complex (Cruciferae). Syst. Bot. 19, 557–574 10.2307/2419777 [DOI] [Google Scholar]
- Minguzzi C., Vegnano O. (1948). Il contenuto di nichel nelle ceneri di Alyssum bertelonii Desv. Atti Soc. Toscana Sci. Nat. 55, 49–74 [Google Scholar]
- Montarges-Pelletier E., Chardot V., Echevarria G., Michot L. J., Bauer A., Morel J. L. (2008). Identification of nickel chelators in three hyperaccumulating plants: an X-ray spectroscopic study. Phytochemistry 69, 1695–1709 10.1016/j.phytochem.2008.02.009 [DOI] [PubMed] [Google Scholar]
- Morat P., Jaffré T., Tronchet F., Munzinger J., Pillon Y., Veillon J. M., et al. (2012). The taxonomic reference base florical and characteristics of the native vascular flora of New Caledonia. Adansonia 34, 179–221 10.5252/a2012n2a1 [DOI] [Google Scholar]
- Morjan C. L., Rieseberg L. H. (2004). How species evolve collectively: implications of gene flow and selection for the spread of advantageous alleles. Mol. Ecol. 13, 1341–1356 10.1111/j.1365-294X.2004.02164.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers N., Mittermeier R. A., Mittermeier C. G., Da Fonseca G. A. B., Kent J. (2000). Biodiversity hotspots for conservation priorities. Nature 403, 853–858 10.1038/35002501 [DOI] [PubMed] [Google Scholar]
- Pelletier B. (2006). Geology of the New Caledonia region and its implications for the study of the New Caledonian biodiversity, in Compendium of Marine Species from New Caledonia, eds Payri C. E., Richer De Forges B. (Nouméa: Centre IRD de Nouméa; ), 17–30 [Google Scholar]
- Persans M. W., Yan X. G., Patnoe J., Krämer U., Salt D. E. (1999). Molecular dissection of the role of histidine in nickel hyperaccumulation in Thlaspi goesingense (Halacsy). Plant Physiol. 121, 1117–1126 10.1104/pp.121.4.1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pianelli K., Mari S., Marques L., Lebrun M., Czernic P. (2005). Nicotianamine over-accumulation confers resistance to nickel in Arabidopsis thaliana. Transgenic Res. 14, 739–748 10.1007/s11248-005-7159-3 [DOI] [PubMed] [Google Scholar]
- Pillon Y. (2012). Time and tempo of diversification in the flora of New Caledonia. Bot. J. Linn. Soc. 170, 288–298 10.1111/j.1095-8339.2012.01274.x [DOI] [Google Scholar]
- Pillon Y., Hopkins H. C. F., Munzinger J., Amir H., Chase M. W. (2009a). Cryptic species, gene recombination and hybridization in the genus Spiraeanthemum (Cunoniaceae) from New Caledonia. Bot. J. Linn. Soc. 161, 137–152 10.1111/j.1095-8339.2009.00997.x [DOI] [Google Scholar]
- Pillon Y., Munzinger J., Amir H., Hopkins H. C. F., Chase M. W. (2009b). Reticulate evolution on a mosaic of soils: diversification of the New Caledonian endemic genus Codia (Cunoniaceae). Mol. Ecol. 18, 2263–2275 10.1111/j.1365-294X.2009.04178.x [DOI] [PubMed] [Google Scholar]
- Pillon Y., Munzinger J., Amir H., Lebrun M. (2010). Ultramafic soils and species sorting in the flora of New Caledonia. J. Ecol. 98, 1108–1116 10.1111/j.1365-2745.2010.01689.x [DOI] [Google Scholar]
- Rascio N., Navari-Izzo F. (2011). Heavy metal hyperaccumulating plants: how and why do they do it? And what makes them so interesting? Plant Sci. 180, 169–181 10.1016/j.plantsci.2010.08.016 [DOI] [PubMed] [Google Scholar]
- Reeves R. D. (2003). Tropical hyperaccumulators of metals and their potential for phytoextraction. Plant Soil 249, 57–65 10.1023/A:1022572517197 [DOI] [Google Scholar]
- Reeves R. D., Baker A. J. M., Borhidi A., Berazain R. (1996). Nickel accumulating plants from the ancient serpentine soils of Cuba. New Phytol. 133, 217–224 10.1111/j.1469-8137.1996.tb01888.x [DOI] [PubMed] [Google Scholar]
- Reeves R. D., Baker A. J. M., Borhidi A., Berazain R. (1999). Nickel hyperaccumulation in the serpentine flora of Cuba. Ann. Bot. 83, 29–38 10.1006/anbo.1998.0786 [DOI] [Google Scholar]
- Sagner S., Kneer R., Wanner G., Cosson J. P., Deus-Neumann B., Zenk M. H. (1998). Hyperaccumulation, complexation and distribution of nickel in Sebertia acuminata. Phytochemistry 47, 339–347 10.1016/S0031-9422(97)00593-1 [DOI] [PubMed] [Google Scholar]
- Schaumlöffel D., Ouerdane L., Bouyssiere B., Lobinski R. (2003). Speciation analysis of nickel in the latex of a hyperaccumulating tree Sebertia acuminata by HPLC and CZE with ICP MS and electrospray MS-MS detection. J. Anal. At. Spectrom. 18, 120–127 10.1039/b209819a [DOI] [Google Scholar]
- Severne B. C., Brooks R. R. (1972). A nickel-accumulating plant from Western Australia. Planta 103, 91–94 10.1007/BF00394610 [DOI] [PubMed] [Google Scholar]
- Swenson U., Munzinger J. (2010). Taxonomic revision of Pycnandra subgenus Trouettia (Sapotaceae), with six new species from New Caledonia. Aust. Syst. Bot. 23, 333–370 10.1071/SB10025 [DOI] [Google Scholar]
- van der Ent A., Baker A. J. M., Reeves R. D., Pollard A. J., Schat H. (2013a). Hyperaccumulators of metal and metalloid trace elements: facts and fiction. Plant Soil 362, 319–334 10.1007/s11104-012-1287-3 [DOI] [Google Scholar]
- van der Ent A., Baker A. J. M., Van Balgooy M. M. J., Tjoa A. (2013b). Ultramafic nickel laterites in Indonesia (Sulawesi, Halmahera): Mining, nickel hyperaccumulators and opportunities for phytomining. J. Geochem. Explor. 128, 72–79 10.1016/j.gexplo.2013.01.009 [DOI] [Google Scholar]
- Verbruggen N., Hermans C., Schat H. (2009). Molecular mechanisms of metal hyperaccumulation in plants. New Phytol. 181, 759–776 10.1111/j.1469-8137.2008.02748.x [DOI] [PubMed] [Google Scholar]
- Whiting S. N., Reeves R. D., Richards D., Johnson M. S., Cooke J. A., Malaisse F., et al. (2004). Research priorities for conservation of metallophyte biodiversity and their potential for restoration and site remediation. Restor. Ecol. 12, 106–116 10.1111/j.1061-2971.2004.00367.x [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.