Abstract
Background
Activation of the extracellular signal-regulated kinases ERK1 and ERK2 in hepatocytes by prostaglandin (PG)F2α was recently found to be inhibited by pertussis toxin (PTX) suggesting a role for Gi proteins.
Results
Targeting the Gi2α expression by a specific ribozyme inhibited the PGF2α -induced ERK1/2 activation in hepatocytes. On the other hand a non-cleaving form of the Gi2α ribozyme did not significantly decrease the ERK1/2 activation. In ribozyme-treated cells the Gi2α protein level was reduced, while the Gqα level was not affected thus confirming the specificity of the ribozyme.
Conclusion
The present data suggest an important role of Gi2 in PGF2α -induced ERK1/2 signaling in hepatocytes.
Introduction
The extracellular regulated kinases ERK1 (p44mapk) and ERK2 (p42mapk) are believed to be implicated in regulation of cell growth and differentiation [1,2]. They are activated in response to stimulation both of heptahelical G protein coupled receptors (GPCRs) and receptor tyrosine kinases (RTKs). Epidermal growth factor (EGF), hepatocyte growth factor (HGF), PGF2α, norepinephrine, and several other agents activate ERK1/2 in hepatocytes [3,4,5,6]. Furthermore, it was observed in these cells that pretreatment with pertussis toxin (PTX) decreased activation of ERK1/2 in response to various agents acting on RTKs or GPCRs [6,7,8,9]. The data suggest an involvement of Gi protein(s) in the mechanisms of ERK1/2 activation in hepatocytes. However, it is not known which Gi protein(s) that mediate this effect. To approach this issue we have targeted the α subunit of Gi2 by a catalytic RNA (ribozyme) [10,11]. The effect of the ribozyme on PGF2α -induced ERK1/2 activation, which is strongly sensitive to PTX, was subsequently assessed.
Results
Inhibition of ERK activation by pertussis toxin
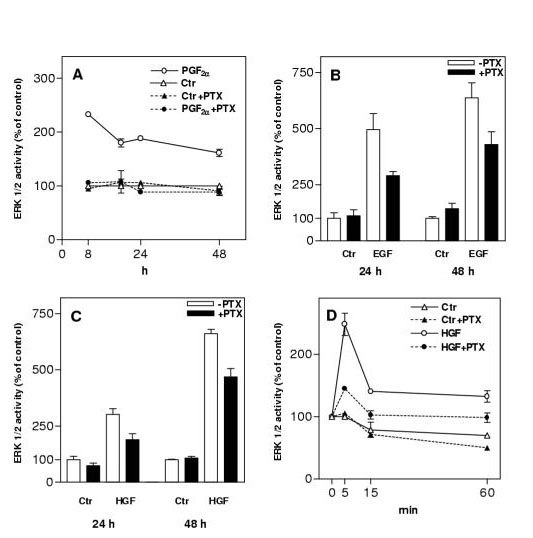
Pretreatment of hepatocytes with pertussis toxin [12] was reported to decrease ERK1/2 activation by agents acting both on heptahelical G protein coupled receptors as well as receptor tyrosine kinases [6,7,8]. These observations are summarized in Fig. 1. In addition these data show the persistence with time of the marked inhibitory effect of PTX on ERK1/2 activation induced by PGF2α . The EGF- and HGF-induced responses are on the other hand only partially decreased. These findings suggest that ERK1/2 activation in hepatocytes involve Gi protein(s).
Figure 1.
Effect of pertussis toxin (PTX) on ERK1/2 activation. PTX was added to cultured hepatocytes at 3 h after the time of seeding. A: ERK1/2 activation in the absence or presence of PTX (400 ng/ml) in response to stimulation with PGF2α (10 μM) for 5 min in the period from 8 h to 48 h. B, C: Effect of PTX (300 ng/ml) on ERK1/2 activation in response to stimulation with EGF (10 nM) (B) or HGF (100 ng/ml) (C) for 5 min at 24 and 48 h. D: Time-course for HGF- (100 ng/ml) induced ERK1/2 activation in the absence or presence of PTX (300 ng/ml). Results in A-D represent means ± S.E.M. from three experiments.
Ribozyme targeting the Gi2α
Although the data shown in Fig. 1 suggest that the activation of ERK1/2 implicated Gi, they do not determine the subtype of Gi involved in this process. Accordingly, we have evaluated the effects of a ribozyme specific for Gi2α upon ERK1/2 activation. The choice of target was based on the knowledge of Gi2 as a major member of the Gi family in hepatocytes, which is also represented by Gi3 in these cells [9,13,14], and furthermore on the α subunit as the unifying part of the heterotrimer which additionally comprises βγ variants of hitherto unknown subtype compositions and G protein specificity.
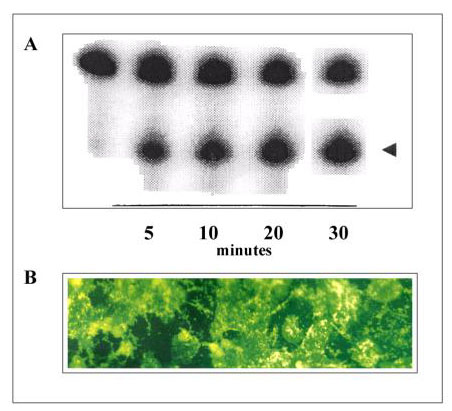
Ribozymes are RNA molecules that specifically cleave mRNAs [10,11]. These molecules have been shown to inhibit gene expression in various cell types [15,16]. To increase the ribozyme stabililty, all hydroxyl pyrimidines were replaced by their 2'-amino analogs. This type of modification was shown to enhance the ribozyme stability without affecting its cleavage activity [15,17]. Fig. 2A shows the cleavage of the RNA substrate by the ribozyme.
Figure 2.
Gi2α ribozyme. A: Ribozyme in vitro cleavage activity. A PhosphorImager (Molecular Dynamics, Sunnyvale, CA, USA) printout of a 15 % polyacrylamide gel with 7 M urea. The 5'-end-labelled RNA substrate (200 nM) was incubated with the ribozyme (75 nM) in reaction buffer as described in Materials and Methods. 10 μl samples were taken at the indicated time, added to 5 μl quenching solution and then analysed by electrophoresis. The arrow indicates cleavage products. B: Ribozyme uptake in cultured hepatocytes. Hepatocytes were transfected with 5'-FITC-labelled ribozyme for 30 h. Following washing with phosphate-buffered saline (PBS), cells were analysed by fluorescence microscopy.
Several approaches have been explored in order to introduce genes into hepatocytes [18,19]. As a first step, we have examined the usefulness of the cationic lipid-mediated ribozyme delivery into hepatocytes. In this respect, the hepatocytes were transfected with a 5'-carboxyfluorescein-conjugated ribozyme and analysed by fluorescence (Fig. 2B). As shown, most cells had taken the ribozyme molecules. Furthermore, no significant cytotoxic effect was observed at the concentration used. Thus DOTAP may represent a versatile transfection reagent for primary hepatocytes.
Inhibition of Gi2α expression and ERK1/2 activation by ribozyme treatment
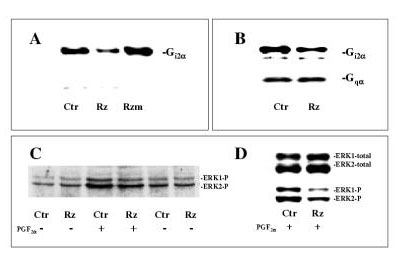
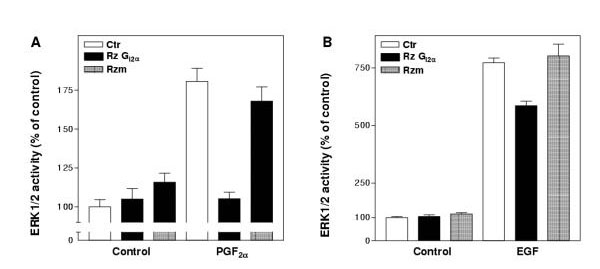
Having demonstrated a cellular uptake of ribozymes into cultured hepatocytes when DOTAP was used as delivery agent, in the next set of experiments we examined the effects of the Gi2α ribozyme on Gi2α protein levels as well as on the total ERK1/2 expression and activation. DOTAP formulated test molecules were added to the hepatocyte cultures at 4–5 hours after the time of seeding. After 30–45 hours transfection time the expression of Gi2α protein was decreased in ribozyme-treated cells, while no significant effect was seen with its non-cleaving form (Fig. 3A). The expression of Gqα (Fig. 3B) or Gsα (not shown) was not affected by the ribozyme treatment thus confirming the specificity of the ribozyme effects upon Gi2α gene expression. To investigate the functional roles of Gi2 on ERK1/2 activation, we examined the phosphorylation of ERK1/2 in ribozyme-treated cells following PGF2α stimulation (Fig. 3C, D). The basal level of ERK1/2 phosphorylation (i.e. in the absence PGF2α stimulation) was not reduced following ribozyme treatment (Fig. 3C). However, the PGF2α-induced phosphorylation of ERK1/2 was decreased (Fig. 3C and 3D, lower panel). In contrast, the total ERK1/2 protein level was not affected by the treatment (Fig. 3D, upper panel). To further confirm the decrease in ERK1/2 phosphorylation, we also assessed their activity in response to PGF2α stimulation (Fig. 4). A marked inhibitory effect was found in ribozyme-treated cells. The activation of ERK1/2 by EGF was, however, reduced to a lesser extent. These results also show the absence of inhibition by the non-cleaving form of the Gi2α ribozyme.
Figure 3.
Western analysis of the effects of ribozyme on Gi2α and ERK1/2. Gi2α ribozyme (Rz) or non-cleaving Gi2α ribozyme (Rzm) complexed with DOTAP giving final ribozyme concentrations of 2.5 μM, or only DOTAP (Ctr) were added to hepatocyte cultures at 4–5 hours after the time of seeding. A: Expression of Gi2α protein was assessed after 45 h of ribozyme treatment using antibody (from Calbiochem) directed against C-terminal end of Gi1/i2α . B: Expression of Gi2α and Gqα protein levels in the same samples subsequent to 30 h of ribozyme treatment using antibodies (from NEN™ Life Science Products) against C-terminal sequences of Gi1/i2α or Gqα, respectively. The polyclonal antibodies used to assess Gi2α recognize both the α subunit of Gi2 and Gi1. As shown previously hepatocytes do not express Gi1α [19], so the reactivity with these antibodies reflects only the Gi2α levels. C, D: After 45 h of ribozyme treatment cells were stimulated with or without PGF2α (10 μM) for 5 min before they were harvested. Immunoblot using antibody against dually phosphorylated ERK1/2 (i.e. ERK1/2-P) (C) is depicted. In Fig. D is developed images from the same immunoblot using antibody detecting total amount of ERK1/2 (i.e. both phosphorylated and unphosphorylated forms) (upper panel) and antibody against dually phosphorylated fractions of ERK1/2 (lower panel).
Figure 4.
Effect of Gi2α ribozyme on ERK1/2 activation. Gi2α ribozyme (Rz) or non-cleaving Gi2α ribozyme (Rzm) complexed with DOTAP giving final ribozyme concentrations of 2.5 μM, or DOTAP without ribozyme, were added to hepatocyte cultures at 4–5 hours after the time of seeding. After 30 h of treatment the cell cultures were stimulated with PGF2α (10 μM) or EGF (10 nM) for 5 min before they were harvested and ERK1/2 activity assessed. Results are expressed as percent of untreated control and represent mean ± S.E.M. from three experiments.
Discussion
In the current study we have investigated the role of Gi2α upon PGF2α -induced ERK1/2 activation in hepatocytes. The data obtained with the ribozyme suggest that Gi2 is an important factor in ERK1/2 activation.
Gi proteins are believed to be involved in regulation of cell growth [20], and a role in activation of ERK1/2 is reported in different cells [21,22,23]. In hepatocytes it has been observed that PTX inhibited activation of ERK1/2 both by agents acting on G protein coupled receptors including vasopressin, angiotensin II, norepinephrine and PGF2α as well as by agents that bind to receptor tyrosine kinases like EGF and HGF [6,7,8], also suggesting roles of Gi. In the present study we explored closer the Gi dependency of PGF2α -induced ERK1/2 activation, which is strongly PTX-sensitive. We used a ribozyme approach [10,11], which has been examined in a variety of experimental models to suppress gene expression [15,24], including in human hepatocytes and hepatoma cells [25,26,27]. Notably, ribozymes were recently reported to effectively suppress the expression of the γ7 subunit of heterotrimeric G proteins in HEK 293 cells [28,29].
Pretreatment of the hepatocytes with the Gi2α ribozyme resulted in a marked inhibition of the PGF2α -induced ERK1/2 activation. The findings might be explained by the ability of PGF2α to act through receptors that couple directly to Gi in these cells [30]. In addition, the Gi2α ribozyme resulted in a partial decrease of the EGF-induced ERK1/2 activation, in accordance with a role of Gi. However, the explanation for an involvement of Gi in this pathway is not known. Observations in different cell types have indicated that Gi might play a role in EGF-induced cell signaling [31], and in hepatocytes a direct coupling of the EGF receptor to Gi has been proposed [32]. One explanation is that receptor-independent functions of Gi may be involved in signaling from receptor tyrosine kinases as well as from seven transmembrane receptors [9,33,34]. Since cyclic AMP was found to exert a negative control of ERK1/2 activation in hepatocytes [35], it can be speculated that the decreases in ERK1/2 responses observed subsequent to inhibition of Gi function might be caused by an elevation of intracellular levels of cyclic AMP. However, in experiments using pertussis toxin there were no detectable alterations of the cyclic AMP level under basal conditions (data not shown). Furthermore, as shown in the present study ribozyme treatment of the hepatocytes did not decrease the basal ERK1/2 activation thus suggesting no significant alteration of cyclic AMP level under these experimental conditions.
The present data show a close correlation between the inhibitory effect on ERK1/2 activation produced by the Gi2α ribozyme compared to the effects obtained with PTX. This suggests that the PTX effects that have been observed, at least on ERK1/2 activation in response to PGF2α as well as EGF stimulation, reflect Gi2-mediated mechanisms. However, the present data do not rule out a possible contribution of Gi3 in ERK1/2 activation. In this regard, it is of note that observations in endothelial cells have suggested a role for Gi2 proteins, but not Gi3, in ERK1/2 activation [36]. Further studies involving specific inhibition of Gi3 proteins will be needed to clarify this issue in the hepatocyte model.
Previous findings have demonstrated that Gi dependent activations of ERK1/2 are mediated through βγ subunits. These results were derived from studies where ERK activation was elicited by overexpression of βγ [37,38], or antagonized by the βγ-inhibitory peptide β1ct (C-terminal fragment of the β-adrenergic receptor kinase-1) [39]. Although a possible role for βγ in ERK1/2 activation can not be ruled out, the present data might be compatible with a role for the α i2 subunit. This interpretation agrees with observations reported by Hedin et al. [40] who found that in Jurkat T lymphocytes the δ-opioid activation of ERK1/2 was PTX-sensitive, but unaffected by β1ct treatment, suggesting an involvement of αi. However, it should be noted that a strategy based on targeting a particular subunit of a G protein might affect the overall function of the heterotrimer.
Conclusion
The present study gives further support to a role of Gi proteins in ERK1/2 activation in hepatocytes and suggests a role of Gi2. On the other hand, the data can not exclude a possible involvement of Gi3 in the mechanisms of ERK1/2 activation in these cells or define the precise contribution of the G protein subunit αi2. The observation that primary hepatocytes are efficiently transfected with ribozymes may facilitate studies of cell signaling in this model system which represents features of normal cells. Thus, it will be interesting to explore the roles of different heterotrimeric G proteins and their subunits in activation of ERK1/2 as well as other mitogen-activated protein kinases by the nucleic acid enzyme strategy.
Materials and Methods
Materials
Dulbecco's modified Eagle's medium, Waymouth's medium MAB 87/3, penicillin and streptomycin were from Gibco, Grand Island, NY, U.S.A. Adenosine 5'-triphosphate, collagen, collagenase, phenylmethylsulfonyl fluoride, benzamidine, leupeptin, pepstatin A, myelin basic protein (MBP), epidermal growth factor, prostaglandin F2α, insulin, pertussis toxin, and 2-mercaptoethanol were from Sigma, St. Louis, MO, USA. Hepatocyte growth factor (human) was a gift from Magne Børset, NTNU, Trondheim, Norway. Sodium(meta)vanadate was from Fluka Chemie AG, Buchs, Switzerland. Phenyl Sepharose CL-4B was from Pharmacia Biotech., Uppsala, Sweden. Dexamethasone was from Norwegian Medicinal Depot, Oslo, Norway. DOTAP was from Boehringer Mannheim, Mannheim, Germany. T7 RNA polymerase and T4 polynucleotide kinase were from Promega Corporation, Madison, WI, USA. [γ-32 P] Adenosine 5'-triphosphate (3000 Ci/mol) was from Amersham International, Buckinghamshire, England.
Isolation and culture of hepatocytes
Male Wistar rats (170–220 g) fed ad libitum were used. Parenchymal liver cells were isolated by in vitro collagenase perfusion and low-speed centrifugation [41] with modifications [42]. Cell viability, measured as the ability to exclude trypan blue, was at least 95 %. The cells were suspended in culture medium and plated in Costar wells at 20.000 cells/cm2. The culture medium (0.2 ml/cm2) was a 1:1 mixture of Dulbecco's modified Eagle's medium and Waymouth's medium MAB 87/3 containing 16.8 mM glucose, supplemented with penicillin (100 U/ml), streptomycin (0.1 mg/ml), dexamethasone (25 nM) and insulin (100 nM). The cultures were gassed with 95 % air 5% CO2 and kept at 37°C.
Measurement of ERK activity
The measurement of ERK1/2 activity was performed as previously described [5,6]. In brief, the hepatocyte cultures were exposed to agonists for 5 minutes before rinsing the cells and scraping them into a buffer containing 10 % ethylene glycol. The lysate was centrifugated (15,800 × g) for 10 minutes and the supernatant mixed with phenyl-Sepharose, which was washed twice in a 10 %, twice in a 35 % ethylene glycol buffer, before finally eluting ERK1/2 with 60 % ethylene glycol buffer [43]. The eluate was assayed for ERK1/2 activity, using MBP as substrate, in the presence of an inhibitor of protein kinase A (Sigma P-0300). The reaction mixture was spotted onto P81 paper (Whatman, Maidstone, UK), which was washed, dried and counted in a liquid scintillation counter. Protein content was determined with the BCA Protein Assay (Pierce, Rockford, IL, U.S.A.).
Immunoblotting
Aliquots with 20 μg cell protein (total cell lysate prepared in Laemmli buffer) were electrophoresed on 10 % polyacrylamide gels (acrylamide:N'N'-bis-methylene acrylamide 30:0.8) followed by protein electrotransfer to nitrocellulose membranes and immunoblotting with a polyclonal MAP kinase antibody against the dually threonine- and tyrosine phosphorylated forms of ERK1 (p44mapk) and ERK2 (p42mapk) or an antibody detecting both the phosphorylated and unphosphorylated forms (Promega Corporation, Madison, WI, USA). Antibodies against α-subunits of Gi1/i2 were from Calbiochem (La Jolla, CA, USA) and NEN™ Life Science Products (Boston, MA, USA). Antibody against the α-subunit of Gq was from NEN™ Life Science Products (Boston, MA, USA). Immunoreactive bands were visualised with ECL Western blotting detection reagents (Amersham International).
In vitro RNA synthesis
A 2'-amino pyrimidine modified hammerhead ribozyme having GUC as cleavage triplet corresponding to the nucleotide number 481 within the rat Gi2α mRNA [44] was synthesized by in vitro transcription using a short DNA template for the T7 RNA polymerase as previously described [15,45]. Subsequent to transcription ribozymes were PAGE gel-purified, ethanol-precipitated and then dissolved in water. The concentration was determined by assessment of absorbency at 260 nm. A non-cleaving form of the ribozyme was made by deleting the G12 from the catalytic core as indicated by lower case letter. The ribozyme short target was synthesized by in vitro transcription of a synthetic DNA template with the T7 RNA polymerase. Subsequent to transcription, the gel-purified RNA was dephosphorylated by alkaline phosphate and then 5'-end labelled using T4 polynucleotide kinase and [γ-32 P]ATP. The ribozyme sequence is: 5'GGCAGCACAGCU GAUGAGUCCGUGAGGACgAAACAGUGCGAACAGC3'. The sequence of the targeted site is: 5'GCUGUUCGCACUGUCCUGUGCUGCC3'. The cleavage site is underlined.
In vitro cleavage activity of the Gi2α ribozyme
Cleavage reactions were performed at 37°C in a buffer containing 50 mM Tris-HCl (pH 7.4) and 10 mM MgCl2. Cleavage products were separated by electrophoresis on a 15 % polyacrylamide gel containing 7 M urea.
Transfection experiments
Cells were transfected with DOTAP- (25 μg/ml) formulated ribozyme 4–5 h after the time of plating. Only single transfections were used giving a ribozyme concentration in the culture medium of 2.5 μM. After 30–45 hours transfection time, immunoblotting experiments and assessment of ERK1/2 activity were performed.
List of abbreviations
Cyclic AMP, Adenosine 3',5'-cyclic monophosphate, DOTAP, N- [1-(2,3-dioleoyloxy)propyl]-N,N,N-trimethyl ammonium-methylsulphate; EGF, epidermal growth factor; ERK1/2, extracellular signal-regulated kinase 1 and 2; G protein, guanine nucleotide binding (regulatory) protein; GPCR, G protein-coupled receptor; HGF, hepatocyte growth factor; MBP, myelin basic protein; PTX, pertussis toxin; PGF2α, prostaglandin F2α; RTK, receptor tyrosine kinase; Rz, ribozyme; Rzm, mutant ribozyme.
Acknowledgments
Acknowledgements
We thank Eva Østby and Ellen Johanne Johansen for technical help. This work was supported by the Norwegian Cancer Society and The Research Council of Norway.
Contributor Information
Øyvind Melien, Email: oyvind.melien@c2i.net.
Thoralf Christoffersen, Email: thoralf.christoffersen@labmed.uio.no.
Mouldy Sioud, Email: mosioud@uirik.uio.no.
References
- Lewis TS, Shapiro PS, Ahn NG. Signal transduction through MAP kinase cascades. Adv Cancer Res. 1998;74:49–139. doi: 10.1016/s0065-230x(08)60765-4. [DOI] [PubMed] [Google Scholar]
- Widmann C, Gibson S, Jarpe MB, Johnson GL. Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol Rev. 1999;79:143–180. doi: 10.1152/physrev.1999.79.1.143. [DOI] [PubMed] [Google Scholar]
- Adachi T, Nakshima S, Saji S, Nakamura T, Nozawa Y. Mitogen-activated protein kinase activation in hepatocyte growth factor-stimulated rat hepatocytes: involvement of protein tyrosine kinase and protein kinase C. Hepatology. 1996;23:1244–1253. doi: 10.1053/jhep.1996.v23.pm0008621160. [DOI] [PubMed] [Google Scholar]
- Spector MS, Auer KL, Jarvis WD, Ishac EJ, Gao B, Kunos G, Dent P. Differential regulation of the mitogen-activated protein and stress-activated protein kinase cascades by adrenergic agonists in quiescent and regenerating adult rat hepatocytes. Mol Cell Biol. 1997;17:3556–3565. doi: 10.1128/mcb.17.7.3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoresen GH, Guren TK, Sandnes D, Peak M, Agius L, Christoffersen T. Response to transforming growth factor alfa (TGFα) and epidermal growth factor (EGF) in hepatocytes: Lower EGF receptor affinity of TGFα is associated with more sustained activation of p42/p44 mitogen-activated protein kinase and greater efficacy in stimulation of DNA synthesis. J Cell Physiol. 1998;175:10–18. doi: 10.1002/(SICI)1097-4652(199804)175:1<10::AID-JCP2>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Melien Ø, Thoresen GH, Sandnes D, Østby E, Christoffersen T. Activation of p42/p44 mitogen- activated protein kinase by angiotensin II, vasopressin, norepinephrine, and prostaglandin F2α in hepatocytes is sustained, and like the effect of epidermal growth factor, mediated through pertussis toxin-sensitive mechanisms. J Cell Physiol. 1998;175:348–358. doi: 10.1002/(SICI)1097-4652(199806)175:3<348::AID-JCP13>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Ginès P, Li X, Brown SES, Nakamura T, Guzelian PS, Heasley LE, Schrier RW, Nemenoff RA. Inhibitory actions of cyclic adenosine monophosphate and pertussis toxin define two distinct epidermal growth factor-regulated pathways leading to activation of mitogen-activated protein kinase in rat hepatocytes. Hepatology. 1996;23:1167–1173. doi: 10.1002/hep.510230535. [DOI] [PubMed] [Google Scholar]
- Adachi T, Nakashima S, Saji S, Nakamura T, Nozawa Y. Possible involvement of pertussis toxin-sensitive G protein in hepatocyte growth factor-induced signal transduction in cultured rat hepatocytes: pertussis toxin treatment inhibits activation of phospholipid signaling, calcium oscillation, and mitogen-activated protein kinase. Hepatology. 1997;26:295–300. doi: 10.1002/hep.510260207. [DOI] [PubMed] [Google Scholar]
- Melien Ø, Sandnes D, Johansen EJ, Christoffersen T. Effects of pertussis toxin on ERK activation in hepatocytes by hormones and receptor-independent agents: evidence suggesting a stimulatory role of Gi proteins at a level distal to receptor coupling. J Cell Physiol. 2000;184:27–36. doi: 10.1002/(SICI)1097-4652(200007)184:1<27::AID-JCP3>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Hasseloff J, Gerlach WL. Simple RNA enzymes with new and highly specific endoribonuclease activities. Nature. 1988;334:585–591. doi: 10.1038/334585a0. [DOI] [PubMed] [Google Scholar]
- Rossi JJ. Controlled, targeted, intracellular expression of ribozymes: progress and problems. Trends Biotech. 1995;13:301–306. doi: 10.1016/S0167-7799(00)88969-6. [DOI] [PubMed] [Google Scholar]
- Murayama T, Ui M. Loss of the inhibitory function of the guanine nucleotide regulatory component of adenylate cyclase due to its ADP ribosylation by islet-activating protein, pertussis toxin, in adipocyte membranes. J Biol Chem. 1983;258:3319–3326. [PubMed] [Google Scholar]
- Bushfield M, Murphy GJ, Lavan BE, Parker PJ, Hruby VJ, Milligan G, Houslay MD. Diabetes- induced alterations in the expression, functioning and phosphorylation state of the inhibitory guanine-nucleotide-binding regulatory protein Gi2. Biochem J. 1990;268:449–457. doi: 10.1042/bj2710365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadrin M, McFarlane-Anderson N, Harper ME, Gaffield J, Begin-Heick N. Comparison of the subcellular distribution of G-proteins in hepatocytes in situ and in primary cultures. J Cell Biochem. 1996;62:334–341. doi: 10.1002/(SICI)1097-4644(199609)62:3<334::AID-JCB4>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Sioud M, Sørensen D. A nuclease-resistant protein kinase Cα ribozyme blocks glioma cell growth. Nature Biotech. 1998;16:556–561. doi: 10.1038/nbt0698-556. [DOI] [PubMed] [Google Scholar]
- Sioud M. Application of preformed hammerhead ribozymes in the gene therapy of cancer. Int J Mol. 1999;3:381–384. doi: 10.3892/ijmm.3.4.381. [DOI] [PubMed] [Google Scholar]
- Leirdal M, Sioud M. High cleavage activity and stability of hammerhead ribozymes with a uniform 2'-amino pyrimidine modification. Biochem Biophys Res Commun. 1998;250:171–174. doi: 10.1006/bbrc.1998.9286. [DOI] [PubMed] [Google Scholar]
- Paquereau L, Le Cam A. Electroporation-mediated gene transfer into hepatocytes: preservation of a growth hormone response. Anal Biochem. 1992;204:147–151. doi: 10.1016/0003-2697(92)90154-y. [DOI] [PubMed] [Google Scholar]
- Hofmann C, Sandig V, Jennings G, Rudolph M, Schlag P, Strauss M. Efficient gene transfer into human hepatocytes by baculovirus vectors. Proc Natl Acad Sci. 1995;92:10099–10103. doi: 10.1073/pnas.92.22.10099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhanasekaran N, Prasad MV. G protein subunits and cell proliferation. Biol Signals Recept. 1998;7:109–117. doi: 10.1159/000014536. [DOI] [PubMed] [Google Scholar]
- Alblas J, van Corven EJ, Hordijk PL, Milligan G, Moolenaar WH. Gi-mediated activation of the p21ras-mitogen-activated protein kinase pathway by α2-adrenergic receptors expressed in fibroblasts. J Biol Chem. 1993;268:22235–22238. [PubMed] [Google Scholar]
- Pace AM, Faure M, Bourne HR. Gi2-mediated activation of the MAP kinase cascade. Mol BiolCell. 1995;6:1685–1695. doi: 10.1091/mbc.6.12.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutkind JS. Cell growth control by G protein-coupled receptors: from signal transduction to signal integration. Oncogene. 1998;17:1331–1342. doi: 10.1038/sj.onc.1202186. [DOI] [PubMed] [Google Scholar]
- Sioud M, Drlica K. Prevention of human immunodeficiency virus type 1 integrase expression in Escherichia coli by a ribozyme. Proc Acad Natl Sci. 1991;88:7303–7307. doi: 10.1073/pnas.88.16.7303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YK, Junn E, Park I, Lee Y, Kang C, Ahn JK. Repression of hepatitis B virus X gene expression by hammerhead ribozymes. Biochem Biophys Res. 1999;257:759–765. doi: 10.1006/bbrc.1999.0537. [DOI] [PubMed] [Google Scholar]
- Ozaki I, Zern MA, Liu S, Wei DL, Pomerantz RJ, Duan L. Ribozyme-mediated specific gene replacement of the α1-antitrypsin gene in human hepatoma cells. J Hepatol. 1999;31:53–60. doi: 10.1016/S0168-8278(99)80163-9. [DOI] [PubMed] [Google Scholar]
- Zern MA, Ozaki I, Duan L, Pomerantz R, Liu SL, Strayer DS. A novel SV40-based vector successfully transduces and expresses an alpha1-antitrypsin ribozyme in a human hepatoma-derived cell line. Gene Ther. 1999;6:114–120. doi: 10.1038/sj.gt.3300793. [DOI] [PubMed] [Google Scholar]
- Wang Q, Mullah B, Hansen C, Asundi J, Robishaw JD. Ribozyme-mediated suppression of the G protein γ7 subunit suggests a role in hormone regulation of adenylyl cyclase activity. J Biol Chem. 1997;272:26040–26048. doi: 10.1074/jbc.272.41.26040. [DOI] [PubMed] [Google Scholar]
- Wang Q, Mullah BK, Robishaw JD. Ribozyme approach identifies a functional association between the G protein β1γ7 subunits in the β-adrenergic receptor signaling pathway. J Biol Chem. 1999;274:17365–17371. doi: 10.1074/jbc.274.24.17365. [DOI] [PubMed] [Google Scholar]
- Melien Ø, Winsnes R, Refsnes M, Gladhaug IP, Christoffersen T. Pertussis toxin abolishes the inhibitory effects of prostaglandins E1, E2, I2 and F2α on hormone-induced cAMP accumulation in cultured hepatocytes. Eur J Biochem. 1988;172:293–297. doi: 10.1111/j.1432-1033.1988.tb13886.x. [DOI] [PubMed] [Google Scholar]
- Ramírez I, Tebar F, Grau M, Soley M. Role of heterotrimeric G proteins in epidermal growth factor signalling. Cell Signal. 1995;7:303–311. doi: 10.1016/0898-6568(95)00001-6. [DOI] [PubMed] [Google Scholar]
- Liang M, Garrison JC. The epidermal growth factor receptor is coupled to a pertussis toxin- sensitive nucleotide regulatory protein in rat hepatocytes. J Biol Chem. 1991;266:13342–13349. [PubMed] [Google Scholar]
- Crouch MF, Belford DA, Milburn PJ, Hendry IA. Pertussis toxin inhibits EGF-, phorbol ester- and insulin-stimulated DNA synthesis in BALB/c3T3 cells: evidence for post-receptor activation of Giα. Biochem Biophys Res Commun. 1990;167:1369–1376. doi: 10.1016/0006-291x(90)90674-c. [DOI] [PubMed] [Google Scholar]
- Takesono A, Cismowski MJ, Ribas C, Bernard M, Chung P, Hazard S, Duzic E, Lanier SM. Receptor-independent activators of heterotrimeric G-protein signaling pathways. J Biol Chem. 1999;274:33202–33205. doi: 10.1074/jbc.274.47.33202. [DOI] [PubMed] [Google Scholar]
- Thoresen GH, Johansen EJ, Christoffersen T. Effects of cAMP on ERK mitogen-activated protein kinase activity in hepatocytes do not parallell the bidirectional regulation of DNA synthesis. Cell Biol Int. 1999;23:13–20. doi: 10.1006/cbir.1998.0314. [DOI] [PubMed] [Google Scholar]
- Jo H, Sipos K, Go Y-M, Law R, Rong J, McDonald JM. Differential effect of shear stress on extracellular signal-regulated kinase and N-terminal Jun kinase in endothelial cells. J Biol Chem. 1997;272:1395–1401. doi: 10.1074/jbc.272.2.1395. [DOI] [PubMed] [Google Scholar]
- Crespo P, Xu N, Simonds WF, Gutkind S. Ras-dependent activation of MAP kinase pathways mediated by G-protein γ subunits. Nature. 1994;369:418–420. doi: 10.1038/369418a0. [DOI] [PubMed] [Google Scholar]
- Faure M, Voyno-Yasenetskaya TA, Bourne HR. cAMP and γ subunits of heterotrimeric G proteins stimulate the mitogen-activated protein kinase pathway in COS-7 cells. J Biol Chem. 1994;269:7851–7854. [PubMed] [Google Scholar]
- van Biesen T, Hawes B, Luttrell DK, Krueger KM, Touhara K, Porfiri E, Sakaue M, Luttrell LM, Lefkowitz RJ. Receptor-tyrosine-kinase and G γ-mediated MAP kinase activation by a common signaling pathway. Nature. 1995;376:781–784. doi: 10.1038/376781a0. [DOI] [PubMed] [Google Scholar]
- Hedin KE, Bell MP, Huntoon CJ, Karnitz LM, McKean DJ. i proteins use a novel γ- and Ras-independent pathway to activate extracellular signal-regulated kinase and mobilize AP-1 transcription factors in Jurkat T lymphocytes. J Biol Chem. 1999;274:19992–20001. doi: 10.1074/jbc.274.28.19992. [DOI] [PubMed] [Google Scholar]
- Seglen PO. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- Christoffersen T, Refsnes M, Brønstad GO, Østby E, Huse J, Haffner F, Sand TE, Hunt NH, Sonne O. Changes in hormone responsiveness and cyclic AMP metabolism in rat hepatocytes during primary culture and effects of supplementing the medium with insulin and dexamethasone. Eur J Biochem. 1984;138:217–226. doi: 10.1111/j.1432-1033.1984.tb07904.x. [DOI] [PubMed] [Google Scholar]
- Anderson NG, Kilgour E, Sturgill TW. Activation of mitogen-activated protein kinase in BC3H1 myocytes by fluoroaluminate. J Biol Chem. 1991;266:10131–10135. [PubMed] [Google Scholar]
- Jones DT, Reed RR. Molecular cloning of five GTP-binding protein cDNA species from rat olfactory neuroepithelium. J Biol Chem. 1987;262:14241–14249. [PubMed] [Google Scholar]
- Aurup H, Williams DM, Eckstein F. 2'-fluoro- and 2'-amino-2'deoxynucleoside 5'- triphosphates as substrates for T7 RNA polymerase. Biochemistry. 1992;31:9636–9641. doi: 10.1021/bi00155a016. [DOI] [PubMed] [Google Scholar]