Abstract
Cytokines play a crucial role in the modulation of inflammatory response in the gastrointestinal tract. Pro-inflammatory cytokines including tumor necrosis factor-α, interferon-γ, interleukin-1β (IL-1β), and interleukin-12 are essential in mediating the inflammatory response, while anti-inflammatory cytokines including interleukin-10 and transforming growth factor-β are important in the attenuation or containment of inflammatory process. It is increasingly recognized that cytokines have an important physiological and pathological effect on intestinal tight junction (TJ) barrier. Consistent with their known pro-inflammatory activities, pro-inflammatory cytokines cause a disturbance in intestinal TJ barrier, allowing increased tissue penetration of luminal antigens. Recent studies indicate that the inhibition of cytokine induced increase in intestinal TJ permeability has an important protective effect against intestinal mucosal damage and development of intestinal inflammation. In this review, the effects of various pro-inflammatory and anti-inflammatory cytokines on intestinal TJ barrier and the progress into the mechanisms that mediate the cytokine modulation of intestinal TJ barrier are reviewed.
Keywords: Tight Junctions, Cytokines, Intestinal Epithelial Cells, Barrier Function, Inflammation, Review
2. INTRODUCTION
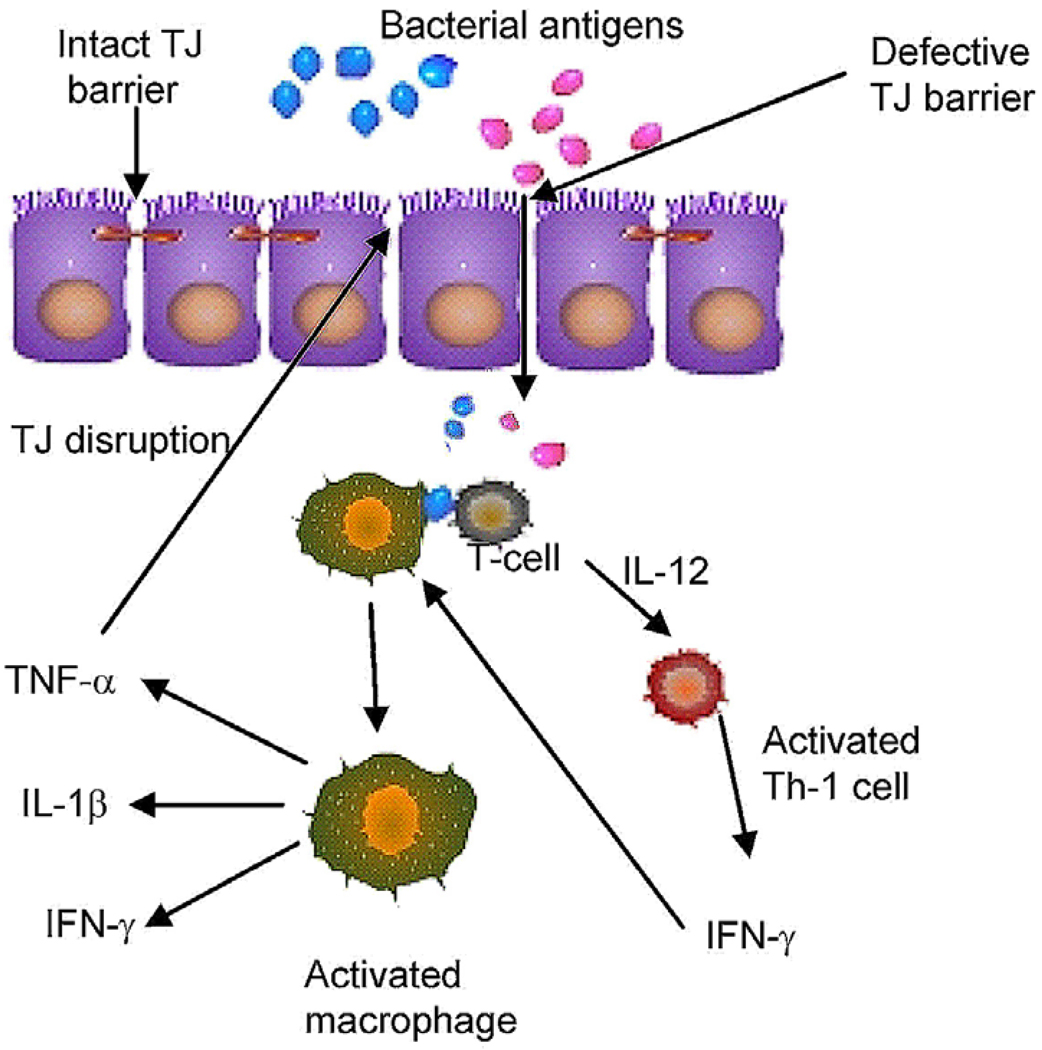
Gastrointestinal epithelial barrier consists of extracellular and intracellular factors that provide barrier function against epithelial penetration of noxious luminal substances (1, 2). The term intrinsic epithelial barrier refers to the physical barrier formed by the elements of intestinal epithelial cells including plasma membrane, intracellular contents and intercellular junctions (1). The apical plasma membrane and intercellular tight junctions form the primary intrinsic epithelial barrier against the luminal contents. The bi-lipid composition of the enterocyte membrane provides an effective barrier against transcellular permeation of water soluble molecules; and the intercellular tight junctions (TJs), located at the apical-most lateral membrane, act as a primary barrier against the paracellular permeation of luminal substances (1). In a normal healthy state, intestinal epithelial TJs provide an effective barrier against paracellular penetration of noxious substances and antigens present in the gastrointestinal lumen, including bacteria, bacterial toxins, bacterial by-products, digestive enzymes, and degraded food products (1, 3). However, in diseased states, such as in Crohn’s disease, ulcerative colitis, non-steroidal anti-inflammatory agent associated enteritis, heat stroke, alcoholic hepatitis, irritable bowel syndrome, and in bacterial infection caused by Escherichia coli, Clostridium difficile, and Vibrio cholera, intestinal TJ barrier is defective allowing increased paracellular permeation of normally excluded luminal antigens (1, 3). The “leaky” intestinal tight junction barrier allows increased antigenic penetration into the underlying intestinal tissue (Figure 1) (1, 3). The foreign antigens are then processed by the antigen presenting cells and helper T-lymphocytes and an inflammatory response is activated (Figure 1); this leads to an increase in the production and secretion of pro-inflammatory cytokines and pro-inflammatory mediators and recruitment of circulating inflammatory cells. The pro-inflammatory cytokines produced during inflammatory response, including tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), interleukin-1β (IL-1β) and IL-12, cause disruption of the intestinal TJ barrier resulting in a further increase in TJ permeability (1, 2, 4, 5). Thus, in addition to their direct effects on immune activation, pro-inflammatory cytokines also exacerbate inflammatory process by allowing increased intestinal permeation of luminal antigens (1, 4, 5). An intriguing possibility exists that anti-inflammatory cytokines such as interleukin-10 and transforming growth factor-β may also attenuate or protect against intestinal inflammation by preserving the TJ barrier function (6, 7).
Figure 1.
Proposed role of defective intestinal epithelial tight junction (TJ) barrier in the pathogenesis of intestinal inflammation.
Since the initial discovery by Madara and Stafford in 1989 that IFN-γ causes a disruption of intestinal epithelial TJ barrier function (8), number of other cytokines have been found to modulate intestinal TJ barrier function (1, 2, 5). Both in-vitro and in-vivo studies have demonstrated the potential importance of cytokine-induced disruption of intestinal TJ barrier as a mechanism contributing to the development of intestinal inflammation (1, 4, 5, 9). Additionally, in-vivo studies have shown that therapeutic preservation of intestinal TJ barrier in animal models of intestinal inflammation prevents the development of intestinal inflammation (9), suggesting a direct causal linkage between TJ barrier defect and development of intestinal inflammation. Understanding the intracellular and molecular mechanisms that mediate the cytokine modulation of intestinal TJ barrier function will be important in developing future therapeutic strategies to preserve the intestinal TJ barrier function during inflammatory conditions. In this review, we summarize the effects of various cytokines in the modulation of intestinal epithelial TJ permeability. Although the primary focus of this review is on intestinal epithelial TJ barrier, the studies in other cell types, including endothelial cells, are also discussed. The effects on IFN-γ and TNF-α are covered in a greater detail due to the large volume of studies related to these cytokines. (The readers are also referred to other excellent reviews on this topic (2, 4, 5)).
3. CYTOKINE MODULATION OF INTESTINAL EPITHELIAL TIGHT JUNCTION BARRIER
3.1. Interferon Gamma (IFN-γ)
Interferon Gamma is a prototypical pro-inflammatory cytokine (10) and is produced primarily by lymphocytes and by antigen presenting cells (dendritic cells, monocytes etc.). Macrophages are a major cellular target of IFN-γ (Figure 1); the activation of macrophages by IFN-γ is a major stimulant to induce a “Th-1” type immune response (10). IFN-γ is important historically as it was the first cytokine shown to affect epithelial TJ barrier function (8). In 1989, Madara and Stafford (8) reported for the first time that IFN-γ causes an increase in epithelial TJ permeability in T84 human derived intestinal epithelial monolayers. The IFN-γ treatment caused a dose-and time-dependent decrease in T84 trans-epithelial resistance (TER) and an increase in transepithelial permeability to extracellular markers mannitol and inulin. They showed that the drop in TER was attributable entirely to an increase in TJ permeability by performing a unidirectional dual flux analysis using 22Na and 3H mannitol (8). The dual flux analysis indicated that the increase in ion flux could be completely accounted for by the increase in paracellular flux (8). Since then, a number of other investigators have confirmed the initial findings by Madara and Stafford and have examined the potential mechanisms through which IFN-γ regulates the intestinal epithelial tight junction barrier. In 1990, Adams et al. demonstrated that the site of interferon-γ action was at the basolateral surface, through the interferon-gamma receptor, as anti-IFN-γ receptor blocking antibodies prevented the IFN-γ induced drop in T84 monolayer TER (11). Using polyethylene glycol molecules of increasing molecular weights, Watson demonstrated that the TJ barrier defect induced by IFN-γ in T84 cells was accompanied by a greater increase in permeability to larger-sized than smaller-sized molecules (12). These investigators postulated that the tight junction barrier had two populations of pores and that IFN-γ selectively causes an increase in larger, non-restrictive pores.
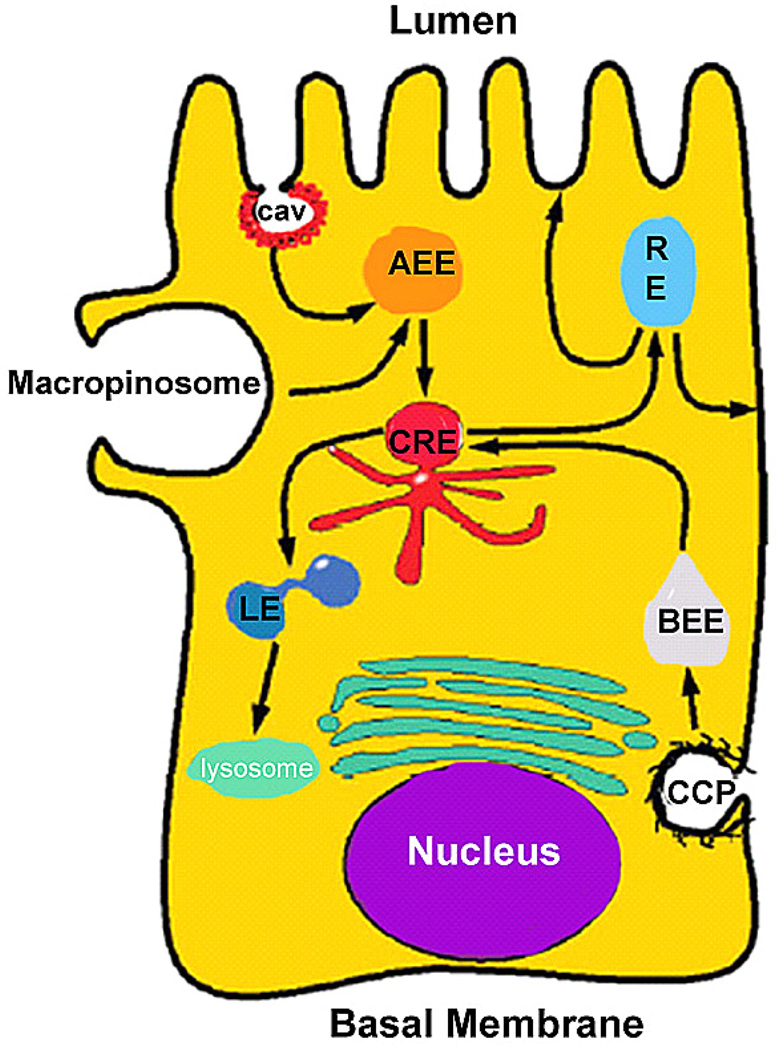
A number of investigators have described the effects of IFN-γ on tight junction protein levels and cellular localization. Nusrat and colleagues in a series of elegant studies described macropinocytosis of tight junction proteins (occludin, JAM-A, claudin-1) as a potential mechanism through which IFN-γ exerts its TJ barrier disrupting effects (13, 14) (Figure 2). Using confocal microscopy, these investigators demonstrated that IFN-γ induces an uptake of occludin protein from junctional complexes into early endosomes through macropinocytosis. In a separate report, they showed that internalization of occludin and claudin-1 colocalizes with large actin-coated vacuoles by confocal microscopy. The inhibitors of clathrin or caveolin mediated endocytic pathways did not affect the TJ protein endocytosis. The IFN-γ induced macropinocytosis required myosin light chain (MLC) phosphorylation. Additionally, the IFN-γ induced TJ protein endocytosis and drop in T84 TER also required activation of Rho kinase (15). Thus, it was concluded that macropinocytosis of TJ proteins may be an important mechanism mediating the IFN-γ modulation of TJ barrier. Other investigators have also examined the effects of IFN-γ on specific TJ proteins. Several investigators have shown that IFN-γ causes a decrease in claudin-2 expression (16–18). Willemsen et al suggested that serine protease cleavage of claudin-2 may lead to the decrease in claudin-2 expression (18), as inhibition of serine proteases protected against IFN-γ induced decrease in claudin-2 and TJ barrier disruption.
Figure 2.
Interferon-γ induced endocytosis of tight junction proteins. (Reproduced from Ivanov (14), by pending permission)
The intracellular signaling pathways that mediate the IFN-γ effect on TJ barrier have been reported by several laboratories. It has been shown that IFN-γ induced barrier disruption was not mediated by cellular necrosis or apoptosis (8, 19). While STAT-1 is an important signaling molecule for IFN-γ, and is activated in T84 cells, the inhibition of STAT-1 activation did not prevent the IFN-γ induced drop in TER, suggesting that STAT-1 pathway is not involved in TJ barrier regulation (20). Studies from our laboratory indicated that the IFN-γ induced increase in T84 TJ permeability was associated with an activation of PI3-kinase pathway; and that the IFN-γ induced drop in TER or increase in paracellular permeability was inhibited by PI3-kinase inhibition (21). The PI3-kinase activation caused a delayed activation of NF-κB. The IFN-γ induced increase in T84 TJ permeability and decrease in occludin protein expression was inhibited by blockade of either PI-3 kinase or NF-κB pathways (21). Similarly, McKay et al also reported that IFN-γ induced increase in TJ permeability and bacterial translocation was inhibited by PI3-kinase inhibitors (22). Together, these studies suggested that PI3-kinase or NF-kB pathways (but not STAT-1 pathway or apoptosis) may be involved in IFN-γ induced modulation of T84 TJ barrier.
IFN-γ also causes an increase in TJ permeability in other intestinal epithelial cell lines including HT-29 and Caco-2 cells (23). Various anti-inflammatory agents including green tea (24), IL-10 (6), TGF-β (25) and 5-ASA (23) have been shown to have a barrier protecting effect on the IFN-γ induced barrier disruption of T84 monolayers. Other factors potentiate the IFN-γ effect on intestinal TJ barrier including TNF-α (26) and hypoxia. IFN-γ treatment also induces an increase in permeability in various other epithelial cell types including retinal (27), cholangiocyte and lung epithelial cells (28).
Animal models of epithelial barrier dysfunction have also provided important supporting evidence for the role of IFN-γ in causing an increase in intestinal permeability. In IL-10 knockout mice, the increase in intestinal permeability correlated with an increase in intestinal tissue levels of IFN-γ (29), (30). Similarly, total parenteral nutrition (TPN) administration induced increase in mouse intestinal permeability also correlated with an increase in IFN-γ expression (31). The TPN induced increase in intestinal permeability was attenuated in an IFN-γ knockout mouse (32), indicating that IFN-γ expression was required for the increase in intestinal permeability. In a mouse model of stress-induced increase in mouse colonic permeability, IFN-γ expression was also required for the increase in colonic permeability (33). Similarly, septic or hemorrhagic shock induced increase in intestinal permeability in mice was also associated with increased levels of IFN-γ (34). The administration of IFN-γ antibodies protected the mice from developing hemorrhagic shock induced intestinal barrier disturbance (35). Two models of obese, leptin deficient mice also have an increase in intestinal permeability and elevated IFN-γ levels (36). Collectively, these in-vivo studies suggested that IFN-γ may play an important pathogenic role in disruption of intestinal TJ barrier under a variety of clinically relevant conditions.
3.2. Tumor Necrosis Factor-α (TNF-α)
Tumor necrosis factor-α was originally described as a circulating factor that causes necrosis of tumors, but has since been identified as a key regulator of the inflammatory response (37–41). TNF is produced predominantly by activated macrophages and T lymphocytes as a 26 kDa protein, pro-TNF, which is expressed on the plasma membrane. The pro-TNF is cleaved in the extracellular domain and released as a soluble 17 kDA TNF-α. Both membrane-associated and soluble TNFs are active in their trimeric forms, and the two forms of TNF may have distinct biological activities. TNF-α is usually not detectable in healthy individuals, but elevated serum and tissue levels are found in inflammatory and infectious conditions and serum levels correlate with the severity of infection and inflammatory response (40). All known responses to TNF-α are triggered by binding to one of two distinct receptors, designated TNFR1 (also known as TNFRSF1A, CD120a, p55) and TNFR2 (also known as TNFRSF1B, CD120b, p75), which are differentially regulated on various cell types in normal and diseased tissue (42). Both the pro-inflammatory and the programmed cell death pathways that are activated by TNF-α are mediated through TNFR1 (40, 43).
Number of studies has shown that TNF-α causes an increase in epithelial TJ permeability in various cell types. In 1993, Mullin et al. first reported that TNF-α causes a decrease in transepithelial resistance (TER) in renal epithelial cell line, LLC-PK1 (44). The TNF-α-induced drop in TER was dose dependent (5–50 ng/ml) and correlated with an increase in transepithelial flux of paracellular marker mannitol. The TNF-α induced decrease in LLC-PK1 TER was inhibited by TNF-α antibody, confirming that TNF-α itself was responsible for the drop in TER. Since this initial report in LLC-PK1 cells, number of other studies have shown that TNF-α causes an increase in TJ permeability in intestinal epithelial monolayers. In human derived intestinal epithelial cell line Caco-2, TNF-α caused a dose- and time-dependent decrease in Caco-2 TER (45, 46). The initial drop in Caco-2 TER occurred after 12 h and the maximal drop occurred at 48–72 h (45, 46). The drop in Caco-2 TER linearly correlated with an increase in epithelial permeability to paracellular marker inulin (m.w.= 5000 g/mol) (46), confirming an inverse relationship between TNF-α effect on TER and paracellular permeability. Similarly, TNF-α also caused an increase in TJ permeability in T84 and HT-29/B6 intestinal epithelial monolayers (26, 47). The TNF-α effect on HT-29/B6 monolayers was more dramatic than in other intestinal cell lines, and TNF-α treatment resulted in an 81% drop in TER over a 24 h treatment period. The morphologic evaluation of the TJ complex using freeze-fracture analysis revealed a decrease in the number and the depth of TJ strands and alteration in TJ complexity in response to TNF-α treatment. The tyrosine kinase inhibitor, genistein, and protein kinase A (PKA) inhibitor, H-8, attenuated the effect of TNF-α on HT-29/B6 TJ permeability, suggesting that tyrosine kinases and PKA may be involved in mediating the TNF-α regulation of TJ barrier function (47).
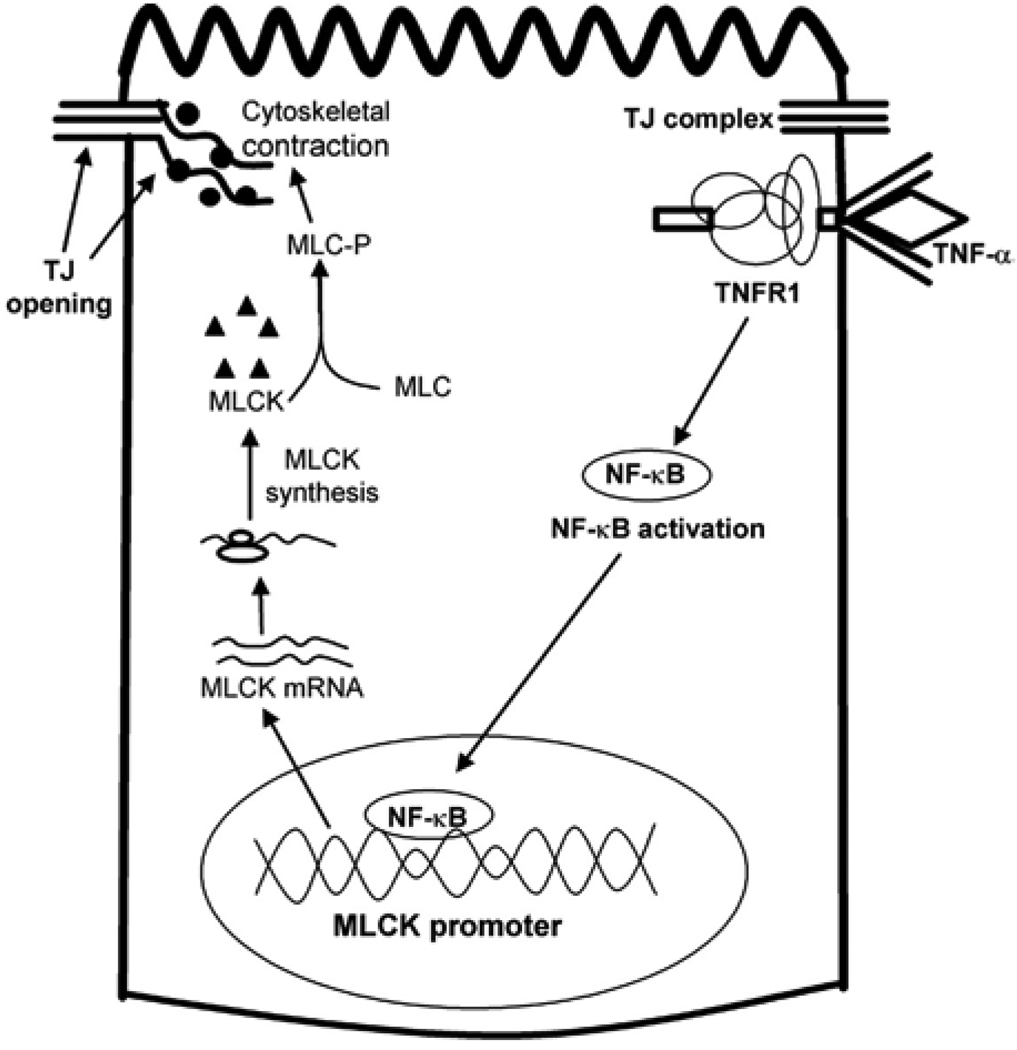
In studies from our laboratory, we elucidated some of the intracellular and molecular mechanisms that mediate the TNF-α regulation of intestinal TJ permeability (46, 48, 49). The time-course of TNF-α-induced increase in Caco-2 TJ permeability closely correlated with an increase in myosin light-chain kinase (MLCK) protein expression and activity (48); and the inhibition of TNF-α induced increase in MLCK protein synthesis (cycloheximide) or MLCK activity (ML-7 or ML-9) inhibited the TNF-α induced increase in Caco-2 TJ permeability. These results suggested that the TNF-α induced increase in MLCK protein expression and activity were required for the increase in TJ permeability. The TNF-α increase in MLCK protein expression was associated with an increase in MLCK mRNA level but not a decrease in protein degradation; and inhibition of MLCK mRNA transcription prevented the increase in Caco-2 TJ permeability (48), suggesting that the TNF-α increase in MLCK expression and TJ permeability was mediated in part by MLCK gene transcription. In subsequent studies, the TNF-α effect on MLCK gene activity was determined by MLCK promoter analysis (49). In these studies, MLCK promoter region was cloned into a pGL3 basic plasmid vector, and transfected into Caco-2 cells, and the TNF-α effect on MLCK promoter activity was determined. The TNF-α treatment resulted in an increase in MLCK promoter activity in the transfected Caco-2 monolayers (49). The TNF-α induced increase in MLCK promoter activity and increase in MLCK protein expression and TJ permeability were mediated in part by activation of nuclear transcription factor NF-κB (48, 49). The TNF-α induced increase in MLCK gene activity and protein expression was preceded by activation of NF-κB; and inhibition of NF-κB activation prevented the increase in MLCK gene and protein expression and Caco-2 TJ permeability. By targeted gene deletion and site-directed mutagenesis of MLCK promoter region, the molecular determinants that mediate the TNF-α regulation of MLCK promoter activity was also delineated (50). These studies indicated that a κB binding site (cis-κB site) located within the minimal MLCK promoter region was an essential element mediating the NF-κB activation of MLCK gene activity (50). Together, these studies demonstrated that the TNF-α induced increase in Caco-2 TJ permeability was mediated in part by activation of nuclear transcription factor NF-κB (Figure 3). The activated NF-κB translocates to the nucleus, binds to the cis-κB binding site on MLCK promoter region, and activates the MLCK gene transcription and protein synthesis process (Figure 3). The increase in MLCK protein level and activity results in MLCK-triggered opening of the TJ barrier.
Figure 3.
Mechanism of TNF-α induced opening of intestinal tight junction (TJ) barrier.
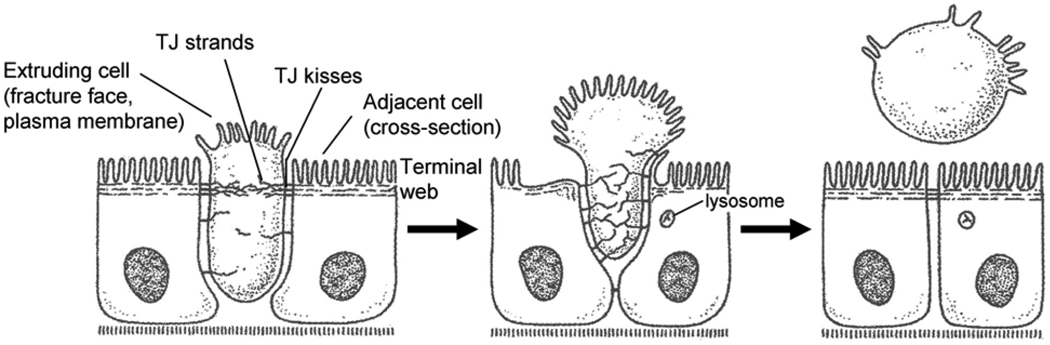
TNF-α is known to have dual effects on cell survival and cell death via selective activation of NF-κB or caspase pathways, respectively (51). The possibility that the TNF-α induced increase in intestinal epithelial permeability may be related to its effect on epithelial cell apoptosis has been explored by several laboratories (52, 53). It has been postulated that the TNF-α induced apoptosis of intestinal epithelial cells may cause a large gap or opening between adjacent intestinal epithelial cells; leading to an increase in permeation through the epithelial gaps caused by the dying cells (53). Gitter et al. reported that TNF-α causes a 2-fold increase in apoptosis of HT-29/B6 intestinal cells. By measuring electrical conductance across the epithelial region in which intestinal cells were undergoing apoptosis, they concluded that 56% of TNF-α induced increase in epithelial permeability in HT-29/B6 monolayers could be accounted for by the increase in cellular apoptosis (53). This finding challenges the earlier reports which demonstrated that epithelial cell apoptosis is a highly regulated process in which the epithelial barrier function is maintained throughout the extrusion or clearance of apoptotic cell (54–56). It had been previously reported by Madara that the healthy adjacent cells rapidly stretch out and maintain epithelial barrier function throughout the extrusion of dying cells (Figure 4) (54, 56, 57). Consistent with such coordinated extrusion process, it has been demonstrated that mechanically induced single cell defects in the epithelial surface are rapidly repaired by extensions of adjacent cells via an actin/myosin contractile ‘purse-string’ mechanism (52, 53, 58, 59). The rapid restitution of single cell defect by purse-string extension of adjacent cells was associated with establishment of intact TJ barrier (52). Additional evidence against the role of apoptosis included the finding that the TNF-α induced increase in Caco-2 TJ permeability was not associated with an increase in Caco-2 cell apoptosis (45, 46). In Caco-2 monolayers, TNF-α induced increase in TJ permeability required an activation of NF-κB, an anti-apoptotic factor (46). Breuewer et al. also examined the role of apoptosis in TNF-α/IFN-γ induced increase in T84 TJ permeability (19). In T84 monolayers, TNF-α and IFN-γ combination treatment for 72 h caused a marked increase in TJ permeability (19). The TNF-α/IFN-γ treatment caused a 3-fold increase in T84 cell apoptosis; however, the inhibition of TNF-α/IFN-γ-induced apoptosis with pharmacologic inhibitor z-val-ala-asp-fluoromethylketone did not affect the increase in TJ permeability (19). Breuewer et al. concluded that these findings clearly “separate the proapoptotic effects of IFN-γ and TNF-α from their ability to disrupt barrier function” (19). While the precise contribution of TNF-α induced apoptosis to the observed increase in epithelial TJ permeability remains to be further elucidated, above reports suggest that apoptosis is not necessary for the TNF-α induced increase in TJ permeability.
Figure 4.
Extrusion of apoptotic cells from intestinal epithelia. (Reproduced from Madara (56), by permission)
TNF-α also causes an increase in endothelial permeability. The TNF-α induced increase in human pulmonary microvascular endothelial permeability has been shown to be associated with the activation of Rho kinase (60). The inhibition of Rho kinase activity by pharmacological inhibitor, Y27632, prevented the TNF-α-induced alteration of actin cytoskeleton and increase in endothelial permeability, suggesting that the TNF-α effect on endothelial barrier function was mediated in part by Rho kinase pathway (60). Similarly, Nwariaku et al. also reported that Rho kinase activation was required for the TNF-α-induced increase in MAPK activity and increase in endothelial permeability (61). Others have implicated MLCK in mediating the endothelial permeability (62). In contrast, McKenzie and Ridley reported that the TNF-α increase in endothelial permeability was unrelated to Rho or MLCK mediated cytoskeletal contractile process but was associated with an alteration in junctional localization of transmembrane proteins occludin and JAM-A (63).
Similar to the studies described above for TNF-α, the cytokine combination (TNF-α and IFN/γ) induced increase in Caco-2 BBE TJ permeability also required an increase in MLCK protein expression and activity (64). The TNF-α/IFN-γ combination induced increase in T84 TJ permeability also correlated with an internalization of apical junctional complex (AJC) including junction adhesion molecule 1 (JAM-1), occludin, and claudin-1 and 4. The treatment with TNF-α/IFN-γ caused a time-dependent drop in T-84 TER over a 72 h experimental period and the drop in TER correlated with a disruption in the junctional localization of tight junction proteins occludin, claudin 1, claudin 4 and JAM-1. The tight junction proteins were internalized in detergent-insoluble “raft-like” membrane microdomains (65, 66). Similarly, Turner and co-workers have also shown that LIGHT (lymphotoxin-like inducible protein that competes with glycoprotein D for herpes virus entry on T cells), a member of TNF core family, and IFN-γ combination induced increase in intestinal epithelial permeability in-vivo and in-vitro was associated with a caveolin-1-dependent endocytosis of junctionally located occludin (9). The inhibition of occludin endocytosis prevented the LIGHT/IFN-γ induced increase in intestinal TJ permeability, suggesting that occludin endocytosis may be an important mechanism mediating the LIGHT/IFN-γ modulation of intestinal TJ barrier function (9).
3.3. Interleukin-1 β (IL-1β)
IL-1β is a pro-inflammatory cytokine released by various immune modulating cells and has been shown to play an important role in the pathogenesis of intestinal inflammation in inflammatory bowel disease (IBD) (67, 68). IL-1 family consists of three members IL-1α, IL-1β and IL-1 receptor antagonist (IL-1ra) (67, 69). IL-1β is synthesized initially as a 31 kD biologically inactive propeptide which is released as the mature active (17 kD) peptide following cleavage by IL-1 converting enzyme (ICE) (70). IL-1β levels are markedly elevated in intestinal tissues in patients with IBD (71, 72), and correlation between increasing levels of IL-1β and the level of intestinal inflammation has been demonstrated (73). Recent studies have shown that IL-1β at physiologically relevant concentrations (1–10 ng/ml) causes an increase in intestinal epithelial TJ permeability (74). IL-1β caused a time dependent drop (0–72 h) in Caco-2 transepithelial resistance which correlated with an increase in paracellular permeability (inulin flux) (74). The IL-1β induced increase in Caco-2 TJ permeability appeared to be modulated in part by regulation of MLCK gene activity. The IL-1β induced increase in Caco-2 TJ permeability was preceded by an increase in MLCK mRNA and protein levels; and targeted inhibition of IL-1β induced MLCK mRNA or MLCK protein expression prevented the increase in TJ permeability (75).
IL-β also caused an increase in retinal pigment and pulmonary epithelial TJ permeability and alteration in TJ protein expression (76, 77). The IL-1β induced increase in retinal pigment epithelial TJ permeability was associated with a down-regulation of occludin protein and increase in claudin-1 expression (76). Other investigators have also shown that IL-1β causes an increase in claudin-2 expression and hyperphosphorylation of occludin in rat hepatocytes, a decrease in occludin expression and increase in claudin-1 expression in human astrocytes, and a decrease in occludin expression in Caco-2 cells (74, 78, 79). IL-1β also caused an increase in pulmonary epithelial permeability (80). IL-1β (100 ng/ml) treatment of pulmonary epithelial monolayers resulted in a drop in TER and increase in dextran flux rate over a 48 h treatment period (80). IL-1β has also been implicated to cause an increase in porcine brain capillary endothelial permeability during hypoxia (77). Yamagata et al. showed that hypoxia caused a time-dependent decrease in the TER in brain capillary endothelial monolayers. The hypoxia-induced decrease in TER was inhibited by anti-IL-1 antibody (77).
3.4. Interleukin-2 (IL-2)
Interleukin-2 is produced by CD4+ lymphocytes, and is a potent T-lymphocyte growth factor (81). IL-2 treatment did not affect T84 monolayer TER. (8). Similarly; IL-2 treatment also did not affect the TER of cholangiocytes (82). The administration of intravenous IL-2 to humans also did not affect intestinal permeability as assessed by lactulose/mannitol flux rates (83). Interestingly, IL-2 administration to sheep causes a severe capillary leak syndrome with increased pulmonary capillary permeability (84). This increase in capillary permeability was also confirmed in humans using conductivity measurements (85). Further studies are needed to better clarify the effects of IL-2 (if any) on epithelial and endothelial TJ permeability.
3.5. Interleukin-4 (IL-4)
Interleukin 4 is an important cytokine that mediates the profile of cytokine production of CD4+ helper T-cells towards a Th2 paradigm cytokine response. IL-4 also induces B-cell activation (86) and IL-10 secretion. It is considered a major mediator of allergic diseases. IL-4 has been shown to cause an increase in epithelial permeability in various cell types. IL-4 treatment of intestinal T84 monolayers caused a 60% decrease in TER at 24 hours and an increase in the transepithelial flux of Dextran 4000 (17). The IL-4 induced decrease in T84 TER was prevented by PI3-kinase inhibitors wortmannin and LY294002 (87). The IL-4 induced increase in T84 TJ permeability was also shown to be associated with an increase in the expression of claudin-2. The IL-4 induced drop in TER was inhibited by TGF-beta (88). Together, these results suggested that the IL-4 effect on T84 TJ barrier may be mediated by activation of PI3-kinase and modulation of claudin-2 expression. IL-4 treatment of kidney glomerular visceral epithelial cells also resulted in a drop in TER but did not effect trans-epithelial flux of horseradish-peroxidase or mannitol (89). In keratinocyte epithelial sheets, IL-4 caused an increase in the trans-epithelial flux of dextran-4000 but had no effect on TER (90). In human umbilical vein endothelial cells, IL-4 treatment resulted in an increase in paracellular permeability to albumin that was present within 6 hours of treatment (91). In animal models, intraperitoneal IL-4 caused a decrease in mouse intestinal tissue TER (measured in an Ussing chamber). STAT-6 (the primary transcription factor induced by IL-4) knockout mice were protected from this effect (92) suggesting a role for STAT6 signaling in-vivo.
3.6. Interleukin-6 (IL-6)
Interleukin-6 is a potent pro-inflammatory cytokine of the Th1 paradigm, secreted by various cell types (including intestinal epithelial cells and liver cells) in response to pro-inflammatory stimuli, particularly TNF-α and IL-1β (93). It had been reported by Tazuke et al. that IL-6 causes a drop in TER at 72 hours and an increase in permeability to mannitol in Caco-2 monolayers (94). However, in a subsequent study, Sitaraman and co-workers showed that IL-6 treatment of Caco-2 monolayers caused a decrease in paracellular flux of Dextran-4000 (95). They also reported that IL-6 knockout mice had a greater increase in intestinal absorption of Dextran 4000 when colitis was induced by to Dextran Sulfate (DSS), and concluded that IL-6 had a barrier protective effect. IL-6 induced up-regulation of Keratin 8 and 18 was postulated as a possible mechanism of barrier protection (95). In a mouse hemorrhage/reperfusion model, oral IL-6 reduced the incidence of bacterial translocation to mesenteric lymph nodes (96, 97). However, not all in-vivo studies have found a barrier protective role for IL-6. In a hemorrhagic shock / reperfusion model (98) and in a sepsis model in mice (99), IL-6 knockout mice were protected from having an increase in intestinal permeability. IL-6 knockout mouse also had less bacterial translocation to mesenteric lymph nodes. Wang et al. suggested that IL-6 knockout mice may be protected from sepsis-induced barrier disruption by over production of IL-10. The effects of IL-6 on epithelial permeability remain controversial and may depend on the specific cell type or model system being studied. Further studies are necessary to better clarify the regulatory role of IL-6 on epithelial barriers.
3.7. Interleukin-10 (IL-10)
IL-10 is primarily secreted by T-cells in response to stimulation by IL-4 and is considered an important anti-inflammatory cytokine (100). Although IL-10 does not appear to affect basal epithelial barrier function, it appears to be protective against TJ barrier disturbance. In T84 monolayers, IL-10 has been shown to prevent the IFN-γ induced increased in paracellular permeability to mannitol and inulin (6). Similar findings have been reported in endothelial cells (30).
IL-10 knockout mice have been widely utilized as an immune-induced model of inflammatory bowel disease (29). These mice develop intestinal inflammation in the presence of bacterial flora (29) or when treated with the non-steroidal anti-inflammatory drug piroxicam (101). An increase in intestinal permeability (as measured by lactulose and mannitol absorption) precedes the histologic signs of intestinal inflammation by several weeks in the IL-10 knockout mouse (29). These data suggested a barrier protective role for IL-10, and support the hypothesis that the defective intestinal barrier plays a key pathogenic role in the development of intestinal inflammation. Increased expression of IL-10 has also been suggested as a mechanism that protects IL-6 knockout mice from sepsis or hemorrhage induced intestinal barrier disruption (98, 99). In a sepsis mouse model of cecal ligation-puncture, intraperitoneal IL-10 treatment abolished the sepsis-induced increase in intestinal epithelial permeability to dextran-4000 and Horseradish peroxidase (99). These investigators showed that the induction of a heat shock response was protective against sepsis induced intestinal barrier disruption; the barrier protective effect of the heat shock response appeared to be mediated by heat shock protein induction of IL-10 (102). IL-10 knockout mice have also been shown to have a disturbance of intrahepatic TJs, with alterations in ZO-1 and claudin-1 localization (103). Increased expression of IL-10 also attenuated the increases in lung epithelial permeability in a model of lung injury (104). In a TPN induced intestinal barrier dysfunction model in mice, TPN caused an increase in intestinal permeability to mannitol and bacterial translocation that was associated with decreased epithelial cell production of IL-10 (105). IL-10 treatment reversed the TPN-induced barrier defect. The disruption of the epithelial barrier by TPN was also associated with a loss of ZO-1, E-cadherin and occludin, and was prevented by treatment with exogenous IL-10 (105). In summary, above studies suggested that IL-10 has an important barrier protective effect against various models of intestinal epithelial barrier damage.
3.8. Interleukin-11 (IL-11)
Interleukin-11 has been shown to be protective against inflammation in rodent models of colitis (HLA-B27 rats, TNBS colitis) but its direct effects on the epithelial barrier remain unclear. IL-11 inhibited the Clostridium difficile toxin induced increase in small intestinal permeability but did not affect cholera toxin induced increase in intestinal permeability. (106).
3.9. Interluekin-13 (IL-13)
Interleukin-13 is structurally very similar to IL-4 and shares many common effects (107). IL-13 treatment of T84 monolayers caused a decrease in TER (108) (16) and an increase in trans-epithelial flux rate of Dextran 4000. IL-13 also caused a decrease in TER in HT-29 cells (109). The IL-13 induced increase in TJ permeability was associated with an increase in claudin-2 levels in both T84 and HT-29 monolayers (16, 109). IL-13 has also been shown to increase bacterial translocation of E.Coli across HT-29 monolayers (110). The IL-13 induced increase in claudin-2 expression and TJ permeability was associated with an increase in AKT activation, and was blocked by the PI-3kinase inhibitor LY294002 (16). IL-13 induced increase in mouse intestinal permeability required STAT6 activation (92). However in a T84 in-vitro model system, the increase in permeability did not require STAT6 activation (111). In the human lung epithelial cell line CaluIII, IL-13 also caused an increase in epithelial permeability and a decrease in expression of ZO-1 and occludin (112).
3.10. Interleukin 15 (IL-15)
Interleukin-15 has been shown to cause a modest increase (10%) in the trans-epithelial resistance in T84 intestinal monolayers (113) and accelerate the development of barrier formation. IL-15 induced barrier enhancing effect was associated with a two-fold increase in the expression of TJ proteins occludin, ZO-1, ZO-2, claudin-1 and claudin-2 (114). This was also associated with an increase in junctional localization of TJ proteins and an increase in occludin phosphorylation.
3.11. Interleukin-17 (IL-17)
Interleukin-17 accelerated the development of a TJ barrier in T84 monolayers and caused an increase in TER and decrease in paracellular permeability to mannitol (115). This barrier enhancing effect was associated with an increase in MEK and phospo-ERK1 levels, and was inhibited by the ERK inhibitor PD98059. IL-17 induced activation of MEK and ERK was accompanied by an increased expression of claudin-2. The increase in claudin-2 expression was also inhibited by PD98059 (115). Thus it appears that IL-17 enhancement of the epithelial TJ barrier may be mediated by ERK activation.
3.12. Other Interleukins
As far as we are aware, there are no published reports supporting a role for interleukin-3, 5, 7,8 (now considered a chemokine), 9 12,14, 16 or 18–35 in regulating TJ permeability.
3.13. Interferon alpha and beta (IFN-α and IFN-β)
Interferons were originally described for their ability to “interfere” with replication of viruses. Currently they are divided into subtypes: Type I interferons (principally IFN-α&β) and Type II (principally IFN-γ) (10). The two subtypes have different receptors and are structurally unrelated (10). IFN-α is frequently given to humans as treatment for several illnesses (including hepatitis C and renal cell carcinoma), and is known to induce a capillary leak syndrome (116). The effect of interferon alpha and beta on permeability has been studied in only a limited manner. Interferon-alpha has been shown to cause an increase in the TER of LLC-PK1 renal epithelial cells (117). This effect was associated with an increase in expression and junctional localization of occludin (117). The IFN-α enhancement of TJ barrier function appeared to be mediated in part by an increase in activation of the ERK pathway (117). Animal studies have also suggested a beneficial role for IFN-α in colitis, whether this is mediated through barrier protective mechanisms is unknown (118). Interferon-beta (IFN-β) had no effect on TER or transcytosis of the marker Rho-123 when studied in Caco-2 cells (119).
3.14. Transforming Growth Factor alpha (TGF-α)
The results of studies examining the effect of TGF-α on intestinal epithelial function have been conflicting. Investigators have reported conflicting results regarding possible protective effect of TGF-α on oxidant induced disruption of Caco-2 TJ barrier function. Forsyth et al reported that TGF-α inhibition (with anti TGF-α antibody) prevented the oxidant induced increase in Caco-2 epithelial permeability (7), while, Rao et al reported the opposite to be the case (120).
3.15. Transforming growth factor beta (TGF-β)
TGF-β appears to cause an enhancement in intestinal epithelial TJ barrier function and protects against TJ barrier disruption. TGF-β has been shown to cause an increase in basal TER in T84 monolayers (25). TGF-β was protective against T84 barrier disruption caused by various noxious agents including IFN-γ, enterohemorrhagic E.Coli (121) and cryptosporidium (122). The TGF-β enhancement of TJ barrier function has been suggested to be mediated by the ERK or PKC pathways. Howe et al. also showed that the TGF-β induced increase in T84 TER was associated with an increase in claudin-1 expression (121). In other cell types, TGF-β has been suggested to have a disruptive effect on the TJ barrier. In breast epithelial cells, TGF-β prevented dexamethasone induced formation of tight junctions by inhibiting junctional localization of ZO-1 protein (123). In sertoli cells of the testes, TGF-β disrupted the epithelial barrier by decreasing the expression of occludin, ZO-1 and claudin-11 (124). Thus, the effect of TGF-β on the tight junction barrier may be tissue specific.
4. SUMMARY AND PERSPECTIVE
Cytokines play an important role in the modulation of intestinal epithelial TJ barrier. The pro-inflammatory cytokine induced increase in intestinal TJ permeability appears to be an important pathogenic mechanism contributing to the development of intestinal inflammation; and therapeutic preservation of intestinal TJ barrier has been shown to prevent the development of intestinal inflammation. Most pro-inflammatory cytokines including IFN-γ, TNF-α, IL-12 and IL-1β cause an increase in TJ permeability, while some anti-inflammatory cytokines such as IL-10 and TGF-β protect against the disruption of intestinal TJ barrier and development of intestinal inflammation. These findings raise the possibility that increasing the tissue level of barrier protective cytokines could be a potential strategy to preserve the TJ barrier and also prevent intestinal inflammation. There have been some but limited advances in delineating the intracellular mechanisms that mediate the cytokine modulation of TJ barrier. The IFN-γ induced increase in intestinal epithelial TJ permeability appears to be mediated in part by endocytosis of TJ proteins (Figure 2); while TNF-α effect on TJ barrier appears to be mediated primarily through activation of MLCK gene activity and increase in MLCK protein expression (Figure 3). The endocytosis of occludin protein (via a calveolin-1 dependent mechanism) also appears to be important in mediating the LIGHT and IFN-γ induced increase in intestinal epithelial permeability in-vivo. For most of the cytokines, the intracellular and molecular mechanisms that mediate the alteration in TJ permeability have not been well elucidated. An important emerging concept is that the cytokines modulate intestinal TJ barrier by distinct intracellular mechanisms and signaling pathways and that inhibition of cytokine induced disturbance in intestinal TJ barrier is an important therapeutic strategy to prevent or attenuate intestinal inflammation. There remains an important opportunity for future investigation into this highly clinically relevant and potentially fruitful area of research.
ACKNOWLEDGEMENT
This research project was supported by a Veterans Affairs Merit Review grant from the Veterans Affairs Research Service and National Institute of Diabetes and Digestive and Kidney Diseases Grant R01-DK-64165-01 (to T.Y.M.)
Abbreviations
- TJ
tight junctions
- IFN
interferon
- TNF
tumor necrosis factor
- IL
interleukin
- MLCK
myosin light chain
- NF-kB
nuclear factor kappa B
REFERENCES
- 1.Ma T, Madara J. Tight Junctions and the intestinal barrier. In: Johnson R, editor. Textbook of Gastrointestinal Physiology. Burlington, Ma: Elsevier Academic Press; 2006. [Google Scholar]
- 2.Nusrat A, Turner JR, Madara JL. Molecular physiology and pathophysiology of tight junctions. IV. Regulation of tight junctions by extracellular stimuli: nutrients, cytokines, and immune cells. Am J Physiol Gastrointest Liver Physiol. 2000;279:G851–G857. doi: 10.1152/ajpgi.2000.279.5.G851. [DOI] [PubMed] [Google Scholar]
- 3.DeMeo M, Mutlu EA, Keshavarzian A, Tobin MC. Intestinal permeation and gastrointestinal disease. J Clin Gastroenterol. 2002;34:385–396. doi: 10.1097/00004836-200204000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Bruewer M, Samarin S, Nusrat A. Inflammatory bowel disease and the apical junctional complex. Ann N Y Acad Sci. 2006;1072:242–252. doi: 10.1196/annals.1326.017. [DOI] [PubMed] [Google Scholar]
- 5.Shen L, Turner JR. Role of epithelial cells in initiation and propagation of intestinal inflammation. Eliminating the static: tight junction dynamics exposed. Am J Physiol Gastrointest Liver Physiol. 2006;290:G577–G582. doi: 10.1152/ajpgi.00439.2005. [DOI] [PubMed] [Google Scholar]
- 6.Madsen K, Lewis SA, Tavernini MM, Hibbard J, Fedorak RN. Interleukin 10 prevents cytokine-induced disruption of T84 monolayer barrier integrity and limits chloride secretion. Gastroenterology. 1997;113:151–159. doi: 10.1016/s0016-5085(97)70090-8. [DOI] [PubMed] [Google Scholar]
- 7.Forsyth C, Banan A, Farhadi A, Fields JZ, Tang Y, Shaikh M, Zhang LJ, Engen PA, Keshavarzian A. Regulation of oxidant-induced intestinal permeability by metalloprotease-dependent epidermal growth factor receptor signaling. J Pharmacol Exp Ther. 2007;321:84–97. doi: 10.1124/jpet.106.113019. [DOI] [PubMed] [Google Scholar]
- 8.Madara J, Stafford J. Interferon-gamma directly affects barrier function of cultured intestinal epithelial monolayers. J Clin Invest. 1989;83:724–727. doi: 10.1172/JCI113938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwarz B, Wang F, Shen L, Clayburgh DR, Su L, Wang Y, Fu YX, Turner JR. LIGHT signals directly to intestinal epithelia to cause barrier dysfunction via cytoskeletal and endocytic mechanisms. Gastroenterology. 2007;132:2383–2394. doi: 10.1053/j.gastro.2007.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75:163–189. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 11.Adams R, Planchon SM, Roche JK. IFN-gamma modulation of epithelial barrier function. Time course, reversibility, and site of cytokine binding. J Immunol. 1993;150:2356–2363. [PubMed] [Google Scholar]
- 12.Watson C, Hoare CJ, Garrod DR, Carlson GL, Warhurst G. Interferon-gamma selectively increases epithelial permeability to large molecules by activating different populations of paracellular pores. J Cell Sci. 2005;118:5221–5230. doi: 10.1242/jcs.02630. [DOI] [PubMed] [Google Scholar]
- 13.Bruewer M, Utech M, Ivanov AI, Hopkins AM, Parkos CA, Nusrat A. Interferon-gamma induces internalization of epithelial tight junction proteins via a macropinocytosis-like process. Faseb J. 2005;19:923–933. doi: 10.1096/fj.04-3260com. [DOI] [PubMed] [Google Scholar]
- 14.Ivanov A, Nusrat A, Parkos CA. Endocytosis of the apical junctional complex: mechanisms and possible roles in regulation of epithelial barriers. Bioessays. 2005;27:356–365. doi: 10.1002/bies.20203. [DOI] [PubMed] [Google Scholar]
- 15.Utech M, Ivanov AI, Samarin SN, Bruewer M, Turner JR, Mrsny RJ, Parkos CA, Nusrat A. Mechanism of IFN-gamma-induced endocytosis of tight junction proteins: myosin II-dependent vacuolarization of the apical plasma membrane. Mol Biol Cell. 2005;16:5040–5052. doi: 10.1091/mbc.E05-03-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prasad S, Mingrino R, Kaukinen K, Hayes KL, Powell RM, MacDonald TT, Collins JE. Inflammatory processes have differential effects on claudins 2, 3 and 4 in colonic epithelial cells. Lab Invest. 2005;85:1139–1162. doi: 10.1038/labinvest.3700316. [DOI] [PubMed] [Google Scholar]
- 17.Wisner D, Harris LR, 3rd, Green CL, Poritz LS. Opposing regulation of the tight junction protein claudin-2 by interferon-gamma and interleukin-4. J Surg Res. 2008;144:1–7. doi: 10.1016/j.jss.2007.03.059. [DOI] [PubMed] [Google Scholar]
- 18.Willemsen L, Hoetjes JP, van Deventer SJ, van Tol EA. Abrogation of IFN-gamma mediated epithelial barrier disruption by serine protease inhibition. Clin Exp Immunol. 2005;142:275–284. doi: 10.1111/j.1365-2249.2005.02906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bruewer M, Luegering A, Kucharzik T, Parkos CA, Madara JL, Hopkins AM, Nusrat A. Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J Immunol. 2003;171:6164–6172. doi: 10.4049/jimmunol.171.11.6164. [DOI] [PubMed] [Google Scholar]
- 20.McKay D, Botelho F, Ceponis PJ, Richards CD. Superantigen immune stimulation activates epithelial STAT-1 and PI 3-K: PI 3-K regulation of permeability. Am J Physiol Gastrointest Liver Physiol. 2000;279:G1094–G1103. doi: 10.1152/ajpgi.2000.279.5.G1094. [DOI] [PubMed] [Google Scholar]
- 21.Roy PM, Bradley A, Kennedy JC, Ma TY. Mechanism of interferon-gamma (IFN-gamma) induced increase in intestinal epithelial tight junction permeability: Cross-talk between PI-3 kinase and NF-kappa B pathways. GASTROENTEROLOGY. 2006 [Google Scholar]
- 22.McKay D, Watson JL, Wang A, Caldwell J, Prescott D, Ceponis PM, Di Leo V, Lu J. Phosphatidylinositol 3'-kinase is a critical mediator of interferon-gamma-induced increases in enteric epithelial permeability. J Pharmacol Exp Ther. 2007;320:1013–1022. doi: 10.1124/jpet.106.113639. [DOI] [PubMed] [Google Scholar]
- 23.Di Paolo M, Merrett MN, Crotty B, Jewell DP. 5-Aminosalicylic acid inhibits the impaired epithelial barrier function induced by gamma interferon. Gut. 1996;38:115–119. doi: 10.1136/gut.38.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watson J, Ansari S, Cameron H, Wang A, Akhtar M, McKay DM. Green tea polyphenol (−)-epigallocatechin gallate blocks epithelial barrier dysfunction provoked by IFN-gamma but not by IL-4. Am J Physiol Gastrointest Liver Physiol. 2004;287:G954–G961. doi: 10.1152/ajpgi.00302.2003. [DOI] [PubMed] [Google Scholar]
- 25.Planchon S, Martins CA, Guerrant RL, Roche JK. Regulation of intestinal epithelial barrier function by TGF-beta 1. Evidence for its role in abrogating the effect of a T cell cytokine. J Immunol. 1994;153:5730–5739. [PubMed] [Google Scholar]
- 26.Fish S, Proujansky R, Reenstra WW. Synergistic effects of interferon gamma and tumour necrosis factor alpha on T84 cell function. Gut. 1999;45:191–198. doi: 10.1136/gut.45.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zech J, Pouvreau I, Cotinet A, Goureau O, Le Varlet B, de Kozak Y. Effect of cytokines and nitric oxide on tight junctions in cultured rat retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1998;39:1600–1608. [PubMed] [Google Scholar]
- 28.Bao S, Knoell DL. Zinc modulates cytokine-induced lung epithelial cell barrier permeability. Am J Physiol Lung Cell Mol Physiol. 2006;291:L1132–L1141. doi: 10.1152/ajplung.00207.2006. [DOI] [PubMed] [Google Scholar]
- 29.Madsen K, Malfair D, Gray D, Doyle JS, Jewell LD, Fedorak RN. Interleukin-10 gene-deficient mice develop a primary intestinal permeability defect in response to enteric microflora. Inflamm Bowel Dis. 1999;5:262–270. doi: 10.1097/00054725-199911000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Oshima T, Laroux FS, Coe LL, Morise Z, Kawachi S, Bauer P, Grisham MB, Specian RD, Carter P, Jennings S, Granger DN, Joh T, Alexander JS. Interferon-gamma and interleukin-10 reciprocally regulate endothelial junction integrity and barrier function. Microvasc Res. 2001;61:130–143. doi: 10.1006/mvre.2000.2288. [DOI] [PubMed] [Google Scholar]
- 31.Yang H, Kiristioglu I, Fan Y, Forbush B, Bishop DK, Antony PA, Zhou H, Teitelbaum DH. Interferon-gamma expression by intraepithelial lymphocytes results in a loss of epithelial barrier function in a mouse model of total parenteral nutrition. Ann Surg. 2002;236:226–234. doi: 10.1097/00000658-200208000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Musch M, Clarke LL, Mamah D, Gawenis LR, Zhang Z, Ellsworth W, Shalowitz D, Mittal N, Efthimiou P, Alnadjim Z, Hurst SD, Chang EB, Barrett TA. T cell activation causes diarrhea by increasing intestinal permeability and inhibiting epithelial Na+/K+-ATPase. J Clin Invest. 2002;110:1739–1747. doi: 10.1172/JCI15695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Demaude J, Salvador-Cartier C, Fioramonti J, Ferrier L, Bueno L. Phenotypic changes in colonocytes following acute stress or activation of mast cells in mice: implications for delayed epithelial barrier dysfunction. Gut. 2006;55:655–661. doi: 10.1136/gut.2005.078675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carrithers M, Tandon S, Canosa S, Michaud M, Graesser D, Madri JA. Enhanced susceptibility to endotoxic shock and impaired STAT3 signaling in CD31-deficient mice. Am J Pathol. 2005;166:185–196. doi: 10.1016/S0002-9440(10)62243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luyer M, Buurman WA, Hadfoune M, Wolfs T, van't Veer C, Jacobs JA, Dejong CH, Greve JW. Exposure to bacterial DNA before hemorrhagic shock strongly aggravates systemic inflammation and gut barrier loss via an IFN-gamma-dependent route. Ann Surg. 2007;245:795–802. doi: 10.1097/01.sla.0000251513.59983.3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brun P, Castagliuolo I, Di Leo V, Buda A, Pinzani M, Palu G, Martines D. Increased intestinal permeability in obese mice: new evidence in the pathogenesis of nonalcoholic steatohepatitis. Am J Physiol Gastrointest Liver Physiol. 2007;292:G518–G525. doi: 10.1152/ajpgi.00024.2006. [DOI] [PubMed] [Google Scholar]
- 37.Atzeni F, Doria A, Carrabba M, Turiel M, Sarzi-Puttini P. Potential target of infliximab in autoimmune and inflammatory diseases. Autoimmun Rev. 2007;6:529–536. doi: 10.1016/j.autrev.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 38.Boehringer N, Hagens G, Songeon F, Isler P, Nicod LP. Differential regulation of tumor necrosing factor-alpha (TNF-alpha) and interleukin-10 (IL-10) secretion by protein kinase and phosphatase inhibitors in human alveolar macrophages. Eur Cytokine Netw. 1999;10:211–218. [PubMed] [Google Scholar]
- 39.Cavaillon J. Contribution of cytokines to inflammatory mechanisms. Pathol Biol (Paris) 1993;41:799–811. [PubMed] [Google Scholar]
- 40.Sekut L, Connolly K. AntiTNF-alpha agents in the treatment of inflammation. Expert Opin Investig Drugs. 1998;7:1825–1839. doi: 10.1517/13543784.7.11.1825. [DOI] [PubMed] [Google Scholar]
- 41.Van der Meide P, Schellekens H. Cytokines and the immune response. Biotherapy. 1996;8:243–249. doi: 10.1007/BF01877210. [DOI] [PubMed] [Google Scholar]
- 42.Kiener P, Marek F, Rodgers G, Lin PF, Warr G, Desiderio J. Induction of tumor necrosis factor, IFN-gamma, and acute lethality in mice by toxic and non-toxic forms of lipid A. J Immunol. 1988;141:870–874. [PubMed] [Google Scholar]
- 43.Shetty A, Forbes A. Pharmacogenomics of response to anti-tumor necrosis factor therapy in patients with Crohn's disease. Am J Pharmacogenomics. 2002;2:215–221. doi: 10.2165/00129785-200202040-00001. [DOI] [PubMed] [Google Scholar]
- 44.Mullin J, Laughlin KV, Marano CW, Russo LM, Soler AP. Modulation of tumor necrosis factor-induced increase in renal (LLC-PK1) transepithelial permeability. Am J Physiol. 1992;263:F915–F924. doi: 10.1152/ajprenal.1992.263.5.F915. [DOI] [PubMed] [Google Scholar]
- 45.Marano C, Lewis SA, Garulacan LA, Soler AP, Mullin JM. Tumor necrosis factor-alpha increases sodium and chloride conductance across the tight junction of CACO-2 BBE, a human intestinal epithelial cell line. J Membr Biol. 1998;161:263–274. doi: 10.1007/s002329900333. [DOI] [PubMed] [Google Scholar]
- 46.Ma T, Iwamoto GK, Hoa NT, Akotia V, Pedram A, Boivin MA, Said HM. TNF-alpha-induced increase in intestinal epithelial tight junction permeability requires NF-kappa B activation. Am J Physiol Gastrointest Liver Physiol. 2004;286:G367–G376. doi: 10.1152/ajpgi.00173.2003. [DOI] [PubMed] [Google Scholar]
- 47.Schmitz H, Fromm M, Bentzel CJ, Scholz P, Detjen K, Mankertz J, Bode H, Epple HJ, Riecken EO, Schulzke JD. Tumor necrosis factor-alpha (TNFalpha) regulates the epithelial barrier in the human intestinal cell line HT-29/B6. J Cell Sci. 1999;112(Pt 1):137–146. doi: 10.1242/jcs.112.1.137. [DOI] [PubMed] [Google Scholar]
- 48.Ma T, Boivin MA, Ye D, Pedram A, Said HM. Mechanism of TNF-{alpha} modulation of Caco-2 intestinal epithelial tight junction barrier: role of myosin light-chain kinase protein expression. Am J Physiol Gastrointest Liver Physiol. 2005;288:G422–G430. doi: 10.1152/ajpgi.00412.2004. [DOI] [PubMed] [Google Scholar]
- 49.Ye D, Ma I, Ma TY. Molecular mechanism of tumor necrosis factor-alpha modulation of intestinal epithelial tight junction barrier. Am J Physiol Gastrointest Liver Physiol. 2006;290:G496–G504. doi: 10.1152/ajpgi.00318.2005. [DOI] [PubMed] [Google Scholar]
- 50.Ye D, Ma TY. Cellular and molecular mechanisms that mediate basal and tumor necrosis factor-alpha induced regulation of myosin light chain kinase gene activity. J Cell Mol Med. 2008 doi: 10.1111/j.1582-4934.2008.00302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saile B, Matthes N, El Armouche H, Neubauer K, Ramadori G. The bcl, NFkappaB and p53/p21WAF1 systems are involved in spontaneous apoptosis and in the anti-apoptotic effect of TGF-beta or TNF-alpha on activated hepatic stellate cells. Eur J Cell Biol. 2001;80:554–561. doi: 10.1078/0171-9335-00182. [DOI] [PubMed] [Google Scholar]
- 52.Florian P, Schoneberg T, Schulzke JD, Fromm M, Gitter AH. Single-cell epithelial defects close rapidly by an actinomyosin purse string mechanism with functional tight junctions. J Physiol. 2002;545:485–499. doi: 10.1113/jphysiol.2002.031161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gitter A, Bendfeldt K, Schulzke JD, Fromm M. Leaks in the epithelial barrier caused by spontaneous and TNF-alpha-induced single-cell apoptosis. Faseb J. 2000;14:1749–1753. doi: 10.1096/fj.99-0898com. [DOI] [PubMed] [Google Scholar]
- 54.Madara J. Epithelial cells develop membrane wounds-- and recover! Gastroenterology. 1989;96:1360–1361. doi: 10.1016/s0016-5085(89)80026-5. [DOI] [PubMed] [Google Scholar]
- 55.Yu A, McCarthy KM, Francis SA, McCormack JM, Lai J, Rogers RA, Lynch RD, Schneeberger EE. Knockdown of occludin expression leads to diverse phenotypic alterations in epithelial cells. Am J Physiol Cell Physiol. 2005;288:C1231–C1241. doi: 10.1152/ajpcell.00581.2004. [DOI] [PubMed] [Google Scholar]
- 56.Madara J. Maintenance of the macromolecular barrier at cell extrusion sites in intestinal epithelium: physiological rearrangement of tight junctions. J Membr Biol. 1990;116:177–184. doi: 10.1007/BF01868675. [DOI] [PubMed] [Google Scholar]
- 57.Moore R, Carlson S, Madara JL. Rapid barrier restitution in an in vitro model of intestinal epithelial injury. Lab Invest. 1989;60:237–244. [PubMed] [Google Scholar]
- 58.Martin P, Lewis J. Actin cables and epidermal movement in embryonic wound healing. Nature. 1992;360:179–183. doi: 10.1038/360179a0. [DOI] [PubMed] [Google Scholar]
- 59.Schulzke J, Bojarski C, Zeissig S, Heller F, Gitter AH, Fromm M. Disrupted barrier function through epithelial cell apoptosis. Ann N Y Acad Sci. 2006;1072:288–299. doi: 10.1196/annals.1326.027. [DOI] [PubMed] [Google Scholar]
- 60.Mong P, Petrulio C, Kaufman HL, Wang Q. Activation of Rho kinase by TNF-alpha is required for JNK activation in human pulmonary microvascular endothelial cells. J Immunol. 2008;180:550–558. doi: 10.4049/jimmunol.180.1.550. [DOI] [PubMed] [Google Scholar]
- 61.Nwariaku F, Rothenbach P, Liu Z, Zhu X, Turnage RH, Terada LS. Rho inhibition decreases TNF-induced endothelial MAPK activation and monolayer permeability. J Appl Physiol. 2003;95:1889–1895. doi: 10.1152/japplphysiol.00225.2003. [DOI] [PubMed] [Google Scholar]
- 62.Vandenbroucke E, Mehta D, Minshall R, Malik AB. Regulation of endothelial junctional permeability. Ann N Y Acad Sci. 2008;1123:134–145. doi: 10.1196/annals.1420.016. [DOI] [PubMed] [Google Scholar]
- 63.McKenzie J, Ridley AJ. Roles of Rho/ROCK and MLCK in TNF-alpha-induced changes in endothelial morphology and permeability. J Cell Physiol. 2007;213:221–228. doi: 10.1002/jcp.21114. [DOI] [PubMed] [Google Scholar]
- 64.Wang F, Graham WV, Wang Y, Witkowski ED, Schwarz BT, Turner JR. Interferon-gamma and tumor necrosis factor-alpha synergize to induce intestinal epithelial barrier dysfunction by up-regulating myosin light chain kinase expression. Am J Pathol. 2005;166:409–419. doi: 10.1016/s0002-9440(10)62264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ivanov A, Nusrat A, Parkos CA. The epithelium in inflammatory bowel disease: potential role of endocytosis of junctional proteins in barrier disruption. Novartis Found Symp. 2004;263:115–124. discussion 124-32, 211-8. [PubMed] [Google Scholar]
- 66.Ivanov A, Nusrat A, Parkos CA. Endocytosis of epithelial apical junctional proteins by a clathrin-mediated pathway into a unique storage compartment. Mol Biol Cell. 2004;15:176–188. doi: 10.1091/mbc.E03-05-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dinarello C. Interleukin-1 and interleukin-1 receptor antagonist production during haemodialysis: which cytokine is a surrogate marker for dialysis-related complications? Nephrol Dial Transplant. 1995;10(Suppl 3):25–28. doi: 10.1093/ndt/10.supp3.25. [DOI] [PubMed] [Google Scholar]
- 68.Dinarello C. Cytokines as mediators in the pathogenesis of septic shock. Curr Top Microbiol Immunol. 1996;216:133–165. doi: 10.1007/978-3-642-80186-0_7. [DOI] [PubMed] [Google Scholar]
- 69.Stylianou E, Saklatvala J. Interleukin-1. Int J Biochem Cell Biol. 1998;30:1075–1079. doi: 10.1016/s1357-2725(98)00081-8. [DOI] [PubMed] [Google Scholar]
- 70.Siegmund B. Interleukin-1beta converting enzyme (caspase-1) in intestinal inflammation. Biochem Pharmacol. 2002;64:1–8. doi: 10.1016/s0006-2952(02)01064-x. [DOI] [PubMed] [Google Scholar]
- 71.Gwee K, Collins SM, Read NW, Rajnakova A, Deng Y, Graham JC, McKendrick MW, Moochhala SM. Increased rectal mucosal expression of interleukin 1beta in recently acquired post-infectious irritable bowel syndrome. Gut. 2003;52:523–526. doi: 10.1136/gut.52.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mahida Y, Wu K, Jewell DP. Enhanced production of interleukin 1-beta by mononuclear cells isolated from mucosa with active ulcerative colitis of Crohn's disease. Gut. 1989;30:835–838. doi: 10.1136/gut.30.6.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Reinecker H, Steffen M, Doehn C, Petersen J, Pfluger I, Voss A, Raedler A. Proinflammatory cytokines in intestinal mucosa. Immunol Res. 1991;10:247–248. doi: 10.1007/BF02919700. [DOI] [PubMed] [Google Scholar]
- 74.Al-Sadi R, Ma TY. IL-1beta causes an increase in intestinal epithelial tight junction permeability. J Immunol. 2007;178:4641–4649. doi: 10.4049/jimmunol.178.7.4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Al-Sadi R, Ye D, Dokladny K, Ma TY. Mechanism of IL-1{beta}-Induced Increase in Intestinal Epithelial Tight Junction Permeability. J Immunol. 2008;180:5653–5661. doi: 10.4049/jimmunol.180.8.5653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Abe T, Sugano E, Saigo Y, Tamai M. Interleukin-1beta and barrier function of retinal pigment epithelial cells (ARPE-19): aberrant expression of junctional complex molecules. Invest Ophthalmol Vis Sci. 2003;44:4097–4104. doi: 10.1167/iovs.02-0867. [DOI] [PubMed] [Google Scholar]
- 77.Yamagata K, Tagami M, Takenaga F, Yamori Y, Itoh S. Hypoxia-induced changes in tight junction permeability of brain capillary endothelial cells are associated with IL-1beta and nitric oxide. Neurobiol Dis. 2004;17:491–499. doi: 10.1016/j.nbd.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 78.Duffy H, John GR, Lee SC, Brosnan CF, Spray DC. Reciprocal regulation of the junctional proteins claudin-1 and connexin43 by interleukin-1beta in primary human fetal astrocytes. J Neurosci. 2000;20:RC114. doi: 10.1523/JNEUROSCI.20-23-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yamamoto T, Kojima T, Murata M, Takano K, Go M, Chiba H, Sawada N. IL-1beta regulates expression of Cx32, occludin, and claudin-2 of rat hepatocytes via distinct signal transduction pathways. Exp Cell Res. 2004;299:427–441. doi: 10.1016/j.yexcr.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 80.Coyne C, Vanhook MK, Gambling TM, Carson JL, Boucher RC, Johnson LG. Regulation of airway tight junctions by proinflammatory cytokines. Mol Biol Cell. 2002;13:3218–3234. doi: 10.1091/mbc.E02-03-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nelson B. IL-2, regulatory T cells, and tolerance. J Immunol. 2004;172:3983–3988. doi: 10.4049/jimmunol.172.7.3983. [DOI] [PubMed] [Google Scholar]
- 82.Hanada S, Harada M, Koga H, Kawaguchi T, Taniguchi E, Kumashiro R, Ueno T, Ueno Y, Ishii M, Sakisaka S, Sata M. Tumor necrosis factor-alpha and interferon-gamma directly impair epithelial barrier function in cultured mouse cholangiocytes. Liver Int. 2003;23:3–11. doi: 10.1034/j.1600-0676.2003.01707.x. [DOI] [PubMed] [Google Scholar]
- 83.Ryan C, Atkins MB, Mier JW, Gelfand JA, Tompkins RG. Effects of malignancy and interleukin-2 infusion on gut macromolecular permeability. Crit Care Med. 1995;23:1801–1806. doi: 10.1097/00003246-199511000-00003. [DOI] [PubMed] [Google Scholar]
- 84.Harms B, Pahl AC, Pohlman TH, Conhaim RL, Starling JR, Storm FK. Effects of interleukin-2 on pulmonary and systemic transvascular fluid filtration. Surgery. 1989;106:339–345. discussion 345-6. [PubMed] [Google Scholar]
- 85.Olthof C, Baars JW, Wagstaff J, Donker AJ, Schneider H, de Vries PM. Determination of capillary leakage due to recombinant interleukin-2 by means of noninvasive conductivity measurements. Eur J Appl Physiol Occup Physiol. 1993;67:168–173. doi: 10.1007/BF00376662. [DOI] [PubMed] [Google Scholar]
- 86.Izuhara K. The role of interleukin-4 and interleukin-13 in the non-immunologic aspects of asthma pathogenesis. Clin Chem Lab Med. 2003;41:860–864. doi: 10.1515/CCLM.2003.130. [DOI] [PubMed] [Google Scholar]
- 87.Ceponis P, Botelho F, Richards CD, McKay DM. Interleukins 4 and 13 increase intestinal epithelial permeability by a phosphatidylinositol 3-kinase pathway. Lack of evidence for STAT 6 involvement. J Biol Chem. 2000;275:29132–29137. doi: 10.1074/jbc.M003516200. [DOI] [PubMed] [Google Scholar]
- 88.Di Leo V, Yang PC, Berin MC, Perdue MH. Factors regulating the effect of IL-4 on intestinal epithelial barrier function. Int Arch Allergy Immunol. 2002;129:219–227. doi: 10.1159/000066778. [DOI] [PubMed] [Google Scholar]
- 89.Van Den Berg J, Aten J, Chand MA, Claessen N, Dijkink L, Wijdenes J, Lakkis FG, Weening JJ. Interleukin-4 and interleukin-13 act on glomerular visceral epithelial cells. J Am Soc Nephrol. 2000;11:413–422. doi: 10.1681/ASN.V113413. [DOI] [PubMed] [Google Scholar]
- 90.Kobayashi J, Inai T, Morita K, Moroi Y, Urabe K, Shibata Y, Furue M. Reciprocal regulation of permeability through a cultured keratinocyte sheet by IFN-gamma and IL-4. Cytokine. 2004;28:186–189. doi: 10.1016/j.cyto.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 91.Kotowicz K, Callard RE, Klein NJ, Jacobs MG. Interleukin-4 increases the permeability of human endothelial cells in culture. Clin Exp Allergy. 2004;34:445–449. doi: 10.1111/j.1365-2222.2004.01902.x. [DOI] [PubMed] [Google Scholar]
- 92.Madden K, Whitman L, Sullivan C, Gause WC, Urban JF, Jr, Katona IM, Finkelman FD, Shea-Donohue T. Role of STAT6 and mast cells in IL-4- and IL-13-induced alterations in murine intestinal epithelial cell function. J Immunol. 2002;169:4417–4422. doi: 10.4049/jimmunol.169.8.4417. [DOI] [PubMed] [Google Scholar]
- 93.Song M, Kellum JA. Interleukin-6. Crit Care Med. 2005;33:S463–S465. doi: 10.1097/01.ccm.0000186784.62662.a1. [DOI] [PubMed] [Google Scholar]
- 94.Tazuke Y, Drongowski RA, Teitelbaum DH, Coran AG. Interleukin-6 changes tight junction permeability and intracellular phospholipid content in a human enterocyte cell culture model. Pediatr Surg Int. 2003;19:321–325. doi: 10.1007/s00383-003-1003-8. [DOI] [PubMed] [Google Scholar]
- 95.Wang L, Srinivasan S, Theiss AL, Merlin D, Sitaraman SV. Interleukin-6 induces keratin expression in intestinal epithelial cells: potential role of keratin-8 in interleukin-6-induced barrier function alterations. J Biol Chem. 2007;282:8219–8227. doi: 10.1074/jbc.M604068200. [DOI] [PubMed] [Google Scholar]
- 96.Rollwagen F, Li YY, Pacheco ND, Dick EJ, Kang YH. Microvascular effects of oral interleukin-6 on ischemia/reperfusion in the murine small intestine. Am J Pathol. 2000;156:1177–1182. doi: 10.1016/S0002-9440(10)64987-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rollwagen F, Li YY, Pacheco ND, Nielsen TB. Systemic bacteraemia following haemorrhagic shock in mice: alleviation with oral Interleukin 6. Cytokine. 1996;8:121–129. doi: 10.1006/cyto.1996.0017. [DOI] [PubMed] [Google Scholar]
- 98.Yang R, Han X, Uchiyama T, Watkins SK, Yaguchi A, Delude RL, Fink MP. IL-6 is essential for development of gut barrier dysfunction after hemorrhagic shock and resuscitation in mice. Am J Physiol Gastrointest Liver Physiol. 2003;285:G621–G629. doi: 10.1152/ajpgi.00177.2003. [DOI] [PubMed] [Google Scholar]
- 99.Wang Q, Fang CH, Hasselgren PO. Intestinal permeability is reduced and IL-10 levels are increased in septic IL-6 knockout mice. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1013–R1023. doi: 10.1152/ajpregu.2001.281.3.R1013. [DOI] [PubMed] [Google Scholar]
- 100.Scumpia P, Moldawer LL. Biology of interleukin-10 and its regulatory roles in sepsis syndromes. Crit Care Med. 2005;33:S468–S471. doi: 10.1097/01.ccm.0000186268.53799.67. [DOI] [PubMed] [Google Scholar]
- 101.Hale L, Gottfried MR, Swidsinski A. Piroxicam treatment of IL-10-deficient mice enhances colonic epithelial apoptosis and mucosal exposure to intestinal bacteria. Inflamm Bowel Dis. 2005;11:1060–1069. doi: 10.1097/01.mib.0000187582.90423.bc. [DOI] [PubMed] [Google Scholar]
- 102.Wang Q, Hasselgren PO. Heat shock response reduces intestinal permeability in septic mice: potential role of interleukin-10. Am J Physiol Regul Integr Comp Physiol. 2002;282:R669–R676. doi: 10.1152/ajpregu.00606.2001. [DOI] [PubMed] [Google Scholar]
- 103.Mazzon E, Puzzolo D, Caputi AP, Cuzzocrea S. Role of IL-10 in hepatocyte tight junction alteration in mouse model of experimental colitis. Mol Med. 2002;8:353–366. [PMC free article] [PubMed] [Google Scholar]
- 104.Kabay B, Aytekin FO, Aydin C, Ozer A, Kabay N, Tekin K, Sungurtekin U, Erdem E, Ozden A. Interleukin-10 gene therapy attenuates pulmonary tissue injury caused by mesenteric ischemia-reperfusion in a mouse model. Tohoku J Exp Med. 2005;207:133–142. doi: 10.1620/tjem.207.133. [DOI] [PubMed] [Google Scholar]
- 105.Sun X, Yang H, Nose K, Nose S, Haxhija EQ, Koga H, Feng Y, Teitelbaum DH. Decline in intestinal mucosal IL-10 expression and decreased intestinal barrier function in a mouse model of total parenteral nutrition. Am J Physiol Gastrointest Liver Physiol. 2008;294:G139–G147. doi: 10.1152/ajpgi.00386.2007. [DOI] [PubMed] [Google Scholar]
- 106.Castagliuolo I, Kelly CP, Qiu BS, Nikulasson ST, LaMont JT, Pothoulakis C. IL-11 inhibits Clostridium difficile toxin A enterotoxicity in rat ileum. Am J Physiol. 1997;273:G333–G341. doi: 10.1152/ajpgi.1997.273.2.G333. [DOI] [PubMed] [Google Scholar]
- 107.Mankertz J, Schulzke JD. Altered permeability in inflammatory bowel disease: pathophysiology and clinical implications. Curr Opin Gastroenterol. 2007;23:379–383. doi: 10.1097/MOG.0b013e32816aa392. [DOI] [PubMed] [Google Scholar]
- 108.Sanders S, Madara JL, McGuirk DK, Gelman DS, Colgan SP. Assessment of inflammatory events in epithelial permeability: a rapid screening method using fluorescein dextrans. Epithelial Cell Biol. 1995;4:25–34. [PubMed] [Google Scholar]
- 109.Heller F, Florian P, Bojarski C, Richter J, Christ M, Hillenbrand B, Mankertz J, Gitter AH, Burgel N, Fromm M, Zeitz M, Fuss I, Strober W, Schulzke JD. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology. 2005;129:550–564. doi: 10.1016/j.gastro.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 110.Troeger H, Richter JF, Beutin L, Gunzel D, Dobrindt U, Epple HJ, Gitter AH, Zeitz M, Fromm M, Schulzke JD. Escherichia coli alpha-haemolysin induces focal leaks in colonic epithelium: a novel mechanism of bacterial translocation. Cell Microbiol. 2007;9:2530–2540. doi: 10.1111/j.1462-5822.2007.00978.x. [DOI] [PubMed] [Google Scholar]
- 111.Ceponis P, McKay DM, Ching JC, Pereira P, Sherman PM. Enterohemorrhagic Escherichia coli O157:H7 disrupts Stat1-mediated gamma interferon signal transduction in epithelial cells. Infect Immun. 2003;71:1396–1404. doi: 10.1128/IAI.71.3.1396-1404.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ahdieh M, Vandenbos T, Youakim A. Lung epithelial barrier function and wound healing are decreased by IL-4 and IL-13 and enhanced by IFN-gamma. Am J Physiol Cell Physiol. 2001;281:C2029–C2038. doi: 10.1152/ajpcell.2001.281.6.C2029. [DOI] [PubMed] [Google Scholar]
- 113.Stevens A, Matthews J, Andres P, Baffis V, Zheng XX, Chae DW, Smith J, Strom TB, Maslinski W. Interleukin-15 signals T84 colonic epithelial cells in the absence of the interleukin-2 receptor beta-chain. Am J Physiol. 1997;272:G1201–G1208. doi: 10.1152/ajpgi.1997.272.5.G1201. [DOI] [PubMed] [Google Scholar]
- 114.Nishiyama R, Sakaguchi T, Kinugasa T, Gu X, MacDermott RP, Podolsky DK, Reinecker HC. Interleukin-2 receptor beta subunit-dependent and -independent regulation of intestinal epithelial tight junctions. J Biol Chem. 2001;276:35571–35580. doi: 10.1074/jbc.M106013200. [DOI] [PubMed] [Google Scholar]
- 115.Kinugasa T, Sakaguchi T, Gu X, Reinecker HC. Claudins regulate the intestinal barrier in response to immune mediators. Gastroenterology. 2000;118:1001–1011. doi: 10.1016/s0016-5085(00)70351-9. [DOI] [PubMed] [Google Scholar]
- 116.Yamamoto K, Mizuno M, Tsuji T, Amano T. Capillary leak syndrome after interferon treatment for chronic hepatitis C. Arch Intern Med. 2002;162:481–482. doi: 10.1001/archinte.162.4.481. [DOI] [PubMed] [Google Scholar]
- 117.Lechner J, Krall M, Netzer A, Radmayr C, Ryan MP, Pfaller W. Effects of interferon alpha-2b on barrier function and junctional complexes of renal proximal tubular LLC-PK1 cells. Kidney Int. 1999;55:2178–2191. doi: 10.1046/j.1523-1755.1999.00487.x. [DOI] [PubMed] [Google Scholar]
- 118.Katakura K, Lee J, Rachmilewitz D, Li G, Eckmann L, Raz E. Toll-like receptor 9-induced type I IFN protects mice from experimental colitis. J Clin Invest. 2005;115:695–702. doi: 10.1172/JCI22996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kawaguchi H, Akazawa Y, Watanabe Y, Takakura Y. Permeability modulation of human intestinal Caco-2 cell monolayers by interferons. Eur J Pharm Biopharm. 2005;59:45–50. doi: 10.1016/j.ejpb.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 120.Rao R, Baker RD, Baker SS. Inhibition of oxidant-induced barrier disruption and protein tyrosine phosphorylation in Caco-2 cell monolayers by epidermal growth factor. Biochem Pharmacol. 1999;57:685–695. doi: 10.1016/s0006-2952(98)00333-5. [DOI] [PubMed] [Google Scholar]
- 121.Howe K, Reardon C, Wang A, Nazli A, McKay DM. Transforming growth factor-beta regulation of epithelial tight junction proteins enhances barrier function and blocks enterohemorrhagic Escherichia coli O157:H7-induced increased permeability. Am J Pathol. 2005;167:1587–1597. doi: 10.1016/s0002-9440(10)61243-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Roche J, Martins CA, Cosme R, Fayer R, Guerrant RL. Transforming growth factor beta1 ameliorates intestinal epithelial barrier disruption by Cryptosporidium parvum in vitro in the absence of mucosal T lymphocytes. Infect Immun. 2000;68:5635–5644. doi: 10.1128/iai.68.10.5635-5644.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Woo P, Cha HH, Singer KL, Firestone GL. Antagonistic regulation of tight junction dynamics by glucocorticoids and transforming growth factor-beta in mouse mammary epithelial cells. J Biol Chem. 1996;271:404–412. doi: 10.1074/jbc.271.1.404. [DOI] [PubMed] [Google Scholar]
- 124.Lui W, Lee WM, Cheng CY. Transforming growth factor-beta3 perturbs the inter-Sertoli tight junction permeability barrier in vitro possibly mediated via its effects on occludin, zonula occludens-1, and claudin-11. Endocrinology. 2001;142:1865–1877. doi: 10.1210/endo.142.5.8116. [DOI] [PubMed] [Google Scholar]