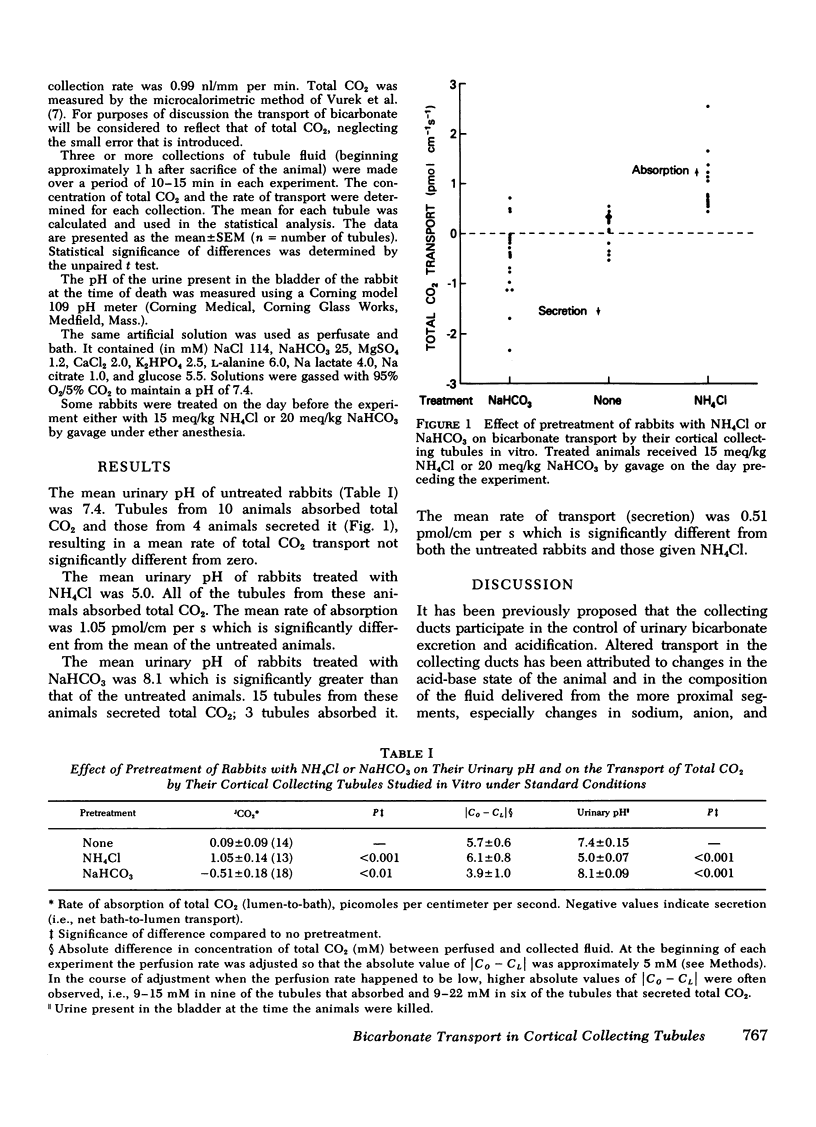
Abstract
Rabbit cortical collecting tubules were perfused in vitro to investigate the control of bicarbonate transport. Bicarbonate was measured by microcalorimetry as total CO2. The perfusate and bath were identical solutions containing 25 mM bicarbonate at pH 7.4. The mean pH of the urine in the bladders of untreated rabbits at the time they were killed was 7.4. Their individual tubules, studied in vitro, either absorbed or secreted bicarbonate, and, combining the results, there was on the average no significant net transport. When the rabbits were treated with NH4Cl the day before the experiment, their urine was acidic and their tubules studied in vitro absorbed bicarbonate (i.e., there was net lumen-to-bath transport). In contrast, when the rabbits were treated with NaHCO3, their urine was significantly more alkaline, and their tubules studied in vitro generally secreted bicarbonate (i.e., net bath-to-lumen transport). Thus, the direction of bicarbonate transport by cortical collecting tubules studied under standard conditions in vitro correlated with the urine pH and was determined by the preceding treatment of the animals in vivo with acidifying or alkalinizing salts. These results demonstrate a previously unrecognized mechanism which contributes to the control of urinary bicarbonate excretion.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernstein B. A., Clapp J. R. Micropuncture study of bicarbonate reabsorption by the dog nephron. Am J Physiol. 1968 Feb;214(2):251–257. doi: 10.1152/ajplegacy.1968.214.2.251. [DOI] [PubMed] [Google Scholar]
- Burg M. B. Perfusion of isolated renal tubules. Yale J Biol Med. 1972 Jun-Aug;45(3-4):321–326. [PMC free article] [PubMed] [Google Scholar]
- Dies F., Lotspeich W. D. Hexose monophosphate shunt in the kidney during acid-base and electrolyte imbalance. Am J Physiol. 1967 Jan;212(1):61–71. doi: 10.1152/ajplegacy.1967.212.1.61. [DOI] [PubMed] [Google Scholar]
- Kurtzman N. A. Regulation of renal bicarbonate reabsorption by extracellular volume. J Clin Invest. 1970 Mar;49(3):586–595. doi: 10.1172/JCI106269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malnic G., De Mello Aires M., Giebisch G. Micropuncture study of renal tubular hydrogen ion transport in the rat. Am J Physiol. 1972 Jan;222(1):147–158. doi: 10.1152/ajplegacy.1972.222.1.147. [DOI] [PubMed] [Google Scholar]
- Steinmetz P. R. Cellular mechanisms of urinary acidification. Physiol Rev. 1974 Oct;54(4):890–956. doi: 10.1152/physrev.1974.54.4.890. [DOI] [PubMed] [Google Scholar]
- Stoner L. C., Burg M. B., Orloff J. Ion transport in cortical collecting tubule; effect of amiloride. Am J Physiol. 1974 Aug;227(2):453–459. doi: 10.1152/ajplegacy.1974.227.2.453. [DOI] [PubMed] [Google Scholar]
- Vurek G. G., Warnock D. G., Corsey R. Measurement of picomole amounts of carbon dioxide by calorimetry. Anal Chem. 1975 Apr;47(4):765–767. doi: 10.1021/ac60354a024. [DOI] [PubMed] [Google Scholar]


