Abstract
The study aim was to explore the anatomy, histopathology, and molecular biological function of the fascias posterior to the interperitoneal colon and its mesocolon to provide information for improving complete mesocolic excision. To accomplish this aim, we performed intraoperative observations in 60 interperitoneal colon-cancer patients accepted for complete mesocolic excision and conducted local anatomy observations for five embalmed cadavers. An additional two embalmed child cadaver specimens were studied with large slices and paraffin sections. Ten of the 60 patients were examined with a lymph node tracer technique in vivo, while fresh specimens from these patients were assessed by histopathological examination and transwell cell migration assays in vitro. The anatomical and histopathological findings showed that the fascias posterior to the interperitoneal colon and its associated mesocolon were composed of two independent layers: the visceral and parietal fascias. These two fascias were primarily composed of collagen fibers, with the parietal fascia containing a small amount of muscle fiber. The in vivo test showed that the visceral fascia surrounded the colon and its associated mesocolon, including vessels and lymphatics, and that it had no lymphatic flow through it into the rear tissues. Moreover, the in vitro assays showed the visceral fascia was able to block tumor cell migration. Although many surgical scholars have known of the existence of fascia tissue posterior to the intraperitoneal colon, the detailed structure has been ignored and been unclear. As shown by our findings, the visceral and parietal fascias are truly formed structures that have not been previously reported. A thorough understanding of fascial structures and the function of the visceral fascia barrier in blocking tumor cells will facilitate surgeons when performing high-quality complete mesocolic excision procedures.
Keywords: anatomy, complete mesocolic excision, histopathology, interperitoneal colon, parietal fascia, visceral fascia
Introduction
As early as 100 years ago, Professor Jamieson proposed the following principles of radical surgery for colon cancer: resection of the lesion and cleaning of the regional lymph nodes to the vascular roots, a step that required dissection of the lymph nodes of the interperitoneal colon, mesocolon, and vascular roots. The achievement of this goal required sufficient excision of the bowels, complete resection of the mesocolon en-bloc, and central ligation of the vessels (Jamieson & Dobson, 1909). This surgical principle still applies to radical surgery for colon cancer. To some extent, simple bowel resection is technically easy to achieve, whereas ensuring dissection of the regional lymph nodes to the vascular roots requires certain skills. In consequence, the excision results achieved by different surgeons and operation techniques differ substantially. Currently, a number of inadequate resections continue to be performed for colon cancer. A recent study that analyzed 399 cases based on colon resection specimens found that less than one-third of operations achieved complete resection of the mesocolon plus central vascular ligation (CVL) (West et al. 2008). Moreover, significant differences were observed in the 5-year prognosis based on the effectiveness of the excision. The effects of the colon cancer resection were not as good as anticipated. One of the primary reasons was the use of the wrong surgical plane. An imprecise approach and plane often caused bleeding, mesocolic residue, and high ligation difficulties.
In 2009, Prof. Hohenberger proposed complete mesocolic excision (CME) as a standardized surgical technique, in which the same principle of total mesorectal excision (TME) in rectal cancer was to be applied to the colon (Hohenberger et al. 2009). He recommended that the operation be performed using a surgical plane based on embryonic anatomy – that is, a high-quality radical operation obtaining an optimal oncological pathological specimen, especially in the interperitoneal colon. A subsequent study showed that the rate of complete mesocolon and central ligation was more than 90% in CME, which was far more than in non-CME surgery (West et al. 2010). Although there is still controversy regarding this procedure, CME has set off a worldwide emphasis on the quality of colon surgery. Reasonable surgical technology should be applied on the basis of anatomical theory, especially for the interperitoneal colon. The proper realization of CME depends on an exact understanding of the interperitoneal colon visceral and parietal fascia. However, the present knowledge of these structures is still controversial because of a lack of systematic anatomical and histopathological evidence. The aims of the study were to confirm that these two fascial layers represent distinct layers and to characterize the barrier function of the intact visceral fascia.
Materials and methods
Anatomy study
This study was approved by our institutional ethics committee and meets the guidelines of the governmental agency responsible. Sixty adult patients suffering from cecum (n = 11; six males, five females), ascending (n = 42; 23 males, 19 females), and descending (n = 7; three males, four females) colon cancers received complete mesocolic excision at Peking University People's Hospital from August 2011 to December 2012, were consecutively enrolled. The median age of the patients was 68 years (range 42–90 years). According to the AJCC 7th tumor staging, patients with stage I (nine cases; five males, four females), stage II (24 cases; 10 males, 14 females), and stage III (27 cases; 17 males, 10 females) were included. All the patients received surgical techniques using CME with CVL as Prof. Hohenberger described (Hohenberger et al. 2009).
Anatomical observations were recorded during the operation and on the postoperative specimens. Intra-operative photographs were taken at various stages, as were photographs of the postoperative specimen. Observations were especially focused on the colon, mesocolon, the plane of the visceral and parietal fascia, as well as on the relevant peritoneal attachments.
Five embalmed cadavers (two male adults, one female adult, and two female children) were also studied. The anatomy, histology, and embryology laboratory at our university offered all the information about the cadavers included in the study. None of the cadavers had a history of abdominal or pelvic diseases or surgery and all had died of chest- or heart-related diseases. Using the CME technique, the gross anatomy with the retroperitoneal space opened was studied in these cadavers to observe and record the location, orientation, and connection of the pelvic peritoneum and fascia. Because the histopathology study described below needed child cadavers, we performed local anatomy studies on two child cadavers to verify the absence of differences between adults and children. The ages of the children at death (the two female children and the two additional cadavers) ranged from 2 to 3 years.
Histopathology study
All the postoperative specimens from the 60 patients underwent a histopathological examination, including gross morphometry and microscope investigations. To investigate the fascias around the entire colon and mesocolon at a finer level of detail, we performed evaluations with large slices. Because the texture of the colon and mesocolon was softer than the rectum, and because the adult specimens were too large to prepare slices, we used large slices from the child cadavers. An additional advantage was that the development of their structures was closer to the adults than the fetuses. The large specimens embedded by paraffin were easily broken during the slicing process because of insufficient hardness. Hence, we chose collodion embedding media. A long preparation time was often required, especially for larger embedded specimens. Since the earlier two child cadavers had undergone local anatomy studies, which could not be derived en-bloc, we selected another two child cadavers to make large slices by collodion embedding. The cadavers' entire colon and mesentery were removed en-bloc, as well as the surrounding tissue, deep into the posterior skeletal muscle, including the stomach and small intestine. After removal of the stomach and small intestine, the en-bloc specimen was separated into two parts along the superior mesenteric artery and inferior mesenteric artery; it was then anatomically dissected in transverse sections. The sections were 10–15 cm thick and embedded in both collodion and paraffin using partial tissues at the same time. The paraffin-embedded sections were selected to provide slices (5 μm in thickness) for histologic analysis with hematoxylin and eosin (HE), immunohistochemistry (SMA), and specific stains (van Gieson staining, Masson trichrome staining). After 3 months for the large-slice preparation, thick slices (30 μm) were generated from the collodion-embedded sections for histologic analysis with HE stain (Fig. 1).
Fig. 1.
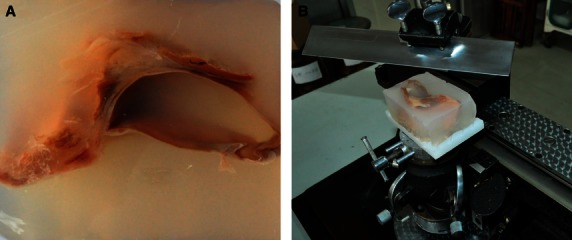
Celloidin embedding of the large tissue slices. (A) Specimen embedded within celloidin. (B) Generation of the sections at a thickness of 30 μm.
Barrier function study
Ten of the 60 patients consented to the lymph node tracer test. The median age of the patients was 64.5 years (range 47–86 years). There were seven ascending patients (four males, three females) and three descending patients (two males, one female). According to the AJCC 7th tumor staging, two stage I cases (one male, one female), three stage II cases (two males, one female), and five stage III cases (three males, two females) were included. One milliliter of nano-carbon lymphatic tracer (Carbon Nanoparticles Suspension Injection, Lummy Pharmaceutical Co., Ltd) was injected during surgery. After exposure of the operative field, 1 mL of tracer was injected into the subserosal layer around the tumor. Photographs of postoperative specimens were taken and the fresh specimens were subsequently processed for paraffin embedding. The paraffin slices (5 μm in thickness) were also analyzed with HE stain. The fresh tissues for the posterior lobe of the mesocolon were examined by transmission electron microscopy and transwell cell migration assays in vitro. For the transwell cell migration assays (transwell compartments, 24-well format, with 8-μm pore size), human colon epithelial adenocarcinoma SW480 and SW620 cells were used. Under sterile conditions, we took a small amount of visceral fascia from the adipose tissue and unfolded the fascia in the bottom of a transwell above the membrane. Each specimen was divided into three subgroups (blank control group, full-cover group, and semi-cover group) for simultaneous assay (Fig. 2). After 12 h, we counted the number of cells on the lower side of the filter under the microscope.
Fig. 2.
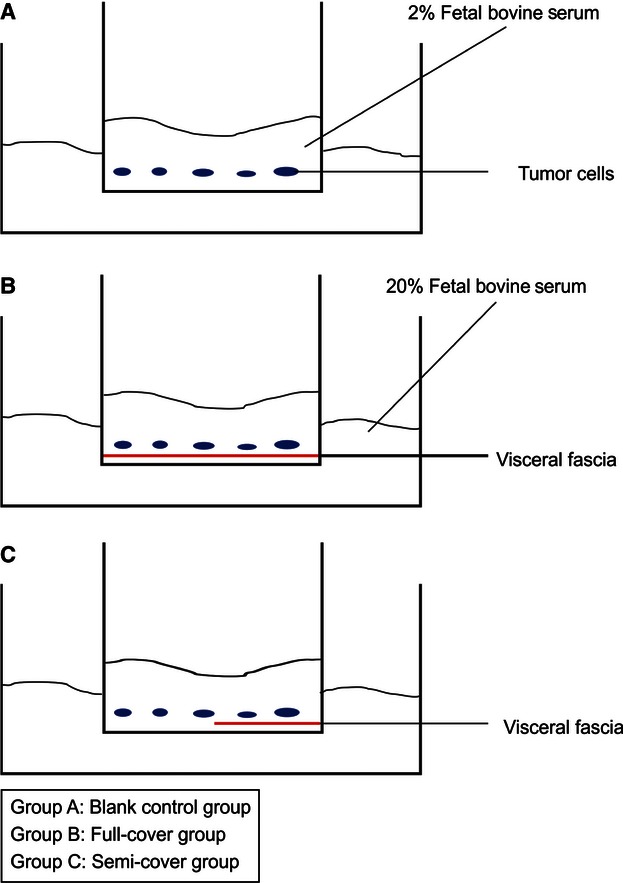
Procedure for the transwell cell migration assays.
Results
Anatomy study
The morphology and distribution of the interperitoneal colon fascias were almost the same in the adults and the children, except that the wall of colon was thinner in the children, especially for the muscularis propria.
Morphological characteristics of the interperitoneal colon fascia
A white line became apparent at the lateral peritoneal reflection as the mobilization approached (Figs 3 and 4). After the sharp incision at Toldt's line, the potential surgical plane formed between the mesocolon and the underlying retroperitoneum was easy to find. By a sharp dissection following the areolar tissue (‘angel's hair’) and complete mobilization of the entire mesocolon, the intact fascias were clearly seen, covering the posterior mesocolon (visceral fascia layer) and the retroperitoneal organs, including the vena cava, aorta, ureter, and genital vasculature (parietal fascia layer) (Figs 5 and 6). No vascular, lymphatic, or nerve distribution was evident in this plane. The visceral fascia became gradually denser laterally and inferiorly, and relatively thinner medially and superiorly. For the cadavers, the visceral and parietal fascias were separated successfully and were independent structures (Fig. 7).
Fig. 3.
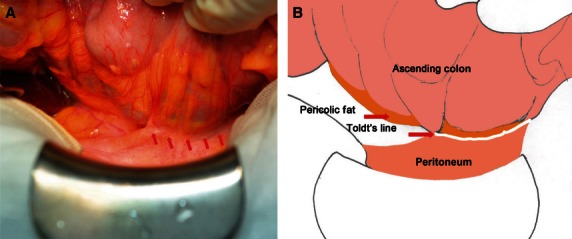
Operative approach of the ascending colon. (A) Somatoscopy photo. (B) Corresponding diagram.
Fig. 4.
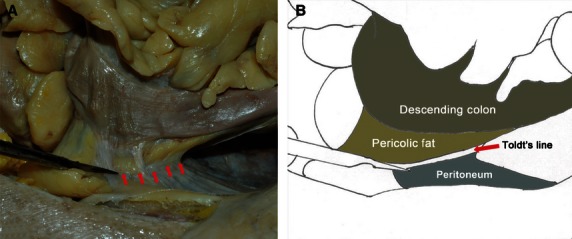
Operative approach of the descending colon. (A) Autopsy photo. (B) Corresponding diagram.
Fig. 5.
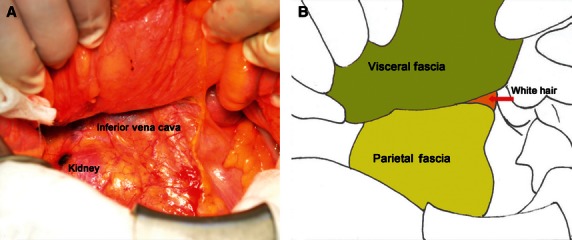
Fascia behind the ascending colon and mesocolon. (A) Somatoscopy photo. (B) Corresponding diagram.
Fig. 6.
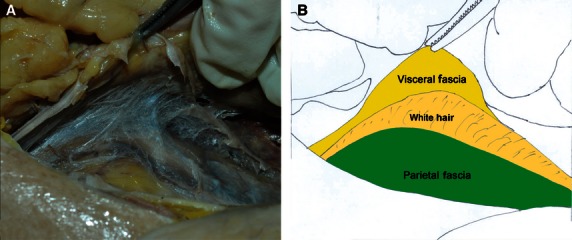
Fascia behind the descending colon and mesocolon. (A) Autopsy photo. (B) Corresponding diagram.
Fig. 7.
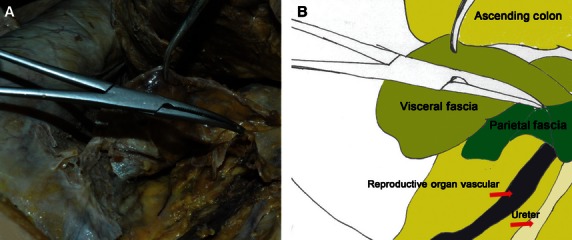
Regional anatomy for the visceral and parietal fascia of the ascending colon and mesocolon. (A) Autopsy photo. (B) Corresponding diagram.
Distribution characteristics of the interperitoneal colon fascia
The mesocolon was covered by the visceral fascia and peritoneum from both sides like an envelope. For the interperitoneal colon, the visceral fascia formed the posterior lobe of the mesocolon and the peritoneum formed the anterior lobe. The visceral fascia was bounded superiorly by the hepatic and splenic flexures, laterally by the lateral peritoneal folds, and was continuous between both sides. On the left side, the fascia extended inferiorly and became continuous with the fascia of the mesorectum. The parietal fascia covered all the retroperitoneal organs and was penetrated by the superior mesenteric vessels and the inferior mesenteric vessels (Fig. 8).
Fig. 8.
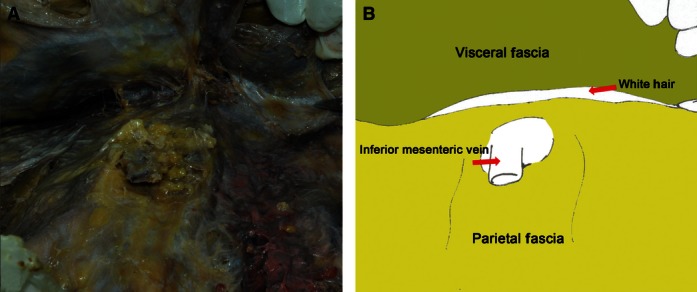
Regional anatomy for the visceral and parietal fascia of the colon and mesocolon. (A) Autopsy photo. (B) Corresponding diagram.
Histopathology study
The gross morphometry of the postoperative specimens showed intact, smooth, thin, and nearly transparent fascia (visceral fascia) covering the posterior of the interperitoneal colon and mesocolon (Fig. 9). For all the specimens, especially those of patients with nano-carbon lymphatic tracer injection, the lymph nodes and supply vasculature clearly appeared to be covered by the visceral fascia (Fig. 10). Observation thorough a microscope confirmed the finding that the peritoneum, mesocolic contents, visceral fascia, parietal fascia, and the organs were between the mesocolon and retroperitoneal organs, the same as behind the colon (Fig. 11). By viewing the operative field after CME, the parietal fascia covering the retroperitoneum was also found to be intact (Fig. 12).
Fig. 9.
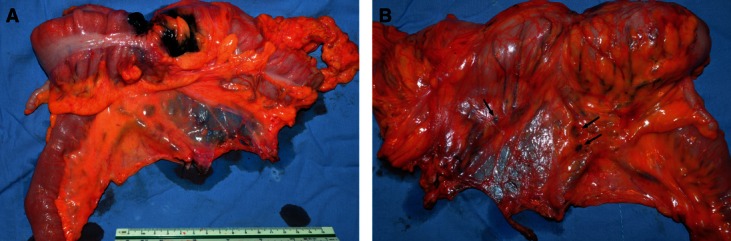
Resected specimen of ascending colon cancer after CME operation. (A) Front view. (B) Dorsal view. Arrows indicate black lymph nodes.
Fig. 10.
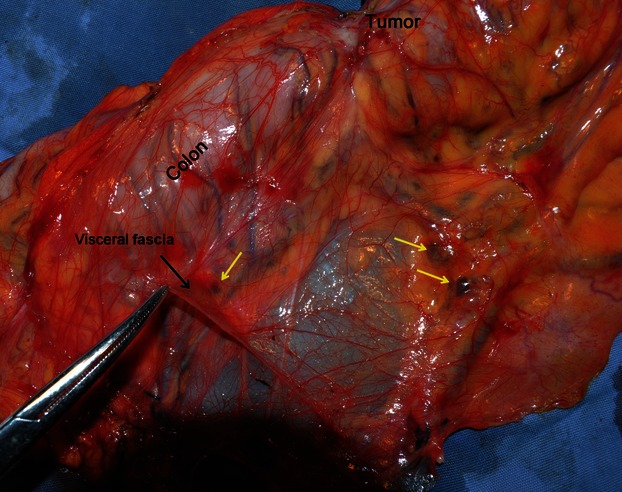
Resected specimen of ascending colon cancer after CME operation (magnification of Fig. 9B). The intact visceral fascia is in the dorsal view. The lymph nodes and supply vasculature are covered by the visceral fascia. The yellow arrows indicate black lymph nodes.
Fig. 11.
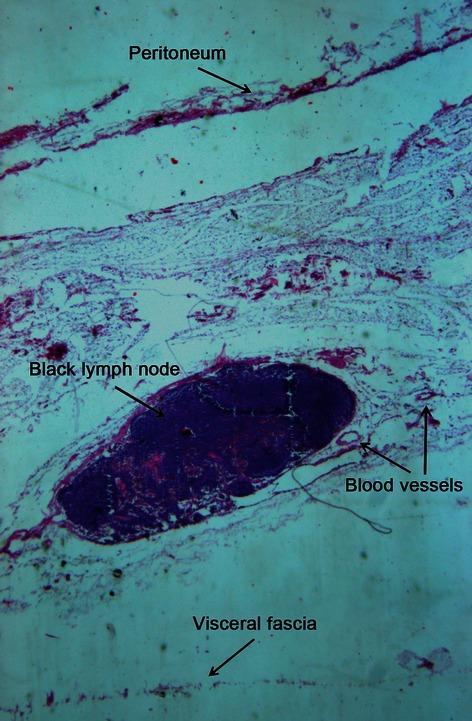
Paraffin section of the mesocolon. HE staining, 16×.
Fig. 12.
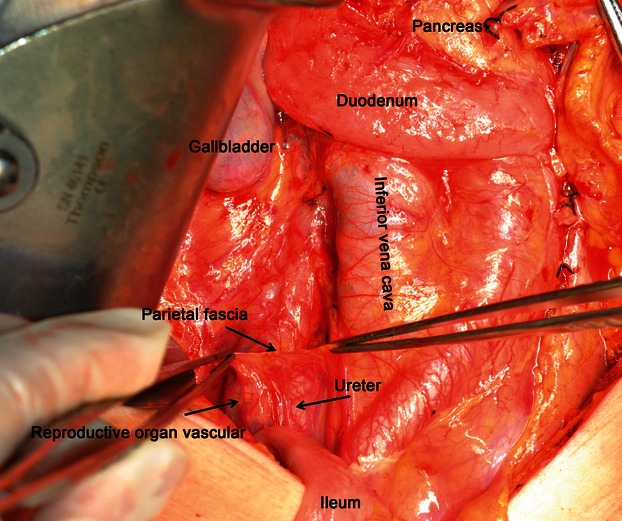
Operative field view afar removing the specimen. An intact parietal fascia in the posterior abdominal wall covers the ureter and reproductive organ vasculature. No black dye (nano-carbon tracer) was detected in the parietal fascia.
An examination of thick slices from the two embalmed male children showed the visceral and parietal fascias were two independent layers between the interperitoneal colon or mesocolon and the retroperitoneal organs (Fig. 13).
Fig. 13.
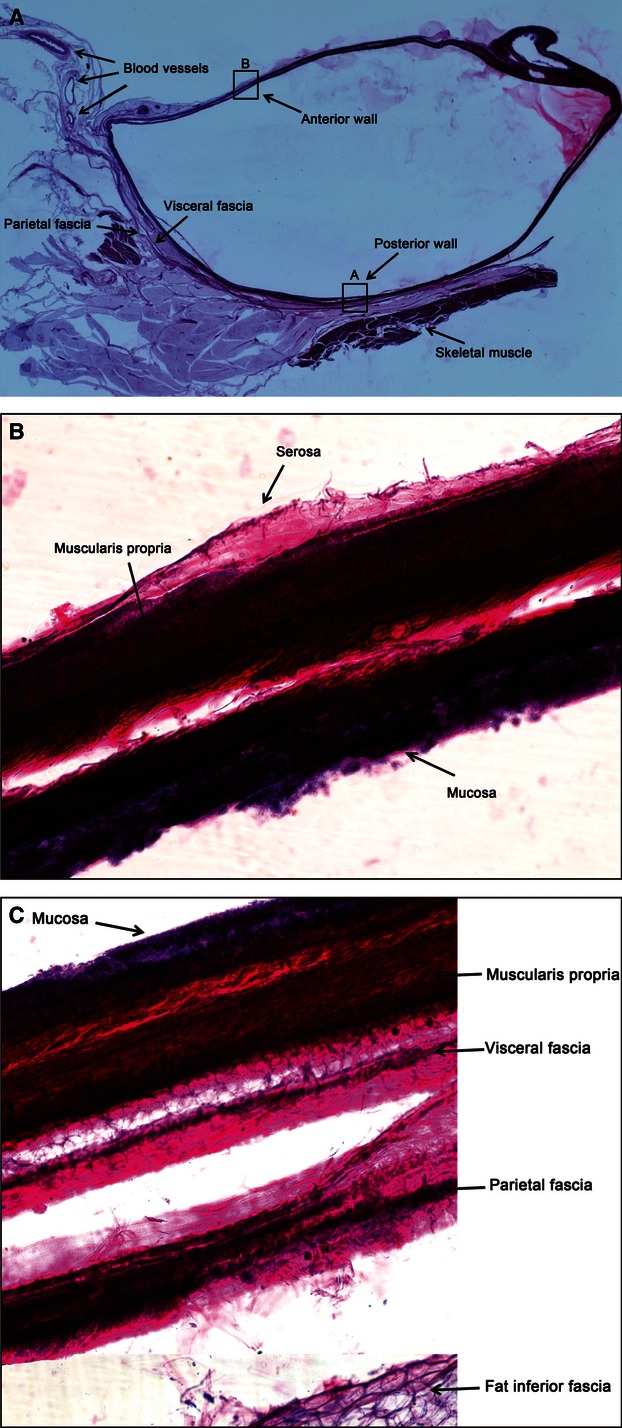
(A) A large tissue slice of the descending colon and mesocolon. HE staining, 1×. (B) A large tissue slice of the descending colon posterior wall. HE staining, 100×. In the posterior wall, the various tissue layers from the muscularis to the outside include the visceral fascia, parietal fascia, fat, etc. (C) A large tissue slice of the descending colon anterior wall. HE staining, 100×.
The sections were divided into the upper and lower parts at the level of the inferior pole of the kidney. The organization of the structures behind the interperitoneal colon and mesocolon were investigated under a microscope. Above the inferior pole of the kidney, the organizational structures included the peritoneum, the anterior and posterior walls of the colon, pericolonic adipose tissue, the visceral fascia and parietal fascia, perirenal fat, and the kidney from the ventral to dorsal side. For the lower part, the peritoneum, colon, pericolonic adipose tissue, visceral fascia, and adipose were behind the fascia, the anterior fascia on the muscle, and skeletal muscle (Fig. 14). By analysis with HE, immunohistochemical (SMA) and specific stains, the visceral fascia and parietal fascias were found to be in the colic adipose tissue and mainly consisted of collagen tissues, while some muscle fiber tissues could be seen in the parietal fascia (Fig. 14). The transmission electron microscopy findings also showed that the visceral fascias were denser laterally and inferiorly. The visceral fascia included a significant amount of collagen fiber tissue; moreover, more myofibroblasts were present in the denser areas (Fig. 15).
Fig. 14.
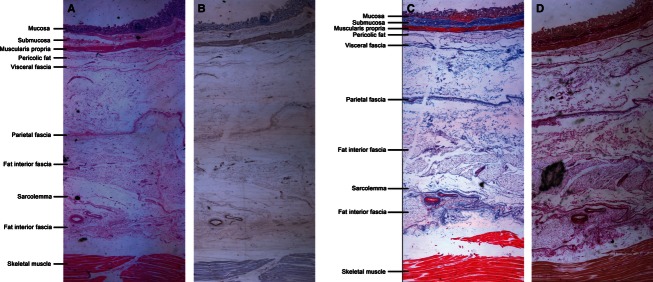
Paraffin sections of tissues behind the ascending colon posterior wall (A) HE staining. (B) SMA immunohistochemical staining (C) Masson staining. (D)VG staining. Magnification is 16×.
Fig. 15.
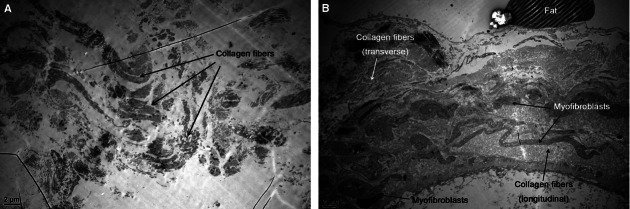
(A) Transmission electron microscopy images of the visceral fascia of the superior inside ascending mesocolon. The main component is collagen fibers. (B) Transmission electron microscopy images of the visceral fascia of the inferior outside ascending mesocolon. The visceral fascia also contains a large amount of collagen fibers, which are denser, and has many myofibroblasts.
Barrier function study
The specimens of all the patients with nano-carbon lymphatic tracer injection showed visceral fascia and peritoneum surrounding the colon and its mesocolon, including the vessels and lymphatics, the dyed lymph nodes, and supply vasculature. No nano-carbon black dye residue was present in the parietal fascia, and no lymphatic flow through the visceral fascia occurred into the rear tissues (Figs 10 and 12).
The transwell assay results showed a large number of the cells on the lower side of the filter in the blank control group, whereas no tumor cells migrated through the fascia into the lower compartment for the full-cover group after 12 h. A similar finding emerged in the semi-cover group. The tumor cells were able to migrate into the lower compartment on the lower side of the uncovered filter area but not the covered area (Fig. 16).
Fig. 16.

The number of SW480 cells on the lower side of the filter in the transwell assay (12 h). (A) Blank control group. (B) Semi-cover group. (C) Full-cover group. Crystal violet staining, small 100×, large 40×.
Discussion
In addition to keeping updated on the latest developments in the treatment and understanding of colorectal cancer, surgeons must thoroughly understand the relationship between the colorectum and its surrounding tissues, and skillfully identify the embryological origin and anatomic variations of these organs to maximize success in removing tumors and their related organization, and the retention of each piece of normal tissue. The visceral and parietal fascias have been identified and described in embryological studies. In 1982, Prof. Heald proposed TME as the standardized radical operation for rectal cancer, which involves a sharp dissection along embryological anatomical planes – namely, with a sharp separation of the visceral fascia from the parietal plane (Heald et al. 1982). Subsequent studies confirmed the presence and detailed structures of perirectal fascias (Fritsch, 1994; Nano et al. 1998; Diop et al. 2003), which provided most surgeons with a more intuitive understanding of TME and enabled them to perform higher quality operations. The survival rate for rectal cancer has improved and is even higher than that of colon cancer because of the development and global use of TME (Birgisson et al. 2005; Siegel et al. 2012). A similar process may now occur with the greater understanding of colon cancer treatment using CME. Prof. Hohenberger proposed the colon and its mesentery-like mesorectum are surrounded by visceral fascia (Fig. 17), with the sharp separation of the visceral fascia from the parietal plane leading to a surgical specimen with intact coverage, including the majority of the regional lymph nodes, the lymph vessels, and the surrounding fat tissue lying within the mesocolon.
Fig. 17.
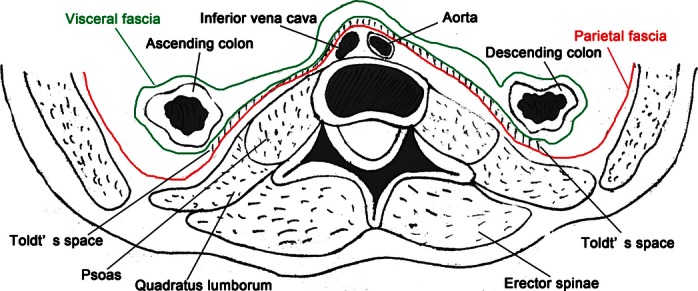
A diagram of the visceral and parietal fascia surrounding the colon and its associated mesocolon.
The classic anatomy definitions stated the interperitoneal colon had no mesocolon, since the posterior lobe of the mesocolon (visceral fascia) integrated with a deep structure (parietal fascia) during embryonic development. However, in the era of modern surgical anatomy, more attention has been paid to the sharp dissection of spaces composed of loose connective tissue following the embryological anatomical planes in the abdominal and pelvic cavity. As early as 100 years ago, Prof. Gerota described the presence of a prerenal fascia in the rear of the colonic adipose by examining frozen sections of corpses for the first time (Gerota, 1895). Subsequent studies by gross anatomy (Congdon, 1941; Lei et al. 1990; Molmenti et al. 1996), radiologic sectional anatomy (Somogyi et al. 1979; Feldberg et al. 1983; Lim et al. 1990), and space perfusion (Marks et al. 1986; Keelnad, 1987; Thornton et al. 2001; O'Connell et al. 2007) in corpses confirmed the existence of the prerenal fascia, and described it as continuing to the pelvis and covering the ureter. Although the above studies described the presence of a prerenal fascia, they did not describe its fine structure and stratification, or its relationship with the colon, which were not explored for a substantial length of time.
In the past 20 years, the rapid development of laparoscopic technology has resulted in better visualization of the anatomical structures in vivo during surgery, and has thereby led to meticulous anatomical surgery becoming a growing trend. Consequently, surgeons has increasingly observed the presence of loose reticular connective tissues between the interperitoneal colon and the prerenal fascia (Gerota's fascia) covering the ureters and kidney. The colon and its associated mesocolon could be resected completely by performing the operation at the level of this space. On the basis of observations during laparoscopic surgery, Zhang et al. (2011) described the fusion of fascia in the retrocolic spaces at the operative level, whereas with surgical separation this fascia would disappear.
Collectively, the findings of the current study showed that the original visceral and parietal fascias could be recovered by sharp separation, despite the fusion of these fascias during embryonic development. The fascia structure became composed of collagen fibers in the retrocolon and formed the posterior lobe of the mesocolon during this developmental period. This layer of the fascia and the preanal fascia constitute the retrocolic space, which is filled with loose connective tissue such as angel's hair, and can serve as a surgical level for CME. In vivo, this potential space needed sharp separation to restore the two-tier structures. Meanwhile, in the specimen slices, the visceral and parietal fascias were naturally stratified during the dehydration and fixation process. With the anatomy of the corpses, we found the left and right sides of the visceral fascia were continuous, whereas the parietal fascia covered the kidneys, ureters, and reproductive blood vessels and passed by the superior mesenteric vasculature and inferior mesenteric vasculature. Several other studies have also confirmed these results (Sato & Hashimoto, 1984; Anidjar et al. 1992).
The traditional anatomical view has considered the interperitoneal colon to have no mesocolon, whereas our study showed the visceral fascia (as the posterior lobe) constituted the mesocolon with the retroperitoneum. By nano-carbon lymph tracer tests, we found all the supply vessels and reflux lymphatic tissues were contained in the mesocolon, and no lymphatic vessel reached the rear tissues through the visceral fascia. Meanwhile, the in vitro transwell assays showed that the visceral fascia was a natural barrier to the migration of tumor cells. Therefore, surgery at this level of the visceral and parietal fascia could ensure the maximum possible removal of the regional lymph nodes. Due to the short follow-up, it was not possible to assess how much more successful CME was with respect to a lower recurrence rate and a better survival rate. A single-center prospective controlled clinical study on CME is ongoing at our center, registered in http://clinicaltrials.gov (SN. NCT01724775). Further studies will clarify these issues.
Concluding remarks
Fascial tissues have long been found to be present between the interperitoneal colon and the prerenal fat or the ureter. The surgical space or plane could be obtained by separating the fascia. Our study further confirmed that this fascia contains two separate layers (the visceral fascia and parietal fascia), with a hair-like fascia (or fusion fascia) between them. On the basis of our findings, we think that the visceral fascia functions as a natural barrier to tumor cells, which therefore cannot pass via the lymphatic vessels into the rear tissues. By fully understanding the structural characteristics of this area, the procedure for an intact excision of the mesocolon can be performed while maintaining the integrity of the visceral fascia; thus, any residual tumor tissue can be removed.
Acknowledgments
We thank the Committee of the Beijing Municipal Science and Technology Commission, the Capital Health Research and Development of Special (2011-4022-05), Clinical Application and Development of Capital Characteristic (Z111107058811046) and Peking University People's Hospital Research and Development Funds (RDB2012-19) of China for the financial support to finish this work. We wish to thank Professor Werner Hohenberger for his assistance in the preparation of this manuscript.
References
- Anidjar M, Delmas V, Villers A, et al. Endo-surgical dissection of the upper urinary tract through the retroperitoneal and transperitoneal route: an experimental study with pigs and cadavers. Prog Urol. 1992;2:592–603. [PubMed] [Google Scholar]
- Birgisson H, Talbäck M, Gunnarsson U, et al. Improved survival in cancer of the colon and rectum in Sweden. Eur J Surg Oncol. 2005;31:845–853. doi: 10.1016/j.ejso.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Congdon ED. The cone of renal fascia in the adult white male. Anat Rec. 1941;80:289–293. [Google Scholar]
- Diop M, Parratte B, Tatu L, et al. ‘Mesorectum’: the surgical value of an anatomical approach. Surg Radiol Anat. 2003;25:290–304. doi: 10.1007/s00276-003-0148-4. [DOI] [PubMed] [Google Scholar]
- Feldberg MA, Koehler PR, van Waes PF. Psoas compartment disease studied by computed tomography. Analysis of 50 cases and subject review. Radiology. 1983;148:505–512. doi: 10.1148/radiology.148.2.6867350. [DOI] [PubMed] [Google Scholar]
- Fritsch H. Topography and subdivision of the pelvic connective tissue in human fetuses and in the adult. Surg Radiol Anat. 1994;16:259–265. doi: 10.1007/BF01627680. [DOI] [PubMed] [Google Scholar]
- Gerota D. Beitrage zur Kenntnis des Befestigungsapparates der Niere. Arch Anat Entwicklungsgesch. 1895;19:265–286. [Google Scholar]
- Heald RJ, Husband EM, Ryall RD. The mesorectum in rectal cancer surgery – the clue to pelvic recurrence? Br J Surg. 1982;69:613. doi: 10.1002/bjs.1800691019. [DOI] [PubMed] [Google Scholar]
- Hohenberger W, Weber K, Matzel K, et al. Standardized surgery for colonic cancer: complete mesocolic excision and central ligation – technical notes and outcome. Colorectal Dis. 2009;11:354–364. doi: 10.1111/j.1463-1318.2008.01735.x. [DOI] [PubMed] [Google Scholar]
- Jamieson JK, Dobson JF. VII. Lymphatics of the colon: with special reference to the operative treatment of cancer of the colon. Ann Surg. 1909;50:1077–1090. doi: 10.1097/00000658-190912000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keelnad JB. Perirenal spaces: CT evidence for communication across the midline. Radiology. 1987;164:657–661. doi: 10.1148/radiology.164.3.3615863. [DOI] [PubMed] [Google Scholar]
- Lei QF, Marks SC, Jr, Touliopoulos P, et al. Fascial planes and compartments of the posterior abdomen: the perirenal and pararenal pathways. Clin Anat. 1990;3:1–15. [Google Scholar]
- Lim JH, Auh YH, Suh SJ, et al. Right perirenal space: computed tomography evidence of communication between the bare area of the liver. Clin Imaging. 1990;14:239–244. doi: 10.1016/0899-7071(90)90082-m. [DOI] [PubMed] [Google Scholar]
- Marks SC, Raptopoulos V, Kleinman P, et al. The anatomic basis for retrorenal extensions of pancreatic effusions: the role of the renal fascia. Surg Radiol Anat. 1986;8:89–97. doi: 10.1007/BF02421375. [DOI] [PubMed] [Google Scholar]
- Molmenti EP, Balfe DM, Kanterman RY, et al. Anatomy of the retroperitoneum: observations of the distribution of pathologic fluid collections. Radiology. 1996;200:95–103. doi: 10.1148/radiology.200.1.8657951. [DOI] [PubMed] [Google Scholar]
- Nano M, Levi AC, Borghi F, et al. Observations on surgical anatomy for rectal cancer surgery. Hepatogastroenterology. 1998;45:717–726. [PubMed] [Google Scholar]
- O'Connell AM, Duddy L, Lee C, et al. CT of pelvic extraperitoneal spaces: an anatomical study in cadavers. Clin Radiol. 2007;62:432–438. doi: 10.1016/j.crad.2006.11.012. [DOI] [PubMed] [Google Scholar]
- Sato T, Hashimoto M. Morphological analysis of the fascial lamination of the trunk. Bull Tokyo Med Dent Univ. 1984;31:21–32. [PubMed] [Google Scholar]
- Siegel R, Naishadham D, Jemal A. Cancer Statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- Somogyi J, Cohen WN, Omar MM, et al. Communication of right and left perirenal spaces demonstrated by computed tomography. J Comput Assist Tomogr. 1979;3:270–273. doi: 10.1097/00004728-197904000-00023. [DOI] [PubMed] [Google Scholar]
- Thornton FJ, Kandiah SS, Monkhouse WS, et al. Helical CT evaluation of the perirenal spaces and its boundaries: a cadaveric study. Radiology. 2001;218:659–663. doi: 10.1148/radiology.218.3.r01fe17659. [DOI] [PubMed] [Google Scholar]
- West NP, Morris EJ, Rotimi O, et al. Pathology grading of colon cancer surgical resection and its association with survival: a retrospective observational study. Lancet Oncol. 2008;9:857–865. doi: 10.1016/S1470-2045(08)70181-5. [DOI] [PubMed] [Google Scholar]
- West NP, Hohenberger W, Weber K, et al. Complete mesocolic excision with central vascular ligation produces an oncologically superior specimen compared with standard surgery for carcinoma of the colon. J Clin Oncol. 2010;28:272–278. doi: 10.1200/JCO.2009.24.1448. [DOI] [PubMed] [Google Scholar]
- Zhang C, Ding ZH, Yu HT, et al. Retrocolic spaces: anatomy of the surgical planes in laparoscopic right hemicolectomy for cancer. Am Surg. 2011;77:1546–1552. [PubMed] [Google Scholar]


