Abstract
Objective
Cannabis use is common among opioid-dependent patients, but studies of its association with treatment outcome are mixed. In this secondary analysis, the association of cannabis use with opioid treatment outcome is assessed.
Methods
In the main study, participants (N=152) aged 15-21 years were randomized to receive psychosocial treatments and either a 12-week course of buprenorphine-naloxone with a dose taper to zero in weeks 9-12, or a 2-week detoxification with buprenorphine-naloxone. Drug use was assessed by self-report and urine drug screen at baseline and during study weeks 1-12. The association between cannabis and opioid use at weeks 4, 8, and 12 was examined using logistic regression models.
Results
Participants reported a median of 3.0 days (range=0-30) cannabis use in the past month; half (50.3%; n=77) reported occasional use, one-third reported no use (33.1%; n=50), and one-sixth reported daily cannabis use (16.6%; n=25). Median lifetime cannabis use was 4.0 years (range=0-11) and median age of initiation of use was 15.0 years (range 9-21). Neither past cannabis use (age of initiation and use in the month prior to baseline) nor concurrent use was associated with level of opioid use.
Conclusions
Overall, cannabis use had no association with opioid use over 12 weeks in this sample of opioid-dependent youth. While cannabis use remains potentially harmful, it was not a predictor of poor opioid treatment outcome.
Keywords: cannabis use, opioid dependence, buprenorphine, adolescent substance abuse
1. INTRODUCTION
Use of non-heroin opioids among young people has almost doubled during the past decade (Johnston et al., 2012) with a 10-fold increase in teenage admissions to publicly-funded substance abuse treatment programs for non-heroin opioid use problems (SAMSHA, 2008). Those seeking treatment for opioid dependence often use cannabis as well (Woody et al., 2008; Subramaniam et al., 2009), so its impact on treatment outcome is of significant clinical interest. Clinicians are likely to want to know the impact of cannabis use, before or during treatment, on outcomes in young opioid-dependent patients.
There is limited research examining the impact of cannabis use on opioid use treatment outcomes, especially among youth. In an earlier secondary analysis from the same data set we used for our analyses, from 152 youths randomized to 12 weeks of buprenorphine/naloxone or up to 2 weeks of buprenorphine/naloxone detoxification, along with counseling (Woody et al. 2008), Subramaniam et al. (2011) found that cannabis use in the 30 days prior to baseline assessment, reported “yes” or “no” as a dichotomous variable, did not predict opioid abstinence during treatment (Subramaniam et al., 2011). Studies in adults examining the prognostic significance of cannabis use during opioid dependence treatment have produced mixed results. Most studies have suggested that cannabis use did not predict treatment outcome, but two studies offered a different view. Both DuPont and Saylor (1989) and Wasserman et al. (1998) reported that patients who used cannabis while receiving methadone maintenance treatment had worse treatment outcomes. Other studies of patients receiving methadone maintenance, buprenorphine maintenance, or naltrexone pharmacotherapy demonstrated that cannabis use was not associated with treatment outcome (Budney et al., 1998; Church et al., 2001; Epstein and Preston, 2003; Nirenberg et al., 1996; Saxon et al., 1993). For example, Budney et al. (1998) studied opioid-dependent patients receiving buprenorphine maintenance, and found that the percentage of cannabinoid-positive urines did not correlate with the number of weeks of heroin abstinence or with treatment retention.
With buprenorphine maintenance emerging as a potentially effective treatment for the growing number of young people with opioid dependence (Marsch et al., 2005; Woody et al., 2008), we became interested in the relationship between cannabis use and treatment outcome in this group. Because the bulk of previous research on the prognostic significance of cannabis use during opioid dependence treatment has focused on adults, we aimed to expand this line of research to adolescents and to also study use prior to treatment (number of days used in the 30 days prior to treatment). We posed the following research question: Is opioid dependence treatment outcome associated with 1) cannabis use during 12 weeks of opioid dependence treatment, 2) age of initiation of cannabis use, and 3) frequency of cannabis use in the 30 days prior to treatment? We hypothesized that cannabis use would be associated with poorer treatment outcome in opioid-dependent youths.
2. METHODS
2.1. Participants
The current analysis sought to identify variables related to cannabis use that may correlate with outcomes in the treatment of opioid-dependent adolescents and young adults. These data were from the 152 patients in the Woody et al. study (2008) that investigated brief vs. extended use of buprenorphine-naloxone (BUP/NAL) for opioid-addicted youth. Participants expressed interest in outpatient treatment, were 15 to 21 years of age, met DSM-IV criteria for opioid dependence with physiological features, had no significant health problems that would make participation hazardous, and provided informed consent. Those under 18 provided assent, and at least one parent gave informed consent. Participants were not excluded if they had any other concurrent substance abuse or dependence diagnosis, except for alcohol or benzodiazepine abuse or dependence.
2.2. Overview of main study design and treatment
Participants were randomized to 1) a 12-week course of BUP/NAL with a taper beginning in week 9 and ending at week 12, or 2) a 2-week detoxification with buprenorphine-naloxone (DETOX). Continued treatment with BUP/NAL resulted in improved outcomes compared with the DETOX group (Woody et al., 2008). Participants were terminated from study medication treatment if they failed to attend psychosocial treatment for two consecutive weeks or if they were not adherent to the medication schedule (see Woody et al., 2008 for details).
2.3. Measures
Demographic and self-report data on drug use, as well as urine drug screens, were collected at the screening and baseline visits and a more extensive drug use history was administered at intake. Throughout the treatment phase, participants completed weekly urine drug screens and a Timeline Followback questionnaire that assessed daily alcohol, opioid, cannabis, and other drug use during the preceding week. Medication adherence and treatment drop-out were documented throughout the study.
2.4. Statistical analysis
Data on opioid use at weeks 4, 8, and 12 were assembled in panel format (i.e., multiple records per subject, with one record for each follow-up period with opioid use data available). The association between cannabis and opioid use was examined using logistic regression models that were fitted by generalized estimating equations (GEE). These analyses modeled the log odds of opioid use in regression models that included cannabis use as a main effect while adjusting for treatment condition and time effects. These analyses appropriately account for the correlation among the repeated measures of opioid use and yield an odds ratio and 95% confidence interval (95%CI) for the association between cannabis and opioid use. Data are reported using the complete N of 152 for the sample baseline description and cannabis use history. However, the associations with opioid use over time are limited to the 115 participants with one or more opioid urine screens at weeks 4, 8, and/or 12.
With a sample size of 115 and prevalence of opioid outcome in the 40-50% range, the study had power of at least 80% to detect an odds ratio of 1.8 or greater (for a 1 standard deviation difference in the cannabis use predictor); this power calculation should be considered conservative for regression analyses of the opioid outcome at three occasions.
3. RESULTS
3.1. Sample description
Most (59.2%, n=90) of the participants (N=152) included in the main outcome trial were male and most were Caucasian (73.7%, n=112), followed by Spanish, Hispanic, or Latino (25.0%, n=38), with a mean age of 19.2 years (sd=1.5, range 15-21). Half (50.0%, n=76) were employed full-time or had been students during the 3 years prior to study enrollment; however, most (65.8%, n=100) reported being neither employed full-time nor students in the 30 days prior to baseline. Comparison of the 115 participants who provided opioid urine screens at weeks 4, 8, or 12 to the remaining participants showed no statistically significant differences in background characteristics, including gender, age, education, race, employment, cannabis use history, and treatment condition.
3.2. Cannabis use history
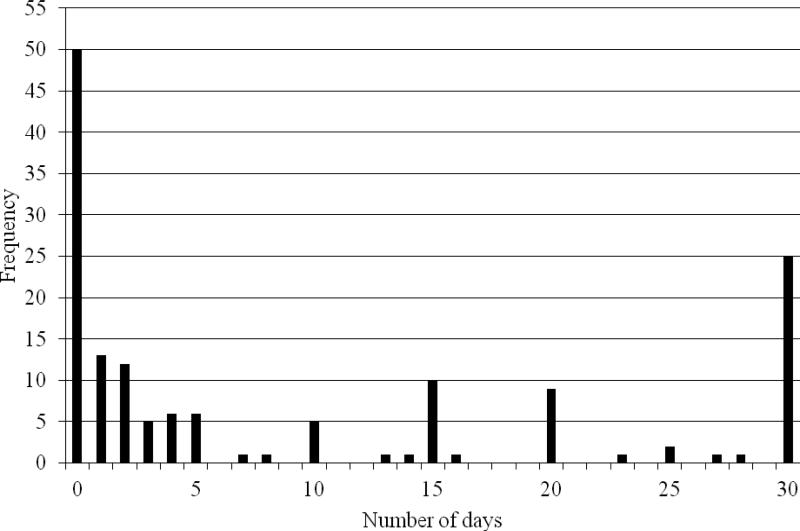
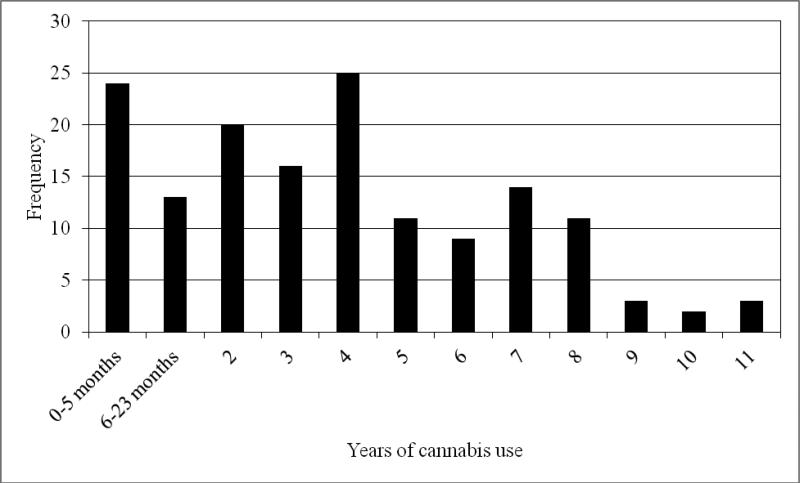
Participants reported a median of 3 days of cannabis use in the past 30 days at baseline, ranging from 0-30 days (mean=9.4, sd=11.5, n=151; see Figure 1). Defining daily use as 5 or more days per week, consistent with the literature (Johnston et al., 2012), one-sixth reported daily use (16.6%; n=25), one-third reported no cannabis use (33.1%; n=50), and half (50.3%; n=77) reported occasional use. The median for lifetime cannabis use was 4 years, ranging from 0-11 years (mean=3.8, sd=2.9, n=151; see Figure 2) with 15.9% (n=24) reporting <6 months of use. Baseline cannabis use was positively associated with cocaine use at baseline (p<.04), but not with alcohol use, sedative use, employment or school status, psychiatric status, or criminal justice involvement. Cannabis use was not associated with cocaine use during treatment, however, as measured by urine drug screens at weeks 4, 8, and 12. Overall, 55.3% (84/152) of participants were considered treatment dropouts. While participants abstinent from cannabis were less likely to drop out of treatment than occasional or daily users (48% vs. 61% and 56%, respectively, p<.38), this difference was not statistically significant. Results were also not significant for the relationship between dropout from treatment and number of days of cannabis use, adjusted for treatment condition (data not shown).
Figure 1.
Frequency distribution of days of cannabis use in the month prior to baseline (N=151).
Figure 2.
Frequency distribution of frequency of years of cannabis use (N=151).
3.3. Was there an association between cannabis use during opioid dependence treatment and positive urine drug screens for opioids?
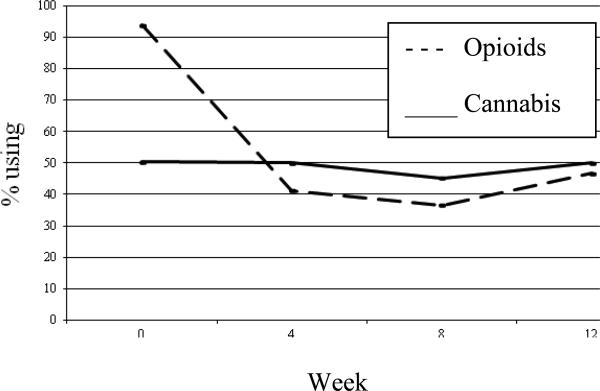
Cannabis use during treatment (assessed at weeks 4, 8, and 12) was examined and no association was found between concurrent cannabis use and opioid use (see Table 1). Participants were able to significantly reduce opioid use after initiating treatment before their level of opioid use stabilized; however, cannabis use was constant throughout (Figure 3). The primary outcome for the main trial was opioid use at baseline and weeks 4, 8, and 12 as measured by urine drug screens: 93.9% (n=113), 41.3% (n=104), 36.6% (n=93), and 46.7% (n=90) had positive urine drug screens at these four time points, respectively. Among all participants, most were stable over time: 30.4% were positive or missing and 28.7% were negative for opioids at all four time points. The percentage of urine drug screens positive for cannabis at these same time points were 50.4%, 50.0%, 45.2%, and 50.0%. Self-report data corroborated the urine drug screen data at baseline and weeks 4, 8, and 12 (data not shown).
Table 1.
Odds ratio (OR), and 95% confidence interval (CI), from logistic regression analysis of opioid use (throughout treatment) on cannabis use.
| Cannabis | OR | 95% CI | p |
|---|---|---|---|
| Age of initiation, years | .96 | .86-1.08 | .53 |
| Days used at baseline1 | .99 | .96-1.01 | .34 |
| Concurrent use2 | 1.56 | .86-2.80 | .14 |
Past 30 days, by self-report
Urine screen
Figure 3.
Prevalence of urine screens positive for opioids or cannabis over time (N=90-150).
We also evaluated whether a participant's change in opioid use over the course of the study was related to the change in that participant's cannabis use. We found that there was no association between opioid use over time and cannabis use over time (data not shown).
3.4. Is there an association between age of initiation of cannabis use and positive urine drug screens for opioids during treatment?
The median age for initiation of cannabis use was 15 years, with a range from 9-21 (mean=15.3, sd=2.9, n=147). Those who abstained from cannabis use during the past 30 days at baseline reported significantly fewer years of lifetime cannabis use than those who used cannabis during the past 30 days (mean=1.7 years, sd=2.0 vs. 4.9, sd=2.6; t (120.7) =8.26, p<.001); however, most cannabis abstainers at baseline (62%, n=31) had used cannabis for more than 5 months in the past.
Logistic regression models, using the GEE approach, assessed the effect of past cannabis use on opioid use during treatment. As shown in Table 1, the association between age of initiation of cannabis use and opioid use over time was not significant. Similarly, when age of initiation was dichotomized as <16 years vs. older, the association was not significant (data not shown).
3.5. Is there an association between cannabis use within the last 30 days and positive urine drug screens for opioids during treatment?
Cannabis use at baseline was measured by urine drug screens at intake and self-report of number of days used during the past month. Results of the regression analyses showed that cannabis use in the 30 days prior to baseline assessment was not related to opioid use over time, adjusted for treatment condition (see Table 1). Frequency of cannabis use over the past month was divided into 3 categories: abstinent, occasional (1-19 days), and regular use (≥20 days); frequency of cannabis use was not associated with opioid use during treatment.
4. DISCUSSION
In our sample of young people with opioid dependence receiving buprenorphine-naloxone maintenance, cannabis use was not associated with treatment outcome as measured by urine drug screens positive for opioids. Our findings support the findings of most previous studies in adults (Budney et al., 1998; Church et al., 2001; Epstein and Preston, 2003; Nirenberg et al., 1996; Saxon et al., 1993) and extend the work of Subramaniam et al., 2011. We examined cannabis use in three ways-- current use patterns, age of initiation of use, and recent history of use-- and none predicted opioid use treatment outcome in our sample. Problems associated with regular cannabis use such as cardiovascular (Mittleman et al., 2001) or respiratory complications (Tashkin, 2005), learning and memory deficits (Pope et al., 2001), and increased risk for the development of psychosis (Keupper et al., 2011; McGrath et al., 2010) are well-documented. However, many clinicians are unsure about whether cannabis use during treatment warrants a higher level of care for the patient. Of note, all analyses were adjusted for treatment condition, which was a consistently significant predictor of opioid use, as reported in the main outcomes paper (Woody et al. 2008); the current paper, however, is focused solely on the prognostic significance of cannabis use in this population.
The age of initiation of cannabis use did not have a significant effect on opioid-use outcomes among this sample of opioid-addicted adolescents and young adults. While other important studies have shown a relationship between early age of initiation of cannabis use and depression, executive function, and psychosis (de Graaf et al., 2010; Gruber et al., 2011; Large et al., 2011), in this sample, the age of initiation of cannabis use did not appear to be associated with opioid dependence treatment outcome. Our findings, given the relatively small sample size and the post-hoc nature of the analysis limited to self-report and urine drug screen data with no information on severity of use (i.e., cannabis abuse/dependence), do not offset the importance of effective education and intervention programs to prevent cannabis use during this critical developmental period.
Baseline cocaine use was associated with baseline cannabis use, but this association did not extend to the treatment period. This association at baseline supports data that shows opioids, cocaine, and marijuana are popular drugs among young people (Johnston et al., 2012). Other studies have shown cocaine and cannabis to be used frequently in young people receiving treatment for substance use disorders (Woody et al., 2008, Bracken et al., 2013). There was no relationship shown between cocaine use and cannabis use during buprenorphine treatment, however. A possible explanation for this is that patients in treatment may have been more committed to decreasing their use of other drugs.
Our investigation describes cannabis use patterns among a treatment-seeking, opioid-dependent sample. About half of the cannabis users in our sample were either using it daily or not at all. Interestingly, a few studies have found that intermittent cannabis use improved retention in naltrexone treatment for opioid dependence (Church et al., 2001, Raby et al., 2009). While cannabis use did not have an effect upon treatment outcome among our sample, even when examining three categories based upon frequency of use, this may be due in part to differences in the effects of opioid antagonists and opioid agonists on the cannabinoid system. Cannabis may mitigate dysphoria and other post-acute opioid withdrawal symptoms in patients receiving naltrexone pharmacotherapy for opioid dependence, thereby improving treatment retention. With buprenorphine possibly treating these symptoms for patients on agonist maintenance regimens, cannabis may have less of an impact on treatment retention in our sample.
Our results add to the growing literature on the effects of cannabis use during treatment for another substance use disorder. It is important that clinicians, when confronted with other drug use during treatment, know how to respond in an evidence-based fashion. While many potential problems may result from regular cannabis use, most studies, including ours, fail to show an association between cannabis use and opioid addiction treatment outcome. We continue to promote abstinence from all drugs, especially while in treatment, but our findings suggest that cannabis use while in treatment for opioid dependence is not necessarily an indicator that treatment is progressing poorly.
Role of the funding source
This study was supported by NIDA K99/R00 DA029115 (Kevin P. Hill, MD, MHS, PI), NIDA K24 DA022288 (Roger D. Weiss, MD, PI), NIDA CTN U10 DA15831 and the Adam Corneel Young Investigator Fellowship from McLean Hospital to Dr. Hill. The publications committee of the CTN gave final approval of the analysis and interpretation of the data and approved the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
All authors contributed to the conceptual design of the article and the analyses plan. HB and MG conducted all statistical analyses. KH, MG, and HB prepared the initial draft of the manuscript. All authors provided content expertise and critical feedback on the manuscript.
Conflict of Interest
All of the authors declare that they have no conflicts of interest.
REFERENCES
- Battisti RA, Roodenrys S, Johnstone SJ, Pesa N, Hermans DF, Solowij N. Chronic cannabis users show altered neurophysiological functioning on Stroop task conflict resolution. Psychopharmacology. 2010;212:613–624. doi: 10.1007/s00213-010-1988-3. [DOI] [PubMed] [Google Scholar]
- Blanco C, Alderson D, Ogburn E, Grant BF, Nunes EV, Hatzenbeuhler ML, Hasin DS. Changes in the prevalence of non-medical prescription drug use and drug use disorders in the United States: 1991-1992 and 2001-2002. Drug Alcohol Depend. 2007;90:252–260. doi: 10.1016/j.drugalcdep.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Bracken BK, Rodolico J, Hill KP. Sex, age, and progression of drug use in adolescents admitted for substance use disorder treatment in the northeastern United States: comparison with a national survey. Subst. 2013 doi: 10.1080/08897077.2013.770424. Abuse epub ahead of print 04 Feb 2013, DOI:10.1080/08897077.2013.770424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budney AJ, Bickel WK, Amass L. Marijuana use and treatment outcome among opioid-dependent patients. Addiction. 1998;93:493–503. doi: 10.1046/j.1360-0443.1998.9344935.x. [DOI] [PubMed] [Google Scholar]
- Church SH, Rothenberg JL, Sullivan MA, Bornstein G, Nunes EV. Concurrent substance abuse and outcome in combined behavioral and naltrexone therapy for opiate dependence. Am. J. Drug Alcohol Abuse. 2001;27:441–452. doi: 10.1081/ada-100104511. [DOI] [PubMed] [Google Scholar]
- Cook CCH. The Minnesota Model in the management of drug and alcohol dependency: miracle, method, or myth? Part I. The philosophy of the program. Br. J. Addict. 1988;83:625–634. doi: 10.1111/j.1360-0443.1988.tb02591.x. [DOI] [PubMed] [Google Scholar]
- de Graaf R, Radovanovic M, van Laar M, Fairman B, Degenhardt L, Aguilar-Gaxiola S, Bruffaerts R, de Girolamo G, Fayyad J, Gureje O, Haro JM, Huang Y, Kostychenko S, Lepine J, Matschinger H, Mora MEM, Neumark Y, Ormel J, Posada-Villa J, Stein DJ, Tachimori H, Wells JE, Anthony JC. Early cannabis use and estimated risk of later onset of depression spells: Epidemiologic evidence from the population-based World Health Organization World Mental Health Survey Initiative. Am. J. Epidemiol. 2010;172:149–159. doi: 10.1093/aje/kwq096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuPont RL, Saylor KE. Marijuana and benzodiazepine in patients receiving methadone maintenance treatment. JAMA. 1989;261:3409. [PubMed] [Google Scholar]
- Epstein DH, Preston KL. Does cannabis use predict poor outcome for heroin-dependent patients on maintenance treatment? A review of past findings, and more evidence against. Addiction. 2003;98:269–279. doi: 10.1046/j.1360-0443.2003.00310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber SA, Sagar KA, Dahlgren MK, Racine M, Lukas SE. Age of onset of marijuana use and executive function. Psychol. Addict. Behav. 2011;26:496–506. doi: 10.1037/a0026269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future National Results on Adolescent Drug Use: Overview of Key Findings, 2011. Institute for Social Research, The University of Michigan; Ann Arbor: 2012. [Google Scholar]
- Kempel P, Lampe K, Parnefjord R, Hennig J, Kunert HJ. Auditory-evoked potentials and selective attention: different ways of information processing in cannabis users and controls. Neuropsychobiology. 2003;48:95–101. doi: 10.1159/000072884. [DOI] [PubMed] [Google Scholar]
- Kuepper R, van Os J, Lieb R, Wittchen H, Hofler M, Henquet C. Continued cannabis use and risk of psychosis and persistence of psychotic symptoms: 10 year follow-up cohort study. BMJ. 2011 doi: 10.1136/bmj.d738. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Large M, Sharma S, Compton MT, Slade T, Nielssen O. Cannabis use and earlier onset of psychosis. Arch. Gen. Psychiatry. 2011;68:555–561. doi: 10.1001/archgenpsychiatry.2011.5. [DOI] [PubMed] [Google Scholar]
- Marsch LA, Bickel WK, Badger GJ, Stothart ME, Quesnel KJ, Stanger C, Brooklyn J. Comparison of pharmacological treatments for opioid-dependent adolescents. Arch. Gen. Psychiatry. 2005;62:1157–1164. doi: 10.1001/archpsyc.62.10.1157. [DOI] [PubMed] [Google Scholar]
- Mattick RP, Kimber J, Breen C, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid maintenance. Cochrane Database Syst. Rev. 2008;16:CD002207. doi: 10.1002/14651858.CD002207.pub3. [DOI] [PubMed] [Google Scholar]
- McGrath J, Welham J, Scott J, Varghese D, Degenhardt L, Hayatbakash MR, Alati R, Williams GM, Bor W, Najman JM. Association between cannabis use and psychosis-related outcomes using sibling pair analysis in a cohort of young adults. Arch. Gen. Psychiatry. 2010;67:440–447. doi: 10.1001/archgenpsychiatry.2010.6. [DOI] [PubMed] [Google Scholar]
- Mittleman MA, Lewis RA, Maclure M, Sherwood JB, Muller JE. Triggering myocardial infarction by marijuana. Circulation. 2001;103:2805–2809. doi: 10.1161/01.cir.103.23.2805. [DOI] [PubMed] [Google Scholar]
- Nirenberg TD, Liepman MR, Cellucci T, Swift RM, Sirota AD. Cannabis versus other illicit drug use among methadone maintenance patients. Psychol. Addict. Behav. 1996;10:222–227. [Google Scholar]
- Novaes MA, Guindalini C, Almedia P, Jungerman F, Bolla K, Laranjeira R. Cannabis use before age 15 is associated with poorer attention and executive function. Biol. Psychiatry. 2008;63:185–195. [Google Scholar]
- Pope HG, Gruber AJ, Hudson JI, Huestis MA, Yurgelun-Todd D. Neuropsychological performance in long-term cannabis users. Arch. Gen. Psychiatry. 2001;58:909–915. doi: 10.1001/archpsyc.58.10.909. [DOI] [PubMed] [Google Scholar]
- Pope HG, Gruber AJ, Hudson JI, Huestis MA, Cohane G, Huestis MA, Yurgelun-Todd D. Early-onset cannabis use and cognitive deficits: what is the nature of the association? Drug Alcohol Depend. 2003;69:303–310. doi: 10.1016/s0376-8716(02)00334-4. [DOI] [PubMed] [Google Scholar]
- Raby WF, Carpenter KM, Rothenberg J, Brooks AC, Jiang H, Sullivan M, Bisaga A, Comer S, Nunes EV. Intermittent marijuana use is associated with improved retention in naltrexone treatment for opiate-dependence. Am. J. Addict. 2009;18:301–308. doi: 10.1080/10550490902927785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxon AJ, Calsyn DA, Greenberg D, Blaes P, Haver VM, Stanton V. Urine screening for marijuana among methadone-maintained patients. Am. J. Addict. 1993;2:207–211. [Google Scholar]
- Subramaniam GA, Stitzer ML, Woody G, Fishman MJ, Kolodner K. Clinical characteristics of treatment-seeking adolescents with opioid versus cannabis/alcohol use disorders. Drug Alcohol Depend. 2009;99:141–149. doi: 10.1016/j.drugalcdep.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam GA, Warden D, Minhajuddin A, Fishman MJ, Stitzer ML, Adinoff B, Trivedi M, Weiss RD, Potter J, Poole SA, Woody GE. Predictors of abstinence: National Institute of Drug Abuse multisite buprenorphine/naloxone treatment trial in opioid-dependent youth. J. Am. Acad. Child Adolesc. Psychiatry. 2011;50:1120–1128. doi: 10.1016/j.jaac.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration . Treatment Episode Data Set (TEDS): 1996-2006. National Admissions to Substance Abuse Treatment Services, DASIS Series: S-43, DHHS Publication No. (SMA) 08-4347. 2008; Rockville, MD: 2008. [Google Scholar]
- Tashkin DP. Smoked marijuana as a cause of lung injury. Monaldi Arch. Chest Dis. 2005;63:92–100. doi: 10.4081/monaldi.2005.645. [DOI] [PubMed] [Google Scholar]
- Washton AM, Stone NS, Hendrickson EC. Cocaine abuse. In: Donovan DM, Marlatt GA, editors. Assessment of Addictive Behaviors. New York. Guilford Press; 1989. pp. 364–369. [Google Scholar]
- Wasserman DA, Weinstein MG, Havassy BE, Hall SM. Factors associated with lapses to heroin use during methadone maintenance. Drug Alcohol Depend. 1998;52:183–192. doi: 10.1016/s0376-8716(98)00092-1. [DOI] [PubMed] [Google Scholar]
- Woody GE, Poole SA, Subramaniam G, Dugosh K, Bogenschutz M, Abbott P, Patkar A, Publicker M, McCain K, Potter JS, Forman R, Vetter V, McNicholas L, Blaine J, Lynch KG, Fudala P. Extended vs. short-term buprenorphine-naloxone for treatment of opioid-addicted youth. JAMA. 2008;300:2003–2011. doi: 10.1001/jama.2008.574. [DOI] [PMC free article] [PubMed] [Google Scholar]