Abstract
Vascularized composite tissue allotransplantation is a viable treatment option for injuries and defects that involve multiple layers of functional tissue. In the past 15 years, over 150 VCA surgeries have been reported for various anatomic locations including – but not limited to –trachea, larynx, abdominal wall, face, and upper and lower extremities. VCA can achieve a level of aesthetic and functional restoration that is currently unattainable using conventional reconstructive techniques.
Although the risks of lifelong immunosuppression continue to be an important factor when evaluating the benefits of VCA, reported short- and long-term outcomes have been excellent, thus far. Acute rejections are common in the early postoperative period, and immunosuppression-related side-effects have been manageable. A multidisciplinary approach to the management of VCA has proven successful. Reports of long-term graft losses have been rare, while several factors may play a role in the pathophysiology of chronic graft deterioration in VCA.
Alternative approaches to immunosuppression such as cellular therapies and immunomodulation hold promise, although their role is so far not defined.
Experimental protocols for VCA are currently being explored. Moving forward, it will be exciting to see if VCA specific aspects of allorecognition and immune responses will be able to help facilitate tolerance induction.
Keywords: vascularized composite tissue allotransplantation, composite tissue transplantation, face transplantation, hand transplantation, immunosuppression
Introduction
The treatment of injuries that destroy multiple functional layers of vascularized tissue including but not limited to skin, muscle, bone or nerves has a long tradition. During World War II, severe burns were treated using skin allografts for wound coverage.[1, 2] Rejection of skin allografts was inevitable and prompted early studies on transplant immunology.[3, 4] Inspired by some of the early attempts of skin transplants, Joseph Murray demonstrated for the first time that clinical organ transplants could be successfully performed in identical twins in 1954.[5, 6] Applying total body irradiation, Murray and co-workers went on to achieve successful kidney transplantation between dizygotic twins in 1959.[7] The emergence of chemical immunosuppression utilization of Azathioprine and Steroids in the early 1960s made the success of renal transplants from deceased donors possible.[8]
Only a few years later, in 1964, the first documented attempt of a vascularized composite allotransplant had been made by an Ecuadorian team, transplanting an upper extremity applying chemical immunosuppression with azathioprine and prednisone.[9] Unfortunately, the forearm had to be removed early after transplantation subsequent to an irreversible acute rejection.[10]
Since the 1960s, advances in immunosuppressive medications have been critical for the progress in solid organ transplantation. In spite of advancements in solid organ transplantation, comparable progress has not been observed in VCA, at least in part related to the assumption that the skin represented the most immunogenic tissue. The emergence of more potent immunosuppressants, including calcineurin inhibitors and anti-proliferative agents such as mycophenolate mofetil (MMF), led to success in animal models of vascularized composite allotransplantation (VCA),[11] allowing subsequent clinical attempts.
The first successful hand transplants were performed 1998 in France and 1999 in North America.[12, 13] The world’s first bilateral upper extremity transplant was performed in 2000.[14] To date, over 50 hands have been transplanted around the world, and patients have experienced substantial improvements in quality of life. The International Registry on Hand and Composite Tissue Transplantation periodically summarizes worldwide outcomes in a thorough and inclusive report generated with data provided by VCA centers across the globe.[15] Considerable improvement in upper extremity sensory and motor function, as determined by the “Disabilities of the Arm, Shoulder, and Hand” outcome measure, has been reported.[16] The accumulating experience in hand transplantation has provided benchmarks for numerous teams and centers that have subsequently pursued VCA.
Clearly, the need for VCA has increased globally. In 1988, the first successful laryngeo-tracheal transplant was performed and numerous centers have since accumulated experience for this procedure.[17–19] A series of knee and femur transplantations were performed in Germany in the 1990s, but despite initial successes, long-term survival was elusive.[20] Centers in Miami, Florida, and Bologna, Italy have reported excellent outcomes for abdominal wall transplants.[21, 22]
Following extensive ethical debates, a French team performed the world’s first face transplant in 2005.[23] Transplants in China and Paris quickly followed, and the first two face transplants in the US were performed in Cleveland in 2008 and Boston in 2009.[24–27] First full face transplants were performed in Spain and Boston in 2010 and 2011, respectively, and the Boston team reported on early outcomes of a three-patient series of full-face transplant recipients in late 2011.[28, 29] Outcomes of face transplantation have so far been excellent, with minimal adverse effects and manageable complications. Centers have reported a consistent return of sensation to the facial allografts along with significant return of motor function to near-normal levels, as patients present improved oral competence, speech, facial expression and social reintegration. Supported by these outcomes, teams around the world are currently working on institutional approval to perform facial VCA.
With over 150 VCAs performed across the globe thus far, the transplantation of vascularized composite tissues has proven to be technically feasible. Aesthetic results after VCA supersede what can be achieved employing staged, conventional reconstructive techniques by far. Most importantly, functional outcomes have been very encouraging.
Induction Immunosupression in VCA
Polyclonal anti-thymocyte globulins (ATG, thymoglobulin), anti-interleukin-2 (IL-2) receptor monoclonal antibodies such as Daclizumab and Basiliximab, anti-CD52 monoclonal antibodies (Alemtuzumab) and anti-CD3 monoclonal antibodies have all been used as immunosuppressive agents during the induction phase. The majority of groups performing clinical VCAs have been using Thymoglobulin at differing doses (1.25–3.0 mg/kg/day over 3–10 days).[14, 23, 25–29]. At our institution, we administer thymoglobulin peri-operatively with a dosage of 1.5 mg/kg × 4 days. Others have used anti-IL-2 receptor monoclonal antibodies peri-operatively with one or two additional boluses in the days/weeks after surgery.[13, 24, 30, 31] Alemtuzumab (humanized anti-CD52 monoclonal antibody) has been used in a cohort of abdominal wall recipients (0.3 mg/kg initiated peri-operatively and re-dosed once to twice during the 1st week).[22] The anti-CD3 monoclonal antibody Muromonab was used in larynx transplants (5mg/day for the first post-operative week).[17] Of note, tracheal transplants have also been performed without antibody induction treatment; however, the trachea may differ from other VCAs as it lacks immunogenic components such as the skin.[19] Experience with induction, maintenance, and rescue immunosuppression in VCA is summarized in Table 1.
Table 1.
Experience of Clinical Immunosuppression in VCA.
| Immunosuppression | VCA Center(s) (in alphabetical order) |
|---|---|
| Induction | |
| Thymoglobulin | Barcelona, Boston, Cleveland, Lyon, Paris |
| Anti-IL-2 mAb | China, Louisville |
| Hematopoietic Stem-cell Transplantation + Serial Extracorporeal photochemotherapy (18 months) | Lyon |
| Alemtuzumab | Louisville |
| Maintenance | |
| Triple Therapy: Tacrolimus + MMF + Prednisone |
Barcelona, Boston, China, Cleveland, Louisville, Lyon, Paris |
| Dual Therapy: Tacrolimus + MMF (after steroid-withdrawal) |
Boston |
| Dual Therapy: Tacrolimus + Prednisone (after MMF − withdrawal) |
Cleveland |
| Steroid Withdrawal | |
| Reported | Boston |
| Rescue treatment | |
| Glucocorticoid bolus | Typical regimen; all centers |
| Temporary Increase in Maintenance Immunossupression (including steroids) | Barcelona, Cleveland, Lyon, Paris |
| Tacrolimus increase alone | Boston |
| Alemtuzumab | Innsbruck |
| Topical Tacrolimus | Louisville |
| Topical Tacrolimus + Clobetasol | Boston |
| Topical Tacrolimus + Clobetasol + Glucocorticoid increase or bolus | China, Louisville, Lyon |
| Rabbit anti-thymocyte globulin | Louisville, Lyon |
| Anti-lymphocyte serum | Paris |
Maintenance Immunosuppression
Animal studies of VCA immunosuppression have been published since the 1980s, when the first successful limb transplants were performed in rats and later in rabbits using monotherapy with cyclosporine.[32, 33] Of note, rat models showed that acute rejection episodes appeared later in the post-transplant period when administering a dual therapy with cyclosporine and MMF in comparison to cyclosporine alone.[34] Success in a porcine model applying cyclosporine and MMF was the catalyst for the first human hand transplant in 1998.[11, 35] In recent years, primate models have successfully explored VCA transplants with tacrolimus monotherapy[36] while others have applied an induction treatment with thymoglobulin followed by a maintenance immunosuppression with tacrolimus and rapamycin.[37]
A triple therapy approach consisting of tacrolimus, an anti-proliferative agent and corticosteroids has been used clinically most frequently as maintenance immunosuppression.[38] At our institution, we administer 1 g of MMF pre-operatively followed by 2 g/d. Peri-operatively, we administer methyl-prednisolone 500 mg IV. Methyl-prednisolone is subsequently tapered and withdrawn in our protocol. On post-operative day 4, we switch from methyl-prednisolone to prednisone, administered as 40 mg PO twice a day and 20 mg PO once a day from post-operative day 5 onwards. Tacrolimus is administered post-operatively with the goal for a trough level of 10–15 ng/ml until month 3. Others have reported on comparable maintenance immunosuppression in VCA [13, 14, 23–26, 28, 30].
Other centers have explored hematopoietic stem-cell transplantation in the early postoperative period, followed by serial extracorporeal photochemotherapy in addition to triple immunosuppression.[23, 39, 40] Microchimerism was detected 2 months after transplantation in few (0.1%) donor cells among the recipient bone marrow cell population. Despite early encouraging result, subsequent analyses failed to show chimerism in the peripheral blood.[41]
Abdominal wall transplants have been performed successfully with an induction immunosuppression of alemtuzumab and maintenance immunosuppression with tacrolimus monotherapy.[21, 22] Cyclosporine monotherapy has been used for maintenance immunosuppression in penile and knee-joint VCAs [20, 31]
Maintenance immunosuppression is tapered at our center with tacrolimus trough levels of 6–8 ng/ml by month 6 and 4–6 ng/ml by month 12. MMF is initially dosed at 2 g per day and subsequently tapered. Gastrointestinal side effects linked to MMF can in some cases be prevented through the substitution with enteric-coated mycophenolate sodium. We were able to withdraw steroids in all our face and hand transplant recipients between months 1–12.
Our steroid-free approach differs from the maintenance immunosuppression used in other centers.[14, 24, 28, 30, 41, 42] One center reports on weaning MMF while continuing with a tacrolimus and prednisone maintenance treatment[43] while others have switched MMF or tacrolimus to mTOR inhibitors. Of interest is the serial extracorporeal chemotherapy in parallel to maintenance immunosuppression up to 18 months post-transplantation in the first French face transplant recipient.[23]
Alternative approaches to VCA immunosuppression, albeit experimental at this stage, have consisted of cellular therapies and immunomodulation in order to induce transplant tolerance.[44, 45] Stable microchimerism was achieved using an immunomodulatory protocol in a swine VCA model.[46] The protocol includes total body and thymic irradiation, bone marrow cell infusion, and co-stimulatory blockade with CTLA4Ig. Stable mixed chimerism has also been achieved in a canine model [47] while sporadic macrochimerism has been reported in non-human primate models.[48]
In solid organ transplantation, tolerance induction has been achieved for a few patients who had received simultaneous renal and bone marrow following a conditioning regimen including immunosuppression and irradiation.[49, 50] Noteworthy is a continuous and detectable humoral response with signs of chronic graft deterioration in some patients treated with this regimen. Most recently, a successful clinical tolerance protocol with the additional use of so called ‘tolerance facilitating cells’ has been presented.[51] If and how those protocols apply to VCA transplants remains unclear at this time.
Of note, minimal immunosuppression has been achieved in some VCA recipients and a better understanding of the biology of VCA in parallel to a thorough immune monitoring may allow achieving safe and low long-term immunosuppression with minimal side-effects in the future. Gene profiles may also help to guide treatment in VCA to achieve a safe reduction and minimization of immunosuppression. Moreover, a better understanding of VCA-specific immune responses may allow for an improved future immunosuppressive treatment.
Scoring Systems for Rejection in VCA
By 2007, 41 VCA procedures had been performed. Meanwhile, four different classification systems detailing pathological changes of rejections in VCA had been published.[52–55] In 2007, VCA pathologists, surgeons, and basic investigators met during the Ninth Banff Conference on Allograft Pathology to evaluate and discuss the need for a universally accepted grading scale for VCA.[56] The main areas discussed were: specimen preparation; scope of acute, chronic, and humoral disease and scoring of acute lesions. These discussions resulted in the Banff 2007 Classification for Acute Rejections in VCAs, which is based on the location and intensity of inflammatory infiltrates (Table 2).[56] A detailed description of Grade I rejection of an upper extremity allograft has been recently published.[57]
Table 2.
The Banff 2007 working classification of skin-containing composite tissue allograft pathology. (With permission from the American Journal of Transplantation / Blackwell Munksgaard)
| Grade 0 | No or rare inflammatory infiltrates. | |
|---|---|---|
| Grade I | Mild Acute Rejection | Mild perivascular infiltration. No involvement of the overlying epidermis. |
| Grade II | Moderate Acute Rejection | Moderate-to-severe perivascular inflammation with or without mild epidermal and/or adnexal involvement (limited to spongiosis and exocytosis). No epidermal dyskeratosis or apoptosis. |
| Grade III | Severe Acute Rejection | Dense inflammation and epidermal involvement with epithelial apoptosis, dyskeratosis and/or keratinolysis. |
| Grade IV | Necrotizing Acute Rejection | Frank necrosis of epidermis or other skin structures. |
Acute Rejections
Indication for treatment
Some centers transplant a sentinel, vascularized and skin-containing composite allograft when performing VCAs while other teams’ protocols do not include a sentinel allograft. At present, the VCA literature is not specific regarding indications for the treatment of acute rejection. At our institution, we treat rejections based on clinical and histopathological evidence of acute rejections ≥ Grade II obtained in both VCA allograft and sentinel graft. As experience grows, evidence on the safety and effectiveness of reported protocols will be examined to determine best possible approaches in treating acute rejections in VCA. The role and significance of sentinel composite allografts in this context will warrant further studies.
Rescue Immunosuppression
Acute rejection episodes are common in the first months following VCA [13, 15, 19, 21, 23–30] and are typically treated with a steroid bolus treatment (Figures 1 & 2). Some acute rejection episodes have also been treated with a temporary increase of maintenance immunosuppression.[20, 21, 25, 26, 28, 58] Topical applications of tacrolimus and clobetasol are of interest to VCA teams, as local immunosuppression with minimal systemic toxicity has yielded encouraging results in the setting of inflammatory dermatoses.[59] Based on effective prevention of rejection in animal models,[60] VCA teams have clinically administered topical tacrolimus and/or clobetasol[27, 30] in combination with a steroid bolus or an increase in steroid maintenance.[24, 30, 58]
Figure 1.
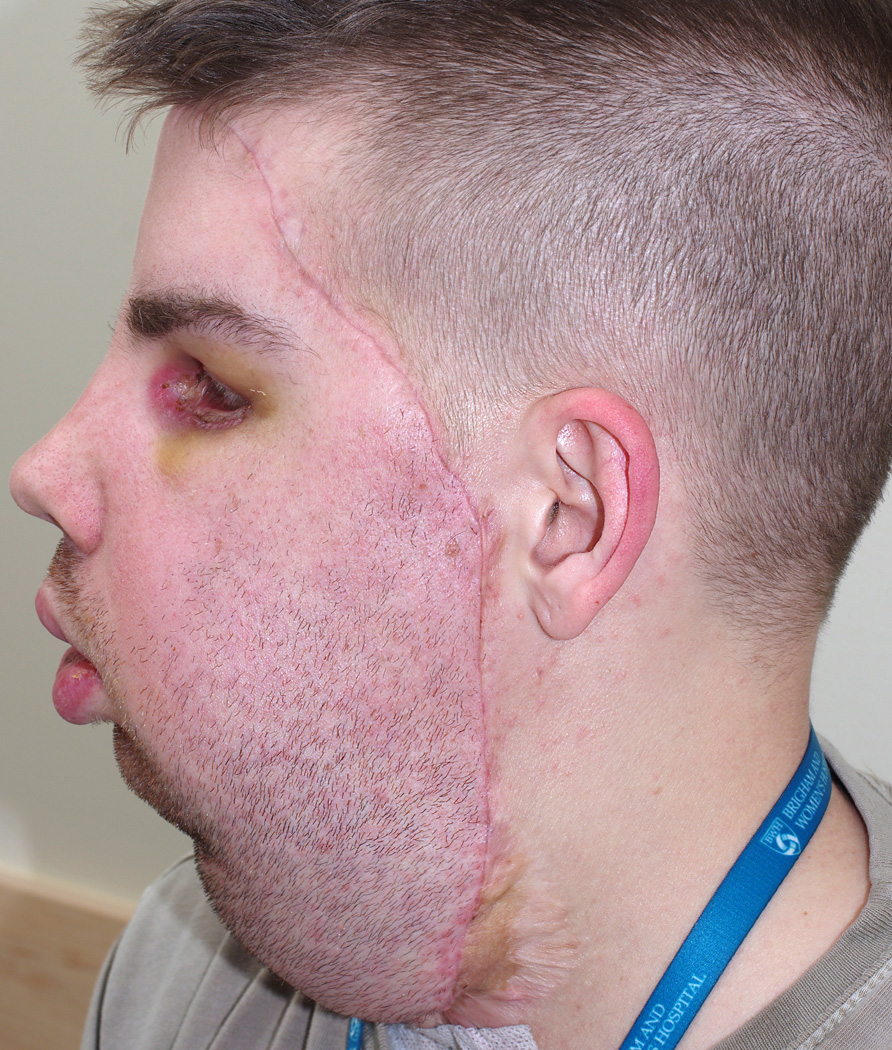
A face transplant recipient with clinically evident acute rejection on postoperative day 20. Generalized erythema is observed on the skin of the facial allograft. (Photograph with permission of the patient)
Figure 2.
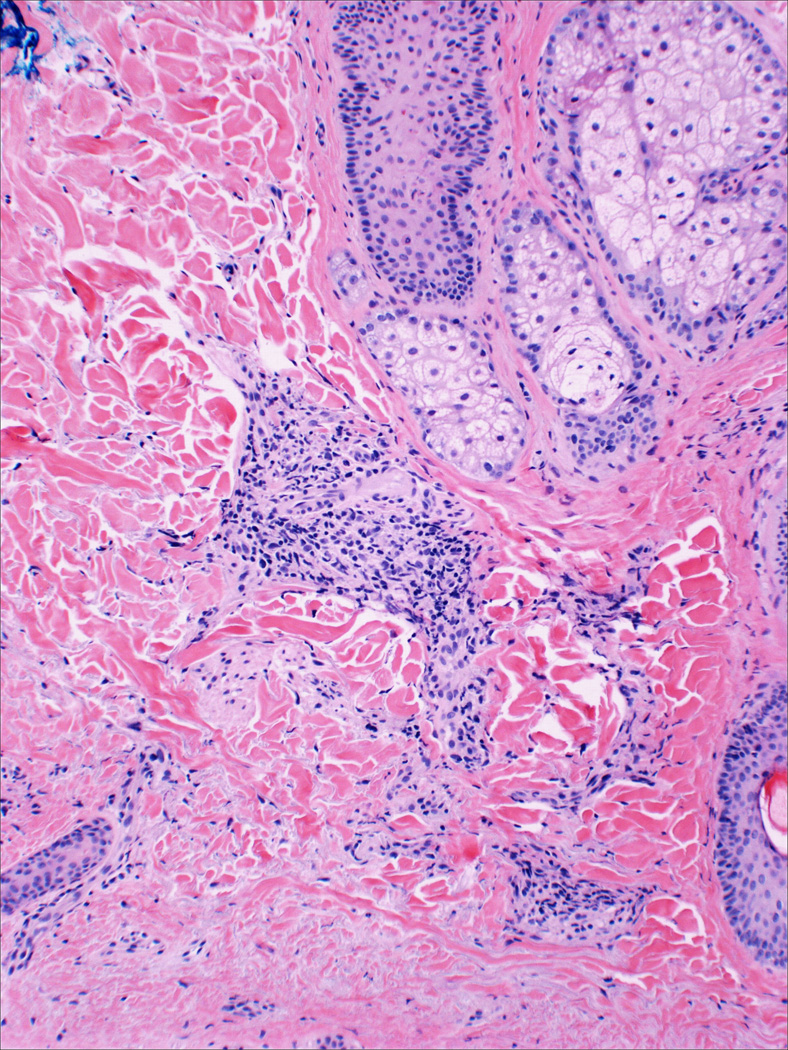
Histology of skin biopsy of the same face transplant recipient, taken on postoperative day 20, shows Grade II rejection. Moderate perivascular infiltrate with mild adnexal involvement is observed.
Late acute rejections have also been observed. At our institution, one patient presented with an acute rejection almost 3 years after transplantation – 2 years after total steroid withdrawal. This acute rejection episode was effectively controlled with a slight increase of the patient’s maintenance tacrolimus. Other agents used to treat acute rejection are rabbit anti-thymocyte globulin [30, 58] and antilymphocyte serum.[25]
Extracorporeal photopheresis (ECP) has been used to treat T-cell lymphoma, Crohn’s disease and steroid-refractory graft-versus-host disease.[61, 62] Recent applications of ECP include the prevention of rejection in cardiac transplantation and the treatment of bronchiolitis obliterans after lung transplantation.[63, 64] Citing its immunomodulatory effects, a VCA team reported using ECP to treat acute rejection in the setting of concomitant ganciclovir-resistant cytomegalovirus viraemia. ECP was continued for 95 days due to persistent grade 1 rejection observed in biopsies of the oral mucosa.[25]
One team reported on a patient who experienced 6 episodes of acute rejections in the first 6 postoperative months. The team used many of the above-mentioned strategies to treat the first few episodes; on day 77 the patient developed a Grade II acute rejection, which responded to topical tacrolimus and clobetasol alone.[30] On day 90, rejection was observed once again, and treated with methylprednisolone.
Chronic graft deterioration
Although the Banff 2007 classification does not specifically describe changes associated with chronic allograft deterioration in VCA,[56] the process is in general defined by the presence of vasculopathy in addition to atrophy and fibrosis of muscles, skin and adnexal structures [65]. Chronic vasculopathy after multiple episodes of acute rejection have been reported in a rat hind-limb allotransplantation model.[66] The first hand transplant recipient in France in 1998 had been assumed to suffer from sequelae of chronic vasculopathy. Following further work-up it seems more likely, however, that the observed graft changes were secondary to repeated and prolonged episodes of acute rejections in the presence of non-adherence to immunosuppressive treatment.[58]
By conventional monitoring and surveillance techniques, there has been one recently confirmed case of chronic allograft deterioration in a compliant patient on a less potent maintenance immunosuppression. This patient presented during routine follow-up for his unilateral hand transplant by month 7 and was found to have a 50–60% stenosis in the area of the brachial artery anastomosis; during attempted surgical repair, severe thickening of all graft arteries was noted and the graft was amputated subsequent to worsening ischemia.
Advanced imaging techniques, including ultrasound biomicroscopy (UBM), allowing a more sensitive identification of intimal thickening may enable earlier detection of chronic changes in the future.[67] An advanced intimal hyperplasia has been identified in another hand transplant recipient 6 months after transplantation by UBM. Subsequent modifications of the immunosuppression in this patient seemed to have prevented a further progress of the observed chronic changes.[67]
Reasons for the infrequent overall occurrence of chronic allograft deterioration in VCA compared to solid organ transplants have been debated. The early diagnosis of acute rejections due to the inclusion of skin in the allograft, the histological confirmation in vascularized sentinel patches, the presence of vascularized bone marrow and potentially VCA specific patterns of allorecognition and neovascularization may all play a role.
Side Effects of Immunosuppression in VCA
Non-compliance to immunosuppressive therapy has resulted in inevitable rejection, followed by allograft loss and even death in some patients.[68, 69] An emphasis on compliance has thus become a continuous priority for the success of VCAs.
VCA recipients have presented with a wide variety of complications associated with immunosuppressive therapy.[15] Opportunistic infections are the most common reported adverse effect, especially cytomegalovirus,[20, 25, 70, 71] herpes simplex[41, 70] and cutaneous mycoses.[30, 70] Some VCA recipients have developed pneumonias. Among the metabolic complications of immunosuppression, post-transplant hyperglycemia has been commonly encountered. Although often reversible, particularly following the discontinuation of steroids, [15] some VCA recipients have developed diabetes mellitus long-term.[24, 27, 30]. VCA recipients have also presented with hypercholesterolemia[30] and hypertriglyceridemia.[41] Complications mostly linked to long-term glucocorticoid treatment have included Cushing syndrome [70], delirium, and bilateral aseptic, avascular necrosis, in one case ultimately requiring bilateral hip replacement surgery.[30]
The first face transplant recipient had developed renal failure 1 year after transplantation,[41] which prompted the gradual withdrawal of tacrolimus and the introduction of an mTOR inhibitor. A temporary and mild thrombotic microangiopathy resolved following the discontinuation of tacrolimus.[23] The same patient also developed a cervical carcinoma in-situ, 4 years after transplantation that was treated by local excision. [41] A laryngotracheal transplant recipient presented with hypertension and elevated serum creatinine on postoperative month 6 which prompted the addition of another hypertensive medication and a reduction in cyclosporine dose.[17] Cyclosporine was replaced with tacrolimus in this patient 9 months later.
Other adverse effects listed on the International Registry on Hand and Composite Tissue Transplantation include Clostridium difficile colitis, herpes zoster infection, cutaneous mycosis, osteitis, arterial hypertension, and basal cell carcinoma.[15]
Conclusions
The worldwide experience in VCA is only beginning to accumulate. Teams around the world have reported their experience in all aspects of VCA, from patient selection, to surgical technique and follow-up care. A sense of collaboration in the VCA community has allowed teams to learn from the experience of more established centers while adapting to the evolving needs of patients in this new and truly restorative field.
Early and long-term outcomes in compliant patients are excellent. Patients have adapted well following VCA with exciting reports of increased functionality and much-improved quality of life. Side effects of immunosuppression, although common, have in most cases not been severe, and have proven to be manageable in a multi-team approach. Acute rejections have been common in the early months post-transplantation and successfully treated with differing approaches. The incidence and significance of chronic graft changes in VCA remains unclear. Moreover, the contribution of humoral components to either acute rejections or chronic changes warrants further explorations.[72]
Most importantly, ongoing and future experimental and clinical investigations into the biology of VCA hold promise to refine immunosuppressive treatment. Considerable resources are being allocated to clinical and pre-clinical investigation of immunological issues related to VCA. Early diagnosis of rejections, close monitoring and VCA specific-advantages of allorecognition, neovascularization and immune responses may help us in the future to design strategies aiming to minimize and potentially avoid immunosuppression.
Acknowledgements
Drs. Diaz-Siso, Bueno, Pomahac and Tullius are supported by a U.S. Department of Defense research contract (W911QY-09-C-0216), under the Biomedical Translational Initiative.
Dr. Tullius’ research is supported by grants from the NIH (R01-AG039449) and the ‘Instituto Carlos Slim de la Salud.’
References
- 1.Brown JB, McDowell F. Massive Repairs of Burns with Thick Split-Skin Grafts: Emergency "Dressings" with Homografts. Ann Surg. 1942;115(4):658–674. doi: 10.1097/00000658-194204000-00017. Epub 1942/04/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tobin GR, Breidenbach WC, 3rd, Ildstad ST, Marvin MM, Buell JF, Ravindra KV. The history of human composite tissue allotransplantation. Transplant Proc. 2009;41(2):466–471. doi: 10.1016/j.transproceed.2009.01.026. Epub 2009/03/31. [DOI] [PubMed] [Google Scholar]
- 3.Brown JB, McDowell F. Epithelial Healing and the Transplantationof Skin. Ann Surg. 1942;115(6):1166–1181. doi: 10.1097/00000658-194206000-00027. Epub 1942/06/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gibson T, Medawar PB. The fate of skin homografts in man. J Anat. 1943;77(Pt 4):299–310. 4. Epub 1943/07/01. [PMC free article] [PubMed] [Google Scholar]
- 5.Harrison JH, Merrill JP, Murray JE. Renal homotransplantation in identical twins. Surg Forum. 1956;6:432–436. Epub 1956/01/01. [PubMed] [Google Scholar]
- 6.Merrill JP, Murray JE, Harrison JH, Guild WR. Successful homotransplantation of the human kidney between identical twins. J Am Med Assoc. 1956;160(4):277–282. doi: 10.1001/jama.1956.02960390027008. Epub 1956/01/28. [DOI] [PubMed] [Google Scholar]
- 7.Murray JE, Merrill JP, Dammin GJ, Dealy JB, Jr, Walter CW, Brooke MS, et al. Study on transplantation immunity after total body irradiation: clinical and experimental investigation. Surgery. 1960;48:272–284. Epub 1960/07/01. [PubMed] [Google Scholar]
- 8.Merrill JP, Murray JE, Takacs FJ, Hager EB, Wilson RE, Dammin GJ. Successful transplantation of kidney from a human cadaver. JAMA. 1963;185:347–353. doi: 10.1001/jama.1963.03060050025015. Epub 1963/08/03. [DOI] [PubMed] [Google Scholar]
- 9.Gilbert R. Transplant is successful with a cadaver forearm. Med Trib Med News. 1964;5:20–22. [Google Scholar]
- 10.Gilbert R. Hand transplanted from cadaver is reamputated. Med Trib Med News. 1964;5:23–25. [Google Scholar]
- 11.Ustuner ET, Zdichavsky M, Ren X, Edelstein J, Maldonado C, Ray M, et al. Long-term composite tissue allograft survival in a porcine model with cyclosporine/mycophenolate mofetil therapy. Transplantation. 1998;66(12):1581–1587. doi: 10.1097/00007890-199812270-00003. Epub 1999/01/12. [DOI] [PubMed] [Google Scholar]
- 12.Dubernard JM, Owen E, Herzberg G, Lanzetta M, Martin X, Kapila H, et al. Human hand allograft: report on first 6 months. Lancet. 1999;353(9161):1315–1320. doi: 10.1016/S0140-6736(99)02062-0. Epub 1999/04/28. [DOI] [PubMed] [Google Scholar]
- 13.Jones JW, Gruber SA, Barker JH, Breidenbach WC. Successful hand transplantation. One-year follow-up. Louisville Hand Transplant Team. N Engl J Med. 2000;343(7):468–473. doi: 10.1056/NEJM200008173430704. Epub 2000/08/19. [DOI] [PubMed] [Google Scholar]
- 14.Petruzzo P, Badet L, Gazarian A, Lanzetta M, Parmentier H, Kanitakis J, et al. Bilateral hand transplantation: six years after the first case. Am J Transplant. 2006;6(7):1718–1724. doi: 10.1111/j.1600-6143.2006.01369.x. Epub 2006/07/11. [DOI] [PubMed] [Google Scholar]
- 15.Petruzzo P, Lanzetta M, Dubernard JM, Landin L, Cavadas P, Margreiter R, et al. The International Registry on Hand and Composite Tissue Transplantation. Transplantation. 2010;90(12):1590–1594. doi: 10.1097/TP.0b013e3181ff1472. Epub 2010/11/06. [DOI] [PubMed] [Google Scholar]
- 16.Landin L, Bonastre J, Casado-Sanchez C, Diez J, Ninkovic M, Lanzetta M, et al. Outcomes with respect to disabilities of the upper limb after hand allograft transplantation: a systematic review. Transpl Int. 2012;25(4):424–432. doi: 10.1111/j.1432-2277.2012.01433.x. Epub 2012/02/16. [DOI] [PubMed] [Google Scholar]
- 17.Strome M, Stein J, Esclamado R, Hicks D, Lorenz RR, Braun W, et al. Laryngeal transplantation and 40-month follow-up. N Engl J Med. 2001;344(22):1676–1679. doi: 10.1056/NEJM200105313442204. Epub 2001/06/02. [DOI] [PubMed] [Google Scholar]
- 18.Duque E, Duque J, Nieves M, Mejia G, Lopez B, Tintinago L. Management of larynx and trachea donors. Transplant Proc. 2007;39(7):2076–2078. doi: 10.1016/j.transproceed.2007.06.072. Epub 2007/09/25. [DOI] [PubMed] [Google Scholar]
- 19.Delaere P, Vranckx J, Verleden G, De Leyn P, Van Raemdonck D. Tracheal allotransplantation after withdrawal of immunosuppressive therapy. N Engl J Med. 2010;362(2):138–145. doi: 10.1056/NEJMoa0810653. Epub 2010/01/15. [DOI] [PubMed] [Google Scholar]
- 20.Hofmann GO, Kirschner MH, Wagner FD, Brauns L, Gonschorek O, Buhren V. Allogeneic vascularized transplantation of human femoral diaphyses and total knee joints--first clinical experiences. Transplant Proc. 1998;30(6):2754–2761. doi: 10.1016/s0041-1345(98)00803-3. Epub 1998/09/24. [DOI] [PubMed] [Google Scholar]
- 21.Levi DM, Tzakis AG, Kato T, Madariaga J, Mittal NK, Nery J, et al. Transplantation of the abdominal wall. Lancet. 2003;361(9376):2173–2176. doi: 10.1016/S0140-6736(03)13769-5. Epub 2003/07/05. [DOI] [PubMed] [Google Scholar]
- 22.Cipriani R, Contedini F, Santoli M, Gelati C, Sgarzani R, Cucchetti A, et al. Abdominal wall transplantation with microsurgical technique. Am J Transplant. 2007;7(5):1304–1307. doi: 10.1111/j.1600-6143.2007.01798.x. Epub 2007/04/14. [DOI] [PubMed] [Google Scholar]
- 23.Dubernard JM, Lengele B, Morelon E, Testelin S, Badet L, Moure C, et al. Outcomes 18 months after the first human partial face transplantation. N Engl J Med. 2007;357(24):2451–2460. doi: 10.1056/NEJMoa072828. Epub 2007/12/14. [DOI] [PubMed] [Google Scholar]
- 24.Guo S, Han Y, Zhang X, Lu B, Yi C, Zhang H, et al. Human facial allotransplantation: a 2-year follow-up study. Lancet. 2008;372(9639):631–638. doi: 10.1016/S0140-6736(08)61276-3. Epub 2008/08/30. [DOI] [PubMed] [Google Scholar]
- 25.Lantieri L, Meningaud JP, Grimbert P, Bellivier F, Lefaucheur JP, Ortonne N, et al. Repair of the lower and middle parts of the face by composite tissue allotransplantation in a patient with massive plexiform neurofibroma: a 1-year follow-up study. Lancet. 2008;372(9639):639–645. doi: 10.1016/S0140-6736(08)61277-5. Epub 2008/08/30. [DOI] [PubMed] [Google Scholar]
- 26.Siemionow M, Papay F, Alam D, Bernard S, Djohan R, Gordon C, et al. Near-total human face transplantation for a severely disfigured patient in the USA. Lancet. 2009;374(9685):203–209. doi: 10.1016/S0140-6736(09)61155-7. Epub 2009/07/18. [DOI] [PubMed] [Google Scholar]
- 27.Pomahac B, Pribaz J, Eriksson E, Annino D, Caterson S, Sampson C, et al. Restoration of facial form and function after severe disfigurement from burn injury by a composite facial allograft. Am J Transplant. 2011;11(2):386–393. doi: 10.1111/j.1600-6143.2010.03368.x. Epub 2011/01/11. [DOI] [PubMed] [Google Scholar]
- 28.Barret JP, Gavalda J, Bueno J, Nuvials X, Pont T, Masnou N, et al. Full face transplant: the first case report. Ann Surg. 2011;254(2):252–256. doi: 10.1097/SLA.0b013e318226a607. Epub 2011/07/21. [DOI] [PubMed] [Google Scholar]
- 29.Pomahac B, Pribaz J, Eriksson E, Bueno EM, Diaz-Siso JR, Rybicki FJ, et al. Three Patients with Full Facial Transplantation. N Engl J Med. 2012;366(8):715–722. doi: 10.1056/NEJMoa1111432. Epub 2011/12/30. [DOI] [PubMed] [Google Scholar]
- 30.Breidenbach WC, Gonzales NR, Kaufman CL, Klapheke M, Tobin GR, Gorantla VS. Outcomes of the first 2 American hand transplants at 8 and 6 years posttransplant. The Journal of hand surgery. 2008;33(7):1039–1047. doi: 10.1016/j.jhsa.2008.02.015. Epub 2008/09/03. [DOI] [PubMed] [Google Scholar]
- 31.Hu W, Lu J, Zhang L, Wu W, Nie H, Zhu Y, et al. A preliminary report of penile transplantation. Eur Urol. 2006;50(4):851–853. doi: 10.1016/j.eururo.2006.07.026. Epub 2006/08/26. [DOI] [PubMed] [Google Scholar]
- 32.Hewitt CW, Black KS, Fraser LA, Howard EB, Martin DC, Achauer BM, et al. Composite tissue (limb) allografts in rats. I. Dose-dependent increase in survival with cyclosporine. Transplantation. 1985;39(4):360–364. doi: 10.1097/00007890-198504000-00004. Epub 1985/04/01. [DOI] [PubMed] [Google Scholar]
- 33.Randzio J, Kniha H, Gold ME, Chang TT, Su LD, Park HH, et al. Growth of vascularized composite mandibular allografts in young rabbits. Ann Plast Surg. 1991;26(2):140–148. doi: 10.1097/00000637-199102000-00006. Epub 1991/02/01. [DOI] [PubMed] [Google Scholar]
- 34.Benhaim P, Anthony JP, Lin LY, McCalmont TH, Mathes SJ. A long-term study of allogeneic rat hindlimb transplants immunosuppressed with RS-61443. Transplantation. 1993;56(4):911–917. doi: 10.1097/00007890-199310000-00026. Epub 1993/10/01. [DOI] [PubMed] [Google Scholar]
- 35.Gordon CR, Nazzal J, Lozano-Calderan SA, Lee SG, Lee WP, Siemionow M, et al. From experimental rat hindlimb to clinical face composite tissue allotransplantation: historical background and current status. Microsurgery. 2006;26(8):566–572. doi: 10.1002/micr.20296. Epub 2006/11/09. [DOI] [PubMed] [Google Scholar]
- 36.Barth RN, Bluebond-Langner R, Nam A, Stanwix M, Shipley S, Bartlett ST, et al. Facial subunit composite tissue allografts in nonhuman primates: I. Technical and immunosuppressive requirements for prolonged graft survival. Plast Reconstr Surg. 2009;123(2):493–501. doi: 10.1097/PRS.0b013e3181954edd. Epub 2009/02/03. [DOI] [PubMed] [Google Scholar]
- 37.Silverman RP, Banks ND, Detolla LJ, Shipley ST, Panda A, Sanchez RA, et al. A heterotopic primate model for facial composite tissue transplantation. Ann Plast Surg. 2008;60(2):209–216. doi: 10.1097/SAP.0b013e318061b792. Epub 2008/01/25. [DOI] [PubMed] [Google Scholar]
- 38.Hautz T, Brandacher G, Zelger B, Gorantla VS, Lee AW, Pratschke J, et al. Immunologic aspects and rejection in solid organ versus reconstructive transplantation. Transplant Proc. 2010;42(9):3347–3353. doi: 10.1016/j.transproceed.2010.09.020. Epub 2010/11/26. [DOI] [PubMed] [Google Scholar]
- 39.Ciancio G, Miller J, Garcia-Morales RO, Carreno M, Burke GW, 3rd, Roth D, et al. Six-year clinical effect of donor bone marrow infusions in renal transplant patients. Transplantation. 2001;71(7):827–835. doi: 10.1097/00007890-200104150-00002. Epub 2001/05/15. [DOI] [PubMed] [Google Scholar]
- 40.Andreu G, Leon A, Heshmati F, Tod M, Menkes CJ, Baudelot J, et al. Extracorporeal photochemotherapy: evaluation of two techniques and use in connective tissue disorders. Transfusion science. 1994;15(4):443–454. doi: 10.1016/0955-3886(94)90178-3. Epub 1994/11/04. [DOI] [PubMed] [Google Scholar]
- 41.Petruzzo P, Testelin S, Kanitakis J, Badet L, Lengele B, Girbon JP, et al. First human face transplantation: 5 years outcomes. Transplantation. 2012;93(2):236–240. doi: 10.1097/TP.0b013e31823d4af6. Epub 2011/12/15. [DOI] [PubMed] [Google Scholar]
- 42.Lantieri L, Hivelin M, Audard V, Benjoar MD, Meningaud JP, Bellivier F, et al. Feasibility, reproducibility, risks and benefits of face transplantation: a prospective study of outcomes. Am J Transplant. 2011;11(2):367–378. doi: 10.1111/j.1600-6143.2010.03406.x. Epub 2011/01/29. [DOI] [PubMed] [Google Scholar]
- 43.Siemionow M, Ozturk C. An update on facial transplantation cases performed between 2005 and 2010. Plast Reconstr Surg. 2011;128(6):707e–720e. doi: 10.1097/PRS.0b013e318230c77b. Epub 2011/11/19. [DOI] [PubMed] [Google Scholar]
- 44.Gorantla VS, Brandacher G, Schneeberger S, Zheng XX, Donnenberg AD, Losee JE, et al. Favoring the risk-benefit balance for upper extremity transplantation--the Pittsburgh Protocol. Hand clinics. 2011;27(4):511–520. doi: 10.1016/j.hcl.2011.08.008. ix-x. Epub 2011/11/05. [DOI] [PubMed] [Google Scholar]
- 45.Gorantla VS, Schneeberger S, Brandacher G, Sucher R, Zhang D, Lee WP, et al. T regulatory cells and transplantation tolerance. Transplant Rev (Orlando) 2010;24(3):147–159. doi: 10.1016/j.trre.2010.04.002. Epub 2010/06/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wachtman GS, Wimmers EG, Gorantla VS, Lin CH, Schneeberger S, Unadkat JV, et al. Biologics and donor bone marrow cells for targeted immunomodulation in vascularized composite allotransplantation: a translational trial in swine. Transplant Proc. 2011;43(9):3541–3544. doi: 10.1016/j.transproceed.2011.10.010. Epub 2011/11/22. [DOI] [PubMed] [Google Scholar]
- 47.Mathes DW, Hwang B, Graves SS, Edwards J, Chang J, Storer BE, et al. Tolerance to vascularized composite allografts in canine mixed hematopoietic chimeras. Transplantation. 2011;92(12):1301–1308. doi: 10.1097/TP.0b013e318237d6d4. Epub 2011/11/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barth RN, Rodriguez ED, Mundinger GS, Nam AJ, Ha JS, Hui-Chou H, et al. Vascularized bone marrow-based immunosuppression inhibits rejection of vascularized composite allografts in nonhuman primates. Am J Transplant. 2011;11(7):1407–1416. doi: 10.1111/j.1600-6143.2011.03551.x. Epub 2011/06/15. [DOI] [PubMed] [Google Scholar]
- 49.Kawai T, Cosimi AB, Spitzer TR, Tolkoff-Rubin N, Suthanthiran M, Saidman SL, et al. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med. 2008;358(4):353–361. doi: 10.1056/NEJMoa071074. Epub 2008/01/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kawai T, Cosimi AB, Sachs DH. Preclinical and clinical studies on the induction of renal allograft tolerance through transient mixed chimerism. Current opinion in organ transplantation. 2011;16(4):366–371. doi: 10.1097/MOT.0b013e3283484b2c. Epub 2011/06/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leventhal J, Abecassis M, Miller J, Gallon L, Ravindra K, Tollerud DJ, et al. Chimerism and tolerance without GVHD or engraftment syndrome in HLA-mismatched combined kidney andhematopoietic stem cell transplantation. Science translational medicine. 2012;4(124):124ra28. doi: 10.1126/scitranslmed.3003509. Epub 2012/03/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bejarano PA, Levi D, Nassiri M, Vincek V, Garcia M, Weppler D, et al. The Pathology of full-thickness cadaver skin transplant for large abdominal defects: a proposed grading system for skin allograft acute rejection. Am J Surg Pathol. 2004;28(5):670–675. doi: 10.1097/00000478-200405000-00016. Epub 2004/04/24. [DOI] [PubMed] [Google Scholar]
- 53.Kanitakis J, Petruzzo P, Jullien D, Badet L, Dezza MC, Claudy A, et al. Pathological score for the evaluation of allograftrejection in human hand (composite tissue) allotransplantation. European journal of dermatology : EJD. 2005;15(4):235–238. Epub 2005/07/29. [PubMed] [Google Scholar]
- 54.Schneeberger S, Kreczy A, Brandacher G, Steurer W, Margreiter R. Steroid- and ATG-resistant rejection after double forearm transplantation responds to Campath-1H. Am J Transplant. 2004;4(8):1372–1374. doi: 10.1111/j.1600-6143.2004.00518.x. Epub 2004/07/23. [DOI] [PubMed] [Google Scholar]
- 55.Cendales LC, Kirk AD, Moresi JM, Ruiz P, Kleiner DE. Composite tissue allotransplantation: classification of clinical acute skin rejection. Transplantation. 2006;81(3):418–422. doi: 10.1097/01.tp.0000185304.49987.d8. Epub 2006/02/16. [DOI] [PubMed] [Google Scholar]
- 56.Cendales LC, Kanitakis J, Schneeberger S, Burns C, Ruiz P, Landin L, et al. The Banff 2007 working classification of skin-containing composite tissue allograft pathology. Am J Transplant. 2008;8(7):1396–1400. doi: 10.1111/j.1600-6143.2008.02243.x. Epub 2008/05/01. [DOI] [PubMed] [Google Scholar]
- 57.Hautz T, Zelger B, Brandacher G, Mueller H, Grahammer J, Lee A, et al. Histopathologic characterization of mild rejection (grade I) in skin biopsies of human hand allografts. Transpl Int. 2012;25(1):56–63. doi: 10.1111/j.1432-2277.2011.01369.x. Epub 2011/10/11. [DOI] [PubMed] [Google Scholar]
- 58.Petruzzo P, Kanitakis J, Badet L, Pialat JB, Boutroy S, Charpulat R, et al. Long-term follow-up in composite tissue allotransplantation: in-depth study of five (hand and face) recipients. Am J Transplant. 2011;11(4):808–816. doi: 10.1111/j.1600-6143.2011.03469.x. Epub 2011/03/31. [DOI] [PubMed] [Google Scholar]
- 59.Sehgal VN, Srivastava G, Dogra S. Tacrolimus in dermatology-pharmacokinetics, mechanism of action, drug interactions, dosages, and side effects: part I. Skinmed. 2008;7(1):27–30. doi: 10.1111/j.1540-9740.2007.06485.x. Epub 2008/01/05. [DOI] [PubMed] [Google Scholar]
- 60.Solari MG, Washington KM, Sacks JM, Hautz T, Unadkat JV, Horibe EK, et al. Daily topical tacrolimus therapy prevents skin rejection in a rodent hind limb allograft model. Plast Reconstr Surg. 2009;123(2 Suppl):17S–25S. doi: 10.1097/PRS.0b013e318191bcbd. Epub 2009/02/21. [DOI] [PubMed] [Google Scholar]
- 61.Reinisch W, Knobler R, Rutgeerts PJ, Ochsenkuhn T, Anderson F, von Tirpitz C, et al. Extracorporeal photopheresis (ECP) in patients with steroid-dependent Crohn's disease: An open-label, multicenter, prospective trial. Inflammatory bowel diseases. 2012 doi: 10.1002/ibd.23012. Epub 2012/05/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Martino M, Fedele R, Cornelio G, Moscato T, Imbalzano L, Ressa G, et al. Extracorporeal photopheresis, a therapeutic option for cutaneous T-cell lymphoma and immunological diseases: state of the art. Expert Opin Biol Ther. 2012 doi: 10.1517/14712598.2012.688025. Epub 2012/05/17. [DOI] [PubMed] [Google Scholar]
- 63.Barr ML, Meiser BM, Eisen HJ, Roberts RF, Livi U, Dall'Amico R, et al. Photopheresis for the prevention of rejection in cardiac transplantation. Photopheresis Transplantation Study Group. N Engl J Med. 1998;339(24):1744–1751. doi: 10.1056/NEJM199812103392404. Epub 1998/12/10. [DOI] [PubMed] [Google Scholar]
- 64.Morrell MR, Despotis GJ, Lublin DM, Patterson GA, Trulock EP, Hachem RR. The efficacy of photopheresis for bronchiolitis obliterans syndrome after lung transplantation. J Heart Lung Transplant. 2010;29(4):424–431. doi: 10.1016/j.healun.2009.08.029. Epub 2009/10/27. [DOI] [PubMed] [Google Scholar]
- 65.Schneeberger S, Ninkovic M, Piza-Katzer H, Gabl M, Hussl H, Rieger M, et al. Status 5 years after bilateral handtransplantation. Am J Transplant. 2006;6(4):834–841. doi: 10.1111/j.1600-6143.2006.01266.x. Epub 2006/03/17. [DOI] [PubMed] [Google Scholar]
- 66.Unadkat JV, Schneeberger S, Horibe EH, Goldbach C, Solari MG, Washington KM, et al. Composite tissue vasculopathy and degeneration following multiple episodes of acute rejection in reconstructive transplantation. Am J Transplant. 2010;10(2):251–261. doi: 10.1111/j.1600-6143.2009.02941.x. Epub 2010/01/01. [DOI] [PubMed] [Google Scholar]
- 67.Kaufman CL, Ouseph R, Blair B, Kutz JE, Tsai TM, Scheker LR, et al. Graft vasculopathy in clinical hand transplantation. Am J Transplant. 2012;12(4):1004–1016. doi: 10.1111/j.1600-6143.2011.03915.x. Epub 2012/02/14. [DOI] [PubMed] [Google Scholar]
- 68.Kanitakis J, Jullien D, Petruzzo P, Hakim N, Claudy A, Revillard JP, et al. Clinicopathologic features of graft rejection of the first human hand allograft. Transplantation. 2003;76(4):688–693. doi: 10.1097/01.TP.0000079458.81970.9A. Epub 2003/09/16. [DOI] [PubMed] [Google Scholar]
- 69.Pomahac B, Nowinski D, Diaz-Siso JR, Bueno EM, Talbot SG, Sinha I, et al. Face transplantation. Curr Probl Surg. 2011;48(5):293–357. doi: 10.1067/j.cpsurg.2011.01.003. Epub 2011/04/06. [DOI] [PubMed] [Google Scholar]
- 70.Francois CG, Breidenbach WC, Maldonado C, Kakoulidis TP, Hodges A, Dubernard JM, et al. Hand transplantation: comparisons and observations of the first four clinical cases. Microsurgery. 2000;20(8):360–371. doi: 10.1002/1098-2752(2000)20:8<360::aid-micr4>3.0.co;2-e. Epub 2001/01/11. [DOI] [PubMed] [Google Scholar]
- 71.Bonatti H, Brandacher G, Margreiter R, Schneeberger S. Infectious complications in three double hand recipients: experience from a single center. Transplant Proc. 2009;41(2):517–520. doi: 10.1016/j.transproceed.2009.01.014. Epub 2009/03/31. [DOI] [PubMed] [Google Scholar]
- 72.Unadkat JV, Schneeberger S, Goldbach C, Solari MG, Washington KM, Afrooz PN, et al. Investigation of antibody-mediated rejection in composite tissue allotransplantation in a rat limb transplant model. Transplant Proc. 2009;41(2):542–545. doi: 10.1016/j.transproceed.2009.01.024. Epub 2009/03/31. [DOI] [PubMed] [Google Scholar]


