Abstract
The agouti (Dasyprocta aguti Linnaeus, 1766) is a wild rodent belonging to the family Dasyproctidae that is found throughout Brazil and feeds on fruits and seeds. The aim of the present study was to describe the following features of the tongue of agouti: its morphological structures, the three-dimensional characteristics of the lingual papillae surface, the connective tissue cores (CTCs) and the epithelial cell ultrastructure. Four types of papillae were observed on the dorsal surface of the tongue with a triangular shape: filiform, fungiform, foliate and vallate. Filiform papillae were distributed throughout the tongue surface, and removal of the epithelial surface revealed conical CTCs and multifilaments. Fungiform papillae were observed in the rostral and middle regions, whereas foliate papillae developed in pairs on the lateral margin of the caudal region. Removal of the epithelium in these regions revealed CTCs with parallel laminar conformation. Vallate papillae were arranged in a V-shape in the caudal region, and their CTCs ranged in shape from elongate to ovoid. The ultrastructural components of the dorsal epithelium were the basal, spinous, granular and keratinised layers. A broad area with cytoplasmic projections was identified in the interface region between the lamina propria and the basal layer. Flattened cells with intermediate filaments were observed in the transitional region between spinous and granular layers. The keratinised layer was composed of superimposed epithelial cells where desmosomes and cell-surface microridges were observed. These structural features, including the three-dimensional aspects of the lingual papillae, the CTCs and the epithelial ultrastructure, indicate that when compared with other animals, particularly other rodent species, the morphological features of the tongue of agouti are relatively well developed, especially regarding foliate and vallate papillae.
Keywords: connective tissue, electron microscopy, epithelium, lingual papillae, tongue
Introduction
The agouti is a wild rodent belonging to the family Dasyproctidae and the genus Dasyprocta. The geographical distribution of agouti extends from southern Mexico to northern Argentina; in Brazil, the specie is ubiquitous and feeds on a herbivorous diet of fruit pulp and seeds (Almeida et al. 2003; Lopes et al. 2004). This terrestrial rodent inhabits the Neotropical forests and contributes significantly to soil aeration and seed dispersal (Pimentel & Tabarelli, 2004).
The tongues of large and small mammals, in both terrestrial and aquatic ecosystems, have distinct morphological and functional features (Guimarães et al. 2011). The main function of the tongue is to facilitate feeding in conjunction with other structures of the oral cavity (Santos et al. 2011).
The morphological features of the tongue and its papillae vary widely among different mammalian species and reflect taxonomic evolution. The development and evolution of tongue structures may also be influenced by habitat (Yoshimura et al. 2007) and functional considerations, including the manipulation of food, swallowing, hygiene and vocalisation (Levin & Pfeiffer, 2002).
The aim of the present study was to describe the following features of the tongue of agouti: the structural morphology, the three-dimensional characteristics of the lingual papillae and their connective tissue cores (CTCs) upon removal of the epithelium, and the epithelial ultrastructure.
Materials and methods
Animals
The tongues of 10 male agoutis (Dasyprocta aguti, Linnaeus, 1766); Order: Rodentia and Suborder: Hystricomorpha (average body length 34 cm, body weight 1.9 kg, age 2.4 years) from the Wild Animal Breeding Centre (Centro de Multiplicação de Animais Silvestres) at the Federal Rural University of Semi-Árido (Universidade Federal Rural do Semi-Árido, UFERSA) were used. This research was authorised by the Ministry of the Environment (Ministério do Meio Ambiente, MMA) (32413-1 SISBIO) and approved by the Animal Ethics Committee of UFERSA (process no. 23091.1972/10).
Light microscopy
The tongues (n = 5) were fixed in a 10% formalin solution, dehydrated in increasing alcohol concentrations, embedded in paraffin and cut into 6-μm-thick sections. The direction planes used for sectioning were frontal sections to observe the structural features of the lingual papillae. To observe the foliate papillae, transversal sections were performed which resulted in perpendicular sections of the ridges. The sections were stained with haematoxylin-eosin (HE) and images were acquired using a light microscope (Carl Zeiss Microimaging; Axiokop 2, Göttingen, Germany).
Scanning electron microscopy
The tongues (n = 5) were immersed in modified Karnovsky's fixative (containing 2.5% glutaraldehyde and 2% paraformaldehyde in sodium phosphate buffer, pH 7.4) at 4 °C for 60 h (Ciena et al. 2011). The samples were washed in buffer solution and then allocated to each of the techniques described below. For the removal of the epithelial surface and analysis of the CTCs, samples were macerated in a 10% sodium hydroxide (NaOH) aqueous solution for 4 days at room temperature and maintained in distilled water with frequent changes for 3 days at 4 °C. The samples were postfixed in a 1% osmium tetroxide aqueous solution for 2 h at 4 °C, dehydrated in increasing alcohol concentrations and dried in a critical point dryer (Balzers CPD-030) with liquid CO2 (Duro et al. 2012; Bolina et al. 2013). The samples were mounted on metal stubs coated with gold ions (Balzers-040 SDC) and examined using a LEO 435 VP scanning electron microscope at the Department of Surgery at the School of Veterinary Medicine and Animal Science, University of São Paulo, São Paulo, Brazil.
Transmission electron microscopy
Dorsal epithelium tongue samples (n = 2) were used for the ultrastructural analysis, and 5-mm3 sections with the same orientation of the light microscopy of the epithelial layer were fixed with modified Karnovsky's solution (Ciena et al. 2012). The samples were postfixed in a 1% osmium tetroxide solution at 4 °C, immersed in 5% uranyl acetate aqueous solution at room temperature, dehydrated in increasing alcohol concentrations, immersed in propylene oxide and embedded in Spurr resin. Semithin sections (0.5 μm) were prepared using a Leica Ultracut® ultramicrotome, and then the area to be analysed was stained with 1% toluidine blue for detection. Ultrathin sections (60 nm) were subsequently prepared and collected in 200-mesh (EMS®) copper grids. These sections were contrasted using a 4% uranyl acetate saturated solution and a 0.4% lead citrate aqueous solution (Ciena et al. 2010; Pícoli et al. 2011). The grids were examined using a Morgagni 268D transmission electron microscope.
Results
Macroscopic overview
The macroscopic observations of the tongue of agouti show that the tongue was elongated 4.5 cm in the rostro-caudal direction and had a triangular shape with a narrowed middle region. The four types of papillae were observed on the dorsal surface of the tongue: filiform, fungiform, foliate and vallate papillae. They were distributed into three regions: rostral (anterior), middle and caudal (posterior). In the rostral region, the apex was present and there was also a median sulcus achieving the intermolar prominence. In the caudal region there were several lingual gland ducts. Well developed foliate papillae were situated in the lateral margin of the caudal region and four vallate papillae were observed in the caudal region surrounded by conical papillae. Filiform papillae were closely distributed over all dorsal surface and among these filiform papillae, numerous fungiform papillae were observed only in the rostral and middle regions (Fig. 1).
Fig. 1.
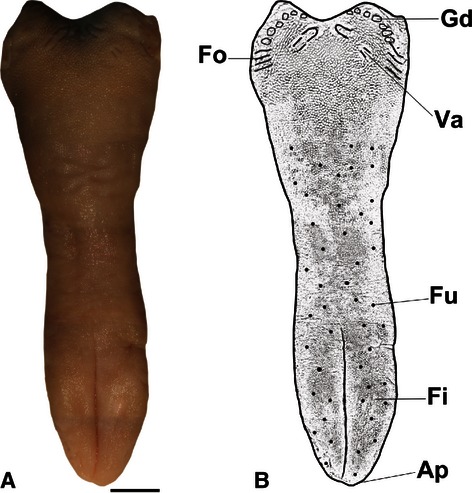
(A) Overview of the dorsal surface of the tongue of agouti, Scale bar: 0.5 cm. (B) Diagram of the tongue: Ap, apex; Fi, filiform papillae; Fu, fungiform papillae; Va, vallate papillae; Fo, foliate papillae; Gd, lingual gland ducts.
Microscopic observations
Light microscopy
The filiform papillae were short, conical and multifilamentous. Furthermore, these papillae were distributed throughout the tongue surface and were present in close proximity to adjacent papillae. The thick layer of epithelium with protrusions on the surface of the tongue contained only keratinised cells. However, no grooves were observed on the surface of the cornea cells. The lamina propria was generally narrow, vascularised and without lingual glands, and remained in contact with the epithelium throughout (Fig. 2A).
Fig. 2.
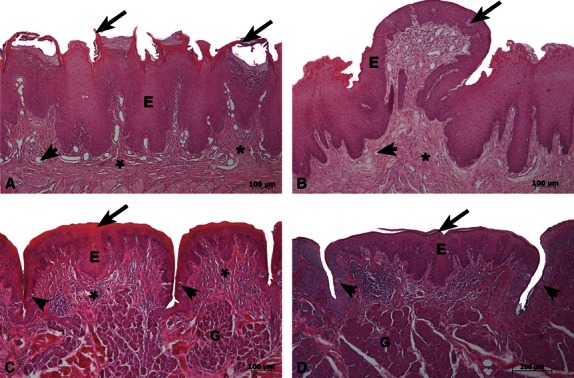
Frontal light micrographs of the tongue of agouti in (A,B,D) stained by HE and transversal micrograph in (C). (A) Filiform papillae were short, conical and bifid and were associated with the following features: a thick epithelial layer (E), superficial keratinised protrusions and processes (arrows), a thin lamina propria (*) and blood vessels (arrowhead). (B) Fungiform papillae had a thin keratinised layer at the epithelial surface (E). Taste buds (arrow) were present in the epithelial dorsal surface, and vascularisation (arrowhead) was observed in the thick lamina propria (*). (C) Foliate papillae had a thick keratinised layer (arrow) in the epithelium (E), and taste buds (arrowheads) were present in the groove walls. The lamina propria (*) had invaginations at the interface and Von Ebner-like glands (G). (D) Vallate papillae had a thin keratinised layer at the epithelial surface (arrow) and taste buds (arrowheads) at the groove walls. The lamina propria (*) had invaginations at the interface and numerous acini groups (Von Ebner-like glands) (G). Scale bars: 100 μm (A,B,C) and 200 μm (D).
The fungiform papillae exhibited a dome-like structure and heterogeneous distribution pattern in the rostral and middle regions. There was a thin layer on the stratum corneum, taste buds were present in the dorsal surface of the epithelium and were absent from the side walls. Some blood vessels were present in the lamina propria of these thick papillae (Fig. 2B).
The foliate papillae were found bilaterally on the lateral margin of the dorsal surface in the caudal region. Thick epithelial keratinised layers were observed and several taste buds were present on the marginal groove wall. The lamina propria had invaginations at the epithelium–connective tissue interface and Von Ebner-like glands (serous-rich lingual glands) located beneath (Fig. 2C).
The vallate papillae showed a deep groove (circumferential groove) in the caudal region. A thin keratinised layer was observed and taste buds were present on both groove walls. The lamina propria below the epithelium had invaginations at the epithelium–connective tissue interface and numerous acini, denominated Von Ebner-like glands (serous-rich lingual glands) (Fig. 2D).
Scanning electron microscopy
The triangular shape of the tongue of agouti was taken into consideration for the three-dimensional characterisation of the dorsal surface. Four vallate papillae arranged in a V-shape were observed in the caudal region; these papillae exhibited an elongated shape and had parallel interposing grooves (Fig. 3A,B). Lingual gland duct projections were also noted in this region (Fig. 3A). Numerous filiform and fungiform papillae were observed in the rostral and middle regions (Fig. 3C,E) and conical papillae in the caudal region (Fig. 3F). On the lateral dorsal surface of the caudal region, bilaterally well developed foliate papillae with 12 ridges separated by deep grooves were seen (Fig. 3D).
Fig. 3.
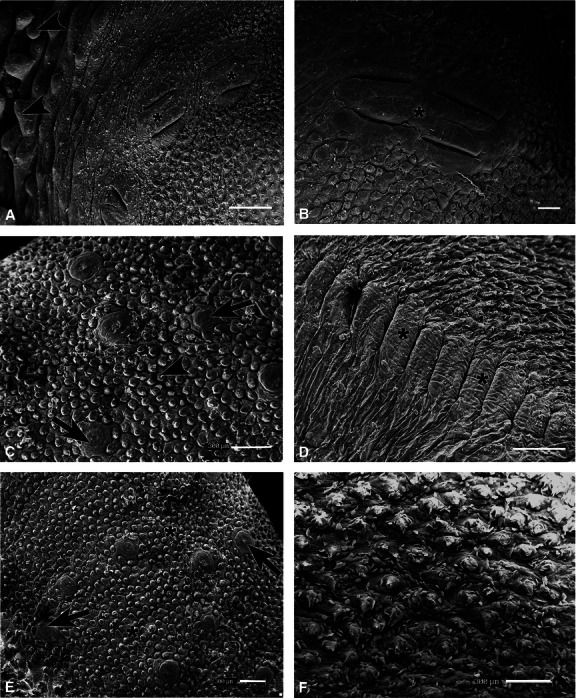
Scanning electron photomicrographs of the dorsal surface of the tongue of agouti. (A) Vallate papillae (*) and lingual glands ducts projections (arrowheads) were observed in the caudal region. (B) Elongated vallate papillae (*) bordered by interposing grooves at higher magnification. (C) Filiform (arrowhead) and fungiform (arrows) papillae in the middle region. (D) Well developed foliate papillae (*) composed of 12 pairs of ridges separated by deep grooves. (E) Three-dimensional characteristics of the rostral region, which had numerous filiform (*) and fungiform (arrows) papillae. (F) Filiform papillae in the caudal region. Scale bars: 1 mm (A,D) and 300 μm (B,C,E,F).
Removal of the epithelium revealed an arrangement of four vallate papillae in the caudal region, which were oval to elongated in shape and encircled by circumferential and parallel grooves surrounded by lingual gland ducts opening caudally and conical papillae opening rostrally (Fig. 4A,B). In the rostral and middle regions, the deep median sulcus and the CTCs of the papillae were seen (Fig. 4C). Among the filiform papillae (Fig. 4C,F) there were heterogeneously distributed volcano-like fungiform papillae (top concavity and running ridges in the lateral wall with protrusions in the upper part) (Fig. 4C,E). At a higher magnification, several apical branches in the upper quarter (multifilaments) of the filiform papillae CTCs were visible in the middle region (Fig. 4F). On the lateral dorsal surface of the caudal region, the CTC of the foliate papillae with thin parallel laminae were observed, and lingual gland duct openings were present in the grooves. Thin projections were seen in the upper part of the caudal surface, and collagen fiber leaflet bundles were found in the lower part of the tubercular organisation (Fig. 4D).
Fig. 4.
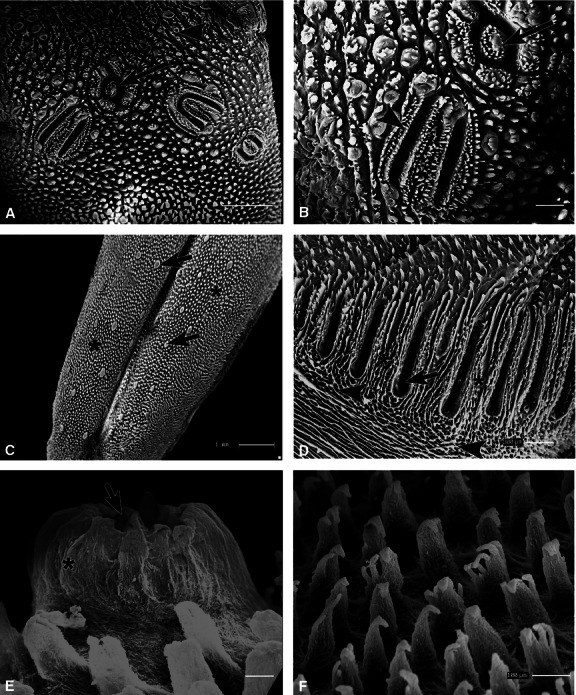
Scanning electron photomicrographs of the dorsal surface of the tongue of agouti treated with NaOH solution. (A) In the caudal region, CTCs of the vallate papillae (arrow) were observed near the lingual gland ducts (arrowhead). (B) Oval (arrow) and elongated (arrowhead) connective vallate papillae at higher magnification (C) CTCs in the rostral region with the arrangement of the filiform (*) and fungiform (arrows) papillae. (D) In the laminar layout of the foliate papillae CTC (*) below the trabecular tissue (arrowheads) and in the grooves, openings of the lingual gland ducts were observed (arrow). (E) In the middle region, the CTCs of the fungiform papillae with top concavity (arrow) and running ridges in the lateral wall with protrusions in the upper part (*) were seen. (F) Numerous CTC of filiform papillae were noted showing several apical branches in the upper quarter. Scale bars: 1 mm (A,C), 300 μm (B,D), 30 μm (E) and 100 μm (F).
Transmission electron microscopy
The dorsal epithelium of the tongue of agouti was composed of keratinised, granular, spinous and basal cell layers (Fig. 5). At the surface of the keratinised layer, superimposed cells arranged in parallel were found to be connected by numerous desmosomes and accentuated microridges in the cell surface (Fig. 5A,B). The features of the flattened cells in the granular layer were noted (Fig. 5C) and, at higher magnification, desmosomes and numerous intermediate filaments that mediate cell adhesion were observed (Fig. 5D). In the interface region between the lamina propria and the basal layer, a sizeable area of subepithelial connective tissue in close contact with adjacent regions was observed (Fig. 5E). This observation allowed the cytoplasmic projections that formed interdigitations with the spinous layer to be identified. The cytoplasm of the keratinocytes in the spinous layer and their volumous oval nuclei were also visible (Fig. 5F).
Fig. 5.
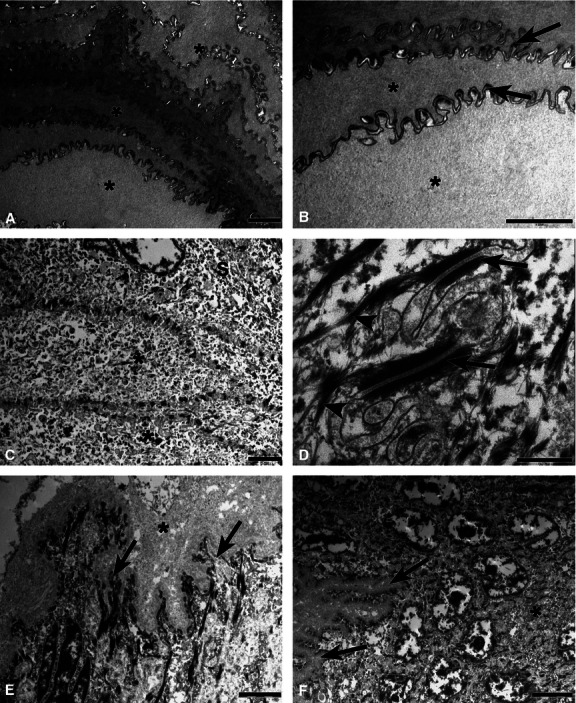
Transmission electron photomicrographs of the dorsal epithelium of the tongue of agouti. (A) In the keratinised layer, epithelial cells (*) were superimposed and arranged in parallel and connected by numerous desmosomes. (B) Epithelial cells (*) and the microridges present on the cell surface (arrows) at a higher magnification. (C) Flattened cells (*) were noted in the granular layer. (D) The desmosomes (arrows) and intermediate filaments (arrowheads) were observed at a higher magnification in the granular layer. (E) Interface region between the lamina propria and basal layer of the epithelium showing the dense collagen layer of the connective papillae (*) and their cytoplasmic projections (arrows). (F) The interdigitation (arrows) between the basal and spinous layers (*) at higher magnification. Scale bars: 2 μm (A,-C,E), 0.5 μm (D) and 5 μm (F).
Discussion
The findings of the present study characterise the following features of the tongue of agouti: the structural morphology, the three-dimensional characteristics of the lingual papillae, their connective tissue cores (CTCs) upon removal of the epithelium and the epithelial ultrastructure.
The tongue of the agouti is triangular in shape, and four types of papillae – filiform, fungiform, foliate and vallate – were observed on the dorsal surface with the median sulcus. The filiform papillae were distributed throughout the dorsal surface of the tongue, and removal of the epithelial surface revealed the conical shape of the filiform papillae CTCs and their multifilaments, as also described by Branco et al. (2011). According to Pastor et al. (2008), the filiform papillae are the most abundant papillae on the tongue and mechanically promote mastication and food ingestion. The morphological features described in the filiform papillae and the CTCs are similar to those seen previously in rodents such as rats (Iino & Kobayashi, 1988) and guinea pig (Kobayashi, 1990). Moreover, Silva et al. (2002) observed, after epithelial removal of the filiform papillae of the lingual prominence, apical branches similar to those of the tongue of agouti. According to Nonaka et al. (2008), the filiform papillae show an evolutive progression, as they become more complex in more developed species.
Fewer fungiform papillae were observed than filiform papillae and they were restricted to the rostral and middle regions. These observations are similar to findings in the tongues of other rodents such as the flying squirrel (Emura et al. 1999), mice (Qin et al. 2010), blind mole rat (Kilinc et al. 2010) and bank vole (Jackowiak & Godynicki, 2005). The CTC aspects revealed the characteristic volcano-like fungiform papillae; other authors have used different nomenclature to describe these papillae: fist-like in the guinea pig (Kobayashi, 1990), flower bud-shaped in the Patagonian cavy (Emura et al. 2011) and bud-like shaped in the capybara (Emura, 2008).
Well developed foliate papillae were distributed on the margin of the dorsal surface of the caudal region, composed of 12 ridges separated by deep grooves with numerous taste buds present on the groove walls. After removal of the epithelium, CTCs of the foliate papillae were revealed, laminar and parallel in shape, as well as salivary gland openings. Silva et al. (2002) described similar structural features in the rabbit, where foliate papillae CTCs were also laminar and parallel in shape.
According to Emura et al. (2011) the foliate papillae are well developed in the Order Rodentia, their arrangement and the number of ridges varying depending on the developmental stage and species. Both the localisation and the structure of these papillae are similar to those in the bank vole (Jackowiak & Godynicki, 2005), mouse (Kobayashi et al. 1989), rat (Iino & Kobayashi, 1988) and gerbil (Grandi et al. 1994). However, the number of ridges in some species may be higher, as in the flying squirrel, which has about 34 ridges in each foliate papillae (Emura et al. 1999).
Four vallate papillae arranged in a V-shape were observed in the caudal region, with numerous taste buds present in the walls of the deep groove. Removal of the epithelium revealed that the CTCs of the vallate papillae were elongated to oval in shape. Vallate papillae have been described widely in rodents, the number and morphology these papillae differing according to the species.
The quantity of vallate papillae in rodents (rat) are reduced according to Iino & Kobayashi (1988), who reported one papilla centrally disposed in the caudal region, similar to the mouse (Kobayashi et al. 1989) and bank vole (Jackowiak & Godynicki, 2005). The guinea pig (Kobayashi, 1990), rabbits (Silva et al. 2002), blind mole rats (Kilinc et al. 2010), Patagonian cavy (Emura et al. 2011) and porcupine (Atalar & Karan, 2011) show two papillae. The flying squirrel (Emura et al. 1999) and American beaver (Shindo et al. 2006) have three papillae, whereas in the present study, four vallate papillae were described with a distinct morphology. The morphological studies of the lingual papillae of rodents, suborder: Hystricomorpha, are limited and recent descriptions concerned the vallate papillae of porcupines. As reported by Atalar & Kalan (2011), porcupines has two vallate papillae located in the caudal region and their structural aspect does not resemble that of the vallate papillae of agouti.
Ultrastructurally, the dorsal epithelium of the tongue was composed of basal, spinous, granular and keratinised cell layers. In the interface region between the lamina propria and the basal layer, there was a sizeable area of subepithelial connective tissue whose cytoplasmic projections formed interdigitations. Flattened cells were present in the transitional region between the spinous and granular layers, and intermediate filaments were observed. Lopes et al. (2009) characterised the ultrastructural features of the epithelial layers of female rat tongue along with the changes that occur with aging. In the present study, the desmosomes and cell surface ridges of epithelial cells at the surface of the keratinised layer were found to contribute to the superimposed and parallel arrangement of these cells. Casteleyn et al. (2010) have reported that the keratinised surface of the lingual tonsil epithelium of sheep is composed of several layers, with the upper layers having apical protrusions and a greater degree of keratinisation. According to Iwasaki (2002), the keratinisation of the lingual epithelium may have been concomitant with the evolution of amniotes.
Concluding remarks
The results from this study showed the structural, three-dimensional features of the lingual papillae, the CTCs and the epithelial ultrastructure and indicate that the morphological features of the tongue of agouti are relatively well developed, especially as regards foliate and vallate papillae, compared with those in other animals, particularly other rodent species.
Conflict of interest
The authors declare that there is no conflict of interest with this manuscript.
References
- Almeida MM, Carvalho MAM, Cavalcante Filho MF, et al. Morfological and morfometric study of the ovary in agoutis (Dasyprocta aguti, Linnaeus, 1766) Braz J Vet Res Anim Sci. 2003;40:55–62. [Google Scholar]
- Atalar O, Meryem Karan. The light and scanning electron microscopic structure of the papila vallate in the porpucine (Hystric cristata. J Anim Vet Adv. 2011;10:3069–3073. [Google Scholar]
- Bolina CS, Bolina-Matos RS, Alves PHM, et al. Three-dimensional aspects of the structural characteristics and kidney angioarchitecture of adult and aged Wistar rats: a scanning electron microscopy study. Microsc Res Tech. 2013;76:538–544. doi: 10.1002/jemt.22197. [DOI] [PubMed] [Google Scholar]
- Branco E, Guimarães JP, Miglino MA, et al. Ultrastructural aspects of lingual papillae in squirrel monkey (Saimiri sciureus. Microsc Res Tech. 2011;74:484–487. doi: 10.1002/jemt.20935. [DOI] [PubMed] [Google Scholar]
- Casteleyn C, Cornelissen M, Simoens P, et al. Ultramicroscopic examination of the ovine tonsillar epithelia. Anat Rec (Hoboken) 2010;293:879–889. doi: 10.1002/ar.21098. [DOI] [PubMed] [Google Scholar]
- Ciena AP, Luques IU, Dias FJ, et al. Ultrastructure of the myotendinous junction of the medial pterygoid muscle of adult and aged Wistar rats. Micron. 2010;41:1011–1014. doi: 10.1016/j.micron.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Ciena AP, Almeida SRY, Alves PHM, et al. Histochemical and ultrastructural changes of sternomastoid muscle in aged Wistar rats. Micron. 2011;42:871–876. doi: 10.1016/j.micron.2011.06.003. [DOI] [PubMed] [Google Scholar]
- Ciena AP, Almeida SRY, Dias FJ, et al. Fine structure of myotendinous junction between the anterior belly of the digastrics muscle and intermediate tendon in adults rats. Micron. 2012;43:258–262. doi: 10.1016/j.micron.2011.08.009. [DOI] [PubMed] [Google Scholar]
- Duro CC, Ciena AP, Almeida SR, et al. Qualitative study of young, adult, and aged Wistar rats temporomandibular synovial membrane employing light, scanning, and transmission electron microscopy. Microsc Res Tech. 2012;75:1522–1527. doi: 10.1002/jemt.22095. [DOI] [PubMed] [Google Scholar]
- Emura S. SEM study of the lingual papillae and their connective tissue cores of the capybara. Med Biol. 2008;152:386–393. [Google Scholar]
- Emura S, Tamada A, Hayakawa D, et al. SEM study on the dorsal lingual surface of the flying squirrel, Petaurista leucogenys. Ann Anat. 1999;181:495–498. doi: 10.1016/S0940-9602(99)80033-8. [DOI] [PubMed] [Google Scholar]
- Emura S, Okumura T, Chen H. Morphology of the lingual papillae in the Patagonian cavy. Okajimas Folia Anat Jpn. 2011;88:121–125. doi: 10.2535/ofaj.88.121. [DOI] [PubMed] [Google Scholar]
- Grandi D, Arcari ML, Azalli G. Ultrastructural aspects of the lingual papillae in the gerbil (Meriones unguiculatus. Ital J Anat Embryol. 1994;99:201–217. [Google Scholar]
- Guimarães JP, Mari Rde B, Marigo J, et al. Light and scanning electron microscopic study of the tongue in the estuarine dolphin (Sotalia guianensis van Bénéden, 1864) Zool Sci. 2011;28:617–622. doi: 10.2108/zsj.28.617. [DOI] [PubMed] [Google Scholar]
- Iino T, Kobayashi K. Morphological studies on the lingual papillae and their connective tissue papillae of rats. Shigaku. 1988;75:1039–1060. [PubMed] [Google Scholar]
- Iwasaki S. Evolution of the structure and function of the vertebrate tongue. J Anat. 2002;201:1–13. doi: 10.1046/j.1469-7580.2002.00073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackowiak H, Godynicki S. The distribution and structure of the lingual papillae on the tongue of the bank vole Clethrinomys glareolus. Folia Morphol. 2005;64:326–333. [PubMed] [Google Scholar]
- Kilinc M, Erdogan S, Ketani S, et al. Morphological study by scanning eléctron microscopy of the lingual papillae in the Middle East blind mole rat (Spalax ehrenbergi, Nehring, 1898) Anat Histol Embryol. 2010;39:509–515. doi: 10.1111/j.1439-0264.2010.01022.x. [DOI] [PubMed] [Google Scholar]
- Kobayashi K. Three-dimensional architecture of the connective tissue core of the lingual papillae in the guinea pig. Anat Embryol. 1990;182:205–213. doi: 10.1007/BF00185514. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Miyata K, Takahashi K, et al. Three-dimensional architecture of the connective tissue papillae of the mouse tongue as viewed by scanning electron microscopy. Kaibogaku Zasshi. 1989;64:523–538. [PubMed] [Google Scholar]
- Levin MJ, Pfeiffer CJ. Gross and microscopic observations on the lingual structure of the Florida Manatee Trichechus manatus latirostris. Anat Histol Embryol. 2002;31:278–285. doi: 10.1046/j.1439-0264.2002.00387.x. [DOI] [PubMed] [Google Scholar]
- Lopes JB, Cavalcante RR, Almeida MM, et al. Performance of agouti (Dasyprocta prymnolopha) bred in captivity according to sex and parturition in Teresina, Piauí. Rev Brasil Zootec. 2004;33:2318–2322. [Google Scholar]
- Lopes MG, Watanabe IS, Soares LE, et al. Ultrastructural aspects of female aging Wistar rat epithelium tongue: a HRSEM and TEM study. Gerontology. 2009;55:442–448. doi: 10.1159/000216829. [DOI] [PubMed] [Google Scholar]
- Nonaka K, Zheng JH, Kobayashi K. Comparative morphological study on the lingual papillae and their connective tissue cores in rabbits. Okajimas Folia Anat Jpn. 2008;85:57–66. doi: 10.2535/ofaj.85.57. [DOI] [PubMed] [Google Scholar]
- Pastor JF, Barbosa M, de Paz FJ. Morphological study of the lingual papillae of the giant panda (Ailuropoda melanoleuca) by scanning electron microscopy. J Anat. 2008;212:99–105. doi: 10.1111/j.1469-7580.2008.00850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pícoli LC, Dias FJ, Issa JPM, et al. Ultrastructure of submandibular salivary glands of mouse: TEM and HRSEM observations. Microsc Res Tech. 2011;74:1154–1160. doi: 10.1002/jemt.21008. [DOI] [PubMed] [Google Scholar]
- Pimentel SP, Tabarelli M. Seed dispersal of Palm Attalea oleifera in a remnant in the Brazilian Atlantic Forest. Biotropica. 2004;36:74–84. [Google Scholar]
- Qin YM, Shi JQ, Zhang GH, et al. A reliable method to obtain cells of taste buds from fungiforme papillae of mice. Acta Histochem. 2010;112:107–112. doi: 10.1016/j.acthis.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Santos TC, Fukuda KY, Guimarães JP, et al. Light and scanning electron microscopy study of the tongue in Rhea americana. Zoolog Sci. 2011;28:41–46. doi: 10.2108/zsj.28.41. [DOI] [PubMed] [Google Scholar]
- Shindo J, Yoshimura K, Kobayashi K. Comparative morphological study on the stereo-structure of the lingual papillae and their connective tissue cores of the American beaver (Castor canadensis. Okajimas Folia Anat Jpn. 2006;82:127–137. doi: 10.2535/ofaj.82.127. [DOI] [PubMed] [Google Scholar]
- Silva MC, Watanabe I, Kronka MC. Three-dimensional architecture of the connective tissue core and surface structures of the lingual papillae in the rabbit. Histol Histopathol. 2002;17:455–461. doi: 10.14670/HH-17.455. [DOI] [PubMed] [Google Scholar]
- Yoshimura K, Shindo J, Miyawaki Y, et al. Scanning electron microscopic study on the tongue and lingual papillae of the adult Spotted Seal, Phoca largha. Okajimas Folia Anat Jpn. 2007;84:83–98. doi: 10.2535/ofaj.84.83. [DOI] [PubMed] [Google Scholar]


