Key Points
Adult survivors of childhood ALL treated with cranial radiation demonstrate a decline in verbal intelligence during an interval of 28 years.
This decline was associated with current attention problems, but not gender, radiation dose, or age at radiation exposure.
Abstract
Survivors of childhood acute lymphoblastic leukemia (ALL) treated with cranial radiation therapy (CRT) are at risk for cognitive impairment, although whether impairment progresses with age into adulthood is unknown. We report change in intelligence for 102 adult survivors of childhood ALL (age range, 26.6-54.7 years) during a median interval of 28.5 years. Survivors demonstrated lower Performance intelligence (mean, 95.3; standard deviation, 16.5; P = .005) but not Verbal IQ (mean, 97.4; standard deviation, 15.44; P = .09) at initial testing. Verbal intelligence declined an average of 10.3 points (P < .0001) during the follow-up interval with no decline in Performance intelligence. Decline was associated with current attention problems (P = .002) but not gender, CRT dose, age at CRT exposure, or years between testing. Results suggest long-term survivors of childhood ALL treated with CRT are at risk for progressive decline in verbal intellect, which may be driven by attention deficits. This trial was registered at clinicaltrials.gov as no. NCT00760656.
Introduction
Long-term survivors of childhood acute lymphoblastic leukemia (ALL) are at risk for cognitive impairment after cranial radiation therapy (CRT).1-4 Previous outcome studies that directly assess survivor intelligence have focused on function during the first 10 years after diagnosis, with relatively few reports extending follow-up into adulthood. During the first decade after treatment, declines in intelligence have been reported with 24 Gy or 18 Gy CRT.3 In addition, adult survivors of childhood cancer have been reported to demonstrate long-term functional deficits, including lower educational attainment5 and self-reported cognitive impairment.6
To our knowledge, no published reports have examined change in cognitive function of ALL survivors during extended intervals into adulthood. It is unknown whether the degree of cognitive impairment increases with time. Here, we report change in a measure of global verbal ability (ie, Verbal intelligence quotient [IQ]) for 2 testing points separated by a median interval of 28.5 years, beginning at least 1 year after completion of CRT for childhood ALL.
Study design
Study population
Adult survivors of childhood leukemia were recruited from the St. Jude Lifetime Cohort (SJLIFE), an institutional protocol designed to examine health outcomes in adult survivors of childhood cancer.7 Survivors enrolled in SJLIFE were previously treated at St. Jude Children's Research Hospital and are now at least 10 years from diagnosis and are at least 18 years old. From this large cohort, survivors who received CRT for childhood ALL and had previously undergone intelligence testing at St. Jude as part of the institution’s standard of care were identified. Initial testing occurred when survivors were ≥1 and <10 years posttherapy. Exclusion criteria included a history of craniotomy, ventriculoperitoneal shunt, subsequent central nervous system neoplasm, traumatic brain injury, genetic disorder associated with neurocognitive impairment (eg, Down syndrome), or active treatment of cancer. Of the 138 participants who met the eligibility criteria, 31 declined participation, 3 were medically unable to participate, and 2 withdrew after consent. This left 102 survivors (73.9%) who participated in follow-up testing. Compared with participants, nonparticipants included slightly more men, although no differences were noted in current age, age at diagnosis, time since diagnosis, or cranial radiation dose. Among participants, 33.3% underwent 18 Gy CRT and 66.7% underwent 24 Gy CRT. Among nonparticipants, 37.1% underwent 18 Gy CRT and 62.9% underwent 24 Gy CRT. Participants did not differ from eligible nonparticipants on initial IQ testing (all P values > .10; Table 1). The study protocol was approved by the St. Jude Children's Research Hospital Institutional Review Board, and all participants provided written informed consent in accordance with the Declaration of Helsinki.
Table 1.
Survivor demographic and treatment characteristics
| Participants | |||
|---|---|---|---|
| N = 102 | |||
| Demographics | N | % | |
| Gender | |||
| Female | 56 | 54.9 | |
| Male | 46 | 45.1 | |
| Race | |||
| White | 96 | 94.1 | |
| Other | 6 | 5.9 | |
| Highest grade | |||
| College graduate | 32 | 31.4 | |
| Noncollege graduate | 70 | 68.6 | |
| Current employment | |||
| Full time | 62 | 60.8 | |
| Part time or unemployed | 38 | 37.3 | |
| Mean | SD | Min-Max | |
| Current age (y) | 38.4 | 6.2 | 27-55 |
| Treatment factors | |||
| IT MTX (mL) | 103.6 | 88.5 | 0-496 |
| IV MTX (g/m2) | 2.22 | 2.90 | 0-21.8 |
| Age (y) | |||
| At diagnosis | 5.0 | 3.2 | 0.8-15.3 |
| At initial testing | 11.0 | 3.8 | 4.3-23.1 |
| At follow-up testing | 38.5 | 6.2 | 26.6-54.7 |
| End of therapy to initial testing (y) | 3.4 | 2.3 | 1.0-9.3 |
| Diagnosis to follow-up testing (y) | 33.5 | 5.7 | 18.8-46.4 |
| Initial to follow-up testing (y) | 27.6 | 5.6 | 14.0-38.4 |
| CRT dose (Gy) | N | % | |
| 18 | 34 | 33.3 | |
| 24 | 68 | 66.7 | |
| Neurocognitive assessment | Mean | SD | |
| Nonparticipants (n = 36) | |||
| IQ at initial testing | |||
| Verbal | 92.7 | 14.6 | |
| Performance | 93.6 | 17.0 | |
| Full Scale | 92.4 | 15.4 | |
| Participants (n = 102) | |||
| IQ at initial testing | |||
| Verbal | 97.4 | 15.4 | |
| Performance | 95.3 | 16.5 | |
| Full Scale | 96.0 | 15.9 | |
| IQ at follow-up testing | |||
| Verbal | 87.1 | 17.1 | |
| Performance | 95.7 | 16.8 | |
| Full Scale | 91.3 | 15.5 | |
| Academic | |||
| Reading | 91.3 | 9.8 | |
| Mathematics | 85.8 | 17.48 | |
| Attention: sustained | 89.7 | 16.99 | |
| Processing speed | 89.5 | 27.45 | |
| Memory | |||
| Total recall | 92.7 | 18.14 | |
| Long-term | 92.4 | 18.94 | |
| Executive function | |||
| Fluency | 89.3 | 16.07 | |
| Flexibility | 77.6 | 32.01 | |
Cumulative doses listed for intrathecal (IT) and intravenous (IV) methotrexate (MTX). Initial testing denotes first testing ≥1 year and ≤10 years after CRT. Follow-up testing denotes current testing. All neurocognitive assessment scores presented in age-adjusted standard scores with an expected mean of 100 and and SD of 15. Participants did not differ from nonparticipants in initial Verbal IQ (P = .12), Performance IQ (P = .60), or Full Scale IQ (P = .24).
IT, intrathecal; IV, intravenous; Min-Max, minimum-maximum; MTX, methotrexate.
Procedures
Survivors completed follow-up neurocognitive testing during a 2-hour session in dedicated testing rooms. All testing was conducted by certified masters-level examiners, under the general supervision of a board-certified clinical neuropsychologist. Assessed domains included intelligence,8 academic skills,9 attention,10 processing speed,11 memory,12 and executive function (ie, cognitive fluency and flexibility).13 Order of testing was standardized, and survivors’ schedules were adjusted to limit the effect from fatigue and extraneous factors. Medical record abstraction was performed to capture previous IQ testing and exposure data including radiation treatment (whole-brain dose), and cumulative doses of intravenous and intrathecal methotrexate, as these chemotherapy agents have been associated with neurocognitive problems.14
Statistical analyses
Descriptive statistics for survivors are provided in Table 1, along with results of initial and follow-up testing conducted as part of SJLIFE. One-sample t tests were used to assess whether Full Scale, Verbal, or Performance IQ scores at both initial and follow-up time points differed from national norms (expected score, 100; standard deviation [SD], 15). One-sample t tests were used to assess whether differences between initial and follow-up Full Scale, Verbal, and Performance IQ scores were equal to zero. Differences in IQ scores (initial and follow-up testing) were modeled using predictors including gender, CRT dose, age at CRT exposure, cumulative intrathecal and intravenous methotrexate dose, years between diagnosis and initial testing, and years between initial testing and follow-up testing. Associations between difference in IQ scores and current neurocognitive performance were also examined.
Results and discussion
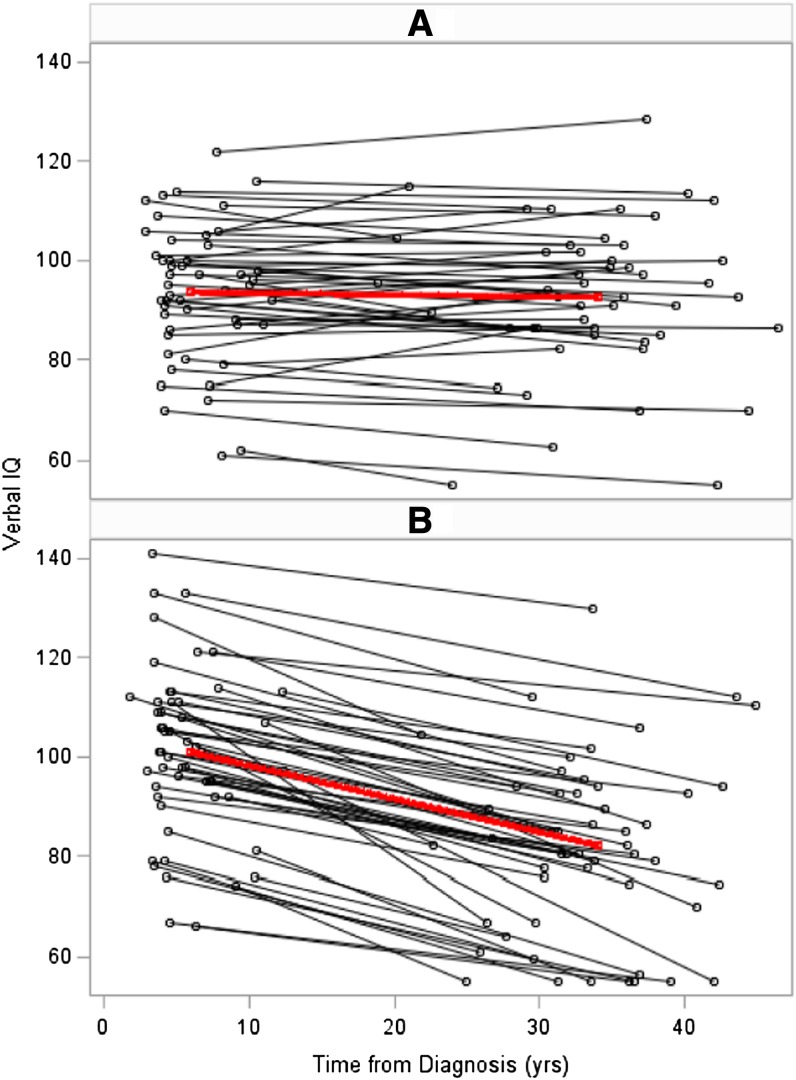
Compared with age-adjusted expectations from national norms, on the initial evaluation survivors demonstrated lower Performance IQ (mean [SD], 95.3 [16.5]; P = .005) and Full Scale IQ (mean [SD], 96.0 [15.9]; P = .01) but not Verbal IQ (mean [SD], 97.4 [15.44]; P = .09). Verbal IQ demonstrated a significant decline at follow-up (mean [SD] change, 10.31; P < .0001); no significant change in Performance IQ was apparent (change, −0.21; P = .88). Full Scale IQ also demonstrated a significant decline (change, 4.75; P < .0001), although, as this is a composite of Verbal and Performance IQs, change is driven by Verbal IQ. Figure 1 presents patterns of decline in Verbal IQ for all survivors as well as group averages for survivors who did and did not demonstrate a decline with time. Of note, 48.0% of survivors did not demonstrate a significant decline in Verbal IQ, suggesting potential protective factors or differential group vulnerability. Linear regression analyses revealed no statistically significant association between decline in Verbal IQ and gender (β, 0.80; P = .73), CRT dose (β, 0.51; P = .15), age at diagnosis (β, −0.51; P = .14), time between diagnosis and initial testing (β, −0.31; P = .50), or time between initial testing and follow-up testing (β, 0.08; P = .66). Decline in Verbal IQ was associated with current impairment on measures of sustained attention (P = .002) and reading (P = .02) but not processing speed, memory, or executive functions.
Figure 1.
Change in Verbal IQ between initial testing and follow-up testing. The horizontal axis depicts testing intervals reflecting time from diagnosis to initial testing, first data point, and time from diagnosis to follow-up testing, second data point. The vertical axis depicts age-adjusted Verbal IQ scores (expected mean, 100; SD, 15), with age identified at time of respective testing. The top box (A) clusters the 49 survivors (black lines) and group average (red line) of those who did not demonstrate a decline in Verbal IQ from initial to follow-up testing, whereas the bottom graph (B) clusters the 53 survivors who demonstrated at least a 10-point decline in Verbal IQ between the 2 testing sessions. Of note, very few survivors demonstrated an increase in Verbal IQ from initial to follow-up testing (A).
The current results indicate that CRT for childhood ALL is associated with an initial decline in performance abilities followed by a late decline in verbal abilities. The initial decline in Performance IQ is consistent with results of previous reports suggesting an earlier decline in nonlanguage reasoning abilities.15,16 The decline in Verbal IQ with time is a novel finding and suggests a progressive effect on brain function from CRT. Traditional variables associated with an early decline in cognitive abilities (eg, CRT dose,17 young age at diagnosis,3 and female gender),18 do not predict change in Verbal IQ during subsequent decades. Instead, a decline in Verbal IQ was associated with current attention and reading problems. Unfortunately, available data from initial testing were generally restricted to IQ scores, and measures of attention were not present in the medical records. Still, persistent problems in attention have been shown to affect verbal intellect during long periods,19,20 and it is certainly plausible that attention problems were present for many years. We have recently identified an association between increased risk for attention problems and increased time from diagnosis in a large cohort of adult survivors of childhood ALL (Krull KR, Brinkman TM, Li C, Armstrong GT, Ness KK, Srivastava DK, Gurney JG, Kimberg C, Krasin MJ, Pui CH, Robison LL, Hudson MM, manuscript submitted to Journal of Clinical Oncology). Thus, the interaction between attention and verbal problems may continue to progress with time and age. Although the decline in Verbal IQ was not associated with current measures of memory, development of verbal abilities is heavily dependent on learning and long-term recall of new information with time.21 We recently demonstrated an increased risk for long-term memory problems in aging adult survivors of ALL treated with CRT,22 suggesting the need for further examination of the effect of memory problems on verbal intellectual abilities.
The findings from our study support several recommendations. These results provide additional evidence for the recommendation of avoiding CRT in the treatment of childhood ALL.23 In addition, because verbal abilities are important to independent success in today’s society, long-term adult survivors should continue to be monitored for declining verbal abilities, with appropriate interventions offered as needed. Ideally, current survivors would be educated and offered cognitive enrichment to prevent future decline in verbal abilities.
Acknowledgments
This work was supported by the grant CA138988 (G.T.A.), the Cancer Center Support (CORE) grant CA21765 from the National Cancer Institute, and by the ALSAC (American Lebanese Syrian Associated Charities). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: K.R.K., D.K.S., M.M.H., L.L.R., and G.T.A. designed the study; K.R.K., A.S., M.M.H., L.L.R., and G.T.A. acquired the data; N.Z. and D.K.S. conducted data analysis; K.R.K., M.J.K., L.E.K., C.-H.P., M.M.H., L.L.R., and G.T.A. interpreted the data; K.R.K., N.Z., A.S., D.K.S., M.J.K., L.E.K., C.-H.P., M.M.H., L.L.R., and G.T.A. conducted manuscript drafts or revisions; K.R.K., N.Z., A.S., D.K.S., M.J.K., L.E.K., C.-H.P., M.M.H., L.L.R., and G.T.A. conducted final approval of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kevin R. Krull, Department of Epidemiology and Cancer Control, St. Jude Children’s Research Hospital, 262 Danny Thomas Place, MS 735, Memphis, TN 38105-3678; email: kevin.krull@stjude.org.
References
- 1.Meadows AT, Gordon J, Massari DJ, Littman P, Fergusson J, Moss K. Declines in IQ scores and cognitive dysfunctions in children with acute lymphocytic leukaemia treated with cranial irradiation. Lancet. 1981;2(8254):1015–1018. doi: 10.1016/s0140-6736(81)91216-2. [DOI] [PubMed] [Google Scholar]
- 2.Ochs J, Mulhern R, Fairclough D, et al. Comparison of neuropsychologic functioning and clinical indicators of neurotoxicity in long-term survivors of childhood leukemia given cranial radiation or parenteral methotrexate: a prospective study. J Clin Oncol. 1991;9(1):145–151. doi: 10.1200/JCO.1991.9.1.145. [DOI] [PubMed] [Google Scholar]
- 3.Jankovic M, Brouwers P, Valsecchi MG, et al. International Study Group on Psychosocial Aspects of Childhood Cancer. Association of 1800 cGy cranial irradiation with intellectual function in children with acute lymphoblastic leukaemia. ISPACC. Lancet. 1994;344(8917):224–227. doi: 10.1016/s0140-6736(94)92997-1. [DOI] [PubMed] [Google Scholar]
- 4.Campbell LK, Scaduto M, Sharp W, et al. A meta-analysis of the neurocognitive sequelae of treatment for childhood acute lymphocytic leukemia. Pediatr Blood Cancer. 2007;49(1):65–73. doi: 10.1002/pbc.20860. [DOI] [PubMed] [Google Scholar]
- 5.Lancashire ER, Frobisher C, Reulen RC, Winter DL, Glaser A, Hawkins MM. Educational attainment among adult survivors of childhood cancer in Great Britain: a population-based cohort study. J Natl Cancer Inst. 2010;102(4):254–270. doi: 10.1093/jnci/djp498. [DOI] [PubMed] [Google Scholar]
- 6.Kadan-Lottick NS, Zeltzer LK, Liu Q, et al. Neurocognitive functioning in adult survivors of childhood non-central nervous system cancers. J Natl Cancer Inst. 2010;102(12):881–893. doi: 10.1093/jnci/djq156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hudson MM, Ness KK, Nolan VG, et al. Prospective medical assessment of adults surviving childhood cancer: study design, cohort characteristics, and feasibility of the St. Jude Lifetime Cohort study. Pediatr Blood Cancer. 2011;56(5):825–836. doi: 10.1002/pbc.22875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wechsler D. San Antonio, TX: Psychological Corporation; 1999. Wechsler Abbreviated Scale of Intelligence. [Google Scholar]
- 9.Woodcock RW, McGrew KS, Mather N, et al. Itasca, IL: Riverside Publishing; 2003. Woodcock-Johnson III. [Google Scholar]
- 10.Conners CK. North Tonawanda, NY: Multi-Health Systems, Inc; 2001. Conners' Continuous Performance Test II. [Google Scholar]
- 11.Wechsler D. 3rd ed. San Antonio, TX: Psychological Corporation; 1997. Wechsler Adult Intelligence Scale. [Google Scholar]
- 12.Delis DC, Kramer JH, Kaplan E, et al. 2nd ed. San Antonio, TX: The Psychological Corporation; 2000. California Verbal Learning Test. [Google Scholar]
- 13.Strauss E, Sherman EM, Spreen O. 3rd ed. Oxford, UK: Oxford University Press; 2006. A Compendium of Neuropsychological Test: Administration, Norms, and Commentary; pp. 562–575. [Google Scholar]
- 14.Conklin HM, Krull KR, Reddick WE, Pei D, Cheng C, Pui CH. Cognitive outcomes following contemporary treatment without cranial irradiation for childhood acute lymphoblastic leukemia. J Natl Cancer Inst. 2012;104(18):1386–1395. doi: 10.1093/jnci/djs344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fletcher JM, Copeland DR. Neurobehavioral effects of central nervous system prophylactic treatment of cancer in children. J Clin Exp Neuropsychol. 1988;10(4):495–537. doi: 10.1080/01688638808408255. [DOI] [PubMed] [Google Scholar]
- 16.Kaemingk KL, Carey ME, Moore IM, Herzer M, Hutter JJ. Math weaknesses in survivors of acute lymphoblastic leukemia compared to healthy children. Child Neuropsychol. 2004;10(1):14–23. doi: 10.1076/chin.10.1.14.26240. [DOI] [PubMed] [Google Scholar]
- 17.Mulhern RK, Fairclough D, Ochs J. A prospective comparison of neuropsychologic performance of children surviving leukemia who received 18-Gy, 24-Gy, or no cranial irradiation. J Clin Oncol. 1991;9(8):1348–1356. doi: 10.1200/JCO.1991.9.8.1348. [DOI] [PubMed] [Google Scholar]
- 18.Waber DP, Tarbell NJ, Fairclough D, et al. Cognitive sequelae of treatment in childhood acute lymphoblastic leukemia: cranial radiation requires an accomplice. J Clin Oncol. 1995;13(10):2490–2496. doi: 10.1200/JCO.1995.13.10.2490. [DOI] [PubMed] [Google Scholar]
- 19.Shaw M, Hodgkins P, Caci H, et al. A systematic review and analysis of long-term outcomes in attention deficit hyperactivity disorder: effects of treatment and non-treatment. BMC Med. 2012;10:99. doi: 10.1186/1741-7015-10-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schatz J, Kramer JH, Ablin A, Matthay KK. Processing speed, working memory, and IQ: a developmental model of cognitive deficits following cranial radiation therapy. Neuropsychology. 2000;14(2):189–200. doi: 10.1037//0894-4105.14.2.189. [DOI] [PubMed] [Google Scholar]
- 21.Alexander JR, Smales S. Intelligence, learning and long-term memory. Pers Individ Dif. 1997;23(5):815–825. [Google Scholar]
- 22.Armstrong GT, Reddick WE, Petersen RC, et al. Evaluation of memory impairment and dementia in adult survivors of childhood acute lymphoblastic leukemia treated with cranial radiotherapy [published online ahead of print April 12, 2013]. J Natl Cancer Inst. doi: 10.1093/jnci/djt089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pui CH. Prophylactic cranial irradiation: going, going, gone. Lancet Oncol. 2009;10(10):932–933. doi: 10.1016/S1470-2045(09)70239-6. [DOI] [PubMed] [Google Scholar]