Abstract
Purine-nucleoside phosphorylase (NP) deficiency is associated with severely defective thymus-derived (T)-cell and normally functioning bone marrow-derived (B)-cell immunity. In this study, two unrelated families with a total of three NP deficient members were investigated.
High pressure liquid chromatography of the plasma of the three patients showed inosine levels greater than 66 μM. This nucleoside was absent from the plasma of their parents and control samples.
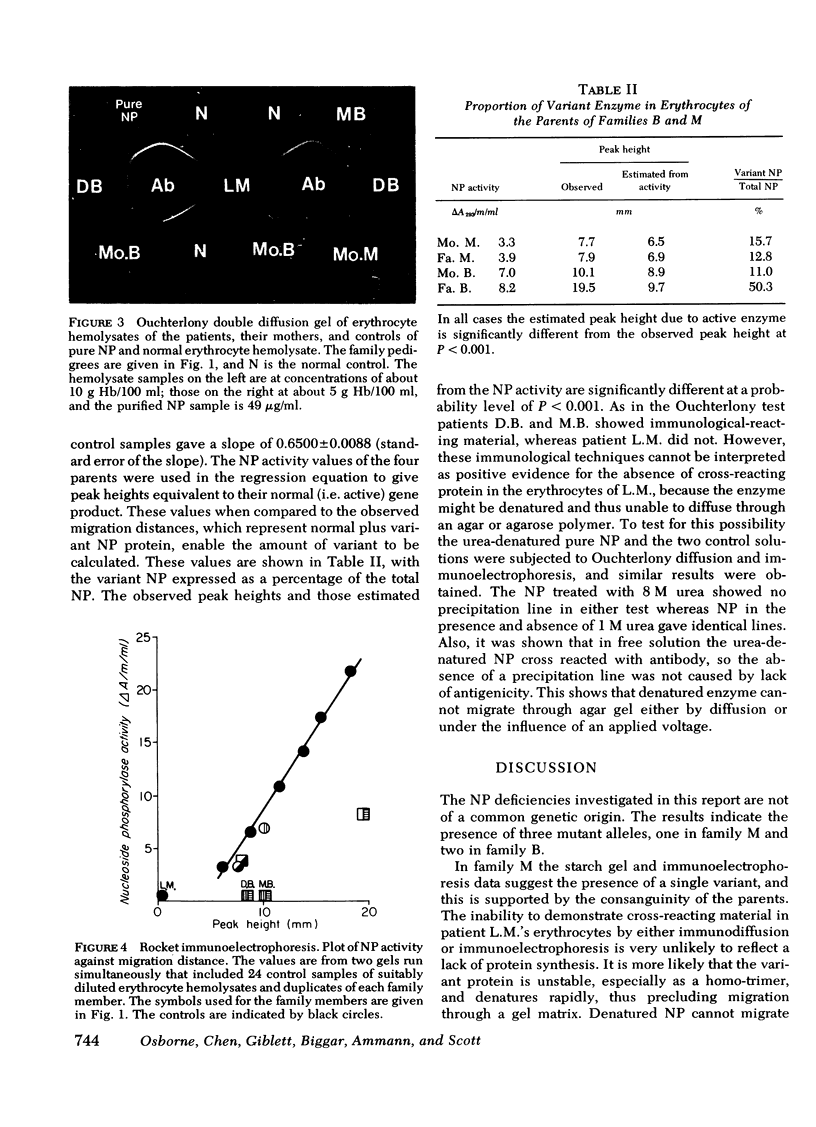
NP was purified from normal human erythrocytes by affinity chromatography and an antiserum prepared in rabbits was used to study the NP variants in the two families.
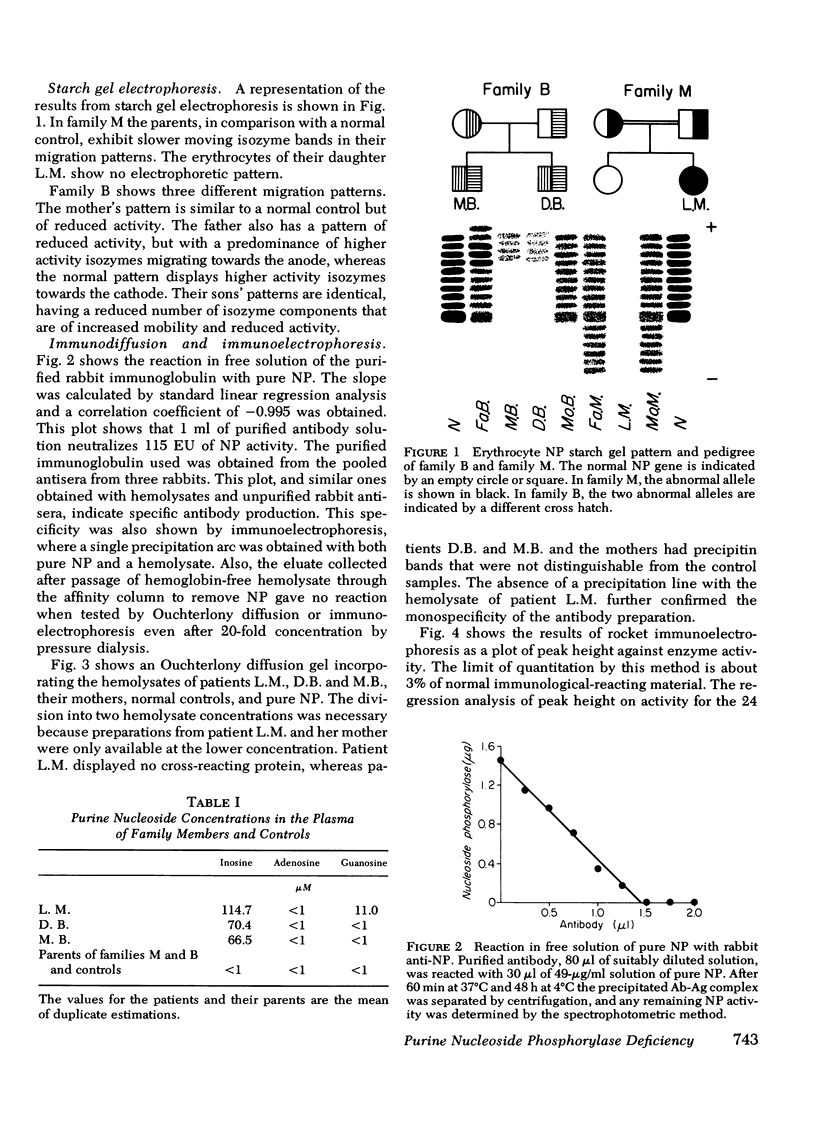
In family M the patient had no detectable erythrocyte NP activity and no detectable immunological-reacting material (irm) to the NP antibody. The parents, who are second cousins, had less than one-half of normal enzyme activity and approximately 14% irm attributable to a variant protein. Their electrophoretic patterns revealed a series of isozymes with slower than normal migration.
In family B the patients had 0.5% residual enzyme activity and about one-half normal irm. Their electrophoretic pattern showed faintly staining bands which migrated faster than normal NP. The mother of the patients had one-half normal enzyme activity, 11% irm attributable to her variant protein, and a normal electrophoretic pattern. The father had less than one-half normal enzyme activity, equal amounts of normal and variant irm, and an electrophoretic pattern that showed increased activity of the more rapidly migrating isozyme bands.
The combined use of immunological and electrophoretic techniques has shown the presence of three separate mutations; one in family M and two in family B associated with severely defective T-cell function.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agarwal R. P., Parks R. E., Jr Purine nucleoside phosphorylase from human erythrocytes. IV. Crystallization and some properties. J Biol Chem. 1969 Feb 25;244(4):644–647. [PubMed] [Google Scholar]
- Cohen A., Doyle D., Martin D. W., Jr, Ammann A. J. Abnormal purine metabolism and purine overproduction in a patient deficient in purine nucleoside phosphorylase. N Engl J Med. 1976 Dec 23;295(26):1449–1454. doi: 10.1056/NEJM197612232952603. [DOI] [PubMed] [Google Scholar]
- Edwards Y. H., Edwards P. A., Hopkinson D. A. A trimeric structure for mammalian purine nucleoside phosphorylase. FEBS Lett. 1973 Jun 1;32(2):235–237. doi: 10.1016/0014-5793(73)80840-3. [DOI] [PubMed] [Google Scholar]
- Edwards Y. H., Hopkinson D. A., Harris H. Inherited variants of human nucleoside phosphorylase. Ann Hum Genet. 1971 May;34(4):395–408. doi: 10.1111/j.1469-1809.1971.tb00252.x. [DOI] [PubMed] [Google Scholar]
- Funakoshi S., Deutsch H. F. Human carbonic anhydrases. II. Some physicochemical properties of native isozymes and of similar isozymes generated in vitro. J Biol Chem. 1969 Jul 10;244(13):3438–3446. [PubMed] [Google Scholar]
- George D. L., Francke U. Gene dose effect: regional mapping of human nuceloside phosphorylase on chromosome 14. Science. 1976 Nov 19;194(4267):851–852. doi: 10.1126/science.824731. [DOI] [PubMed] [Google Scholar]
- Giblett E. R., Ammann A. J., Wara D. W., Sandman R., Diamond L. K. Nucleoside-phosphorylase deficiency in a child with severely defective T-cell immunity and normal B-cell immunity. Lancet. 1975 May 3;1(7914):1010–1013. doi: 10.1016/s0140-6736(75)91950-9. [DOI] [PubMed] [Google Scholar]
- Giblett E. R. Immune cell function and recycling of purines. N Engl J Med. 1976 Dec 9;295(24):1375–1376. doi: 10.1056/NEJM197612092952409. [DOI] [PubMed] [Google Scholar]
- Harboe N., Ingild A. Immunization, isolation of immunoglobulins, estimation of antibody titre. Scand J Immunol Suppl. 1973;1:161–164. doi: 10.1111/j.1365-3083.1973.tb03798.x. [DOI] [PubMed] [Google Scholar]
- Kim B. K., Cha S., Parks R. E., Jr Purine nucleoside phosphorylase from human erythroyctes. II. Kinetic analysis and substrate-binding studies. J Biol Chem. 1968 Apr 25;243(8):1771–1776. [PubMed] [Google Scholar]
- Krenitsky T. A., Elion G. B., Henderson A. M., Hitchings G. H. Inhibition of human purine nucleoside phosphorylase. Studies with intact erythrocytes and the purified enzyme. J Biol Chem. 1968 Jun 10;243(11):2876–2881. [PubMed] [Google Scholar]
- Osborne W. R., Spencer N. Partial purification and properties of the common inherited forms of adenosine deaminase from human erythrocytes. Biochem J. 1973 May;133(1):117–123. doi: 10.1042/bj1330117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott C. R., Chen S. H., Giblett E. R. Detection of the carrier state in combined immunodeficiency disease associated with adenosine deaminase deficiency. J Clin Invest. 1974 Apr;53(4):1194–1196. doi: 10.1172/JCI107658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoop J. W., Zegers B. J., Hendrickx G. F., van Heukelom L. H., Staal G. E., de Bree P. K., Wadman S. K., Ballieux R. E. Purine nucleoside phosphorylase deficiency associated with selective cellular immunodeficiency. N Engl J Med. 1977 Mar 24;296(12):651–655. doi: 10.1056/NEJM197703242961203. [DOI] [PubMed] [Google Scholar]
- Turner B. M., Fisher R. A., Harris H. An association between the kinetic and electrophoretic properties of human purine-nucleoside-phosphorylase isozymes. Eur J Biochem. 1971 Dec;24(2):288–295. doi: 10.1111/j.1432-1033.1971.tb19684.x. [DOI] [PubMed] [Google Scholar]
- Ullman B., Cohen A., Martin D. W. Characterization of a cell culture model for the study of adenosine deaminase- and purine nucleoside phosphorylase-deficient immunologic disease. Cell. 1976 Oct;9(2):205–211. doi: 10.1016/0092-8674(76)90111-2. [DOI] [PubMed] [Google Scholar]
- Weeke B. A manual of quantitative immunoelectrophoresis. Methods and applications. 1. General remarks on principles, equipment, reagents and procedures. Scand J Immunol Suppl. 1973;1:15–35. doi: 10.1111/j.1365-3083.1973.tb03776.x. [DOI] [PubMed] [Google Scholar]
- Weeke B. Rocket immunoelectrophoresis. Scand J Immunol Suppl. 1973;1:37–46. doi: 10.1111/j.1365-3083.1973.tb03777.x. [DOI] [PubMed] [Google Scholar]
- van Heukelom L. H., Staal G. E., Stoop J. W., Zegers B. J. An abnormal form of purine nucleoside phosphorylase in a family with a child with severe defective T-cell-and normal B-cell immunity. Clin Chim Acta. 1976 Oct 1;72(1):117–124. doi: 10.1016/0009-8981(76)90042-5. [DOI] [PMC free article] [PubMed] [Google Scholar]



