Abstract
Objectives
To evaluate performance characteristics of routine echo for LV thrombus (LVT).
Background
While the utility of dedicated echocardiography (echo) for LVT is established, echo is widely used as a general test for which LVT is rarely the primary indication. We used delayed enhancement cardiac magnetic resonance (DE-CMR) as a reference to evaluate LVT detection by routine echo.
Methods
Dedicated LVT assessment using DE-CMR was prospectively performed in patients with LV systolic dysfunction. Echoes were done as part of routine clinical care. Echo and CMR were independently read for LVT and related indices of LVT size, shape, and image quality/diagnostic confidence. Follow-up was done for embolic events and pathology validation of LVT.
Results
243 patients had routine clinical echo and dedicated CMR within 1 week without intervening events. Follow-up supported DE-CMR as a reference standard, with >5-fold difference in endpoints between patients with vs. without LVT by DE-CMR (p=0.02). LVT prevalence was 10% by DE-CMR. Echo contrast was used in 4% of patients. Echo sensitivity and specificity were 33% and 91%, with positive and negative predictive values of 29% and 93%. Among patients with possible LVT as the clinical indication for echo, sensitivity and positive predictive value were markedly higher (60%, 75%). Regarding sensitivity, echo performance related to LVT morphology and mirrored cine-CMR, with protuberant thrombus typically missed when small (p≤0.02). There was also a strong trend to miss mural thrombus irrespective of size (p=0.06). Concerning positive predictive value, echo performance related to image quality, with lower diagnostic confidence scores for echoes read positive for LVT in discordance with DE-CMR compared to echoes concordant with DE-CMR (p<0.02).
Conclusions
Routine echo with rare contrast use can yield misleading results concerning LVT. Echo performance is improved when large protuberant thrombus is present and when the clinical indication is specifically for LVT assessment.
Keywords: thrombus, cardiovascular magnetic resonance, echocardiography
Introduction
In clinical practice, echocardiography (echo) is widely accepted as the primary screening test for left ventricular (LV) thrombus.1, 2 This approach is supported by multiple studies showing that echo performs well as a test for LV thrombus when imaging is tailored for this purpose.3–5 More recently, sonographic contrast has been shown to further improve diagnosis of LV thrombus.6, 7 Indeed, prior research by our group and others has demonstrated that a routine strategy of echo contrast use in at-risk patients can markedly improve LV thrombus assessment, reducing both false positives and false negatives.8, 9
While the utility of dedicated echo for LV thrombus is established, echo is widely performed as a general screening test of cardiac structure and function for which thrombus is rarely the primary indication.10 Echo contrast use also remains low,11 possibly attributable to recent FDA mandated product safety label warnings12 and resultant controversy surrounding widespread utilization.11, 13, 14 Thus, for many patients at risk for LV thrombus such as those with systolic dysfunction, both the primary indication and the use of echo contrast for LV thrombus is rare. As echo is the most common imaging test in the United States,15 better understanding of its performance characteristics in a real life clinical setting is of substantial importance.
Delayed enhancement cardiac magnetic resonance (CMR) can establish LV thrombus based on avascular tissue characteristics, an approach that has been shown to be highly accurate in multiple validation studies.8, 16–18 As DE-CMR is non-invasive, it holds the added utility of studying LV thrombus in broad at-risk populations for whom an invasive standard such as surgical pathology would be impractical. To date, DE-CMR has not been used to study factors that influence performance of routine clinical echo for detection of LV thrombus.
We have previously reported the results of a prospective registry in which patients with LV dysfunction underwent dedicated CMR thrombus imaging and were thereafter followed for prognostic assessment.18 In this prior study, only cine- and DE-CMR were compared and echo was not part of the research protocol. For the current study, we evaluated registry patients in whom echo was performed as part of clinical care. The goal was to employ DE-CMR as a means to investigate real life practice patterns and performance characteristics of routine echo for LV thrombus.
Methods
Population
The study population was accrued from an ongoing CMR registry of consecutive patients at Duke University with LV systolic dysfunction. The sole criterion for registry participation was impaired systolic function, defined as a left ventricular ejection fraction (LVEF) below 50% measured quantitatively on cine-CMR. No patients were excluded based on clinical characteristics or other criteria. As previously reported,18 the CMR imaging protocol entailed tailored (cine and delayed enhancement [DE]) imaging for dedicated LV thrombus assessment. Comprehensive clinical data was collected at the time of CMR, including coronary risk factors, revascularization history, and medication regimen. Echocardiography was not a component of the registry protocol.
For the current study, clinical records were queried for all registry patients who underwent echo within 1 week of CMR. Echo was performed as part of routine care at the discretion of treating clinicians. To standardize interpretation of thrombus and assess imaging factors that could potentially impact echo performance, echoes were retrieved from image archives and interpreted for the express purpose of this study by experienced (AHA/ACC level III) readers blinded to patient identifiers or CMR results. Echoes were also reviewed for factors that could potentially impact performance characteristics, including sonographic contrast use and clinical indication for echo.
In accordance with the established registry protocol,18 prospective follow-up was performed for endpoints consistent with presence or absence of thrombus by imaging. For the current investigation, follow-up was examined in the sub-cohort of registry patients that underwent CMR and echo. The follow-up protocol consisted of two components: First, all records were carefully reviewed in patients who had direct inspection and pathology evaluation of the left ventricle (i.e. patients who underwent heart transplantation, LV aneurysmectomy, or necropsy) within 6 months of imaging. Second, follow-up was performed for identification of clinical embolic events that were highly suggestive of the presence of LV thrombus. These events consisted of a documented cerebrovascular accident (CVA) or transient ischemic attack (TIA) that occurred within 6 months of imaging. Concordant with established criteria,19, 20 CVA was defined as an acute neurologic deficit of presumed vascular origin lasting ≥24 hours, and TIA a deficit lasting < 24 hours. Clinical information was obtained via: (1) telephone interview with the patient, or, if deceased, with family members, (2) contact with the patient’s physician, and (3) hospital records. All reported clinical events (CVA, TIA) were confirmed based on medical documentation by a treating physician. Death was not considered evidence of LV thrombus unless attributed to a cerebrovascular embolic event.
This study was performed with the approval of the Institutional Review Board at Duke University; all patients provided written informed consent.
Image Acquisition
Cardiac Magnetic Resonance
CMR was performed using 1.5-Tesla scanners (Siemens Sonata or Avanto). The pre-specified CMR protocol consisted of two components - cine-CMR for anatomical/functional assessment and DE-CMR for tissue characterization. Cine-CMR used a steady-state free-precession sequence (typical TR, 3.0 ms; TE, 1.5 ms; in-plane spatial resolution, 1.7×1.4 mm; temporal resolution, 35–40 ms). DE-CMR, performed 10–30 minutes after gadolinium (0.15 mmol/kg) administration, used a segmented inversion-recovery sequence (in-plane spatial resolution, 1.8×1.3 mm, temporal resolution 160–200 ms). Cine and DE-CMR were obtained in matching short and long-axis planes (slice thickness 6 mm). Short-axis images were acquired every one centimeter (gap, 4 mm) throughout the entire LV. Long-axis images were obtained in standard two, three, and four-chamber orientations.
On standard DE-CMR (tailored to null viable myocardium; inversion time [TI] 250–350 msec) thrombus typically had an etched appearance (grey centrally, black border) whereas viable myocardium was black and infarcted myocardium white. As we have previously reported,18 the diagnosis of thrombus by standard DE-CMR can sometimes be challenging as both viable myocardium and thrombus appear relatively dark and are difficult to distinguish from one another. Although contrast uptake is low in viable compared to infarcted myocardium, it is not zero as is the case with avascular tissue such as thrombus and the difference in contrast uptake between viable myocardium and thrombus can be used to improve the conspicuity of thrombus. Thus, a tailored DE-CMR sequence was designed in which the inversion time was increased from that needed to null viable myocardium (250–350 msec) to a fixed time (600 msec) needed to selectively null avascular tissue such as thrombus.21 With this “long inversion time” (long-TI) sequence, regions with contrast uptake (i.e. LV cavity and myocardium) appear bright, thrombus appears homogeneously black, and there is improved thrombus delineation.18
Echocardiography
Transthoracic 2D-echocardiograms were obtained by experienced sonographers on commercially available equipment (Sonos-5500 or 7500, Philips Healthcare Andover MA) with phased and sector array transducers. Echoes were acquired in standard parasternal short- and long-axis as well as apical 2-, 3- and 4-chamber imaging planes in accordance with American Society of Echocardiography consensus guidelines.22
All echoes were performed as part of routine clinical practice; sonographer protocols were not altered for the current study. Echo contrast agents (perflutren lipid [Definity] or protein [Optison] microspheres) were selectively utilized for cavity opacification and endocardial border delineation if deemed clinically necessary at the time of imaging. Images were digitally stored, viewed, and analyzed using Xcelera workstations (Philips Healthcare, Andover MA). In accordance with echo lab standards at our institution, images were displayed at a typical frame rate of 30 frames per second.
Data Analysis
Thrombus Assessment
Images were interpreted by consensus of two experienced readers (level-3 trained in CMR and echo) who were blinded to subject identifiers, clinical history, and all prior imaging tests (echo and CMR). A pre-designated third reader was consulted in cases of interpretive discordance (cine-CMR 1%, DE-CMR 4%, echo 11%). Studies were read in random order. Each modality was interpreted independently of the others.
For DE-CMR, thrombus was identified as an LV mass with post-contrast inversion-recovery characteristics consistent with avascular tissue;17, 18 Thrombus appeared as a low signal intensity mass surrounded by high-signal intensity structures such as intracavitary blood and/or hyperenhanced myocardial infarction on DE-CMR. Established criteria21, 23 were used to distinguish thrombus from acute myocardial infarction with microvascular obstruction, which may also appear as a filling defect. Differentiating features included: (a) surrounding structures (no-reflow zones should be completely encompassed by hyperenhanced myocardium or LV cavity); (b) appearance (no-reflow occurs within the myocardium, thrombus in the LV cavity); and (c) stability of size on consecutive DE-CMR acquisitions (no-reflow size shrinks from contrast fill-in at the periphery, thrombus size is stable). When thrombus was identified, morphology was classified as protuberant (if borders were distinct from endocardial contours with protrusion into LV cavity) or mural (borders were contiguous with adjacent endocardial contours),24 with exams independently re-interpreted to assess intra- and inter-reader reproducibility. Thrombus volume was measured quantitatively via planimetry. Thrombus location was scored based on the nearest myocardial tissue using a standard 17-segment LV model.
For echo and cine-CMR, thrombus was diagnosed using established anatomic criteria.25 Thrombus was defined as a mass within the LV cavity with margins distinct from ventricular endocardium and distinguishable from papillary muscles, chordae, trabeculations, or technical artifact. Thrombus was diagnosed based on review of parasternal short and long axis images, and apical 2-, 3-, and 4-chamber images. Echoes interpreted as positive for thrombus were scored for diagnostic confidence on a 3-point scale (low, medium, high confidence) based on clarity of thrombus definition (distinct borders, independent mobility pattern) and overall image quality (endocardial border definition, LV cavity artifacts).
Left Ventricular Quantification
LVEF and LV volumes were quantified on the basis of end-diastolic and end-systolic endocardial contours from the stack of short-axis cine-CMR images. Regional wall motion and scarring were assessed on a standard 17-segment model using previously described methods.26 Regional function on cine-CMR was graded on a 5-point scale as follows: 0=normal contraction; 1=mild-to-moderate hypokinesia; 2=severe hypokinesia; 3=akinesia; 4=dyskinesia. Regional scarring based on area of hyperenhanced (bright) myocardium on DE-MRI was graded on a 5-point scale as follows: 0=no hyperenhancement; 1=1–25%; 2=26–50%; 3=51–75%; 4=76–100%. Global scar size as a percentage of LV myocardium was calculated by summing the segmental scores (each weighted by the midpoint of the range of hyperenhancement) and dividing by the total number of regions.27
Statistical Methods
Continuous data (expressed as mean±SD) were compared using two-sampled t-tests. Non-normally distributed data (expressed as median and 25th–75th percentile) were compared using the Wilcoxon rank-sum test. Comparison of thrombus volumes were made after logarithmic transformation; results are expressed as the antilog of the mean and 95% confidence intervals. Chi square tests were used to compare discrete data between groups; in those cases where the expected cell count was <5, Fisher’s exact test was used. McNemar’s test was used for paired comparisons of discrete variables. All statistical tests were two tailed; p-values <0.05 were regarded as significant.
Results
Population Characteristics
The study population consisted of 243 patients who underwent routine clinical echo and dedicated registry CMR within a 1 week (1.5±2.7 day) interval. The most common clinical indications for echo were to assess LV (92%) and/or valve (29%) function. Assessment following CVA/TIA/systemic embolism was uncommon (2.5%; n=6), as was evaluation for possible LV thrombus (5%; n=13; known LV aneurysm/apical dysfunction, n=5; prior documented LV thrombus, n=4; recent CVA, n=3; anterior MI, n=1).
Table 1 details patient characteristics, with comparison between the current study population and registry patients. Patients with echo were relatively similar to those without echo based on age, prior thrombo-embolic events, and rates of warfarin use. However, they were more often female and were less likely to have prior coronary revascularization and ischemic cardiomyopathy. Additionally, echo patients were more likely to have advanced systolic dysfunction as measured by LVEF or regional wall motion score (both p<0.0001). Consistent with this, prevalence of thrombus by DE-CMR was slightly greater among the echo study population vs. registry patients who did not undergo echocardiography (10% vs. 6%, p=0.04).
Table 1.
Patient Characteristics
| Parameter | Study Population (n=243) |
Registry Patients without Echo (n=541) |
P |
|---|---|---|---|
| CLINICAL | |||
| Age (year) | 60 ± 15 | 61 ± 14 | 0.86 |
| Male gender | 63% (154) | 74% (398) | 0.004 |
| Atherosclerosis Risk Factors | |||
| Diabetes Mellitus | 35% (86) | 26% (142) | 0.009 |
| Hypertension | 61% (149) | 59% (320) | 0.57 |
| Tobacco Use | 24% (58) | 29% (156) | 0.15 |
| Hypercholesterolemia | 39% (94) | 57% (306) | <0.0001 |
| Prior Myocardial Infarction | 44% (108) | 50% (269) | 0.17 |
| Coronary Revascularization | 28% (67) | 40% (268) | <0.0001 |
| Percutaneous Intervention | 16% (39) | 31% (169) | <0.0001 |
| Coronary Artery Bypass Grafting | 17% (42) | 27% (147) | 0.003 |
| Ischemic Cardiomyopathy | 63% (154) | 74% (401) | 0.002 |
| Atrial Fibrillation | 19% (45) | 14% (74) | 0.08 |
| Lifetime History of Prior Cerebrovascular Event | |||
| Cerebrovascular Accident (CVA) | 9% (22) | 7% (37) | 0.28 |
| Transient Ischemic Attack (TIA) | 7% (16) | 4% (22) | 0.13 |
| Therapeutic Regimen | |||
| Aspirin | 58% (140) | 69% (371) | 0.003 |
| Warfarin | 14% (35) | 19% (101) | 0.14 |
| Thienopryidines | 6% (16) | 18% (98) | <0.0001 |
| Beta-blocker | 51% (125) | 71% (386) | < 0.0001 |
| ACE-Inhibitor | 46% (111) | 63% (343) | <0.0001 |
| Angiotensin Receptor Blocker | 6% (15) | 13% (70) | 0.005 |
| Loop diuretic | 31% (76) | 45% (244) | 0.0003 |
| Spironolactone | 11% (26) | 18% (96) | 0.02 |
| Digoxin | 16% (39) | 28% (150) | 0.0004 |
| Nitroglycerin | 28% (150) | 18% (44) | 0.004 |
| CARDIOVASCULAR MAGNETIC RESONANCE | |||
| Left Ventricular Thrombus (DE-CMR) | 10% (24) | 6% (31) | 0.04 |
| Left Ventricular Function and Morphology | |||
| Ejection Fraction (%) | 28 ± 11 | 33 ± 10 | < 0.0001 |
| Wall motion score | 1.7 ± 0.7 | 1.5 ± 0.7 | < 0.0001 |
| % LV with akinesis or dyskinesis* | 24% (6 – 41) | 24% (6 – 35) | 0.054 |
| End-diastolic volume (ml) | 210 ± 89 | 206 ± 85 | 0.50 |
| End-systolic volume (ml) | 156 ± 83 | 142 ± 79 | 0.03 |
| Aneurysm present | 16% (38) | 12% (63) | 0.12 |
| Left Ventricular Infarction | |||
| Myocardial Infarction (presence) | 71% (172) | 75% (404) | 0.25 |
| Infarct size (% LV)* | 10% (0 – 25) | 16% (0 – 29) | 0.03 |
| % LV with >50% transmural infarction* | 0% (0 – 24) | 12% (0 – 29) | 0.002 |
Numbers in boldface indicate P values < 0.05
Expressed as median (25th–75th percentile)
Follow-up Validation
Follow-up was performed as part of the registry protocol for endpoints supporting the imaging diagnosis of thrombus (CVA, TIA, pathology verification). Of the 243 patients with echo, 216 (89%) had complete follow-up for the entire 6-months after imaging. Patients with follow-up were similar to those without follow-up (n=27) based on prevalence of thrombus by DE-CMR or echo, LVEF, or clinical indices such as age, diabetes, or hypertension (all p=NS).
Figure 1 shows the rate of study endpoints for groups stratified by the presence or absence of thrombus as determined by imaging. Previously reported results of the overall registry are shown for comparison. For DE-CMR, patients with thrombus had over a 5-fold higher rate of endpoints than those without thrombus (16.7% vs. 3.1%, p=0.02), a proportion similar to the > 7-fold difference in the overall registry.18 There was a 1.8-fold difference in endpoints when patents were stratified by presence or absence of thrombus by echo (7.7% vs. 4.2%, p=0.34). Rate of endpoints was lower among patients with thrombus by echo compared to DE-CMR despite the fact that patients with thrombus by echo tended less likely to be anticoagulated than were patients with thrombus by DE-CMR (36% vs. 63%, p=0.054).
Figure 1. Follow-up Endpoints in Relation to Imaging Findings.
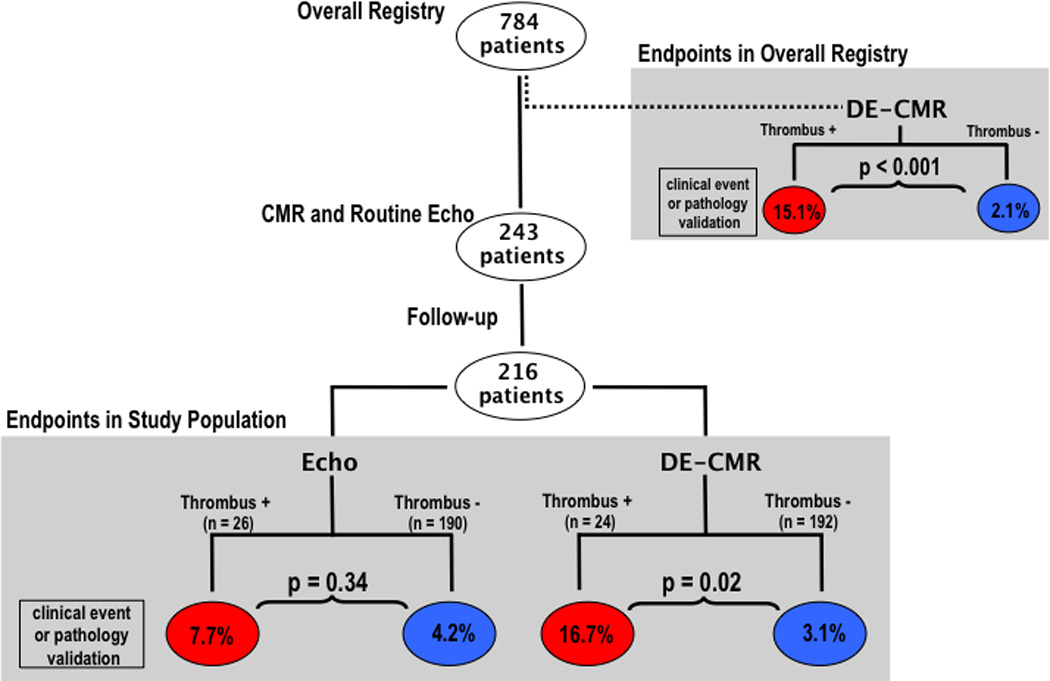
Stratification of patients with follow-up according to presence or absence of thrombus by DE-CMR yielded over a 5-fold difference in study endpoints (TIA, CVA, or pathology-verified thrombus) between groups whereas stratification according to echo thrombus yielded a 1.8 fold difference (red = thrombus +, blue = thrombus−).
Prevalence of LV Thrombus
DE-CMR identified LV thrombus in 10% (n=24) of patients whereas echo was read as positive in 12% (n=28). Despite similar overall prevalence by echo and DE-CMR (p=0.5), there was substantial discordance between modalities, as evidenced by the fact that only 8 patients had thrombus concordantly detected by echo and DE-CMR. Figure 2 provides a representative example of discordance between techniques, with echo read as negative and DE-CMR read as positive in a patient with thrombus verified by pathology.
Figure 2. Typical Example of LV Thrombus Assessment by Routine Echo and Dedicated DE-CMR.
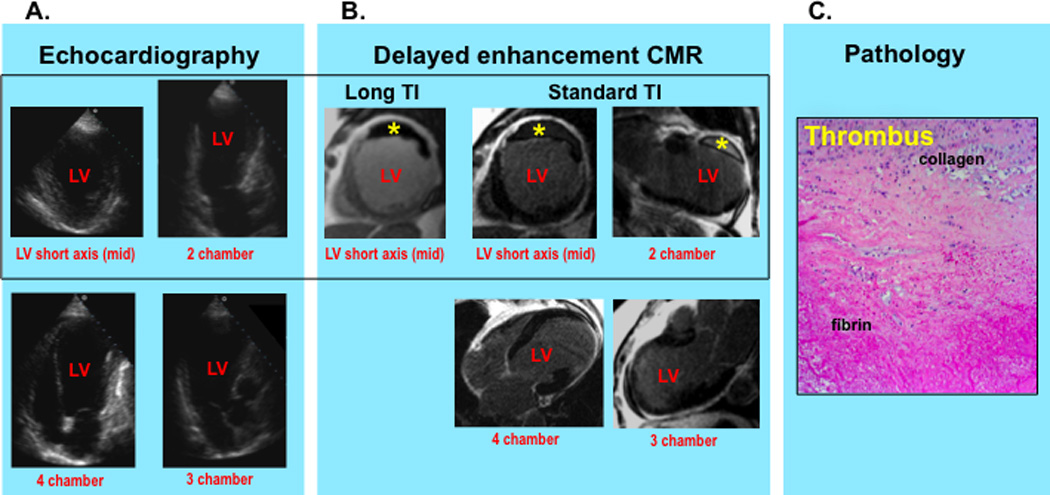
Routine echo (2A) demonstrates absence of thrombus but prominent near field artifact within the LV apex resulting in suboptimal image quality. DE-CMR (2B) demonstrates a large mural thrombus adherent to the LV anterior wall (asterisk) as well as absence of apical thrombus. Note that thrombus on DE-CMR appears black on long TI (left) and etched on standard TI (right) imaging.
DE-CMR findings were confirmed by direct surgical inspection of the LV and histopathology examination (2C) (H&E stain, high power) of surgically resected material, which demonstrated a thrombus with organizing features (prominent collagen and fibrin content) adjacent to the LV anterior wall.
Echo Contrast Use
Echo contrast was administered in 4% (n=10) of patients. Of the 10 patients who received echo contrast, 2 had thrombus by DE-CMR and eight were negative. Echo was negative for thrombus in all 10 of these cases (accuracy 80%).
Diagnostic Performance
Table 2 reports diagnostic performance of echo and cine-CMR using the reference of DE-CMR. Echo yielded a sensitivity of 33% and specificity of 91%, and positive and negative predictive values of 29% and 93% respectively. Although overall sensitivity and positive predictive value were limited, echo performance varied based on clinical indication, with sensitivity increased more than 2-fold (60% vs. 26%) and positive predictive value more than 3-fold (75% vs. 21%) for echoes performed for the clinical indication of LV thrombus assessment compared to those performed for non-thrombus indications.
Table 2.
Thrombus Diagnosis by Routine Echo and Cine-CMR
| Sensitivity | Specificity | Accuracy | Positive Predictive Value |
Negative Predictive Value |
||
|---|---|---|---|---|---|---|
| Echo (overall) | 33% (8/24) | 91% (199/219) | 85% (207/243) | 29% (8/28) | 93% (199/215) | |
| Clinical Indication | LV Thrombus | 60% (3/5) | 88% (7/8) | 77% (10/13) | 75% (3/4) | 78% (7/9) |
| Other* | 26% (5/19) | 91% (192/211) | 86% (197/230) | 21% (5/24) | 93% (192/206) | |
| Cine-CMR (overall) | 58% (14/24) | 99% (218/219) | 95% (232/243) | 93% (14/15) | 96% (218/228) |
Excluding patients with echo done for stated indication of possible LV thrombus assessment (n=13).
Cine-CMR, which was acquired using a tailored thrombus protocol and analyzed for thrombus using the same criteria as echo, was used to provide insight into echo performance by testing the degree to which optimized anatomic imaging might impact diagnosis of LV thrombus. As shown in Figure 3A, echo sensitivity was 50% among cases in which cine-CMR detected thrombus whereas sensitivity was only 10% in which cine-CMR missed thrombus (p=0.08). Regarding positive predictive value, Figure 3B stratifies the diagnosis of thrombus first by echo and then by cine-CMR. Among cases where cine-CMR diagnosed thrombus, positive predictive value of echo was 88%: When cine-CMR was negative for thrombus, positive predictive value of echo was 5% (p<0.0001).
Figure 3. Imaging Results Concerning LV Thrombus.
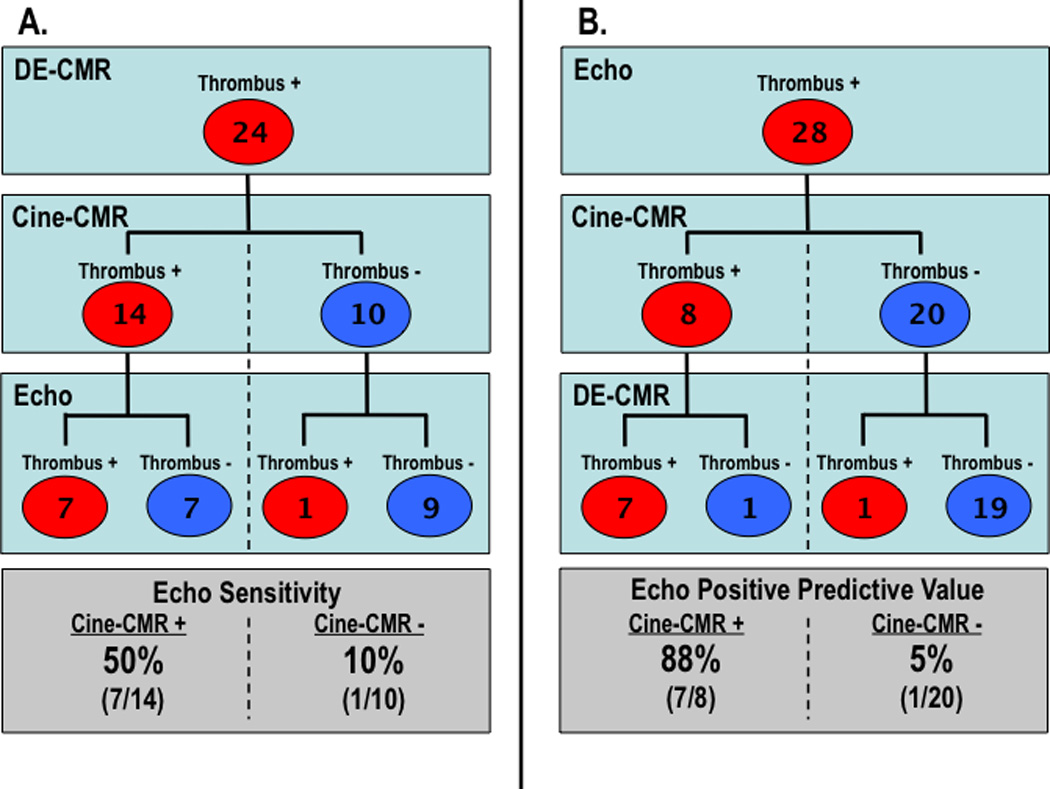
Echo results concerning the diagnosis of thrombus stratified by cine- and DE-CMR findings (red = thrombus +, blue = thrombus −). Both echo sensitivity (3A) and positive predictive value (3B) were higher among cases in which thrombus was also evidenced by cine-CMR vs. those in which cine-CMR was negative. While cine-CMR appropriately detected thrombus in an additional 7 patients with negative echo, both tests were negative in 9/24 patients with thrombus by DE-CMR tissue characterization.
Echo results also demonstrated that positive predictive value was related to image quality/diagnostic confidence. Echoes read for thrombus in discordance with DE-CMR had lower diagnostic confidence scores (assigned at the time of blinded interpretation) than echoes with concordant thrombus assessment to DE-CMR (1.95±0.69 vs. 2.63±0.51, p < 0.02).
Thrombus Morphology
Among the 24 cases of DE-CMR evidenced thrombus, 17 were classified as protuberant and 7 as mural. Reproducibility was high for both inter (23/24) and intra-reader (23/24) classification of thrombus morphology (kappa=0.90, 95% CI = 0.69–1.0). Table 3 reports echo performance according to thrombus morphology (3A) and size (3B). As shown, echo was far less likely to identify mural thrombus, with nearly all protuberant thrombi detected (7/8; p=0.06). Improved detection of protuberant thrombus occurred despite the fact that protuberant and mural thrombi were, on average, virtually identical in size (2.9cm3). Further stratification demonstrated that, for protuberant thrombus, size was a major determinant of echo detection. As shown in Table 3B, protuberant thrombi detected by echo were over 4–fold larger than those missed (6.6 vs. 1.3 cm3, p=0.02). Echo performance paralleled that of cine-CMR, for which detected protuberant thrombi were over 6-fold larger than those missed (4.9 vs. 0.8 cm3, p<0.01).
Table 3.
Thrombus Morphology in Relation to Echo Detection
| A. | |||
|---|---|---|---|
| LV Thrombus by DE-CMR (n=24) |
|||
| Echo + (n=8) |
Echo − (n=16) |
P | |
| Type | |||
| Protuberant | 7 | 10 | 0.06 |
| Mural | 1 | 6* | |
| B. | |||
|---|---|---|---|
| Thrombus Size (cm3) | |||
| Thrombus Detected | Thrombus Missed | P | |
| Echo | |||
| Overall | 6.6 [2.0, 21.4] | 2.0 [1.2, 3.5] | 0.054 |
| Protuberant Thrombus | 6.6 [2.0, 21.4] | 1.3 [0.8, 2.3] | 0.02 |
| Cine-CMR | |||
| Overall | 4.9 [2.6, 9.3] | 1.4 [0.6, 3.0] | 0.02 |
| Protuberant Thrombus | 4.9 [2.2, 11.0] | 0.8 [0.5, 1.1] | <0.01 |
Indices reported as antilog of mean [95% confidence intervals] of log transformed data. Numbers in boldface indicate p values <0.05
In one case, DE-CMR detected mural thrombus adherent to mid anterior wall and excluded apical thrombus; echo was negative for anterior wall thrombus but positive for apical thrombus (P value based on thrombus detection by correct location).
Discussion
Echocardiography is the most common cardiac imaging test in the United States,15 with over 21 million exams performed annually.28 While the utility of echo for the dedicated purpose of diagnosing LV thrombus is established,3–5 echo is commonly used as a general screening test and rarely performed for the specific indication of LV thrombus.10 We believe the current study is the first to assess performance characteristics of routine echo for detection of LV thrombus. Major new findings include (1) how routine echo performs as a test for LV thrombus in context of everyday clinical practice, (2) factors that impact LV thrombus assessment – including fixed (i.e. thrombus morphology) and potentially modifiable (i.e. image quality) indices, and (3) magnitude of improvement to be expected if echo protocols were optimized for detection of LV thrombus based on anatomical appearance.
It is important to recognize that this study should not be construed as an equivalent comparison between diagnostic tests – echo vs. DE-CMR. This would require sonographic contrast use in all patients, a strategy previously tested in our prior research.8 For our current study, DE-CMR was performed in a dedicated manner using tailored LVT imaging to make sure that our reference standard was optimized, whereas echo was performed according to routine clinical practice. With this approach, we sought to examine current clinical practice patterns for echo, and to determine factors that impact echo performance for assessment of LVT. One of the primary findings is that echo performance varied by indication for testing, as evidenced by over a 2-fold higher sensitivity (60% vs. 26%) and 3-fold higher positive predictive value (75% vs. 21%) among exams performed for the specific clinical indication of LV thrombus.
In this real life clinical cohort, echo contrast was rarely used (4%). It is highly likely that echo performance in this study would have substantially improved were sonographic contrast used more frequently.6 This low rate of echo contrast use is reflective of national practice patterns. In a recent multicenter study encompassing over 4.3 million patients, echo contrast was utilized in only 1.4% of exams.11 However, this study was a survey of a general population and may not apply to our cohort of patients with systolic dysfunction, in whom echo contrast may be particularly useful for semi-quantitative evaluation of LV systolic performance as well as thrombus.
The importance of echo contrast is well established. In prior research by our group among patients at risk for thrombus,8 an obligate strategy of echo contrast yielded nearly a 2-fold increase in echo sensitivity (33% vs. 61%) for thrombus as established by the reference standard of DE-CMR. This concept was also demonstrated by Kurt et al,9 who studied patients with technically difficult echo and reported that use of contrast excluded thrombus in 34 of 35 patients in whom non-contrast echo was positive, while detecting thrombus in 5 additional patients in whom non-contrast echo was negative. Whereas consensus guidelines recommend that echo contrast be used in cases of suboptimal image quality,22, 29 low echo contrast utilization rates persist despite the fact that 15% of echoes without contrast have been reported to be technically difficult9 and up to 46% are inconclusive for LV thrombus.6 Our results demonstrate the importance of diagnostic uncertainty concerning LV thrombus: Echoes read positive for thrombus in discordance with DE-CMR were assigned lower diagnostic confidence scores than those read in concordance with DE-CMR (p<0.02). Taken together, these findings demonstrate the importance of optimized imaging protocols, including the frequent use of contrast, when echo is to be used to diagnose LV thrombus.
To better elucidate the magnitude of improvement in echo performance that could be expected if imaging protocols were optimized, our analysis included comparison of echo to cine-CMR – a test that provides excellent endocardial border definition30 while identifying thrombus using the same anatomical criteria as echo. Echo results generally tracked cine-CMR, with both echo sensitivity (p=0.08) and positive predictive value (p<0.0001) improved when limited to patients in whom cine-CMR also detected thrombus. Cine-CMR did identify thrombus in an additional 7 patients in whom routine echo was negative, and it is likely that echo would have also detected these if imaging were tailored for optimized thrombus assessment. On the other hand, even cine-CMR missed nearly half (42%) of thrombi detected by DE-CMR, suggesting that echo limitations are not modality specific but are partially attributable to detection of thrombus based on anatomic rather than tissue-characteristic based criteria. Regarding this point, it is important to recognize that perfusion echo can be also used for tissue characterization, and this approach has been shown to be useful for assessment of thrombus.31 While promising, perfusion echo is not performed as part of routine clinical practice at our center and thus was not incorporated in the current study.
Stratification of thrombus detection based on morphology and size demonstrated parallels between echo and cine-CMR. For both echo and cine-CMR, protuberant thrombus was more likely to be detected when large (p≤0.02). Mural thrombus was less likely to be detected by echo irrespective of size (p=0.06), and this finding paralleled results for the overall registry,18 in which cine-CMR was shown to miss over half (58%) of all mural thrombi detected by DE-CMR.
Our current findings extend results of prior studies comparing echo to DE-CMR. Srichai et al,17 who studied patients with aneurysms undergoing LV reconstruction surgery, reported that sensitivity of transthoracic echo for thrombus was 23% compared with 88% for CMR. All patients in this study had pathology validation of the imaging diagnosis of thrombus. However, in contradistinction to our study, all patients were at high pre-test probability for LV thrombus, imaging reports were retrospectively reviewed instead of primary interpretation of images, and neither cine-CMR nor DE-CMR were analyzed as independent tests. Mollet et al23 reported that the sensitivity of echo was 42% vs. the reference of DE-CMR. However, this study evaluated a small cohort of 57 patients with CAD and no independent standard for thrombus was applied. In a separate study by our group, conducted primarily among patients with acute MI, sensitivity of contrast echo was 61%.8 However, these echoes were performed as part of a research protocol that required obligate sonographic contrast use in all patients.
A central aspect of our study concerns our use of prospective follow-up to validate the imaging detection of LVT. Whereas dedicated DE-CMR yielded over a 5-fold difference in endpoints between patients with and without LV thrombus (p=0.02), routine echo yielded a 1.8 fold difference (p=0.34). Prior echo studies have yielded conflicting results regarding the clinical risks of LV thrombus, with some reporting that thrombus does32, 33 and others that thrombus does not34, 35 increase embolic events risk. Our results may potentially explain the conflicting prior data, demonstrating that while LV thrombus is inherently associated with increased thrombo-embolic event risk, this association may be missed if imaging is suboptimal. As thrombus can be effectively treated with anticoagulation, our findings regarding the clinical risks of thrombus and the importance of optimized imaging for LV thrombus assessment bear important clinical and therapeutic implications. However, one limitation of our data concerns the fact that although anticoagulation status was obtained after imaging, long-term status was not serially assessed throughout follow-up. It is possible that the timing and intensity of warfarin treatment may have been suboptimal in some patients with thrombus, and this may have impacted risk for thrombo-embolic events.
In summary, our study provides new data concerning routine clinical echo as a screening test for LV thrombus. Among this diverse cohort of patients with systolic dysfunction, routine echo with rare use of contrast often yielded misleading results concerning presence or absence of thrombus; Echo performance improved when imaging was performed for the specific indication of LV thrombus. Diagnostic performance of echo paralleled cine-CMR, with higher echo sensitivity and positive predictive value among patients in which cine-CMR was positive for thrombus. Like cine-CMR, echo was less likely to detect protuberant thrombus when small, although mural thrombus was frequently missed independent of size.
Results of this study add to a growing body of literature that has demonstrated the utility of DE-CMR for LV thrombus assessment in high-risk populations, such as individuals with advanced LV systolic dysfunction, aneurysms, and/or large myocardial infarctions. A dedicated DE-CMR protocol, including use of long-TI imaging, could be used to confirm the diagnosis of LV thrombus by routine echo in cases when diagnostic confidence is low or sonographic contrast use is not used. However, these findings should be confirmed by other groups before recommending broad changes in practice patterns regarding imaging for LV thrombus. Future research is also necessary to compare the relative utility of dedicated thrombus imaging by tailored echo and DE-CMR for prognostic assessment and therapeutic management of patients at risk for LV thrombus.
Acknowledgments
Source of Funding
Supported by R01-HL64726 (RJK), R01-HL63268 (RMJ), Doris Duke Clinical Scientist Development Award (JWW), 1 K23 HL102249-01 (JWW).
Footnotes
Conflicts of Interests Disclosure
Drs Kim and Judd are inventors of a US patent on Delayed Enhancement MRI, which is owned by Northwestern University. Dr Weinsaft is a recipient of a research grant from Lantheus Medical Imaging on LV thrombus (echo contrast manufacturer).
References
- 1.Cheitlin MD, Armstrong WF, Aurigemma GP, Beller GA, Bierman FZ, Davis JL, Douglas PS, Faxon DP, Gilliam LD, Kimball TR, Kussmaul WG, Pearlman AS, Philbrick JT, Rakowski H, Thys DM. ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography: a report of the Amrican College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASE Committee to Update the 1997 Guidelines for the Clinical Application of Echocardiography) Circulation. 2003;108:1146–1162. doi: 10.1161/01.CIR.0000073597.57414.A9. [DOI] [PubMed] [Google Scholar]
- 2.Cheitlin MD, Alpert JS, Armstrong WF, Aurigemma GP, Beller GA, Bierman FZ, Davidson TW, Davis JL, Douglas PS, Gillam LD. ACC/AHA Guidelines for the Clinical Application of Echocardiography. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Clinical Application of Echocardiography). Developed in collaboration with the American Society of Echocardiography. Circulation. 1997;95(6):1686–1744. doi: 10.1161/01.cir.95.6.1686. [DOI] [PubMed] [Google Scholar]
- 3.Visser CA, Kan G, David GK, Lie KI, Durrer D. Two dimensional echocardiography in the diagnosis of left ventricular thrombus. A prospective study of 67 patients with anatomic validation. Chest. 1983;83(2):228–232. doi: 10.1378/chest.83.2.228. [DOI] [PubMed] [Google Scholar]
- 4.Ezekowitz MD, Wilson DA, Smith EO, Burow RD, Harrison LH, Jr, Parker DE, Elkins RC, Peyton M, Taylor FB. Comparison of Indium-111 platelet scintigraphy and two-dimensional echocardiography in the diagnosis of left ventricular thrombi. N Engl J Med. 1982;306(25):1509–1513. doi: 10.1056/NEJM198206243062502. [DOI] [PubMed] [Google Scholar]
- 5.Stratton JR, Lighty GW, Jr, Pearlman AS, Ritchie JL. Detection of left ventricular thrombus by two-dimensional echocardiography: sensitivity, specificity, and causes of uncertainty. Circulation. 1982;66(1):156–166. doi: 10.1161/01.cir.66.1.156. [DOI] [PubMed] [Google Scholar]
- 6.Thanigaraj S, Schechtman KB, Perez JE. Improved echocardiographic delineation of left ventricular thrombus with the use of intravenous second-generation contrast image enhancement. J Am Soc Echocardiogr. 1999;12(12):1022–1026. doi: 10.1016/s0894-7317(99)70097-0. [DOI] [PubMed] [Google Scholar]
- 7.Mansencal N, Nasr IA, Pilliere R, Farcot JC, Joseph T, Lacombe P, Dubourg O. Usefulness of contrast echocardiography for assessment of left ventricular thrombus after acute myocardial infarction. Am J Cardiol. 2007;99(12):1667–1670. doi: 10.1016/j.amjcard.2007.01.046. [DOI] [PubMed] [Google Scholar]
- 8.Weinsaft JW, Kim RJ, Ross M, Krauser D, Manoushagian S, LaBounty TM, Cham MD, Min JK, Healy K, Wang Y, Parker M, Roman MJ, Devereux RB. Contrast-enhanced anatomic imaging as compared to contrast-enhanced tissue characterization for detection of left ventricular thrombus. JACC Cardiovasc Imaging. 2009;2(8):969–979. doi: 10.1016/j.jcmg.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kurt M, Shaikh KA, Peterson L, Kurrelmeyer KM, Shah G, Nagueh SF, Fromm R, Quinones MA, Zoghbi WA. Impact of contrast echocardiography on evaluation of ventricular function and clinical management in a large prospective cohort. J Am Coll Cardiol. 2009;53(9):802–810. doi: 10.1016/j.jacc.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 10.Okrah K, Vaughan-Sarrazin M, Cram P. Trends in echocardiography utilization in the Veterans Administration Healthcare System. Am Heart J. 2010;159(3):477–483. doi: 10.1016/j.ahj.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Main ML, Ryan AC, Davis TE, Albano MP, Kusnetzky LL, Hibberd M. Acute mortality in hospitalized patients undergoing echocardiography with and without an ultrasound contrast agent (multicenter registry results in 4,300,966 consecutive patients) Am J Cardiol. 2008;102(12):1742–1746. doi: 10.1016/j.amjcard.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 12.New U.S. Food and Drug Administration prescribing information for Definity approved October 10, 2007. [Accessed October 20, 2007]; Available at http://www.fda.gov/cder/foi/label/2007/021064s007lbl.pdf.
- 13.Main ML, Goldman JH, Grayburn PA. Thinking outside the "box" - the ultrasound contrast contraversy. Journal of the American College of Cardiology. 2007;50(25):2434–2437. doi: 10.1016/j.jacc.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 14.Kusnetzky LL, Khalid A, Khumri TM, Moe TG, Jones PG, Main ML. Acute mortality in hospitalized patients undergoing echocardiography with and without an ultrasound contrast agent: results in 18,671 consecutive studies. J Am Coll Cardiol. 2008;51(17):1704–1706. doi: 10.1016/j.jacc.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 15.Pearlman AS, Ryan T, Picard MH, Douglas PS. Evolving trends in the use of echocardiography: a study of Medicare beneficiaries. J Am Coll Cardiol. 2007;49(23):2283–2291. doi: 10.1016/j.jacc.2007.02.048. [DOI] [PubMed] [Google Scholar]
- 16.Barkhausen J, Hunold P, Eggebrecht H, Schuler WO, Sabin GV, Erbel R, Debatin JF. Detection and characterization of intracardiac thrombi on MR imaging. AJR Am J Roentgenol. 2002;179(6):1539–1544. doi: 10.2214/ajr.179.6.1791539. [DOI] [PubMed] [Google Scholar]
- 17.Srichai MB, Junor C, Rodriguez LL, Stillman AE, Grimm RA, Lieber ML, Weaver JA, Smedira NG, White RD. Clinical, imaging, and pathologic characteristics of left ventricular thrombus: A comparison of contrast enhanced magnetic resonance imaging, transthoracic echocardiography and transesophageal echocardiography with surgical or pathological validation. American Heart Journal. 2006;152(1):75–84. doi: 10.1016/j.ahj.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 18.Weinsaft J, Kim H, Shah DJ, Klem I, Crowley A, Brosnan R, James OG, Patel M, Heitner J, Parker M, Velazquez EJ, Steenbergen C, Judd RM, Kim RJ. Detection of Left Ventricular Thrombus by Delayed-Enhancement CMR: Prevalence and Markers in Patients with Systolic Dysfunction. J Am Coll Cardiol. 2008;52(2):148–157. doi: 10.1016/j.jacc.2008.03.041. [DOI] [PubMed] [Google Scholar]
- 19.Karas MG, Francescone S, Segal AZ, Devereux RB, Roman MJ, Liu JE, Hahn RT, Kizer JR. Relation between mitral annular calcium and complex aortic atheroma in patients with cerebral ischemia referred for transesophageal echocardiography. Am J Cardiol. 2007;99(9):1306–1311. doi: 10.1016/j.amjcard.2006.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sacco RL, Adams R, Albers G, Alberts MJ, Benavente O, Furie K, Goldstein LB, Gorelick P, Halperin J, Harbaugh R, Johnston SC, Katzan I, Kelly-Hayes M, Kenton EJ, Marks M, Schwamm LH, Tomsick T. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: co-sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline. Stroke. 2006;37(2):577–617. doi: 10.1161/01.STR.0000199147.30016.74. [DOI] [PubMed] [Google Scholar]
- 21.Shah DJ, Judd RM, Kim RJ. Myocardial Viability. In: Edelman RRHJR, Zlatkin MB, Crues JV, editors. Clinical Magnetic Resonance Imaging (3rd ed.) 3rd Edition ed. New York: Elsevier; 2006. [Google Scholar]
- 22.Lang RM, Biereg M, Devereux RM, Flachskampf FA, Foster E, Pellikka PA, Picard MA, Roman MJ, Seward S, Shanewise JS, Solomon SD, Spencer KT, St John Sutton M, Stewart WJ. Recommendations for Chamber Quantification: A Report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, Developed in Conjunction with the European Association of Echocardiography, a Branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18(12):1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 23.Mollet NR, Dymarkowski S, Volders W, Wathiong J, Herbots L, Rademakers FE, Bogaert J. Visualization of ventricular thrombi with contrast-enhanced magnetic resonance imaging in patients with ischemic heart disease. Circulation. 2002;106(23):2873–2876. doi: 10.1161/01.cir.0000044389.51236.91. [DOI] [PubMed] [Google Scholar]
- 24.Domenicucci S, Chiarella F, Bellotti P, Bellone P, Lupi G, Vecchio C. Long-term prospective assessment of left ventricular thrombus in anterior wall acute myocardial infarction and implications for a rational approach to embolic risk. Am J Cardiol. 1999;83(4):519–524. doi: 10.1016/s0002-9149(98)00906-0. [DOI] [PubMed] [Google Scholar]
- 25.Asinger RW, Mikell FL, Elsperger J, Hodges M. Incidence of left-ventricular thrombosis after acute transmural myocardial infarction. Serial evaluation by two-dimensional echocardiography. N Engl J Med. 1981;305(6):297–302. doi: 10.1056/NEJM198108063050601. [DOI] [PubMed] [Google Scholar]
- 26.Wagner A, Mahrholdt H, Holly TA, Elliott MD, Regenfus M, Parker M, Klocke FJ, Bonow RO, Kim RJ, Judd RM. Contrast-enhanced MRI and routine single photon emission computed tomography (SPECT) perfusion imaging for detection of subendocardial myocardial infarctions: an imaging study. Lancet. 2003;361:374–379. doi: 10.1016/S0140-6736(03)12389-6. [DOI] [PubMed] [Google Scholar]
- 27.Sievers B, Elliott MD, Hurwitz LM, Albert TS, Klem I, Rehwald WG, Parker MA, Judd RM, Kim RJ. Rapid detection of myocardial infarction by subsecond, free-breathing delayed contrast-enhancement cardiovascular magnetic resonance. Circulation. 2007;115(2):236–244. doi: 10.1161/CIRCULATIONAHA.106.635409. [DOI] [PubMed] [Google Scholar]
- 28.Office USGA. Medicare Ultrasound Procedures - Consideration of Payment Reforms and Technician Qualification Requirements. 2007 [Google Scholar]
- 29.Quinones MA, Douglas PS, Foster E, Gorcsan J, 3rd, Lewis JF, Pearlman AS, Rychik J, Salcedo EE, Seward JB, Stevenson JG, Thys DM, Weitz HH, Zoghbi WA, Creager MA, Winters WL, Jr, Elnicki M, Hirshfeld JW, Jr, Lorell BH, Rodgers GP, Tracy CM. American College of Cardiology/American Heart Association clinical competence statement on echocardiography: a report of the American College of Cardiology/American Heart Association/American College of Physicians--American Society of Internal Medicine Task Force on Clinical Competence. Circulation. 2003;107(7):1068–1089. doi: 10.1161/01.cir.0000061708.42540.47. [DOI] [PubMed] [Google Scholar]
- 30.Thiele H, Nagel E, Paetsch I, Schnackenburg B, Bornstedt A, Kouwenhoven M, Wahl A, Schuler G, Fleck E. Functional cardiac MR imaging with steady-state free precession (SSFP) significantly improves endocardial border delineation without contrast agents. J Magn Reson Imaging. 2001;14(4):362–367. doi: 10.1002/jmri.1195. [DOI] [PubMed] [Google Scholar]
- 31.Kirkpatrick JN, Wong T, Bednarz JE, Spencer KT, Sugeng L, Ward RP, DeCara JM, Weinert L, Krausz T, Lang RM. Differential diagnosis of cardiac masses using contrast echocardiographic perfusion imaging. J Am Coll Cardiol. 2004;43(8):1412–1419. doi: 10.1016/j.jacc.2003.09.065. [DOI] [PubMed] [Google Scholar]
- 32.Stratton JR, Resnick AD. Increased embolic risk in patients with left ventricular thrombi. Circulation. 1987;75(5):1004–1011. doi: 10.1161/01.cir.75.5.1004. [DOI] [PubMed] [Google Scholar]
- 33.Crawford TC, Smith WTt, Velazquez EJ, Taylor SM, Jollis JG, Kisslo J. Prognostic usefulness of left ventricular thrombus by echocardiography in dilated cardiomyopathy in predicting stroke, transient ischemic attack, and death. Am J Cardiol. 2004;93(4):500–503. doi: 10.1016/j.amjcard.2003.10.056. [DOI] [PubMed] [Google Scholar]
- 34.Nihoyannopoulos P, Smith GC, Maseri A, Foale RA. The natural history of left ventricular thrombus in myocardial infarction: a rationale in support of masterly inactivity. J Am Coll Cardiol. 1989;14(4):903–911. doi: 10.1016/0735-1097(89)90463-4. [DOI] [PubMed] [Google Scholar]
- 35.Ciaccheri M, Castelli G, Cecchi F, Nannini M, Santoro G, Troiani V, Zuppiroli A, Dolara A. Lack of correlation between intracavitary thrombosis detected by cross sectional echocardiography and systemic emboli in patients with dilated cardiomyopathy. Br Heart J. 1989;62(1):26–29. doi: 10.1136/hrt.62.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]


