Abstract
The insulin-like growth factor (IGF) family and the IGF-1 receptor (IGF-1R) play an important role in cancer. This intricate and complex signaling pathway provides many opportunities for therapeutic intervention, and several novel therapeutics aimed at the IGF-1R, particularly monoclonal antibodies and small molecule tyrosine kinase inhibitors, are under clinical investigation. This article provides a patent overview of the IGF signaling pathway and its complexity, addresses the justification for the use of IGF-1R-targeted therapy, and reviews the results of in vivo and in vitro novel therapeutics. Over the past year, the completion of several phase I, II, and III trials have provided interesting new information about the clinical activity of these novel compounds, particularly CP-751,871, IMC-A12, R1507, AMG-479, AVE-1642, MK-0646, XL-228, OSI-906, and BMS-754807. We review the important preliminary results from clinical trials with these compounds and conclude with a discussion about future therapeutic efforts.
Keywords: cancer, clinical trials, insulin-like growth factor, insulin receptor, IGF-1R, IGF-1R inhibitor, monoclonal antibody, targeted therapy, tyrosine kinase inhibitor
1. Introduction
The insulin like growth factor (IGF) family comprises a complex molecular signaling pathway, now well-recognized as an important factor in oncogenesis, disease progression, metastasis, and chemoresistance. Involvement of the IGF pathway—particularly the receptor IGF-1R—has been documented in many malignancies [1], including colorectal, breast, pancreatic, lung, head and neck, prostate, renal, ovarian, and endometrial cancer, as well as sarcomas [2-11]. IGF-1R has become the target of novel therapeutics, specifically monoclonal IGF-1R antibodies, and small molecules targeting inhibition of the IGF-1R tyrosine kinase, as reviewed by Hewish et al. [12]. In this review, we will discuss recent preliminary results from clinical trials with a few promising compounds, with a focus on efficacy and toxicity, and conclude with a discussion about future therapeutic efforts, including targeted therapy and predictive biomarkers.
1.1 The IGF Family
The IGF signaling system family itself is composed of the three ligands IGF-1, IGF-2, and insulin; three cell membrane receptors—type 1 and 2 IGF receptors (IGF-1R and IGF-2R) and the insulin receptor (IR); seven high-affinity binding proteins (IGFBP 1-7); and several associated proteins, namely IRS and shc. These lead to two main signaling cascades: the PI3K/Akt and Ras/Raf/MEK/ERK pathways, which ultimately result in suppressed apoptosis, cell proliferation and invasion, and enhanced cell survival.
The IGF-1R is a heterotetrameric transmembrane receptor, composed of two α and two β subunits linked by disulfide bonds. The extracellular α subunits are responsible for ligand binding, while the transmembrane β subunits contain tyrosine kinase and C-terminal domains [13]. Although the IGF-2 receptor participates in ligand binding, the absence of a tyrosine kinase domain prevents participation in the signaling cascade [14]. IGF-2R thereby scavenges circulating IGF-2 and may in fact act as a tumor suppressor [15]. The IGF-1R is structurally related to the insulin receptor, with 60% homology in its amino acid sequence [16], allowing formation of hybrid receptors (IGF-1R/IR-A or IGF-1R/IR-B) [17]. Hybrid receptors can bind both IGF ligands and act as mitogens, especially IR-A, which is overexpressed in several cancers [18, 19]. IGF-1 binds IGF-1R with the greatest affinity, yet both IGF-2 and insulin may bind IGF-1R (IGF-2 with much greater affinity than insulin).
The IGF-binding proteins serve to modulate the bioactivities of the circulating IGFs and bind with differing affinities [20]. The IGFBPs may inhibit the signaling pathway by binding circulating IGFs, thus blocking receptor binding, but they may also maintain IGFs in circulation and lengthen their circulating half-life [21]. In this sense, they perform dual but opposing roles and concomitantly regulate IGF function.
1.2 Activation and Signal Transduction
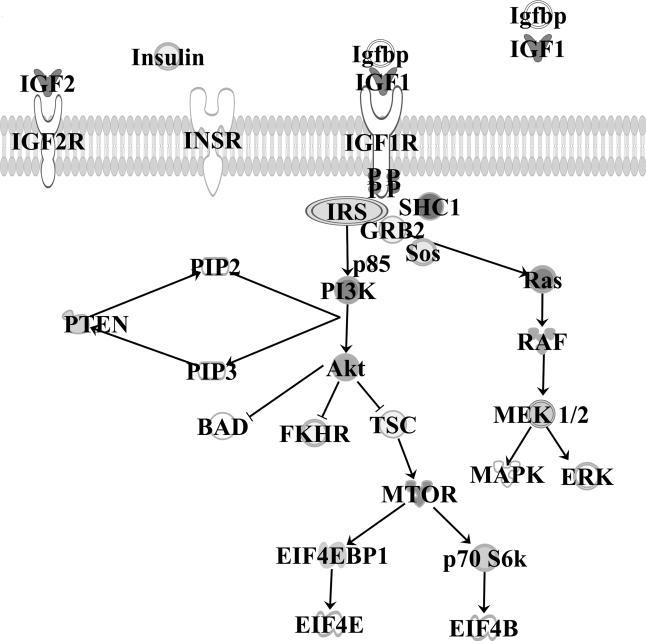
Upon ligand binding of IGF-1 or IGF-2 to the IGF-1R, conformational changes lead to auto-phosphorylation in the tyrosine kinase domain. This conformational change provides docking sites for receptor substrate proteins, including shc, insulin receptor substrate proteins (IRS 1-4), and p85 Fig. (1). After phosphorylation, IRS-1 activates the p85 regulatory subunit of PI3-kinase (PI3K), which in turn activates the enzyme protein kinase B(Akt) [22]. Activated downstream Akt in turn activates the mammalian target of rapamycin (mTOR) pathway, which promotes cell growth and survival, production of transcription factors, and protein synthesis, and regulates the cell cycle. This is effected through Akt-dependent phosphorylation of the tumor-suppressor gene tuberous sclerosis 1 (TSC1), which relieves the TSC1-driven inhibition on the mTOR pathway [23]. PTEN regulates this pathway via dephosphorylation of PIP3, and ultimately inhibits the PI3K signaling pathway; correspondingly, loss of PTEN suppressor activity results in a constitutively active mTOR pathway. Additionally, Akt phosphorylates several downstream mediators, including propapoptotic protein BAD, forkhead in human rhabdomyosarcoma (FKHR), and other important targets that contribute to inhibition of apoptosis, cell proliferation, and mitogenic activity. Eukaryotic initiation factors (EIFs) then initiate mRNA translation, which supports cell survival and proliferation.
Fig. (1).
The IGF pathway.
Concomitantly, phosphorylation of shc or IRS-1 leads to activation of the mitogen-activated protein kinase (MAPK) signaling pathway. Grb2 docks to shc and binds Ras-GEF SOS, thereby activating the Raf-1/MEK/ERK pathway [24]. Extracellular signal-related kinase (ERK) promotes cell growth and transcription and cellular differentiation.
A third pathway has emerged involving the Janus kinases (JAK)-1 and JAK-2, which upon phosphorylation through IGF-1R, activate signal transducers and activators of transcription (STAT) proteins [25]. Although their exact function has not yet been elucidated, the JAK/STAT pathway has been implicated in IGF-1 feedback signaling, receptor cross-talk, and cytokine signaling [26].
Insulin, IGF-1, or IGF-2 can bind to IR, IGF-1R, or IGF-2R. IGFBPs may act as sinks for excess IGF. Upon ligand binding and phosphorylation of IGF-1R, downstream networks including the RAS/RAF/MEK/ERK pathway and PI3K/AKT/TOR pathway are activated. This leads to upregulation of nuclear transcription factors, decreased apoptosis, and increased cellular survival. IGF-1 insulin-like growth factor 1; IGF-2 Insulin-like growth factor 2; IGF-1R insulin-like growth factor 1 receptor, IGF-2R insulin-like growth factor 2 receptor; IR insulin receptor; IGFBP insulin-like growth factor binding protein; IRS insulin receptor substrate protein; GRB2 growth factor receptor-bound protein 2; SHC SRC homology and collagen; SOS son of sevenless; PI3K phosphatidlylinositol-3kinase; PIP 2 and 3 phosphatidlylinositol 4,5 biphosphate/3,4,5 triphosphate; AKT protein kinase B; PTEN phosphatase and tensin homolog; TSC2 tuberous sclerosis complex; FHKR forkhead in human rhabdomyosarcoma; mTOR molecular target of rapamycin; eIF4EBP1 eukaryotic translation initiation factor binding protein 1; eIF4E/B eukaryotic translation initiation factor 4E/4B; p 70 S6K p70S6 kinase; MAPK mitogen-activated protein kinase; MEK MAPK extracellular-signal-related kinase; ERK extracellular-signal-related kinase.
1.3 Functional Role in Cancer
Under normal conditions, IGF is responsible for childhood growth and development, and it continues to mediate autocrine and paracrine functions throughout adulthood. In the endocrine system, growth hormone (GH) stimulates liver synthesis of IGF-1, which in turn stimulates growth and development. IGF-1 deficiency results in short stature in humans [27]; mice with targeted IGF-2 deficiency have severe growth retardation and delayed lung development [28].
It is generally accepted that the IGF family plays a crucial role in the development, progression, and dissemination of multiple cancer types [29]. As reviewed by Bruchim, upregulated IGF-1R expression, both in vitro and in vivo, has been associated with cancer in several organ systems, including the reproductive tract (ovary, endometrial, prostate), GI system (colorectal, gallbladder, hepatocellular, pancreatic), respiratory (lung), skin (melanoma), genitourinary tract (renal and bladder), and various other sites (breast, thyroid) [30].
Although the role of the IGF family in oncogenesis is well-established, the utility of the IGF-1/2 ligands and IGFBPs as a marker for risk and prognosis in cancer remains undefined, with conflicting data. A group recently genotyped 24 IGF-1 gene variants in 6300 cases of breast cancer compared with 8100 controls and found no significant association between IGF-1 germline variations between the two groups [31]. Although Key et al. noted that increased levels of IGF-1 were associated with increased breast cancer risk [32], this association may differ by ethnicity [33]. In ovarian cancer, for which no reliable screening method exists and is desperately needed, results have also been inconsistent. Whereas one cohort exhibited a trend towards increased IGF-1 levels and risk of ovarian cancer in younger patients [34], a larger cohort exhibited no association between IGFBP3, IGFBP2, IGF-1 levels or ratios and ovarian cancer risk [35]. Although the value of IGF ligands remains controversial in determining risk, they may predict prognosis, especially in the case of IGF-1 and/or IGF-2. In ovarian cancer, increased levels of IGF-2 are associated with poor prognosis, decreased survival, and taxol resistance [6, 36]. Escalating IGF-1 or -2 levels may indicate tumor progression, as has been documented in colorectal and hepatocellular carcinoma [37-40].
A relationship also exists between IGFBPs and clinical outcomes, but it is inconsistent. Higher levels of IGFBP1 and 2 have been associated with a poorer prognosis or higher grade tumors in glioblastomas [41], colorectal cancer [39], and ovarian cancer [6]. In prostate cancer cell lines, IGFBP2 promoted growth and decreased sensitivity to docetaxel [42]. Increased circulating levels of IGFBP3, on the other hand, seem to indicate a more favorable prognosis in colorectal and prostate cancer [43, 44]. It has been suggested that IGFBP3 may inhibit tumor growth and that it is downregulated in hepatocellular cancer [45]. Mehta et al. crossed IGFBP3 knockout mice with a prostate cancer mouse that typically develops nonmetastatic tumors, and found that loss of tumor suppression by IGFBP3 accelerated tumor growth and resulted in metastases [46]. One of the mechanisms by which IGFBP3 functions as a tumor suppressor in prostate cancer appears to involve degradation of NF-κB [47]. Recently IGFBP7 has gained attention as a favorable prognostic marker in thyroid cancer, breast cancer, and melanoma [48-50]. Similar to the relationship of IGF ligands to risk and prognosis, results for the IGFBPs have varied. Some studies have found a significant association, but these results are not always reproducible. Rinaldi et al. determined that the strength of association between IGFBP3 and risk of breast cancer depended on the assay used [51]. Although it is widely accepted that the IGF family plays an important role in many cancer types—a relationship validated both in vivo and in vitro—the exact correlation between the circulating ligands and binding proteins varies by individual disease subsets and patient cohorts and may depend on the type of assays and methods used in analysis. Interestingly, these variations may aid in selection of patients who would benefit most from IGF-targeted therapy and biomarker development.
1.4 Receptor Cross-Talk
Receptor cross-talk can occur through ligand interactions or via common downstream molecular pathways and modulation. These interactions provide tumors with additional signaling opportunities and the potential to evade regulatory checkpoints, especially in the context of chemoresistance. Consequently, many of the newer investigational agents target the receptor cross-talk system, and clinical trials have begun employing multiple targeted agents in order to overcome drug resistance and improve efficacy.
While IGF-1R can heterodimerize with members of its own receptor family, it also participates in dimerization with other receptors; heterodimerization with the epidermal growth factor receptor (EGFR) is well-established [52]. Inhibition of one receptor may shift the signaling pathway in favor of an available counterpart receptor; for example, inhibition of IGF-1R leads to EGFR upregulation and phosphorylation. Likewise, EGFR inhibition with erlotinib results in IGF-1R phosphorylation and upregulation [53]. The IGF-1R/EGFR cross-talk is further potentiated through intracellular downstream signaling via the PI3K/Akt and Ras/Raf/MEK/ERK pathways. Evidence exists that IGF-1R can independently activate downstream EGFR pathways with subsequent EFGR tyrosine kinase inhibitor (TKI) resistance [54]. IGF-1R can also trans-activate G protein-coupled receptor signaling systems (GPCRs) via chemokine receptors, which in turn activates EGFR [55]. Correspondingly, lung cancer patients treated with gefitinib, an EGFR inhibitor, had a worse prognosis and a diminished disease response when they exhibited elevated circulating levels of IGF-1 [56].
The EGFR/IGF-1R chemoresistance relationship has been observed in the setting of tamoxifen as well [57]. In addition to EGFR, IGF-1R collaborates with the estrogen receptor (ER) in signaling pathways. While IGF can activate the ER in the absence of estrogen ligands, downstream pathways also take part in cross-talk. When bound to estradiol, the ER collaborates with IGF-1R to cause EGFR upregulation. In breast cancer cells, estrogen-induced proliferation has been shown to upregulate the MAP kinase cascade, which is mediated by autocrine stimulation of IGF-1R and has been linked to tamoxifen resistance [58]. Upregulation of the IGF-1 pathway causes breast cancer cell line resistance to tamoxifen and fulvestrant via upregulation of MAPK and PI3K signaling [59]. Trastuzumab resistance similarly arises from receptor cross-talk, and it has been shown that the ErbB2 receptor heterodimerizes with IGF-1R [60].
Others have demonstrated a relationship between the platelet-derived growth factor receptor (PDGFR) and IGF-1R. Similar to the above observations, inhibition of both receptors resulted in increased cell death in high-grade glioma cell lines [61]. This likely occurs through the PI3K/Akt /mTOR-mediated pathway. Zhang et al. found decreased Akt signaling in PDGFR-depleted cells in the presence of IGF and EGF, suggesting that PDGFR is required for downstream signaling [62].
In pancreatic cancer, GPCRs are overexpressed and stimulate p90RSK/ERK, which then activates the mTOR pathway [63]; as opposed to the IGF-1R pathway, which activates mTOR through PI3K. It is hypothesized that mTOR is the point of pathway coalescence, thereby allowing IGF-1R/GCPR interactions to potentiate cell survival and proliferation. Metformin can disrupt this crosstalk and is gaining recognition as a potential anticancer agent in the IGF-1R arena [64].
1.5 Angiogenesis and Invasion
IGF has been linked to angiogenesis, which is essential for tumor metastasis and nutrient recruitment. In nonmalignant disease such as diabetic retinopathy, IGF-1 has been linked to neovascularization. Logically, concerns exist that insulin compounds could stimulate angiogenesis in human cancers, and recent evidence exists supporting this theory [65]. Both hypoxia-induced angiogenesis and crosstalk between IGF-1R and other receptors mediate neovascularization in the IGF pathway. Hypoxia-inducible factor 1α (HIF-1α) is expressed under hypoxic conditions; IGF-1 and -2 have been shown to provoke HIF-1α expression [66]. In response to HIF-1α expression, vascular endothelial growth factor (VEGF) is recruited—an entity that is well-established in angiogenesis and tumor growth. Reciprocity and cross-talk also exist in the IGF/HIF-1α relationship, where IGFs can cause direct HIF-1α expression through the MAPK/PI3K pathway, and IGF-1R synthesis increases in hypoxia [67].
Through a separate mechanism, IGF-1 also appears to regulate migration and angiogenesis of endothelial cells [68]. Concordantly, EGFR is well-recognized as a contributor to neovascularization and hematologic metastasis, and again the cross-talk relationship plays a role in IGF-mediated angiogenesis. New evidence supports the contribution of the insulin receptor (IR) to angiogenesis as well. In mice with preserved IGF-1R but downregulated IR, less cellular proliferation, angiogenesis, lymphangiogenesis, and metastasis were observed [69]. On the other hand, certain IGFBPs that have been protective against oncogenesis have also proven protective against angiogenesis. IGFBP7, for example, blocks VEGF-induced angiogenesis in vascular endothelial cells [70].
Although neovascularization and vessel recruitment are essential for hematogenous dissemination, IGF also supports direct invasion and metastasis via matrix metalloproteinases (MMPs), particularly MMP 2. IGF-1R is a factor in MMP regulation, including synthesis and activation, and when partnered with MMP-2 participates in extracellular matrix degradation and tumor invasion [71]. Both in vitro and in vivo models support this observation: In a study of colon adenocarcinoma Wu et al demonstrated that IGF-1 promoted liver metastasis and angiogenesis in mice [37]. Conversely, with inhibition of IGF-1R, fewer numbers of circulating tumor cells and pulmonary metastases were observed [72].
2. Targeting the IGF Pathway
Given its function in tumor growth, progression, angiogenesis, and invasion, the IGF pathway is an appealing target for cancer therapy. However, apprehension surrounding the potential toxicity of inhibiting the IGF pathway has resulted in a delay in therapeutic development. Because the IGF pathway is nearly ubiquitous in healthy and malignant cells alike, and because the IGF-1R shares considerable homology with the insulin receptor, the potential for metabolic dysfunction including hyperglycemia is real and has been observed in human trials.
The mainstay of IGF-targeted therapy is directed at IGF-1R and falls into two categories: small molecule inhibitors aimed at the tyrosine kinase domain of IGF-1R (TKI), and monoclonal antibodies (mAb) directed at IGF-1R. There are at least 30 such drugs in clinical or laboratory testing and over 60 clinical trials evaluating IGF-1R-directed therapy Table 1. The small molecule inhibitors compete for the ATP-binding sites on the tyrosine kinase domains, thereby decreasing receptor phosphorylation. Although highly effective, receptor specificity is poor, and concurrent insulin receptor inhibition occurs. Interestingly, this lack of specificity may provide added benefit in the case of hybrid receptors, which are overexpressed in certain tumor types (although their exact role in malignancy remains undefined). Several TKIs (tyrphostins, picropodophyllins, INSM-18, and BMS-754807; see below) inhibit IGF-1R via non-ATP-competitive binding to the insulin receptor. This is postulated to decrease untoward side effects and result in less resistance.
Table 1.
Results of recent clinical trials within the last two years.
| Compound | Category | Phase | Population | Results | Toxicities (n) |
|---|---|---|---|---|---|
| II [85] | Refractory ES or desmoplastic small round cell tumors | ORR 6% 2/35 PR 7/35 with clinical benefit (PR or SD) | G3/4 thrombocytopenia (14%), neutropenia (9%), hyperglycemia (6%) | ||
| II [84] | Metastatic pancreatic cancer +gemcitabine | 1/38 PR 19/38 SD | G3/4 neutropenia 18%, G3/4 thrombocytopenia 15%, G3/4 fatigue 10%, G3/4 hyperglycemia 15% | ||
| I [82] | Solid tumors, non-Hodgkin's Lymphoma | One CR (ES), PR (carcinoid) | G3 thrombocytopenia(8), G3 transaminitis (1), G1/2 thrombocytopenia, hyperglycemia, fatigue, fever, rash | ||
| BIIB-022 | Fully human IgG4. Blocks IGF-1, IGF-2 binding | I [104] | Sarcoma (10), colorectal (8), lung (3), pancreas(2), adrenal (1) | One SD (sarcoma) | Headache, fatigue, nausea; G3 hypertension, fatigue, dyspnea, QTc interval prolongation (1 each) |
| CP-751,871 | Fully human IgG2 | I [128] | ACC, metastatic refractory | 8/14 SD | G4 hyperuricemia, proteinuria, elevated LFTs (1 each), hyperglycemia, nausea, fatigue |
| II [99] | Advanced NSCLC +paclitaxel/carboplatin | 54% ORR (vs. 42% of patients treated with standard) | G3/4 neutropenia, hyperglycemia, thrombocytopenia, infection | ||
| II [101] | mCRC | Did not meet 6-mo OS goal | G3/4 hyperglycemia, asthenia, fatigue | ||
| III [102] | Advanced NSCLC +paclitaxel/carboplatin | Accrual suspended—no survival advantage | Hyperglycemia, dehydration, hemoptysis | ||
| IMC-A12 | Fully human IgG1 | I [114] | Advanced cancer, +temsirolimus | 18/42 SD | G3 mucositis, G3 febrile neutropenia, G3 hyperglycemia, G3 hypertriglyceridemia, G4 thrombocytopenia |
| II [110] | mCRPC | In q2wk dosing, 9/31 SD. In q3wk dosing, 3/10 SD | G3 fatigue, hyperglycemia, thrombocytopenia; G4 leukencephalopathy, hyperkalemia; G5 pneumonia | ||
| II [112] | Advanced hepatocellular carcinoma | Inactive as monotherapy 0/22 OR 7/22 SD | G3/4 hyperglycemia (46%), elevated liver functions tests, hyponatremia (25%), lymphopenia (12%) | ||
| II [111] | Sarcomas | 1 PR ES, 1 PR LIP, SD 24-57% | G3/ 4 pain (7), hyperglycemia (6), thrombocytopenia (5), asthenia (5) | ||
| MK-0646 | Humanized IgG1 | I [161] | 13 colorectal, 11 breast, 5 ES, 19 other | Three SD | G3 purpura, G1-2 hyperglycemia |
| I [91] | Advanced solid tumors | Median days on treatment 64, two patients remain on study > 1 yr | G4 thrombocytopenia (1), fatigue, vomiting, nausea, constipation, diarrhea, abdominal pain | ||
| II [92] | Stage IV pancreatic cancer, +gemcitabine and/or erlotinib | PR 20% gemcitabine + MK-0646 (A), PR 25% in triple combination (B) | G3/4 neutropenia 9/A, 26/B; thrombocytopenia 5/A, 10/B; anemia 2/B; hyponatremia 1/A, 5/B; hyperglycemia 3/A, 3/B; dehydration 1/B; thrombus 2/B; infection 2/B | ||
| II/III [93] | mCRC chemorefractory, + irinotecan and cetuximab | Addition of MK-0646 worsened PFS/OS | Decreased weight, dehydration, hyperglycemia, skin exfoliation, neuropathy, and stomatitis in MK-0646 group. | ||
| R-1507 | Fully human IgG1 | I [117] | Advanced solid tumors | PR in two patients (ES), SD in 13 patients | G3/4 fatigue, anorexia, abdominal pain, anemia, diarrhea, hyperglycemia, lymphopenia, CVA, nausea, thrombocytopenia, hyperbilirubinemia |
| II [118] | Recurrent or refractory ES | 18/125 with CR or PR | G3/4 diarrhea (2), anemia (2), thrombocytopenia (2), and neutropenia (2) | ||
| II [120] | Advanced NSCLC, + erlotinib | Failed to improve efficacy of erlotinib | G3/4 rash, fatigue, hyperglycemia | ||
| AXL-1717 (PPP) | TKI | I [137] | Solid tumors | SD in 4 (NSCLC) | Neutropenia, no DLTs in four NSCLC patients |
| BMS-754807 | TKI (IR and IGF-1R) | I [144] | Solid tumors | SD in 7 (SCLC, osteosarcoma) | G3 hyperglycemia (1), fatigue (<G3) |
| OSI-906 | TKI (IR and IGF-1R) | I [126] | Advanced solid tumors | SD in 24 patients. In ACC patients, 1 PR, 4 SD | G3 hyperglycemia (4), G4 fatigue (1), G3 QTc interval prolongation, and G3 vomiting |
| I [129] | Advanced solid tumors + paclitaxel | 11/55 on study for ≥24 wks. In ovarian cancer (n=29), 5 with PR, 11 with SD | DLTs: G3 neutropenia, G2 neuropathy, G3 deep vein thrombosis, G3 hyperglycemia, G3 fatigue, G4 pulmonary embolism | ||
| I [131] | Refractory mCRC + everolimus | 57% SD as best response, “no significant sign of clinical activity” | G3 mucositis (14%), nausea (7%), vomiting (7%), and hypokalemia (7%); G4 thrombocytopenia (7%) | ||
| I [127] | Advanced cancer | SD in 18/43 pts (thymic, ACC, colon, melanoma) | G3 hyperglycemia (3), G3 QTc interval prolongation (1), G3 transaminitis (2). Nausea, vomiting, fatigue | ||
| XL-228 | TKI, + activity against src kinases, Bcr-Abl variant T315I, Aurora kinases, FGFR1-3 | I [141] | CML or Ph+ ALL | CR in two (CML), PR in two (ALL) | Fatigue, nausea, hyperglycemia |
| I [140] | Solid tumors, lymphoma | PR (NSCLC), 9 SD | G3/ 4 neutropenia, G3 vomiting (1); G1 nausea, fatigue, decreased appetite, flushing; G1/2 hyperglycemia |
G=grade, ACC=adrenocortial carcinoma, ALL=acute lymphoblastic leukemia, CML=chronic myelogenous leukemia, CR=complete response, CVA=cerebrovascular accident, DLT=dose-limiting toxicity, ES=Ewing's sarcoma, LIP=adipocytic sarcoma, LMS=leiomyosarcoma, mCRPC=metastatic castration-resistant prostate cancer, mCRC=metastatic colorectal cancer, NSCLC=non-small cell lung carcinoma, ORR=objective response rate, PR=partial response, RMS=rhabdomyosarcoma, SCLC=small cell lung carcinoma, SD=stable disease, SYN=synovial sarcoma
Furthermore, TKIs may also inhibit the receptor crosstalk pathway, and in vivo studies have demonstrated synergistic effects in combination with other targeted therapies [73-75], as well as benefit in chemoresistant or EGFR/HER-2 inhibitor refractory tumors [76-78].
Antibodies, on the other hand, are more attractive owing to their higher selectivity and lack of reactivity with the insulin receptor. They bind the extracellular domain of the IGF-1R, block ligand binding, and cause receptor internalization and degradation. Dose-limiting toxicity (DLT) is rare, and half-life ranges from 7 to 11 days. Most pharmaceuticals employ an IgG1 base, with the exception of CP-751,871 (Pfizer, NY, NY, US7700742) [79], which uses an IgG2 base, and BIIB-022 (Biogen, Cambridge, MA, US7612178) [80], which employs an IgG4 base.
2.1 Monoclonal Antibodies
AMG-479 (ganitumab, Amgen, Thousand Oaks, CA, US7700742) [79] is a fully human monoclonal antibody with an IgG1 base. AMG-479 acts on IGF-1R by uniquely inhibiting ligand binding of IGF-1 and -2 with resultant inhibition of IGF-1R phosphorylation. In vivo and in vitro studies have demonstrated IGF-1R inhibition in several tumor types, particularly sarcoma cell lines and pancreatic carcinoma xenografts [81]. Tolcher et al. conducted a phase I study in 53 patients with solid tumors or Hodgkin's lymphoma at a dose of 1-20mg/kg every 2 weeks [82]. The most common mild toxicities included fatigue, thrombocytopenia, fever, rash, chills, and anorexia, while severe toxicities included grade 3 thrombocytopenia (n = 8) and transaminitis (n = 1). Durable complete responses were seen in two patients, who remained disease-free at 28 months. A phase Ib study of AMG-479 in combination with panitumumab or gemcitabine reported grade 3/4 toxicities of neutropenia and hyperglycemia [83]. A phase II study has recently validated the efficacy of AMG-479 in combination with gemcitabine in 121 patients with pancreatic cancer [84] and Ewing's family tumors (EFT) or desmoplastic small round cell tumors (DSRCT) [85]. Combination therapy resulted in an increased survival rate (57% in combination vs. 50% in gemcitabine alone at 6 months) and longer progression-free survival (5.1 vs. 2.1 months). Grade 3/4 events included neutropenia (18%), thrombocytopenia (15%), and fatigue (13%). There are currently ongoing phase I and II trials in small cell lung cancer, epithelial ovarian cancer, pancreatic cancer, colorectal cancer, and other solid tumors (NCT0079154, NCT0071852, NCT01024387, NCT01061788, NCT01122119). Additional trials are examining the efficacy of AMG-479 in combination with AMG-655, a fully human monoclonal antibody targeting the TRAIL (tumor necrosis factor-related apoptosis-inducing ligand) receptor 2 (NCT00819169, NCT00813615). Several other phase II trials have completed accrual but have not yet published results.
AVE-1642 (Sanofi-Aventis, Paris, France) binds IGF-1R with high affinity and is another IgG1-based mAb. Two phase I studies have documented its safety, and phase II trials are currently underway. In the setting of refractory multiple myeloma, Moreau et al. documented two grade 3 hyperglycemias in a total of 14 patients, two of which were in diabetic patients [86]. Tolcher et al. followed with a phase I study at 3mg/kg every 3 weeks in combination with docetaxel in patients with solid tumors, and they reported no severe toxicities in patients with advanced solid tumors [87]. Moreau et al. then combinated AVE-1642 with bortezomib, a protease inhibitor, in a phase I trial in patients with multiple myeloma, and unfortunately concluded that despite the favorable toxicity profile, there was insufficient activity in multiple myeloma to merit further development of AVE-1642 in this subgroup [88].
MK-0646 (h7C10, dalotuzumab, Merck, Whitehouse Station, NJ) is a humanized monoclonal antibody that inhibits IGF-1R in a dose-dependent manner and inhibits the downstream PI3K/AKT and MAPK pathways, causing receptor internalization and degradation [89]. Following documented activity in multiple tumor xenografts, phase I trials established safety in patients with IGF-1R-expressing tumors on immunohistochemistry [90]. Unlike other IGF-1R monoclonal antibodies, where no DLT was achieved, grade 3 thrombocytopenia was observed at a dose of 5 mg/kg in another phase I study [91], as well as gastrointestinal bleeding, pneumonitis, and transaminitis. Another phase I study evaluating the role of MK-0646 in breast cancer is open to enrollment (NCT00759785), and phase II trials verifying single-agent efficacy in metastatic neuroendocrine tumors and metastatic nonsquamous lung cancer are ongoing (NCT00610129, NCT00799240). Phase II trials are also investigating MK-0646 in combination with other agents in non-small cell lung cancer (NSCLC) (erlotinib), and advanced metastatic cancers (ridaforolimus) (NCT00614393, NCT00729742, NCT00730379, NCT00769483). A recent trial evaluating gemcitabine and erlotinib in stage IV pancreatic cancer yielded a partial response in 20% in patients who received MK-0646 with gemcitabine, and in 25% of patients who received triple combination therapy [92]. A phase II/III trial investigated the utility of MK-0646 in metastatic colorectal cancer with wild-type KRAS mutations in combination with cetuximab and irinotecan in 345 patients who underwent unsuccessful treatment with oxaliplatin and irinotecan. Unfortunately, compared to patients on irinotecan and cetuximab alone, the addition of MK-0646 to the regimen worsened progression-free and overall survival, which could not be explained by adverse events alone, and the trial was stopped after first interim analysis [93].
Although other agents such as AMG-479 and MK-0646 harbor dose-limiting hematologic toxicities such as grade 3 thrombocytopenia and grade 3/4 thrombocytopenia, CP-751,871 (figitumumab) was designed with an IgG2 backbone in the hopes that reduced complement fixation and immune-mediated cytotoxicity would circumvent hematologic toxicity. CP-751,871 competes for the IGF-1R receptor and causes internalization with degradation and subsequent downregulation of IGF-1R, and it exhibits activity against xenograft tumor growth in several different cancer types [94]. It also boosts antitumor activity in combination with other standard chemotherapeutic agents [94]. In combination with the m-TOR inhibitor rapamycin, CP-751,871 enhances rapamycin activity and suppresses VEGF [95]. A phase I dose-escalation study was initiated in patients with multiple myeloma [96], followed by a phase I dose-escalation study in combination with carboplatin and taxol in patients with solid tumors [97], and a phase I dose-escalation study in combination with docetaxel in patients with solid tumors [98]. Doses were generally well-tolerated, with mild adverse events including anemia, hyperglycemia, anorexia, nausea, diarrhea, asthenia, and elevated liver function tests. Grade 3 or 4 events included DVT, hyperuricemia, neutropenia, febrile neutropenia, fatigue, diarrhea, and cellulitis. Several partial responses and disease stabilizations were observed. IGF-1R inhibition was demonstrated by downregulated IGF-1R levels, increased circulating IGF-1, and hyperglycemia. Interestingly, hyperglycemia was most prevalent in those patients with robust responses. Karp et al. followed with a phase II trial of CP 751,871 (10-20mg/kg, q3 weeks) with or without paclitaxel and carboplatin in 156 patients with advanced non-small-cell lung cancer (NSCLC) [99]. Of the patients treated with combination therapy, 54% had a documented response, compared with 42% of patients treated with carboplatin and taxol alone, with the highest response rate (78%) in patients with squamous cell histology in the combination arm. All-cause grade 3/4 toxicities were equal in all arms, with the exception of hyperglycemia in patients who received figitumumab (15% in combination arm, 8% in carboplatin/taxol alone arm). Another phase II trial [100] demonstrated activity in patients with prostate cancer, with one diabetic patient experiencing grade 3 hyperglycemia. On the other hand, a phase II trial of figitumumab in patients with refractory, metastatic colorectal cancer did not show any clinical benefit [101]. Results of a phase III trial in advanced NSCLC with figitumumab in combination with carboplatin and paclitaxel versus carboplatin/paclitaxel alone were unfortunately disappointing and resulted in trial discontinuation [102]. There was no survival benefit in the figitumumab group with an increased number of patient deaths, and significantly more side effects including dehydration, hyperglycemia, and hemoptysis. However, a subset of patients with elevated circulating IGF-1 levels appeared to benefit more than others, and endured less toxicity than patients with lower circulating IGF-1 levels. Another phase III study (NCT00596830) comparing erlotinib with or without figitumumab in patients with NSCLC was recently discontinued after interim analysis concluded that patient survival on figitumumab was not superior to erlotinib. However, combination with mTOR inhibition in solid tumors yielded one partial response and 15 stable disease responses out of 18 patients, and no DLTs in a recent phase I trial [103]. Unfortunately, the disappointing results of these recent phase III trials have led to the termination of several trials employing figitumumab.
Similar to figitumumab, BIIB-022 is not based on IgG1. It is another fully human IGF-1R mAb, based on IgG4, and is non-glycosylated. A phase I trial in solid tumors with dosing at 1.5 to 30mg/kg found grade 3 toxicities in 4/24 patients, including hypertension, fatigue, dyspnea, and QTc interval prolongation; two patients experienced DLTs [104]. Although there were no objective responses, three patients had metabolic PET responses. Another phase I trial is in progress assessing BIIB-022 in the setting of stage IIIB/IV NSCLC in combination with carboplatin and paclitaxel (NCT00970580), and a phase I/II trial assessing the efficacy of BIIB-022 in hepatocellular carcinoma is underway (NCT00956436).
IMC-A12 (cixutumumab, Imclone, New York, NY, US7638605) [105] is a fully-human monoclonal antibody with an IgG1 backbone specific to IGF-1R. Both in vitro and in vivo research has validated its activity against breast, pancreatic, colon, and prostate cancers [106, 107]. In prostate cancer xenografts, IMC-A12 was efficacious in both androgen-dependent and androgen-independent carcinomas, exhibiting activity and apoptosis at different stages of the cell cycle depending on androgen status [108]. An early phase I study of 11 patients with advanced solid refractory tumors treated with 3-10mg/kg weekly revealed no DLTs and no grade 3/4 toxicities; mild side effects included rash, pruritis, anemia, hyperglycemia, and infusion reaction [109]. A phase II study for patients with metastatic castration-resistant prostate cancer was then launched using single-agent IMC-A12, and stable disease for >6 months was achieved in 9/31 and 3/10 patients in the 10mg/kg/q2 weeks and 20mg/kg q3 weeks cohorts, respectively [110]. Grade 3/4 toxicities included thrombocytopenia, hyperglycemia (five patients required insulin), fatigue, leukoencephalopathy, hyperkalemia, and one grade 5 pneumonia, with most of the severe toxicities occurring in the frequently dosed group. Schoffski et al. examined cixutumumab in sarcoma patients in a phase II setting, and documented objective responses in Ewing's sarcoma and adipocytic sarcoma (1 each), with disease stabilization in 57% (21/37) patients with adipocytic sarcoma, suggesting this particular subtype of sarcoma may benefit the most from IGF-directed therapy [111]. Another phase II trial in advanced hepatocellular carcinoma failed to find IMC-A12 effective as monotherapy [112]. Several clinical trials are examining combination targeted therapy. In a phase II trial, Reidy et al [113] examined concurrent cixutumumab and cetuximab in patients with metastatic colorectal cancer refractory to anti-EGFR therapy, in the hopes of overcoming EGFR resistance via the crosstalk pathway. Disappointingly, single or combination therapy with cixutumumab did not yield significant improvement in disease status. However, combination with the mTOR inhibitor temsirolimus in a phase I trial in patients with advanced solid tumors yielded promising results (18/46 had stable disease, 9 of whom experienced SD ≥5 months, and 2/3 Ewing's sarcoma patients had SD for 8 months and 14 months, respectively) [114]. Currently several phase II trials are evaluating the efficacy of cixutumumab as a single agent or in combination chemotherapy in thymic, prostate, lung, adrenocortical, esophageal, head and neck, breast, and liver cancers; sarcoma; carcinoid or islet cell cancer; and mesothelioma (www.clinicaltrials.gov).
R-1507 (robatumumab, Roche, Basel, Switzerland, US20100158919) [115], a fully human IgG1 type monoclonal antibody, expresses in vivo potency against multiple tumor types, including lung, prostate, and breast cancers, and has gained interest in use against sarcoma. Initial phase I studies [116] confirmed the safety of robatumumab in solid tumors or lymphomas and sarcomas [117]. Grade 3 hyperglycemia and lymphopenia developed in 21 patients at a dose escalation of 1-16mg/kg/weekly, while a second study of 37 patients reported one cerebrovascular accident and one case of hyperbilirubinemia (in a patient with hepatic metastases) at a dose of 9 mg/kg. Two patients with Ewing's sarcoma in the second study experienced an objective response, and an additional two had stable disease. These findings have prompted a phase II trial in patients with refractory or recurrent Ewing's sarcoma; 18 of 125 patients experienced a complete or partial response, with a median duration of response of 25 weeks [118]. Robatumumab also seems to have value in combination with rapamycin in osteosarcoma xenografts and merits further evaluation in patients [119]. In a phase II trial in patients with advanced NSCLC, R-1507 did not improve the efficacy of erlotinib [120].
SCH-717454 (Schering-Plough, Kenilworth, NJ, US7667021)[121] is another fully human monoclonal antibody with documented efficacy in multiple tumor xenografts [122]. A phase IB trial is ongoing in pediatric patients with solid tumors comparing SCH-717454 in combination with temozolomide and irinotecan; or cyclophosphamide, doxorubicin, and vincristine; or ifosfamide and etoposide. Several phase II trials have completed recruitment but have not yet published study results; these include an evaluation of SCH-717454 in patients with relapsed or refractory colorectal cancer (NCT005551213), relapsed or refractory Ewing's sarcoma or osteosarcoma (NCT00617890), and patients with advanced solid tumors (NCT00954512).
2.2 Small Molecule Tyrosine Kinase Inhibitors (TKIs)
OSI-906 (OSI Pharmaceuticals, Melville, NY, US7459554) [123] is a small-molecular-weight TKI that attaches to the ATP-binding pocket of tyrosine kinase receptors, causing dual inhibition of both IR and IGF-1R. In vitro studies revealed activity against colorectal, NSCLC, pancreatic, and breast cancer cell lines [124]; subsequent in vivo investigations confirmed its antitumor properties in colon cancer xenografts [125]. This led to two phase I clinical trials, both of which supported the safety of OSI-906. Carden et al. [126] reported on a dose-escalation study in 61 patients with advanced solid tumors and found that 24 patients (39%) experienced stable disease, several of whom continued on the study. Seven DLTs were recorded, including grade 3 hyperglycemia, grade 4 fatigue, grade 3 QTc prolongation, and grade 3 vomiting, all of which occurred at doses >600mg daily. Evans et al. [127] administered escalating doses at 10-450mg/day and 20-200mg/twice daily in 57 patients with advanced cancers, achieving stable disease in 18 patients. Six DLTs were observed; all occurred at doses greater than 300mg total/day and included grade 3 hyperglycemia, grade 3/4 transaminitis, and grade 3 elevated QTc. A phase II expansion of the study is planned at 150 mg/twice daily in patients with colorectal cancer and non-insulin dependent diabetes mellitus. Robust activity was noticed in patients with adrenocortical carcinoma, and an ensuing phase III trial launched in patients with adrenocortical carcinoma, which is ongoing [128]. In a phase I trial of OSI-906 in patients with recurrent epithelial ovarian cancer in combination with paclitaxel, results are promising: 5/29 patients experienced a partial response, while 11/29 had stable disease [129]. A phase II portion of this study is also currently underway. Other trials include an ongoing phase II trial in patients with advanced hepatocellular carcinoma (NCT 01101906) and a phase I trial in patients with KRAS mutant-positive colorectal cancer in combination with irinotecan (NCT01016860). Two additional phase I trials exploit the potential receptor cross-talk pathway and combine OSI-906 with erlotinib (NCT00739453) in patients with advanced solid tumors and everolimus in patients with advanced refractory colorectal cancer (NCT01154335). Preliminary results from NCT00739453 are promising—21% of patients have experienced stable disease, while two partial responses have been reported [130]. Unfortunately, results from NCT01154335 are less encouraging—the investigators concluded that there were no signs of significant clinical activity in this refractory colorectal cancer cohort, and several dose-limiting toxicities were observed with escalation of everolimus, which precluded dose escalation and may be partially responsible for the clinically insignificant results [131].
Picropodophyllin (PPP) is a member of the cyclolignan family and selectively inhibits IGF-1R without affecting the other insulin receptors. Its proposed selectivity stems from inhibition of IGF-1R phosphorylation at the substrate level rather than the kinase domain [132]. In vivo observations have shown that PPP inhibits several tumor types, including melanoma, myeloma, and multidrug resistant osteosarcoma [133-135]. AXL-1717 (Axelar, Stockholm, Sweden, US7662851) [136] is an orally dosed picropodophyllin-derived compound under evaluation in phase I/II trials (NCT 01062620). Ekman et al. reported on a subset of the above patients, four of whom had progressive NSCLC and were treated with AXL-1717 [137]. Over a treatment period of 7 months, none of the patients have developed metastases and there have been no DLTs, although one patient did develop neutropenia requiring a dose reduction. BVP-51004 (Biovitrum) is another small molecule PPP-based TKI that causes IGF-1R downregulation, it is currently in preclinical development.
XL-228 (Exelixis, San Francisco, CA, US20090232828)[138] is a multitarget TKI that inhibits IGF-1R (IC50 2 nm), Src (IC50 5 nm), and Bcr-abl kinases (IC50 7 nm) [139]. Particularly promising in the field of leukemia, XL-228 harbors activity against a mutant form of Abl, T315I, which is largely resistant to imatinib and dasatinib in chronic myelogenous leukemia (CML). Smith et al. presented the results of a phase I trial [140] in 36 patients who were dosed at 0.45 to 8.0mg/kg weekly via IV infusions. DLTs at 8mg/kg included grade 3/4 neutropenia and grade 3 vomiting, while milder side effects included nausea, fatigue, and grade 1/2 hyperglycemia. Nine patients had stable disease, and one patient with NSCLC experienced a partial response. Another phase I study examined XL-228 in CML and Bcr-Abl positive acute lymphoid leukemia (ALL) in 35 patients, at doses of 0.45-10.8mg/kg once weekly and 3.6mg/kg twice weekly [141]. Two CML patients experienced a complete cytogenic response, one of whom expressed the mutant Abl T315I, and two patients had a major cytogenic response, one of whom was also T315I-positive. Side effects consisted of tumor lysis syndrome, thrombocytopenia, neutropenia, anemia, fever, and infection.
BMS-754807(Bristol-Myers-Squibb, New York, NY, US7534792) [142] is a dual IGF-1R/IR TKI that inhibits in vivo growth of breast, lung, pancreatic, colon, and colon cancers as well as multiple myeloma, leukemia, and sarcomas [143]. Initial phase I results have been reported for 19 subjects at doses of 4, 10, 20, 30, 50, or 70mg/day. The only reported grade 3 event was hyperglycemia in three patients, without any DLTs. Partial metabolic responses and stable metabolic disease were reported in one and 11 patients, respectively, on PET-CT [144]. In vivo evidence also suggests that BMS-754807 can overcome tamoxifen and letrozole resistance in breast cancer and that it exhibits synergistic effects in combination with tamoxifen and letrozole [77]. This is currently under evaluation in another phase I/II trial in which BMS-754807 is combined with trastuzumab in patients with metastatic or advanced Her-2-positive breast cancer (NCT007883333). Several other phase I trials are also recruiting patients with solid tumors.
INSM-18 (NDGA, Insmed, Richmond, VA) is a nordihydroguaiaretic acid (NDGA)-derived dual TKI, active against IGF-1R and HER2. A phase I study in 15 patients with prostate cancer at oral doses of 750-2500 mg resulted in a positive effect on PSA levels [145]. Six of 15 patients experienced transaminitis, two of which were grade 3. The phase II portion of this trial was recently completed and results are pending.
Other promising IGF-1R TKIs are in preclinical development and have not yet undergone investigation in human trials. AG-1024 (Tyrphostin, Calbiochem-EMD biosciences, La Jolla, CA) has been extensively studied in the preclinical phase. It is effective at inhibiting several cancer cell lines and shows synergistic effects with gefitinib [73], and tamoxifen and fulvestrant [74] in breast cancer cell lines. It also enhances the effects of chemotherapy in mesothelioma [146]. PQIP (OSI Pharmaceuticals, Melville, NY) is a highly-selective IGF-1R TKI that is effective against colorectal cancer cell lines [147], and it exhibited independent and synergistic activity in combination with erlotinib in pancreatic cancer cell lines [148].
The Novartis-produced compounds NVP-AEW541 and NVP-ADW742 are ATP-competitive inhibitors that prevent IGF-IR autophosphorylation and have been extensively studied in the laboratory setting. NVP-AEW541 disrupts the HIF-1 pathway in human glioblastoma cells [149] and acts synergistically with dasatinib to block human glioma cell lines [150]. It also increased the response in taxol-resistant ovarian carcinoma cells [36]. NVP-ADW742 acts synergistically with etoposide and carboplatin in small cell lung cancer cell lines [151]. Combined with imatinib, doxorubicin, or vincristine, NVP-ADW742 successfully induced apoptosis and reduced VEGF expression in Ewing's sarcoma cells [152].
Epigallocatechin gallate is a catechin polyphenol component of green tea, and emerging laboratory data implicate its potential activity as a TKI. Kang et al. demonstrated its ability to inhibit IGF-1R via autophosphorylation of IGF-1R tyrosine kinase, with downstream inhibition and resultant cell cycle arrest and apoptosis in Ewing's sarcoma cells [153]. Others have demonstrated its efficacy in multiple tumor types, including breast and colorectal cell lines [154]. It is still in preclinical development and has not yet been used in humans. Abbott has also released a new compound, A-928605, which is a dual IR and IGF-1R inhibitor. Although there are no human clinical trials, in vivo the novel inhibitor was efficacious against neuroblastoma, pancreatic cancer, and NSCLC [155].
2.3 Others
Resveratrol, a phytoalexin compound found in the skin of red grapes, has been found to suppress colon cancer cell lines via IGF-1R and p53 activation [156], with documented activity in ovarian cancer as well [157]. GlaxoSmithKline (London, United Kingdom, WO2008098917) [158] employed the resveratrol-based compound SRT-501 in a phase I/II trial of multiple myeloma (NCT00920556) with or without bortezomib at a dose of 5 g daily in 21-day cycles. The study was suspended after 5/24 (21%) of patients developed kidney failure. The etiology of the renal failure remains unclear, as patients with multiple myeloma are prone to renal failure as a result of cast nephropathy. Given its in vivo activity against colon cancer, phase I/II trials are underway assessing the safety and efficacy of SRT-501 use in colon cancer with hepatic metastases (NCT00920803).
ATL1101 (Antisense Therapeutics, Victoria, Australia, US7468356)[159] is a second-generation antisense oligonucleotide inhibitor of IGF-1R. When tested on castration-resistant and paclitaxel-resistant prostate cancer, ATL1101 suppressed tumor growth in both cell lines and increased paclitaxel sensitivity in vitro and in vivo, with concurrent downregulation of IGF-IR mRNA and protein expression [160]. It has not yet entered clinical phase trials.
2.4 Safety and Toxicity
Most IGF inhibitors are well tolerated, with both general class-specific and individual drug-specific toxicities. The most common milder reactions included skin toxicities (rash, flushing, pruritis) and fatigue. As predicted, hyperglycemia has emerged as a significant side effect, but most cases are grade 1 or 2 and are easily managed with oral hypoglycemics. Grade 3 or 4 reactions tended to occur in diabetic patients and were reversible. Not surprisingly, hyperglycemia was more severe in regimens including corticosteroids [97], an important consideration when combining IGF inhibitors with chemotherapy. Interestingly, despite the improved specificity of the IGF-1R-directed monoclonal antibodies, hyperglycemia was also observed in those patient cohorts as well. It is hypothesized that hyperglycemia in those cases develops through the hypothalamic-pituitary axis and GH-related mechanisms. Given the longer half-lives of IGF-1R directed antibodies, hyperglycemia is more prevalent, and recently has raised concerns about toxicity. In combination with cytotoxic agents and other targeted therapies, toxicities are generally more frequent and severe.
Hematological toxicity has resulted as well, particularly thrombocytopenia in the cases of MK-0646 and AMG-479, as noted above [87, 161], but less commonly in figitumumab, possibly owing to the IgG2-based pharmacokinetics and cell-mediated cytotoxicity. However, lymphopenia has also been reported in both figitumumab and R1507. Hence caution is warranted when these are administered in combination with chemotherapy.
Because IGF-targeted therapy is still young, long-term effects are unknown. Important considerations include the pediatric population, especially in light of the promising outcomes with IGF-targeted therapy in Ewing's sarcoma. Given the interaction between IGF and GH, growth delay could potentially occur. Small molecule TKIs may also traverse the blood-brain barrier, and although no neurologic side effects have been reported, long-term effects are unknown. Similarly, IGF inhibitors may have unforeseen enduring effects on metabolism, body fat, muscle mass, and bone density.
3. Biomarkers for predicting response to IGF inhibitors
The focus in oncology therapeutics is shifting from general systemically toxic agents to targeted, personalized medicine. Imatinib (Gleevec), trastuzumab (Herceptin), erlotinib (Tarceva), and others have paved the way for IGF-mediated targeting. Amongst these, the IGF pathway is unique. It is ubiquitously expressed in cells, with multiple complex signaling conduits, and much remains unknown and ill-defined. Yet ubiquitous expression may not translate to a global response to IGF-targeted therapeutics, with IGF-1R overexpression differing between tumor types and individual patients. Sarcomas, for example, exhibit high levels of IGF-1R overexpression and have demonstrated encouraging responses to IGF inhibitors, whereas other solid tumors have varied in responsiveness. In parallel to IGF-1R development, efforts at identifying predictive biomarkers have increased. IGF-1R overexpression has been reported as a potential biomarker in rhabdomyosarcoma and breast cancer [162, 163], while IGF ligands may also predict response to therapy [164]. A trend has emerged that tumor differentiation types predict response along the lines of the epithelial-mesenchymal axis, where epithelial-like tumors respond better to IGF inhibition than do mesenchymal tumors. As an example, the squamous subset of the NSCLC tumors had the highest response rate to CP-751,871, whereas mesenchymal-expressing NSCLC did not exhibit as robust a response, with concurrently low circulating ligand levels [165]. Pretreatment circulating levels of IGF-1 were also predictive of response [166]. Thus, mesenchymal markers may identify which patients are sensitive to IGF-mediated therapy. In BMS-536924, insulin receptor substrates were a useful marker for sensitivity [167]. Zha et al. examined the IGF-1R-directed monoclonal antibody h10H5 (Genentech, South San Francisco, CA) in breast and colon cancers and determined that IGF-2, IRS1, and IRS2 have predictive value for response, as well as IGF-1R overexpression [168]. In breast cancer, higher levels of phosphorylated IGF-1R and IR portended poor prognosis, and some have therefore suggested that levels of receptor phosphorylation could serve as biomarkers [169]. Use of FDG-PET, which is dependent upon fluorodeoxyglucose uptake, has been employed in several clinical trials to assess response to therapy—however it remains uncertain if decreased tumor signal originates from actual growth inhibition or the anti-glucose effects of IGF-1R.
4. Cross-talk between receptors leads to IGF inhibitor resistance
The receptor cross-talk pathways between IGF-1R and other receptors including EGFR, HER-2, ER, and PDGFR represent crucial relationships through which resistance may evolve, both to IGF-related therapies and to other receptor-specific drugs. IGF inhibitor resistance may occur via receptor cross-talk: using BMS-754807, Huang et al. induced IGF inhibitor resistance in breast, sarcoma, and colon cancer cell lines, with this resistance occurring through an EGFR-mediated mechanism [167]. Logically, it follows that combining these agents could prevent and/or overcome resistance. Both in vivo and in vitro studies utilizing such combinations have yielded affirmative results. In vivo, BMS-754807 can circumvent tamoxifen and letrozole resistance in breast cancer [77]. AG-1024 likewise shows synergistic effects with gefitinib [73] and with tamoxifen and fulvestrant [74] in breast cancer cell lines. Other dual-kinase inhibitors exhibit activity via multiple pathways; one such example is XL-228, which targets IGF-1R, Src, and Bcr-abl kinases. Another dual inhibitor, di-diabody (Imclone), binds both IGF-1R and EGFR and has been active in tumor xenografts [170] but has not yet undergone human testing. Another bispecific molecule with distinct EGFR and IGFR binding sites is currently under patent application (US20100179094) [171]. In vivo success has resulted in the launch of several clinical trials combining IGF-1R inhibitors and erlotinib, trastuzumab, exemestane, letrozole, gefitinib, and others. Initial results from combination dalotuzumab and the mTOR inhibitor ridaforolimus in a phase I study with ER-positive breast cancer patients are encouraging [172].
Targeting the point of cross-talk convergence could yield even greater levels of inhibition. To this effect, the mTOR pathway has elicited interest. In combination with the mTOR inhibitor rapamycin, CP-751,871 enhances rapamycin activity and suppresses VEGF [95]. Inhibitors of the MEK and PI3K pathway have also undergone in vitro testing in combination with IGF inhibitors and offer additional downstream interventions [173]. New patents in pyrazole derivatives promote activity against VEGF-2, the angiopoeitin receptor TIE-2, and raf kinases (US7767696) [174]. It is largely unknown whether continuous or intermittent regimens provide the most benefit. In vivo and in vitro studies have also confirmed the role of receptor cross-talk in chemoresistance and have proven that IGF-1R inhibitors can overcome cytotoxic resistance. Several phase II/III trials are underway examining the effectiveness of both IGF-directed TKIs and monoclonal antibodies in combination with chemotherapy in resistant tumors.
5. Current & Future Developments
Other classes of drugs are also in development, including antisense oligonucleotides, peptides, and proteins. In addition to IGF-1R, other members of the IGF family are upregulated in cancer, including IR, IR-A, and IGF-II. A-928605, a dual IR and IGF-1R inhibitor, is currently under investigation. Targeting hybrid receptors and IGF ligands is also currently under development (US7553485)[175], as is targeting IGF-II as a specific anticancer agent (US7824681)[176]. Medi-573 (Medimmune, Gaithersburg, MD) is a fully-human monoclonal antibody which targets the ligands IGF-1 and IGF-2, and thereby inhibits both IGF-1R and IR-A downstream signaling. A phase I clinical trial investigating its efficacy in solid tumors is currently underway (NCT00816361). Similarly, IGF-binding proteins affect ligand bioavailability and may serve as valuable targets, acting as a sink for circulating ligands. Kirman and Whelan proposed a patent utilizing IGFBP3 to prevent tumor cell growth and metastasis during surgery (US20070142289) [177], while Oh et al. have developed IGFBP-3 receptor-binding molecules which do not cross-react with the IGFs [178]. Another method proposes the use of antisense oligonucleotides which inhibit the tumorigenic activity of IGFBP-2 and IGFBP5 [179].
In the field of IGF-1R-directed monoclonal antibodies, efforts continue to develop IGF-1R antibodies with reduced immunogenicity (US7575746) [180]. This patent in particular describes altering the germline framework within the antibody structure, which lends a reduced immunogenicity and potentially improved activity. Another patent proposes cytokine conjugation of IGF-1R, with improved pharmacokinetics and a potentially longer serum half-life (US20100189689) [181]. Improvements evolve within current antibodies as well: a new and improved version of AVE-1642 promises IGF-1R-specific inhibition rather than dual IGF-1R and IR inhibition and thus an improved side effect profile (US7538195) [182]. Another novel antibody specifically binds IGF-1R and not IR, and also allows for ligand-receptor binding but neutralizes IGF-1R activation (US20100226884) [183]. However, a recent study determined that IGF-1R translocates to the nucleus of tumor cells and suggested that the size of antibodies may limit their ability to enter the cell membrane, thus favoring the mechanism of smaller TKIs [184].
Several novel compounds including oxindoles, pyrrolo[2,3,-B]pyridine derivatives, pyrollo and cyanoquinolones harbor potential activity against multiple tyrosine kinases including IGF-1R [185-187]. Heinrich et al. describe a potent novel class of non-ATP competitive allosteric IGF-1R inhibitors, which selectively bind IGF-1R [188]. This improved selectivity may potentially avoid chemoresistance and some of the side effects encountered with nonspecific TKIs. The use of siRNA to silence overexpressed genes has gained much attention, and Khvorova et al. have explored using siRNA targeted against IGF-1R (US7638621) [189]. In humans, successful therapeutic use of siRNA has posed many challenges and certainly merits further investigation in the context of IGF.
It is now generally accepted that the IGF family, particularly the IGF-1 receptor, participates in oncogenesis, tumor progression, invasion, and metastasis, although the exact mechanisms remain largely undefined. After an initial lag, largely due to concern about metabolic side effects, the development of IGF-1R inhibitors rapidly grew, with several novel small molecule TKIs and IGF-1R monoclonal antibodies. Initial success in in vivo and in vitro studies have prompted several phase I studies, many of which have progressed to stage II or III trials. As expected, hyperglycemia has emerged as a toxicity but is generally mild and easily managed. While initial results of safety and efficacy are encouraging, long-term effects remain unknown, and optimal dosing and scheduling are under investigation. Not surprisingly, combination with other targeted therapies or cytotoxic chemotherapeutics increases toxicity.
In addition, new pathways and potential therapeutic targets have emerged and warrant further study. Recent efforts directed at new drug classes, utilization of IGF-1R inhibitors in chemoresistance, and exploiting the receptor cross-talk mechanism will surely optimize efficacy. While some phase II and III results have been disappointing—even resulting in trial termination—others have been encouraging, which highlights the need for reliable biomarkers to aid in selection of those patients and tumor types most likely to benefit from IGF-targeted therapy, such as sarcoma. As the era of “personalized medicine” continues, so will developments in IGF-targeted drug discovery.
Acknowledgements
Supported in part by grants from the National Institutes of Health, The University of Texas MD Anderson Cancer Center Specialized Program of Research Excellence in Ovarian Cancer (P50 CA08369), and Hera Foundation. Author ERK is supported by the NCI-DHHS-NIH T32 Training Grant (T32 CA101642). The authors thank Michael Worley for his help in critical revision of the article.
Footnotes
Conflict of Interest:
The authors declare no potential conflicts of interest.
REFERENCES
- 1.Furstenberger G, Senn HJ. Insulin-like growth factors and cancer. Lancet Oncol. 2002;3:298–302. doi: 10.1016/s1470-2045(02)00731-3. [DOI] [PubMed] [Google Scholar]
- 2.Chan JM, Stampfer MJ, Giovannucci E, Gann PH, Ma J, Wilkinson P, et al. Plasma insulin-like growth factor-I and prostate cancer risk: a prospective study. Science. 1998;279:563–6. doi: 10.1126/science.279.5350.563. [DOI] [PubMed] [Google Scholar]
- 3.Donovan EA, Kummar S. Role of insulin-like growth factor-1R system in colorectal carcinogenesis. Crit Rev Oncol Hematol. 2008;66:91–8. doi: 10.1016/j.critrevonc.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scotlandi K, Picci P. Targeting insulin-like growth factor 1 receptor in sarcomas. Curr Opin Oncol. 2008;20:419–27. doi: 10.1097/CCO.0b013e328302edab. [DOI] [PubMed] [Google Scholar]
- 5.Gunter MJ, Hoover DR, Yu H, Wassertheil-Smoller S, Manson JE, Li J, et al. A prospective evaluation of insulin and insulin-like growth factor-I as risk factors for endometrial cancer. Cancer Epidemiol Biomarkers Prev. 2008;17:921–9. doi: 10.1158/1055-9965.EPI-07-2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sayer RA, Lancaster JM, Pittman J, Gray J, Whitaker R, Marks JR, et al. High insulin-like growth factor-2 (IGF-2) gene expression is an independent predictor of poor survival for patients with advanced stage serous epithelial ovarian cancer. Gynecol Oncol. 2005;96:355–61. doi: 10.1016/j.ygyno.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 7.Wu X, Zhao H, Do KA, Johnson MM, Dong Q, Hong WK, et al. Serum levels of insulin growth factor (IGF-I) and IGF-binding protein predict risk of second primary tumors in patients with head and neck cancer. Clin Cancer Res. 2004;10:3988–95. doi: 10.1158/1078-0432.CCR-03-0762. [DOI] [PubMed] [Google Scholar]
- 8.Yu H, Spitz MR, Mistry J, Gu J, Hong WK, Wu X. Plasma levels of insulin-like growth factor-I and lung cancer risk: a case-control analysis. J Natl Cancer Inst. 1999;91:151–6. doi: 10.1093/jnci/91.2.151. [DOI] [PubMed] [Google Scholar]
- 9.Bergmann U, Funatomi H, Yokoyama M, Beger HG, Korc M. Insulin-like growth factor I overexpression in human pancreatic cancer: evidence for autocrine and paracrine roles. Cancer Res. 1995;55:2007–11. [PubMed] [Google Scholar]
- 10.Parker A, Cheville JC, Lohse C, Cerhan JR, Blute ML. Expression of insulin-like growth factor I receptor and survival in patients with clear cell renal cell carcinoma. J Urol. 2003;170:420–4. doi: 10.1097/01.ju.0000071474.70103.92. [DOI] [PubMed] [Google Scholar]
- 11.Hankinson SE, Willett WC, Colditz GA, Hunter DJ, Michaud DS, Deroo B, et al. Circulating concentrations of insulin-like growth factor-I and risk of breast cancer. Lancet. 1998;351:1393–6. doi: 10.1016/S0140-6736(97)10384-1. [DOI] [PubMed] [Google Scholar]
- 12.Hewish M, Chau I, Cunningham D. Insulin-like growth factor 1 receptor targeted therapeutics: novel compounds and novel treatment strategies for cancer medicine. Recent Pat Anticancer Drug Discov. 2009;4:54–72. doi: 10.2174/157489209787002515. [DOI] [PubMed] [Google Scholar]
- 13.Favelyukis S, Till JH, Hubbard SR, Miller WT. Structure and autoregulation of the insulin-like growth factor 1 receptor kinase. Nat Struct Biol. 2001;8:1058–63. doi: 10.1038/nsb721. [DOI] [PubMed] [Google Scholar]
- 14.Yee D. Targeting insulin-like growth factor pathways. Br J Cancer. 2006;94:465–8. doi: 10.1038/sj.bjc.6602963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Gorman DB, Weiss J, Hettiaratchi A, Firth SM, Scott CD. Insulin-like growth factor-II/mannose 6-phosphate receptor overexpression reduces growth of choriocarcinoma cells in vitro and in vivo. Endocrinology. 2002;143:4287–94. doi: 10.1210/en.2002-220548. [DOI] [PubMed] [Google Scholar]
- 16.Ullrich A, Gray A, Tam AW, Yang-Feng T, Tsubokawa M, Collins C, et al. Insulin-like growth factor I receptor primary structure: comparison with insulin receptor suggests structural determinants that define functional specificity. Embo J. 1986;5:2503–12. doi: 10.1002/j.1460-2075.1986.tb04528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frattali AL, Treadway JL, Pessin JE. Insulin/IGF-1 hybrid receptors: implications for the dominant-negative phenotype in syndromes of insulin resistance. J Cell Biochem. 1992;48:43–50. doi: 10.1002/jcb.240480108. [DOI] [PubMed] [Google Scholar]
- 18.Frasca F, Pandini G, Scalia P, Sciacca L, Mineo R, Costantino A, et al. Insulin receptor isoform A, a newly recognized, high-affinity insulin-like growth factor II receptor in fetal and cancer cells. Mol Cell Biol. 1999;19:3278–88. doi: 10.1128/mcb.19.5.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Denley A, Wallace JC, Cosgrove LJ, Forbes BE. The insulin receptor isoform exon 11-(IR-A) in cancer and other diseases: a review. Horm Metab Res. 2003;35:778–85. doi: 10.1055/s-2004-814157. [DOI] [PubMed] [Google Scholar]
- 20.Clemmons DR. Role of insulin-like growth factor binding proteins in controlling IGF actions. Mol Cell Endocrinol. 1998;140:19–24. doi: 10.1016/s0303-7207(98)00024-0. [DOI] [PubMed] [Google Scholar]
- 21.Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev. 1995;16:3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- 22.Hermanto U, Zong CS, Wang LH. Inhibition of mitogen-activated protein kinase kinase selectively inhibits cell proliferation in human breast cancer cells displaying enhanced insulin-like growth factor I-mediated mitogen-activated protein kinase activation. Cell Growth Differ. 2000;11:655–64. [PubMed] [Google Scholar]
- 23.Levine AJ, Feng Z, Mak TW, You H, Jin S. Coordination and communication between the p53 and IGF-1-AKT-TOR signal transduction pathways. Genes Dev. 2006;20:267–75. doi: 10.1101/gad.1363206. [DOI] [PubMed] [Google Scholar]
- 24.Grey A, Chen Q, Xu X, Callon K, Cornish J. Parallel phosphatidylinositol-3 kinase and p42/44 mitogen-activated protein kinase signaling pathways subserve the mitogenic and antiapoptotic actions of insulin-like growth factor I in osteoblastic cells. Endocrinology. 2003;144:4886–93. doi: 10.1210/en.2003-0350. [DOI] [PubMed] [Google Scholar]
- 25.Zong CS, Chan J, Levy DE, Horvath C, Sadowski HB, Wang LH. Mechanism of STAT3 activation by insulin-like growth factor I receptor. J Biol Chem. 2000;275:15099–105. doi: 10.1074/jbc.M000089200. [DOI] [PubMed] [Google Scholar]
- 26.Himpe E, Kooijman R. Insulin-like growth factor-I receptor signal transduction and the Janus Kinase/Signal Transducer and Activator of Transcription (JAK-STAT) pathway. Biofactors. 2009;35:76–81. doi: 10.1002/biof.20. [DOI] [PubMed] [Google Scholar]
- 27.Backeljauw P, Bang P, Clayton PE, Geffner M, Woods KA. Diagnosis and management of primary insulin-like growth factor-I deficiency: current perspectives and clinical update. Pediatr Endocrinol Rev. 2010;7(Suppl 1):154–71. [PubMed] [Google Scholar]
- 28.Silva D, Venihaki M, Guo WH, Lopez MF. Igf2 deficiency results in delayed lung development at the end of gestation. Endocrinology. 2006;147:5584–91. doi: 10.1210/en.2006-0498. [DOI] [PubMed] [Google Scholar]
- 29.Pollak M. Insulin and insulin-like growth factor signaling in neoplasia. Nature Rev Cancer. 2008;8:915–928. doi: 10.1038/nrc2536. [DOI] [PubMed] [Google Scholar]
- 30.Bruchim H, Attias Z, Werner H. Targeting the IGF1 axis in cancer proliferation. Expert Opin Ther Targets. 2009;13:1179–1192. doi: 10.1517/14728220903201702. [DOI] [PubMed] [Google Scholar]
- 31.Canzian F, Cox DG, Setiawan VW, Stram DO, Ziegler RG, Dossus L, et al. Comprehensive analysis of common genetic variation in 61 genes related to steroid hormone and insulin-like growth factor-I metabolism and breast cancer risk in the NCI breast and prostate cancer cohort consortium. Hum Mol Genet. 2010;19:3873–84. doi: 10.1093/hmg/ddq291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Key TJ, Appleby PN, Reeves GK, Roddam AW. Insulin-like growth factor 1 (IGF1), IGF binding protein 3 (IGFBP3), and breast cancer risk: pooled individual data analysis of 17 prospective studies. Lancet Oncol. 2010;11:530–42. doi: 10.1016/S1470-2045(10)70095-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rollison DE, Giuliano AR, Risendal BC, Sweeney C, Boulware D, Laronga C, et al. Serum insulin-like growth factor (IGF)-1 and IGF binding protein-3 in relation to breast cancer among Hispanic and white, non-Hispanic women in the US Southwest. Breast Cancer Res Treat. 2010;121:661–9. doi: 10.1007/s10549-009-0609-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peeters PH, Lukanova A, Allen N, Berrino F, Key T, Dossus L, et al. Serum IGF-I, its major binding protein (IGFBP-3) and epithelial ovarian cancer risk: the European Prospective Investigation into Cancer and Nutrition (EPIC). Endocr Relat Cancer. 2007;14:81–90. doi: 10.1677/erc.1.01264. [DOI] [PubMed] [Google Scholar]
- 35.Tworoger SS, Lee IM, Buring JE, Pollak MN, Hankinson SE. Insulin-like growth factors and ovarian cancer risk: a nested case-control study in three cohorts. Cancer Epidemiol Biomarkers Prev. 2007;16:1691–5. doi: 10.1158/1055-9965.EPI-07-0319. [DOI] [PubMed] [Google Scholar]
- 36.Huang GS, Brouwer-Visser J, Ramirez MJ, Kim CH, Hebert TM, Lin J, et al. Insulin-like growth factor 2 expression modulates Taxol resistance and is a candidate biomarker for reduced disease-free survival in ovarian cancer. Clin Cancer Res. 2010;16:2999–3010. doi: 10.1158/1078-0432.CCR-09-3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu Y, Yakar S, Zhao L, Hennighausen L, LeRoith D. Circulating insulin-like growth factor-I levels regulate colon cancer growth and metastasis. Cancer Res. 2002;62:1030–5. [PubMed] [Google Scholar]
- 38.Zhao R, Berho M, Nogueras J, Sands D, Weiss E, Wexner S, et al. Positive correlation of insulin-like growth factor-II with proliferating cell index in patients with colorectal neoplasia. Cancer Epidemiol Biomarkers Prev. 2005;14:1819–22. doi: 10.1158/1055-9965.EPI-04-0803. [DOI] [PubMed] [Google Scholar]
- 39.Renehan AG, Jones J, Potten CS, Shalet SM, O'Dwyer ST. Elevated serum insulin-like growth factor (IGF)-II and IGF binding protein-2 in patients with colorectal cancer. Br J Cancer. 2000;83:1344–50. doi: 10.1054/bjoc.2000.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dong ZZ, Yao DF, Yao DB, Wu XH, Wu W, Qiu LW, et al. Expression and alteration of insulin-like growth factor II-messenger RNA in hepatoma tissues and peripheral blood of patients with hepatocellular carcinoma. World J Gastroenterol. 2005;11:4655–60. doi: 10.3748/wjg.v11.i30.4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Santosh V, Arivazhagan A, Sreekanthreddy P, Srinivasan H, Thota B, Srividya MR, et al. Grade-specific expression of insulin-like growth factor-binding proteins-2, -3, and -5 in astrocytomas: IGFBP-3 emerges as a strong predictor of survival in patients with newly diagnosed glioblastoma. Cancer Epidemiol Biomarkers Prev. 2010;19:1399–408. doi: 10.1158/1055-9965.EPI-09-1213. [DOI] [PubMed] [Google Scholar]
- 42.Uzoh CC, Holly JM, Biernacka KM, Persad RA, Bahl A, Gillatt D, et al. Insulin-like growth factor-binding protein-2 promotes prostate cancer cell growth via IGF-dependent or -independent mechanisms and reduces the efficacy of docetaxel. Br J Cancer. 2011;104:1587–93. doi: 10.1038/bjc.2011.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ingermann AR, Yang YF, Han J, Mikami A, Garza AE, Mohanraj L, et al. Identification of a novel cell death receptor mediating IGFBP-3-induced anti-tumor effects in breast and prostate cancer. J Biol Chem. 2010;285:30233–46. doi: 10.1074/jbc.M110.122226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ma J, Pollak MN, Giovannucci E, Chan JM, Tao Y, Hennekens CH, et al. Prospective study of colorectal cancer risk in men and plasma levels of insulin-like growth factor (IGF)-I and IGF-binding protein-3. J Natl Cancer Inst. 1999;91:620–5. doi: 10.1093/jnci/91.7.620. [DOI] [PubMed] [Google Scholar]
- 45.Subramaniam K, Ooi LL, Hui KM. Transcriptional down-regulation of IGFBP-3 in human hepatocellular carcinoma cells is mediated by the binding of TIA-1 to its AT-rich element in the 3'-untranslated region. Cancer Lett. 2010;297:259–68. doi: 10.1016/j.canlet.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 46.Mehta HH, Gao Q, Galet C, Paharkova V, Wan J, Said J, et al. IGFBP-3 Is a Metastasis Suppression Gene in Prostate Cancer. Cancer Res. 2011;71:5154–63. doi: 10.1158/0008-5472.CAN-10-4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Han J, Jogie-Brahim S, Harada A, Oh Y. Insulin-like growth factor-binding protein-3 suppresses tumor growth via activation of caspase-dependent apoptosis and cross-talk with NF-kappaB signaling. Cancer Lett. 2011;307:200–10. doi: 10.1016/j.canlet.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 48.Amemiya Y, Yang W, Benatar T, Nofech-Mozes S, Yee A, Kahn H, et al. Insulin like growth factor binding protein-7 reduces growth of human breast cancer cells and xenografted tumors. Breast Cancer Res Treat. 2011;126:373–84. doi: 10.1007/s10549-010-0921-0. [DOI] [PubMed] [Google Scholar]
- 49.Vizioli MG, Sensi M, Miranda C, Cleris L, Formelli F, Anania MC, et al. IGFBP7: an oncosuppressor gene in thyroid carcinogenesis. Oncogene. 2010;29:3835–44. doi: 10.1038/onc.2010.136. [DOI] [PubMed] [Google Scholar]
- 50.Chen RY, Chen HX, Lin JX, She WB, Jiang P, Xu L, et al. In-vivo transfection of pcDNA3.1-IGFBP7 inhibits melanoma growth in mice through apoptosis induction and VEGF downexpression. J Exp Clin Cancer Res. 2010;29:13. doi: 10.1186/1756-9966-29-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rinaldi S, Toniolo P, Muti P, Lundin E, Zeleniuch-Jacquotte A, Arslan A, et al. IGF-I, IGFBP-3 and breast cancer in young women: a pooled re-analysis of three prospective studies. Eur J Cancer Prev. 2005;14:493–6. doi: 10.1097/00008469-200512000-00001. [DOI] [PubMed] [Google Scholar]
- 52.Morgillo F, Woo JK, Kim ES, Hong WK, Lee HY. Heterodimerization of insulin-like growth factor receptor/epidermal growth factor receptor and induction of survivin expression counteract the antitumor action of erlotinib. Cancer Res. 2006;66:10100–11. doi: 10.1158/0008-5472.CAN-06-1684. [DOI] [PubMed] [Google Scholar]
- 53.Barr S, Russo S, Buck E, Thomson S, Haley J, Ji Q, et al. The EGFR antagonist, erlotinib, combined with a small molecule inhibitor of IGF-1R acts synergistically to inhibit proliferation and induce apoptosis in ovarian and HNSCC cells.. AACR-NCIEOR1111TC International Conference on Molecular Targets and Cancer Therapeutics; San Francisco, CA. 2007. [Google Scholar]
- 54.Guix M, Faber AC, Wang SE, Olivares MG, Song Y, Qu S, et al. Acquired resistance to EGFR tyrosine kinase inhibitors in cancer cells is mediated by loss of IGF-binding proteins. J Clin Invest. 2008;118:2609–19. doi: 10.1172/JCI34588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van der Veeken J, Oliveira S, Schiffelers RM, Storm G, van Bergen En Henegouwen PM, Roovers RC. Crosstalk between epidermal growth factor receptor and insulin-like growth factor-1 receptor signaling: implications for cancer therapy. Curr Cancer Drug Targets. 2009;9:748–60. doi: 10.2174/156800909789271495. [DOI] [PubMed] [Google Scholar]
- 56.Masago K, Fujita S, Togashi Y, Kim YH, Hatachi Y, Fukuhara A, et al. Clinical significance of epidermal growth factor receptor mutations and insulin-like growth factor 1 and its binding protein 3 in advanced non-squamous non-small cell lung cancer. Oncol Rep. 2011;26:795–803. doi: 10.3892/or.2011.1354. [DOI] [PubMed] [Google Scholar]
- 57.Knowlden JM, Hutcheson IR, Barrow D, Gee JM, Nicholson RI. Insulin-like growth factor-I receptor signaling in tamoxifen-resistant breast cancer: a supporting role to the epidermal growth factor receptor. Endocrinology. 2005;146:4609–18. doi: 10.1210/en.2005-0247. [DOI] [PubMed] [Google Scholar]
- 58.Song RX, Chen Y, Zhang Z, Bao Y, Yue W, Wang JP, et al. Estrogen utilization of IGF-1-R and EGF-R to signal in breast cancer cells. J Steroid Biochem Mol Biol. 2010;118:219–30. doi: 10.1016/j.jsbmb.2009.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang Y, Moerkens M, Ramaiahgari S, de Bont H, Price L, Meerman J, et al. Elevated insulin-like growth factor 1 receptor signaling induces antiestrogen resistance through the MAPK/ERK and PI3K/Akt signaling routes. Breast Cancer Res. 2011;13:R52. doi: 10.1186/bcr2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nahta R, Yuan LX, Du Y, Esteva FJ. Lapatinib induces apoptosis in trastuzumab-resistant breast cancer cells: effects on insulin-like growth factor I signaling. Mol Cancer Ther. 2007;6:667–74. doi: 10.1158/1535-7163.MCT-06-0423. [DOI] [PubMed] [Google Scholar]
- 61.Carapancea M, Cosaceanu D, Budiu R, Kwiecinska A, Tataranu L, Ciubotaru V, et al. Dual targeting of IGF-1R and PDGFR inhibits proliferation in high-grade gliomas cells and induces radiosensitivity in JNK-1 expressing cells. J Neurooncol. 2007;85:245–54. doi: 10.1007/s11060-007-9417-0. [DOI] [PubMed] [Google Scholar]
- 62.Zhang H, Bajraszewski N, Wu E, Wang H, Moseman AP, Dabora SL, et al. PDGFRs are critical for PI3K/Akt activation and negatively regulated by mTOR. J Clin Invest. 2007;117:730–8. doi: 10.1172/JCI28984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rozengurt E, Sinnett-Smith J, Kisfalvi K. Crosstalk between insulin/insulin-like growth factor-1 receptors and G protein-coupled receptor signaling systems: a novel target for the antidiabetic drug metformin in pancreatic cancer. Clin Cancer Res. 2010;16:2505–11. doi: 10.1158/1078-0432.CCR-09-2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kisfalvi K, Eibl G, Sinnett-Smith J, Rozengurt E. Metformin disrupts crosstalk between G protein-coupled receptor and insulin receptor signaling systems and inhibits pancreatic cancer growth. Cancer Res. 2009;69:6539–45. doi: 10.1158/0008-5472.CAN-09-0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rensing KL, Houttuijn Bloemendaal FM, Weijers EM, Richel DJ, Buller HR, Koolwijk P, et al. Could recombinant insulin compounds contribute to adenocarcinoma progression by stimulating local angiogenesis? Diabetologia. 2010;53:966–70. doi: 10.1007/s00125-010-1687-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Feldser D, Agani F, Iyer NV, Pak B, Ferreira G, Semenza GL. Reciprocal positive regulation of hypoxia-inducible factor 1alpha and insulin-like growth factor 2. Cancer Res. 1999;59:3915–8. [PubMed] [Google Scholar]
- 67.Peretz S, Kim C, Rockwell S, Baserga R, Glazer PM. IGF1 receptor expression protects against microenvironmental stress found in the solid tumor. Radiat Res. 2002;158:174–80. doi: 10.1667/0033-7587(2002)158[0174:irepam]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 68.Shigematsu S, Yamauchi K, Nakajima K, Iijima S, Aizawa T, Hashizume K. IGF-1 regulates migration and angiogenesis of human endothelial cells. Endocr J. 1999;46(Suppl):S59–62. doi: 10.1507/endocrj.46.suppl_s59. [DOI] [PubMed] [Google Scholar]
- 69.Zhang H, Fagan DH, Zeng X, Freeman KT, Sachdev D, Yee D. Inhibition of cancer cell proliferation and metastasis by insulin receptor downregulation. Oncogene. 2010;29:2517–27. doi: 10.1038/onc.2010.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tamura K, Hashimoto K, Suzuki K, Yoshie M, Kutsukake M, Sakurai T. Insulin-like growth factor binding protein-7 (IGFBP7) blocks vascular endothelial cell growth factor (VEGF)-induced angiogenesis in human vascular endothelial cells. Eur J Pharmacol. 2009;610:61–7. doi: 10.1016/j.ejphar.2009.01.045. [DOI] [PubMed] [Google Scholar]
- 71.Zhang D, Samani AA, Brodt P. The role of the IGF-I receptor in the regulation of matrix metalloproteinases, tumor invasion and metastasis. Horm Metab Res. 2003;35:802–8. doi: 10.1055/s-2004-814143. [DOI] [PubMed] [Google Scholar]
- 72.Sachdev D, Zhang X, Matise I, Gaillard-Kelly M, Yee D. The type I insulin-like growth factor receptor regulates cancer metastasis independently of primary tumor growth by promoting invasion and survival. Oncogene. 2010;29:251–62. doi: 10.1038/onc.2009.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Camirand A, Zakikhani M, Young F, Pollak M. Inhibition of insulin-like growth factor-1 receptor signaling enhances growth-inhibitory and proapoptotic effects of gefitinib (Iressa) in human breast cancer cells. Breast Cancer Res. 2005;7:R570–9. doi: 10.1186/bcr1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chakraborty AK, Welsh A, Digiovanna MP. Co-targeting the insulin-like growth factor I receptor enhances growth-inhibitory and pro-apoptotic effects of anti-estrogens in human breast cancer cell lines. Breast Cancer Res Treat. 2010;120:327–35. doi: 10.1007/s10549-009-0382-5. [DOI] [PubMed] [Google Scholar]
- 75.Kaulfuss S, Burfeind P, Gaedcke J, Scharf JG. Dual silencing of insulin-like growth factor-I receptor and epidermal growth factor receptor in colorectal cancer cells is associated with decreased proliferation and enhanced apoptosis. Mol Cancer Ther. 2009;8:821–33. doi: 10.1158/1535-7163.MCT-09-0058. [DOI] [PubMed] [Google Scholar]
- 76.Browne BC, Crown J, Venkatesan N, Duffy MJ, Clynes M, Slamon D, et al. Inhibition of IGF1R activity enhances response to trastuzumab in HER-2-positive breast cancer cells. Ann Oncol. 2010;22:68–73. doi: 10.1093/annonc/mdq349. [DOI] [PubMed] [Google Scholar]
- 77.Haluska P, Hou X, Huang F, Harrington S, Greer A, Macedo L, et al. Complete IGF signaling blockade by the dual-kinase inhibitor, BMS-754807, is sufficient to overcome tamoxifen and letrozole resistance in vitro and in vivo. Thirty-Second Annual CTRCAACR San Antonio Breast Cancer Symposium. Cancer Res. 2009;69 Abstract 402. [Google Scholar]
- 78.Patel BB, Gupta D, Elliott AA, Sengupta V, Yu Y, Majumdar AP. Curcumin targets FOLFOX-surviving colon cancer cells via inhibition of EGFRs and IGF-1R. Anticancer Res. 2010;30:319–25. [PMC free article] [PubMed] [Google Scholar]
- 79.Cohen B, Beebe J, Miller P, Moyer J, Corvalan J, Gallo M. Antibodies to insulin-like growth factor I receptor. 2010 US7700742.
- 80.Kandasamy H, Glaser S, Garber E, Graff C, Reyes C, Demarest S. Anti-IGF-1R antibodies and uses thereof. 2009 US7612178.
- 81.Beltran PJ, Mitchell P, Chung YA, Cajulis E, Lu J, Belmontes B, et al. AMG 479, a fully human anti-insulin-like growth factor receptor type I monoclonal antibody, inhibits the growth and survival of pancreatic carcinoma cells. Mol Cancer Ther. 2009;8:1095. doi: 10.1158/1535-7163.MCT-08-1171. [DOI] [PubMed] [Google Scholar]
- 82.Tolcher AW, Sarantopoulos J, Patnaik A, Papadopoulos K, Lin CC, Rodon J, et al. Phase I, pharmacokinetic, and pharmacodynamic study of AMG 479, a fully human monoclonal antibody to insulin-like growth factor receptor 1. J Clin Oncol. 2009;27:5800–7. doi: 10.1200/JCO.2009.23.6745. [DOI] [PubMed] [Google Scholar]
- 83.Sarantopoulos J, Mita A, Mulay M, Romero O, Lu J, Capilla F. A phase IB study of AMG 479, a type 1 insulin-like growth factor receptor (IGF1R) antibody, in combination with panitumumab (P) or gemcitabine (G). J Clin Oncol. 2008;26 abstr 3583. [Google Scholar]
- 84.Kindler H, Richards D, Stephenson J, Garbo L, Lima CR, Safran H. A placebo-controlled, randomized phase II study of conatumumab (C) or AMG 479 (A) or placebo (P) plus gemcitabine (G) in patients (pts) with metastatic pancreatic cancer (mPC). J Clin Oncol. 2010;28:15s. [Google Scholar]
- 85.Tap WD, Demetri GD, Barnette P, Desai J, Kavan P, Tozer R, et al. AMG 479 in relapsed or refractory Ewing's family tumors (EFT) or desmoplastic small round cell tumors (DSRCT): Phase II results.. 2010 ASCO Annual Meeting.; Chicago, IL. 2010. [Google Scholar]
- 86.Moreau P, Hulin C, Facon T, Boccadoro M, Mery-Mignard D, Deslandes A. Phase I Study of AVE1642 Anti IGF-1R monoclonal antibody in patients with advanced multiple myeloma. Blood. 2007;110:1166. [Google Scholar]
- 87.Tolcher A, Patnaik A, Till E, Takimoto C, Papadopoulos K, Massard C. A phase I study of AVE1642, a humanized monoclonal antibody IGF-1R (insulin like growth factor1 receptor) antagonist, in patients (pts) with advanced solid tumor (ST). J Clin Oncol. 2008;26:3582. [Google Scholar]
- 88.Moreau P, Cavallo F, Leleu X, Hulin C, Amiot M, Descamps G, et al. Phase I study of the anti insulin-like growth factor 1 receptor (IGF-1R) monoclonal antibody, AVE1642, as single agent and in combination with bortezomib in patients with relapsed multiple myeloma. Leukemia. 2011;25:872–4. doi: 10.1038/leu.2011.4. [DOI] [PubMed] [Google Scholar]
- 89.Broussas M, Dupont J, Gonzalez A, Blaecke A, Fournier M, Corvaia N, et al. Molecular mechanisms involved in activity of h7C10, a humanized monoclonal antibody, to IGF-1 receptor. Int J Cancer. 2009;124:2281–93. doi: 10.1002/ijc.24186. [DOI] [PubMed] [Google Scholar]
- 90.Atzori F, Tabernero J, Cervantes A, Prudkin L, Andreu J, Rodriguez-Braun E, et al. A Phase I, Pharmacokinetic and Pharmacodynamic Study of Dalotuzumab (MK-0646), an Anti-IGF-1R Monoclonal Antibody, in Patients with Advanced Solid Tumors. Clin Cancer Res. 2011 doi: 10.1158/1078-0432.CCR-10-3336. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 91.Hidalgo M, M MTG, Lewis N, JL JV, Taylor G, Hayburn J, et al. A phase I study of MK-0646, a humanized monoclonal antibody against the insulin-like growth factor receptor type 1 (IGF1R) in advanced solid tumor patients in a q2 wk schedule. J Clin Oncol. 2008;26:3520. [Google Scholar]
- 92.Javle MM, Varadhachary GR, Fogelman DR, Shroff RT, Overman MJ, Ukegbu L, et al. Randomized phase II study of gemcitabine (G) plus anti-IGF-1R antibody MK-0646, G plus erlotinib (E) plus MK-0646 and G plus E for advanced pancreatic cancer.. 2011 ASCO Annual Meeting.; Chicago, IL. 2011. [Google Scholar]
- 93.Watkins D, Tabernero J, Schmoll H, Trarbach T, Ramos F, Hsu K, et al. A randomized phase II/III study of the anti-IGF-1R antibody MK-0646 (dalotuzumab) in combination with cetuximab (Cx) and irinotecan (Ir) in the treatment of chemorefractory metastatic colorectal cancer (mCRC) with wild-type (wt) KRAS status.. 2011 ASCO Annual Meeting.; 2011. [Google Scholar]
- 94.Cohen BD, Baker DA, Soderstrom C, Tkalcevic G, Rossi AM, Miller PE, et al. Combination therapy enhances the inhibition of tumor growth with the fully human anti-type 1 insulin-like growth factor receptor monoclonal antibody CP-751,871. Clin Cancer Res. 2005;11:2063–73. doi: 10.1158/1078-0432.CCR-04-1070. [DOI] [PubMed] [Google Scholar]
- 95.Kurmasheva RT, Dudkin L, Billups C, Debelenko LV, Morton CL, Houghton PJ. The insulin-like growth factor-1 receptor-targeting antibody, CP-751,871, suppresses tumor-derived VEGF and synergizes with rapamycin in models of childhood sarcoma. Cancer Res. 2009;69:7662–71. doi: 10.1158/0008-5472.CAN-09-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lacy MQ, Alsina M, Fonseca R, Paccagnella ML, Melvin CL, Yin D, et al. Phase I, pharmacokinetic and pharmacodynamic study of the anti-insulinlike growth factor type 1 Receptor monoclonal antibody CP-751,871 in patients with multiple myeloma. J Clin Oncol. 2008;26:3196–203. doi: 10.1200/JCO.2007.15.9319. [DOI] [PubMed] [Google Scholar]
- 97.Karp DD, Pollak MN, Cohen RB, Eisenberg PD, Haluska P, Yin D, et al. Safety, pharmacokinetics, and pharmacodynamics of the insulin-like growth factor type 1 receptor inhibitor figitumumab (CP-751,871) in combination with paclitaxel and carboplatin. J Thorac Oncol. 2009;4:1397–403. doi: 10.1097/JTO.0b013e3181ba2f1d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Molife LR, Fong PC, Paccagnella L, Reid AH, Shaw HM, Vidal L, et al. The insulin-like growth factor-I receptor inhibitor figitumumab (CP-751,871) in combination with docetaxel in patients with advanced solid tumours: results of a phase Ib dose-escalation, open-label study. Br J Cancer. 2010;103:332–9. doi: 10.1038/sj.bjc.6605767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Karp DD, Paz-Ares LG, Novello S, Haluska P, Garland L, Cardenal F, et al. Phase II study of the anti-insulin-like growth factor type 1 receptor antibody CP-751,871 in combination with paclitaxel and carboplatin in previously untreated, locally advanced, or metastatic non-small-cell lung cancer. J Clin Oncol. 2009;27:2516–22. doi: 10.1200/JCO.2008.19.9331. [DOI] [PubMed] [Google Scholar]
- 100.Chi K, Gleave M, Fazli L, Goldenberg S, So A, Kollmannsberger C, et al. A phase II study of preoperative figitumumab (F) in patients (pts) with localized prostate cancer (PCa). J Clin Oncol. 2010;28:15s. doi: 10.1158/1078-0432.CCR-12-0482. [DOI] [PubMed] [Google Scholar]
- 101.Becerra C, Salazar R, Garcia-Carbonero R, Thomas AL, Vázquez-Mazón F, Cassidy J, et al. Phase II trial of figitumumab in patients with refractory, metastatic colorectal cancer (mCRC).. 2011 ASCO Annual Meeting.; Chicago, IL. 2011; [DOI] [PubMed] [Google Scholar]
- 102.Jassem J, Langer C, Karp D, Mok T, Benner R, Green S, et al. Randomized, open label, phase III trial of figitumumab in combination with paclitaxel and carboplatin versus paclitaxel and carboplatin in patients with non-small cell lung cancer (NSCLC). J Clin Oncol. 2010;28:7500. doi: 10.1200/JCO.2013.54.4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Quek R, Wang Q, Morgan JA, Shapiro GI, Butrynski JE, Ramaiya N, et al. Combination mTOR and IGF-1R inhibition: phase I trial of everolimus and figitumumab in patients with advanced sarcomas and other solid tumors. Clin Cancer Res. 2011;17:871–9. doi: 10.1158/1078-0432.CCR-10-2621. [DOI] [PubMed] [Google Scholar]
- 104.vonMehren M, Britten C, Lear K, Camidge D, Wainberg Z. Phase I, dose-escalation study of BIIB022 (anti-IGF-1R antibody) in advanced solid tumors. J Clin Oncol. 2010;28:2612. al E. [Google Scholar]
- 105.Ludwig D. Fully human antibodies directed against the human insulin-like growth factor-1 receptor. 2009 US7638605.
- 106.Burtrum D, Zhu Z, Lu D, Anderson DM, Prewett M, Pereira DS, et al. A fully human monoclonal antibody to the insulin-like growth factor I receptor blocks ligand-dependent signaling and inhibits human tumor growth in vivo. Cancer Res. 2003;63:8912–21. [PubMed] [Google Scholar]
- 107.Rowinsky EK, Youssoufian H, Tonra JR, Solomon P, Burtrum D, Ludwig DL. IMC-A12, a human IgG1 monoclonal antibody to the insulin-like growth factor I receptor. Clin Cancer Res. 2007;13:5549s–55s. doi: 10.1158/1078-0432.CCR-07-1109. [DOI] [PubMed] [Google Scholar]
- 108.Wu JD, Haugk K, Coleman I, Woodke L, Vessella R, Nelson P, et al. Combined in vivo effect of A12, a type 1 insulin-like growth factor receptor antibody, and docetaxel against prostate cancer tumors. Clin Cancer Res. 2006;12:6153–60. doi: 10.1158/1078-0432.CCR-06-0443. [DOI] [PubMed] [Google Scholar]
- 109.Higano C, Yu E, Whiting S, Gordon M, LoRusso P, Fox F, et al. A phase I, first in man study of weekly IMC-A12, a fully human insulin like growth factor-I receptor IgG1 monoclonal antibody, in patients with advanced solid tumors. J Clin Oncol. 2007;25:3505. [Google Scholar]
- 110.Higano C, Alumkal J, Ryan C, Yu E, Beer T, Fox F, et al. A phase II study of cixutumumab (IMC-A12), a monoclonal antibody (MAb) against the insulin-like growth factor 1 receptor (IGF-IR), monotherapy in metastatic castration-resistant prostate cancer (mCRPC): Feasibility of every 3-week dosing and updated results.. ASCO 2010 Genitourinary Cancers Symposium.; San Francisco, CA. 2010. [Google Scholar]
- 111.Schoffski P, Adkins D, Blay J, Gil T, Elias AD, Rutkowski P, et al. Phase II trial of anti-IGF-IR antibody cixutumumab in patients with advanced or metastatic soft-tissue sarcoma and Ewing family of tumors.. 2011 ASCO Annual Meeting.; Chicago, IL. 2011; [DOI] [PubMed] [Google Scholar]
- 112.Abou-Alfa GK, Gansukh B, Chou JF, Shia J, Capanu M, Kalin M, et al. Phase II study of cixutumumab (IMC-A12, NSC742460; C) in hepatocellular carcinoma (HCC).. 2011 ASCO Annual Meeting.; Chicago, IL. 2011; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Reidy DL, Vakiani E, Fakih MG, Saif MW, Hecht JR, Goodman-Davis N, et al. Randomized, phase II study of the insulin-like growth factor-1 receptor inhibitor IMCA12, with or without cetuximab, in patients with cetuximab- or panitumumab-refractory metastatic colorectal cancer. J Clin Oncol. 2010;28:4240–6. doi: 10.1200/JCO.2010.30.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Naing A, Kurzrock R, Burger AM, Gupta S, Lei X, Busaidy NL, et al. Phase I Trial of Cixutumumab Combined with Temsirolimus in Patients with Advanced Cancer. Clin Cancer Res. 2011 doi: 10.1158/1078-0432.CCR-10-2979. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bauer-Dauphin I, Mahler H. Pharmaceutical composition. 2010 US20100158919.
- 116.Rodon J, Patnaik A, Stein M, Tolcher A, Ng C, Dias C, et al. A phase I study of q3W R1507, a human monoclonal antibody IGF-1R antagonist in patients with advanced cancer. J Clin Oncol. 2007;25:3590. [Google Scholar]
- 117.Kurzrock R, Patnaik A, Aisner J, Warren T, Leong S, Benjamin R, et al. A phase I study of weekly R1507, a human monoclonal antibody insulin-like growth factor-I receptor antagonist, in patients with advanced solid tumors. Clin Cancer Res. 2010;16:2458–65. doi: 10.1158/1078-0432.CCR-09-3220. [DOI] [PubMed] [Google Scholar]
- 118.Pappo AS, Patel S, Crowley J, Reinke DK, Staddon AP, Kuenkele K, et al. Activity of R1507, a monoclonal antibody to the insulin-like growth factor-1 receptor (IGF1R), in patients (pts) with recurrent or refractory Ewing's sarcoma family of tumors (ESFT): Results of a phase II SARC study.. 2010 ASCO Annual Meeting.; Chicago, IL. 2010; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kolb EA, Kamara D, Zhang W, Lin J, Hingorani P, Baker L, et al. R1507, a fully human monoclonal antibody targeting IGF-1R, is effective alone and in combination with rapamycin in inhibiting growth of osteosarcoma xenografts. Pediatr Blood Cancer. 2010;55:67–75. doi: 10.1002/pbc.22479. [DOI] [PubMed] [Google Scholar]
- 120.Ramalingam SS, Spigel DR, Steins M, Engelman JA, Schneider C, Novello S, et al. Randomized, double-blind, phase II study of erlotinib in combination with placebo or R1507, a monoclonal antibody to insulin-like growth factor receptor-1 (IGF-1R), for advanced-stage non-small cell lung cancer (NSCLC). 2011 ASCO Annual Meeting. 2011 doi: 10.1200/JCO.2011.36.6799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang Y, Greenberg R, Presta L, Pachter J, Hailey J, Brams P, et al. Neutralizing human anti-IGFR antibody. 2010 US7667021.
- 122.Kolb EA, Gorlick R, Houghton PJ, Morton CL, Lock R, Carol H, et al. Initial testing (stage 1) of a monoclonal antibody (SCH 717454) against the IGF-1 receptor by the pediatric preclinical testing program. Pediatr Blood Cancer. 2008;50:1190–7. doi: 10.1002/pbc.21450. [DOI] [PubMed] [Google Scholar]
- 123.Dong H, Foreman K, Mulvihill M, Nigro A, Steinig A, Werner D, et al. Imidazopyrazine tyrosine kinase inhibitors. 2008 US7459554.
- 124.Mulvihill MJ, Ji QS, Coate HR, Cooke A, Dong H, Feng L, et al. Novel 2-phenylquinolin-7-yl-derived imidazo[1,5-a]pyrazines as potent insulin-like growth factor-I receptor (IGF-IR) inhibitors. Bioorg Med Chem. 2008;16:1359–75. doi: 10.1016/j.bmc.2007.10.061. [DOI] [PubMed] [Google Scholar]
- 125.Flanigan SA, Pitts TM, Eckhardt SG, Tentler JJ, Tan AC, Thorburn A, et al. The insulin-like growth factor I receptor/insulin receptor tyrosine kinase inhibitor PQIP exhibits enhanced antitumor effects in combination with chemotherapy against colorectal cancer models. Clin Cancer Res. 2010;16:5436–46. doi: 10.1158/1078-0432.CCR-10-2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Carden C, Kim E, Jones RL, Alam S, Johnson F, Stephens A, et al. Phase I study of intermittent dosing of OSI-906, a dual tyrosine kinase inhibitor of insulin-like growth factor-1 receptor (IGF- 1R) and insulin receptor (IR) in patients with advanced solid tumors. J Clin Oncol. 2010;28:15s. doi: 10.1158/1078-0432.CCR-14-0265. [DOI] [PubMed] [Google Scholar]
- 127.Evans T, Lindsay C, Chan E, Campbell S, Bell P, Stephens A, et al. Phase I dose-escalation study of continuous oral dosing of OSI-906, a dual tyrosine kinase inhibitor of insulin-like growth factor-1 receptor (IGF-1R) and insulin receptor (IR), in patients with advanced solid tumors. J Clin Oncol. 2010;28:15s. [Google Scholar]
- 128.Haluska P, Worden F, Olmos D, Yin D, Schteingart D, Batzel GN, et al. Safety, tolerability, and pharmacokinetics of the anti-IGF-1R monoclonal antibody figitumumab in patients with refractory adrenocortical carcinoma. Cancer Chemother Pharmacol. 2010;65:765–73. doi: 10.1007/s00280-009-1083-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Harb WA, Sessa C, Hirte HW, Kaye SB, Simantov R, Banerjee SN, et al. A phase I study evaluating the combination of OSI-906, a dual inhibitor of insulin growth factor-1 receptor (IGF-1R) and insulin receptor (IR) with weekly paclitaxel (PAC) in patients with advanced solid tumors.. 2011 ASCO Annual Meeting.; Chicago, IL. 2011. [Google Scholar]
- 130.Macaulay VM, Middleton MR, Eckhardt SG. Phase I study of OSI-906, dual tyrosine kinase inhibitor of insulin-like growth factor-1 receptor (IGF-1R) and insulin receptor (IR) in combination with erlotinib (E) in patients with advanced solid tumors.. ASCO 2010 Annual Meeting.; Chicago, IL. 2010. [Google Scholar]
- 131.Hart L, Burris HA, Infante JR, Jones SF, Spigel DR, Bendell JC. mTOR inhibitor everolimus (Ev) and IGFR inhibitor OSI-906 (OSI) for the treatment of patients (pts) with refractory metastatic colorectal cancer (mCRC): A Sarah Cannon Research Institute phase I trial.. 2011 ASCO Annual Meeting.; Chicago, IL. 2011. [Google Scholar]
- 132.Vasilcanu D, Girnita A, Girnita L, Vasilcanu R, Axelson M, Larsson O. The cyclolignan PPP induces activation loop-specific inhibition of tyrosine phosphorylation of the insulin-like growth factor-1 receptor. Link to the phosphatidyl inositol-3 kinase/Akt apoptotic pathway. Oncogene. 2004;23:7854–62. doi: 10.1038/sj.onc.1208065. [DOI] [PubMed] [Google Scholar]
- 133.Menu E, Jernberg-Wiklund H, Stromberg T, De Raeve H, Girnita L, Larsson O, et al. Inhibiting the IGF-1 receptor tyrosine kinase with the cyclolignan PPP: an in vitro and in vivo study in the 5T33MM mouse model. Blood. 2006;107:655–60. doi: 10.1182/blood-2005-01-0293. [DOI] [PubMed] [Google Scholar]
- 134.Karasic TB, Hei TK, Ivanov VN. Disruption of IGF-1R signaling increases TRAIL-induced apoptosis: a new potential therapy for the treatment of melanoma. Exp Cell Res. 2010;316:1994–2007. doi: 10.1016/j.yexcr.2010.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Duan Z, Choy E, Harmon D, Yang C, Ryu K, Schwab J, et al. Insulin-like growth factor-I receptor tyrosine kinase inhibitor cyclolignan picropodophyllin inhibits proliferation and induces apoptosis in multidrug resistant osteosarcoma cell lines. Mol Cancer Ther. 2009;8:2122–30. doi: 10.1158/1535-7163.MCT-09-0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Larsson O, Axelson M. Use of specific cyklolignans. 2010 US7662851.
- 137.Ekman S, Frodin JE, Harmenberg J, Bergman A, Hedlund A, Dahg P, et al. Clinical Phase I study with an Insulin-like Growth Factor-1 Receptor Inhibitor: Experiences in patients with squamous non-small cell lung carcinoma. Acta Oncol. 2011;50:441–7. doi: 10.3109/0284186X.2010.499370. [DOI] [PubMed] [Google Scholar]
- 138.Zhang W. Methods of IGF1R and ABL kinase modulators. 2009 US20090232828.
- 139.Shah N, Kasap C, Paquette R. Targeting drug-resistant CML and Ph+-ALL with the spectrum selective protein kinase inhibitor XL228. Blood. 2007;110:474. [Google Scholar]
- 140.Smith D, Britten C, Clary D, Nguyen L, Woodard P, Hurwitz H. A phase I study of XL228, a potent IGF1R/AURORA/SRC inhibitor, in patients with solid tumors or hematologic malignancies. J Clin Oncol. 2009;27:15s. [Google Scholar]
- 141.Shah P, Asatiani E, Cortes J, Paquette R, Pinilla-Ibarz J, Kasap K, et al. Interim results from a phase I clinical trial of the BCR-ABL inhibitor XL228 in drug-resistant PH+ leukemias. Haematologica. 2008;93:47. [Google Scholar]
- 142.Wittman M, Mastalerz H, Zimmermann K, Saulnier M, Velaparthi U, Vyas D, et al. Pyrollotriazine kinase inhibitors. 2011 US7534792.
- 143.Carboni JM, Wittman M, Yang Z, Lee F, Greer A, Hurlburt W, et al. BMS-754807, a small molecule inhibitor of insulin-like growth factor-1R/IR. Mol Cancer Ther. 2009;8:3341–9. doi: 10.1158/1535-7163.MCT-09-0499. [DOI] [PubMed] [Google Scholar]
- 144.Desai J, Solomon B, Davis I, Lipton L, Hicks R, Scott A, et al. Phase I dose-escalation study of daily BMS-754807, an oral, dual IGF-1R/insulin receptor (IR) inhibitor in subjects with solid tumors. J Clin Oncol. 2010;28:15s. [Google Scholar]
- 145.Harzstark ALRC, Diamond M, Jones J, Zavodovskaya M, Maddux B, Claros C, Goldfine I, Small EJ. A phase I trial of nordihydroguareacetic acid (NDGA) in patients with non-metastatic prostate cancer and rising PSA. J Clin Oncol. 2007;25:15500. [Google Scholar]
- 146.Kai K, D'Costa S, Sills RC, Kim Y. Inhibition of the insulin-like growth factor 1 receptor pathway enhances the antitumor effect of cisplatin in human malignant mesothelioma cell lines. Cancer Lett. 2009;278:49–55. doi: 10.1016/j.canlet.2008.12.023. [DOI] [PubMed] [Google Scholar]
- 147.Ji QS, Mulvihill MJ, Rosenfeld-Franklin M, Cooke A, Feng L, Mak G, et al. A novel, potent, and selective insulin-like growth factor-I receptor kinase inhibitor blocks insulin-like growth factor-I receptor signaling in vitro and inhibits insulin-like growth factor-I receptor dependent tumor growth in vivo. Mol Cancer Ther. 2007;6:2158–67. doi: 10.1158/1535-7163.MCT-07-0070. [DOI] [PubMed] [Google Scholar]
- 148.Vendluri V, Wright J, Coppola D, Buck E, Iwata K, Mokenze M. A small molecule inhibitor of insulin-like growth factor 1 receptor (PQIP) inhibits human pancreatic cancer cell proliferation in vitro and synergizes with erlotinib.. ASCO 2008 GI Cancers Symposium.; Orlando, FL. 2008. [Google Scholar]
- 149.Gariboldi MB, Ravizza R, Monti E. The IGFR1 inhibitor NVP-AEW541 disrupts a pro-survival and pro-angiogenic IGF-STAT3-HIF1 pathway in human glioblastoma cells. Biochem Pharmacol. 2010;80:455–62. doi: 10.1016/j.bcp.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 150.Premkumar DR, Jane EP, Pollack IF. Co-administration of NVP-AEW541 and dasatinib induces mitochondrial-mediated apoptosis through Bax activation in malignant human glioma cell lines. Int J Oncol. 2010;37:633–43. doi: 10.3892/ijo_00000712. [DOI] [PubMed] [Google Scholar]
- 151.Warshamana-Greene GS, Litz J, Buchdunger E, Garcia-Echeverria C, Hofmann F, Krystal GW. The insulin-like growth factor-I receptor kinase inhibitor, NVP-ADW742, sensitizes small cell lung cancer cell lines to the effects of chemotherapy. Clin Cancer Res. 2005;11:1563–71. doi: 10.1158/1078-0432.CCR-04-1544. [DOI] [PubMed] [Google Scholar]
- 152.Martins AS, Mackintosh C, Martin DH, Campos M, Hernandez T, Ordonez JL, et al. Insulin-like growth factor I receptor pathway inhibition by ADW742, alone or in combination with imatinib, doxorubicin, or vincristine, is a novel therapeutic approach in Ewing tumor. Clin Cancer Res. 2006;12:3532–40. doi: 10.1158/1078-0432.CCR-05-1778. [DOI] [PubMed] [Google Scholar]
- 153.Kang HG, Jenabi JM, Liu XF, Reynolds CP, Triche TJ, Sorensen PH. Inhibition of the insulin-like growth factor I receptor by epigallocatechin gallate blocks proliferation and induces the death of Ewing tumor cells. Mol Cancer Ther. 2010;9:1396–407. doi: 10.1158/1535-7163.MCT-09-0604. [DOI] [PubMed] [Google Scholar]
- 154.Chen ZP, Schell JB, Ho CT, Chen KY. Green tea epigallocatechin gallate shows a pronounced growth inhibitory effect on cancerous cells but not on their normal counterparts. Cancer Lett. 1998;129:173–9. doi: 10.1016/s0304-3835(98)00108-6. [DOI] [PubMed] [Google Scholar]
- 155.Pappano WN, Jung PM, Meulbroek JA, Wang YC, Hubbard RD, Zhang Q, et al. Reversal of oncogene transformation and suppression of tumor growth by the novel IGF1R kinase inhibitor A-928605. BMC Cancer. 2009;9:314. doi: 10.1186/1471-2407-9-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Vanamala J, Reddivari L, Radhakrishnan S, Tarver C. Resveratrol suppresses IGF-1 induced human colon cancer cell proliferation and elevates apoptosis via suppression of IGF-1R/Wnt and activation of p53 signaling pathways. BMC Cancer. 2010;10:238. doi: 10.1186/1471-2407-10-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lee MH, Choi BY, Kundu JK, Shin YK, Na HK, Surh YJ. Resveratrol suppresses growth of human ovarian cancer cells in culture and in a murine xenograft model: eukaryotic elongation factor 1A2 as a potential target. Cancer Res. 2009;69:7449–58. doi: 10.1158/0008-5472.CAN-09-1266. [DOI] [PubMed] [Google Scholar]
- 158.Burden M, Ellis J, Hamblin P, Lewis A, Shah R. Novel antibodies against IGF-1R. 2008 WO2008098917.
- 159.Wraight C, Werther G, Dean J, Dobie K. Modulation of insulin like growth factor 1 receptor expression. 2008 US7468356.
- 160.Furukawa J, Wraight C, Monia B, Gleave M, Cox M. Antisense oligonucleotide targeting insulin-like growth factor-1 receptor (IGF-1R) enhances paclitaxel sensitivity in a castrate-resistant and paclitaxel-resistant prostate cancer model. American Association for Cancer Research (AACR). 101st Annual Meeting 2010.; Washington, DC. 2010. [Google Scholar]
- 161.Atzori F, Tabernero J, Cervantes A, Botero M, Hsu K, Brown H, et al. A phase I, pharmacokinetic (PK) and pharmacodynamic (PD) study of weekly (qW) MK-0646, an insulin-like growth factor-1 receptor (IGF1R) monoclonal antibody (MAb) in patients (pts) with advanced solid tumors. J Clin Oncol. 2008;26:3519. [Google Scholar]
- 162.Cao L, Yu Y, Darko I, Currier D, Mayeenuddin LH, Wan X, et al. Addiction to elevated insulin-like growth factor I receptor and initial modulation of the AKT pathway define the responsiveness of rhabdomyosarcoma to the targeting antibody. Cancer Res. 2008;68:8039–48. doi: 10.1158/0008-5472.CAN-08-1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Fu P, Ibusuki M, Yamamoto Y, Hayashi M, Murakami K, Zheng S, et al. Insulin-like growth factor-1 receptor gene expression is associated with survival in breast cancer: a comprehensive analysis of gene copy number, mRNA and protein expression. Breast Cancer Res Treat. 2011 doi: 10.1007/s10549-011-1605-0. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 164.Gualberto A, Karp DD. Development of the monoclonal antibody figitumumab, targeting the insulin-like growth factor-1 receptor, for the treatment of patients with non-small-cell lung cancer. Clin Lung Cancer. 2009;10:273–80. doi: 10.3816/CLC.2009.n.038. [DOI] [PubMed] [Google Scholar]
- 165.Gualberto A, Dolled-Filhart M, Gustavson M, Christiansen J, Wang YF, Hixon ML, et al. Molecular analysis of non-small cell lung cancer identifies subsets with different sensitivity to insulin-like growth factor I receptor inhibition. Clin Cancer Res. 2010;16:4654–65. doi: 10.1158/1078-0432.CCR-10-0089. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 166.Gualberto A, Hixon ML, Karp DD, Li D, Green S, Dolled-Filhart M, et al. Pre-treatment levels of circulating free IGF-1 identify NSCLC patients who derive clinical benefit from figitumumab. Br J Cancer. 2011;104:68–74. doi: 10.1038/sj.bjc.6605972. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 167.Huang F, Greer A, Hurlburt W, Han X, Hafezi R, Wittenberg GM, et al. The mechanisms of differential sensitivity to an insulin-like growth factor-1 receptor inhibitor (BMS-536924) and rationale for combining with EGFR/HER2 inhibitors. Cancer Res. 2009;69:161–70. doi: 10.1158/0008-5472.CAN-08-0835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zha J, O'Brien C, Savage H, Huw LY, Zhong F, Berry L, et al. Molecular predictors of response to a humanized anti-insulin-like growth factor-I receptor monoclonal antibody in breast and colorectal cancer. Mol Cancer Ther. 2009;8:2110–21. doi: 10.1158/1535-7163.MCT-09-0381. [DOI] [PubMed] [Google Scholar]
- 169.Law JH, Habibi G, Hu K, Masoudi H, Wang MY, Stratford AL, et al. Phosphorylated insulin-like growth factor-i/insulin receptor is present in all breast cancer subtypes and is related to poor survival. Cancer Res. 2008;68:10238–46. doi: 10.1158/0008-5472.CAN-08-2755. [DOI] [PubMed] [Google Scholar]
- 170.Lu D, Zhang H, Koo H, Tonra J, Balderes P, Prewett M, et al. A fully human recombinant IgG-like bispecific antibody to both the epidermal growth factor receptor and the insulin-like growth factor receptor for enhanced antitumor activity. J Biol Chem. 2005;280:19665–72. doi: 10.1074/jbc.M500815200. [DOI] [PubMed] [Google Scholar]
- 171.Emanuel S, Engle L, Camphausen R, Wright M, Chao G, Gottardis M, et al. Bispecific EGFR/IGF1R binding molecule. 2010 US20100179094.
- 172.Di Cosimo S, Bendell JC, Cervantes A. A phase I study of the oral mTOR inhibitor ridaforolimus (RIDA) in combination with the IGF-1R antibody dalotuzumab (DALO) in patients (pts) with advanced solid tumors. J Clin Oncol. 2010;28 abstract 3008. [Google Scholar]
- 173.Bertrand FE, Steelman LS, Chappell WH, Abrams SL, Shelton JG, White ER, et al. Synergy between an IGF-1R antibody and Raf/MEK/ERK and PI3K/Akt/mTOR pathway inhibitors in suppressing IGF-1R-mediated growth in hematopoietic cells. Leukemia. 2006;20:1254–60. doi: 10.1038/sj.leu.2404217. [DOI] [PubMed] [Google Scholar]
- 174.Hoelzemann G, Crassier H, Rautenberg W. Pyrazole derivatives. 2010 US7767696.
- 175.Goetsch L, Corvaia N, Duflos A, Haeuw J-F, Leger O, Beck A. Anti-IGF-IR and/or anti-insulin/IGF-I hybrid receptors antibodies and uses thereof. 2009 US7553485.
- 176.Dmitrov D, Feng Y. Human monoclonal antibodies that specifically bind IGF-II. 2010 US7824681.
- 177.Kirman I, Whelan R. Use of insulin-like growth factor binding protein 3 (IGF-BP3) for inhibition of tumor growth. 2007 US20070142289.
- 178.Oh Y, Rosenfeld R, Buckway C. Mutant IGFBP-3 molecules that do not bind to IGFs, but retain their ability to functionally bind IGFBP-3 receptor. 2010 US7714102.
- 179.Gleave M, Signaevsky M. Bispecific antisense oligonucleotides that inhibit IGFBP-2 and IGFBP-5 and methods of using same. 2010 US20100267808.
- 180.Cohen B, Bedian V. Modified human IGF-1R antibodies. 2009 US7575746.
- 181.Chang C, Goldenberg D, Rossi E. Tetrameric cytokines with improved biological activity. 2010 US20100189689.
- 182.Singh R, Tavares D, Dagdigan N. Anti-IGF-1 receptor antibody. 2009 US7538195.
- 183.Chang C, Losman M, Goldenberg D. Novel class of monospecific and bispecific humanized antibodies that target the insulin-like growth factor type I receptor (IGF-1R). 2010 US20100226884.
- 184.Aleksic T, Chitnis MM, Perestenko OV, Gao S, Thomas PH, Turner GD, et al. Type 1 insulinf-like growth factor receptor translocates to the nucleus of human tumor cells. Cancer Res. 2010;70:6412–9. doi: 10.1158/0008-5472.CAN-10-0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Salom B, D'Anello M, Brasca M, Giordano P, Martina K, Angelucci F, et al. Pyrrolo[2,3-b]pyridine derivatives active as kinase inhibitors and pharmaceutical compositions comprising them. 2011 US20100210476.
- 186.Burgdorf L, Bruge D, Greiner H. Oxindoles as kinase inhibitors. 2010 US7781475.
- 187.Mayer S, Miller L. 3-Cyanoquinolines, methods for preparation and use as insulin-like growth factor inhibitors. 2009 US20090264427.
- 188.Heinrich T, Gradler U, Bottcher H, Blaukat A, Shutes A. Allosteric IGF-1R inhibitors. ACS Med Chem Lett. 2010;1:199–203. doi: 10.1021/ml100044h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Khvorova A, Reynolds A, Leake D, Marshall W, Read S, Scaringe S. siRNA targeting insulin-like growth factor 1 receptor (IGF-1R). 2009 US7638621.