Abstract
Mast cells (MCs) contribute to atherogenesis by releasing pro-inflammatory mediators to activate vascular cells and other inflammatory cells. This study examined whether MC activation or stabilization affects diet-induced atherosclerosis in low-density lipoprotein receptor-deficient (Ldlr−/−) mice. When Ldlr−/− mice consumed an atherogenic diet for 3 or 6 months, MC activation with compound 48/80 (C48/80) increased aortic arch intima and total lesion areas, and plasma total cholesterol, LDL, and triglyceride levels, whereas MC stabilization with cromolyn reduced these parameters. There were significant differences in arch intima and total lesion areas, and plasma total cholesterol, LDL, and triglyceride levels between C48/80-treated and cromolyn-treated mice. To examine a therapeutic application of cromolyn in atherosclerosis, we fed Ldlr−/− mice an atherogenic diet for 3 months followed by giving mice cromolyn for additional 3 months. Cromolyn did not affect aortic arch intima area, but significantly reduced lipid deposition in the thoracic-abdominal aortas. In aortic arches, however, cromolyn treatment significantly reduced lesion contents of Mac-3+ macrophages, CD4+ T cells, activated MCs, and lesion cell proliferation. While plasma total cholesterol and LDL levels increased and high-density lipoprotein (HDL) levels decreased from 3 months to 6 months of an atherogenic diet, cromolyn treatment decreased significantly plasma total cholesterol, LDL, and triglyceride levels and increased HDL levels above those of 3-month time point. These observations demonstrate that MC stabilization reduces lesion inflammation, ameliorates plasma lipid profiles, and may serve as a potential therapy for this cardiovascular disease.
Keywords: mast cell, atherosclerosis, cromolyn, C48/80, LDL receptor-deficient mice
1. Introduction
Accumulating evidences suggest an essential role of mast cells (MCs) in the initiation and progression of atherosclerosis. Since the original detection of MCs in human atherosclerotic lesions [1, 2], possible mechanisms of MC participation in atherosclerosis have been postulated from in vitro cell cultures and experimental models. After activation, MCs release pro-inflammatory mediators, including cytokines, proteases, histamine, proteoglycans, and chemokines, all which participate directly or indirectly in atherogenesis [3-6]. By releasing cytokines, MCs induce endothelial cell (EC) expression of adhesion molecules to recruit blood-borne leukocytes [7], or induce EC and vascular smooth muscle cell (VSMC) expression of cathepsins [8] that consequently mediate arterial wall extracellular matrix protein degradation [9, 10]. By releasing MC-specific chymases and tryptases, MCs promote VSMC apoptosis [11-13] and EC apoptosis and desquamation [14] that enhance intima formation and plaque vulnerability or rupture. MC chymase and tryptase also cleave high-density lipoprotein (HDL) components (apolipoproteins A-1, A-2, and E) [15, 16] and regulate nuclear receptor LXRα (liver × receptor α) activation [17], thereby reducing the ability of HDL in cholesterol efflux from lipid-loaded cells [18, 19] and the expression of lipid metabolizing genes ABCG1 (ATP-binding cassette transporter G1), ABCA1, and SREBP-1 (sterol regulatory element-binding protein-1) [20, 21]. In atherosclerosis-prone apolipoprotein E-deficient (Apoe−/−) mice, oral administration of a chymase inhibitor reduced spontaneous thoracic atherosclerosis, prevented repetitive perivascular MC activation-induced carotid atherosclerosis, including reduced lesion necrotic core sizes, enhanced lesion collagen contents, and normalized the increased frequency and sizes of intraplaque hemorrhages [22].
MC-deficient KitW-sh/W-sh mice provided important reagent in testing a direct participation of MCs in atherosclerosis. At least three groups, including our own used both Apoe−/− mice and another atherosclerosis-prone low-density lipoprotein receptor-deficient (Ldlr−/−) mice and demonstrated that absence of MCs reduced atherosclerotic lesions in thoracic-abdominal aorta, aortic arch, or aortic root, along with significant suppression of lesion inflammatory cell accumulation and matrix remodeling [8, 23, 24]. Therefore, MC activation or stabilization may affect the growth of atherosclerotic lesions in Apoe−/− and Ldlr−/− mice. In carotid artery semiconstrictive collar placement-induced atherosclerosis in Apoe−/− mice, MC activation with dinitrophenyl (DNP)-albumin [25] or substance P [26] greatly increased leukocyte adhesion, atherosclerotic lesion areas, lesion apoptosis, and intraplaque hemorrhage incidences. In mouse vein graft-induced carotid artery intimal thickness, MC stabilization with cromolyn reduced lesion area by 22% and total vessel area by 19%, without affecting lumen areas [27].
This current study was designed to test whether MC activation with compound 48/80 (C48/80) or MC stabilization with cromolyn expedites or prevents atherogenesis in Ldlr−/− mice and whether MC stabilization with cromolyn attenuates the progression of pre-established atherosclerosis in Ldlr−/− mice.
2. Materials and Methods
2.1. Experimental atherosclerosis in Ldlr−/− mice
To test whether MC activation or stabilization affects atherogenesis, we fed six-week-old Ldlr−/− males (C57BL/6, N11, The Jackson Laboratory, Bar Harbor, ME) an atherogenic diet (Research Diets, Inc., New Brunswick, NJ) for 3 months or 6 months while giving mice intraperitoneal administration of 25 mg/kg/day disodium cromoglycate (DSCG, also known as cromolyn) or 4 mg/kg/day C48/80 (Sigma-Aldrich, St. Louis, MO). The same age male Ldlr−/− mice consumed the same atherogenic diet for 3 months or 6 months from an independent experiment were used as experimental controls.
To examine a possible therapeutic application of cromolyn in atherosclerosis, we fed Ldlr−/− mice an atherogenic diet for 3 months followed by giving mice cromolyn for additional 3 months. Control groups treated with vehicles used same age male mice consumed the same atherogenic diet in an independent experiment. We analyzed mouse atherosclerotic lesions in longitudinal sections from a 3-mm segment of the lesser curvature of the aortic arch (defined using a perpendicular line dropped from the right side of the innominate artery) using previously published approaches [28].
2.2. Atherosclerotic lesion characterization
Lesion characterizations, including thoracic-abdominal aorta oil red O staining, aortic arch lesion intima and media areas, lesion macrophages (Mac-3), T cells (CD4), SMC (α-actin), MHC class II–positive cells, proliferating cells (Ki67), and TUNEL-positive apoptotic cells (ApopTag Plus Peroxidase In Situ Apoptosis Kit), were performed as previously described [29]. Lesion MCs were detected using horseradish peroxidase (HRP)-conjugated avidin (Life Technologies, Grand Island, NY) as previously reported [30]. Images were captured, the staining area was measured using computer-assisted image quantification system (Image-Pro Plus software, Media Cybernetics), and immunopositive cells were counted manually. All mouse experiments were performed, and data were analyzed in a blinded fashion, by at least 3 observers. All animal procedures conform with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health and were approved by the Harvard Medical School Standing Committee on Animals (protocol # 03759).
2.3. Plasma lipid determination
Blood samples were collected by retro-orbital venous plexus puncture or by heart punctuation at the end of each time point. Plasma total cholesterol, triglyceride, and high-density lipoprotein (HDL) were determined using kits from Pointe Scientific. Inc. Canton, MI. Low-density lipoprotein (LDL) cholesterol was calculated as follows: serum LDL cholesterol concentration (mg/dL) = total cholesterol – HDL cholesterol – (triglycerides/5).
2.4. Statistical analysis
All data in the study were presented as means ± SEM. Due to our small sample sizes and often skewed data distributions among all continuous variables, we performed a pairwise non-parametric Mann-Whitney test followed by Bonferroni corrections to examine the statistical significances.
3. Results
3.1. MC stabilization reduces atherogenesis in Ldlr−/− mice
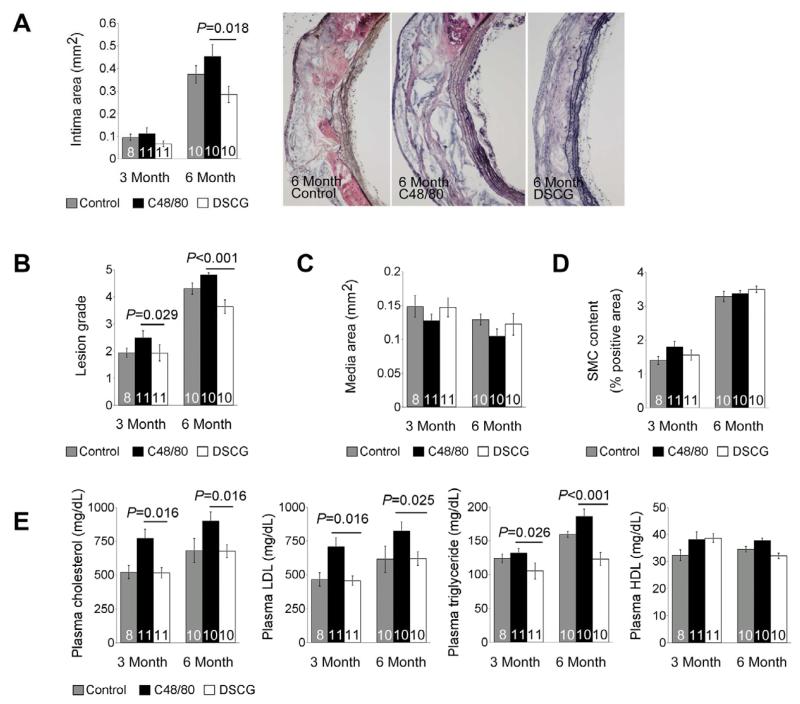
In this study, we fed Ldlr−/− mice an atherogenic diet for 3 and 6 months while giving mice daily intraperitoneal administration of either MC activator C48/80 or MC stabilizer DSCG to test whether MC activation or inhibition affects diet-induced atherosclerosis. While C48/80 increased aortic arch intima area and lesion grade at both 3 and 6 months time points, DSCG reduced aortic arch intima size and lesion grade (Figure 1A and 1B). Compared with untreated control Ldlr−/− mice, increase of atherosclerosis in C48/80-treated mice and decrease of atherosclerosis in DSCG-treated mice did not reach statistical significances. However, there were significant differences in both intima areas and lesion grades between C48/80-treated (MC activation) and DSCG-treated (MC stabilization) groups at 6-month time point. At 3-month time point, only lesion grades reached statistical significance between the MC stabilization and activation groups (Figure 1A and 1B). Neither C48/80 nor DSCG affected significantly aortic arch media areas (Figure 1C) or α-actin-positive SMC areas (Figure 1D).
Figure 1.
MC activation and stabilization in atherosclerosis-prone Ldlr−/− mice. A. Aortic arch atherosclerotic lesion intima area in Ldlr−/− mice received daily intraperitoneal administration of C48/80 or DSCG or without treatment (Control) for 3 and 6 months while mice were consuming an atherogenic diet. Representative aortic arch lesions are shown to the right. B. Aortic arch lesion grade. C. Aortic arch lesion media area. D. Aortic arch media α-actin-positive SMC area in percentage. E. ELISA determined plasma lipid profile. Data were mean ± SE. The number of mice per group was indicated in each bar.
Although we did not measure lipid depositions in thoracic-abdominal aortas from these mice, we found that plasma total cholesterol, LDL, and triglyceride levels were significantly lower in DSCG-treated mice than those in C48/80-treated mice at both 3-month and 6-month time points. In contrast, MC activation or stabilization did not affect significantly plasma HDL levels at both time points (Figure 1E).
3.2. MC stabilization attenuate progression of pre-established atherosclerosis
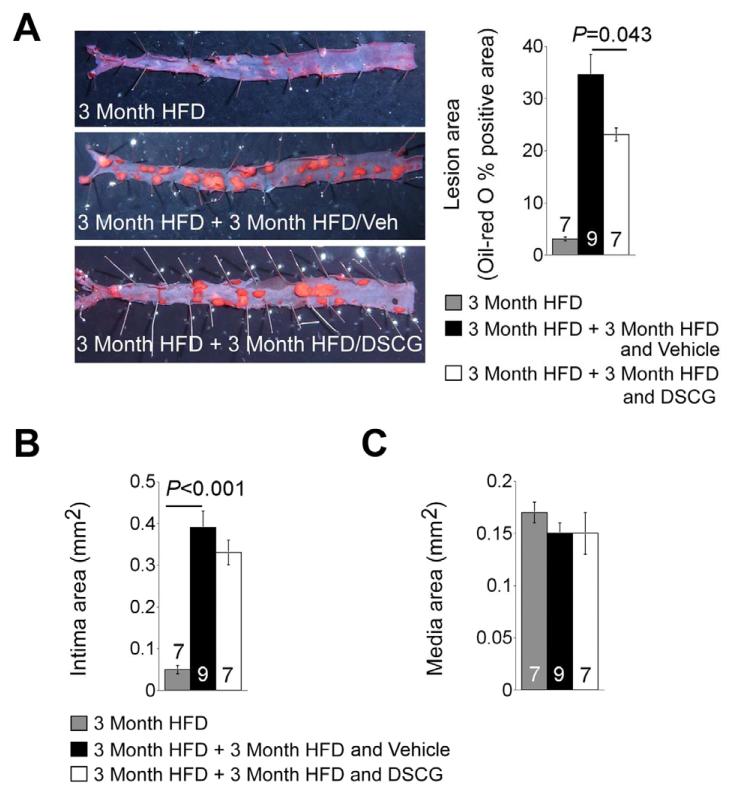
DSCG did reduce both the aortic arch intima area and lesion grade, but did not reach statistic significance, compared with untreated control group at both 3 months and 6 months of atherogenic diet consumptions. To test further a role of DSCG in atherogenesis, we fed Ldlr−/− mice an atherogenic diet for 3 months, and then gave mice daily intraperitoneal administration of DSCG for 3 months while mice remained on the same diet. Enface preparation and oil-red O staining of thoracic-abdominal aorta detected lipid deposition that represents thoracic-abdominal aorta atherosclerotic lesions in Ldlr−/− mice after 3 months of an atherogenic diet. Three more months of an atherogenic diet while mice received vehicle administration significantly increased thoracic-abdominal aorta lipid deposition in Ldlr−/− mice by more than 11-fold. In contrast, such increase of thoracic-abdominal aorta lipid deposition was reduced to about 7-fold in those received daily DSCG intraperitoneal administration, significantly lower than the vehicle-treated mice (Figure 2A). However, we did not detect significant differences in aortic arch intima areas between the vehicle and DSCG groups, although both groups had significantly larger aortic arch intima areas than those consumed 3 months of an atherogenic diet (Figure 2B). There were also no significant differences in aortic arch media areas between the groups (Figure 2C).
Figure 2.
MC stabilization in Ldlr−/− mice with established atherosclerosis. Ldlr−/− mice were fed with an atherogenic diet for 3 months and then continued consuming another 3 weeks of the same diet while receiving intraperitoneal administration of DSCG or vehicle alone. A. Thoracic-abdominal aorta en-face preparation and oil-red O staining-positive lesion area. Representative aortas were shown to the left panels. B. Aortic arch atherosclerotic lesion intima and media areas. C. Aortic arch atherosclerotic lesion media areas. The number of mice per group was indicated in each bar.
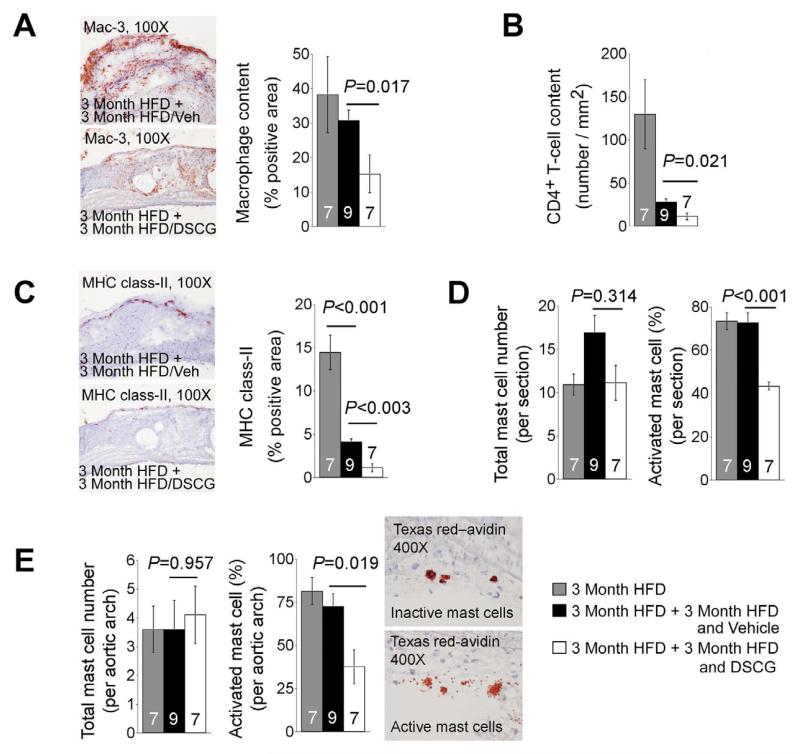
Despite the insignificant differences in atherosclerotic lesion sizes in aortic arches between the two treatment groups, we found that the atherosclerotic lesions in aortic arches from DSCG-treated Ldlr−/− mice contained significantly fewer macrophages (Figure 3A) and CD4+ T cells (Figure 3B), and smaller major histocompatibility complex (MHC) class-II-positive areas (Figure 3C) than those from vehicle-treated Ldlr−/− mice. Using HRP-conjugated avidin, we detected no significant differences in numbers of total MCs in the whole aortic section (containing aortic arch, brachiocephalic artery, left common carotid artery, and left subclavian artery) (Figure 3D, left panel) or in the aortic arch alone (Figure 3E, left panel) among all three groups. However, when degranulated MCs were considered as activated MCs (Figure 3E, right panel), we found that DSCG significantly reduced the percentages of activated MCs from both the whole aortic section and aortic arch (Figure 3D, right panel; Figure 3E, middle panel).
Figure 3.
Inflammatory cell contents in aortic lesions from Ldlr−/− mice that consumed 3 months of an atherogenic diet followed by another 3 months of the same diet while receiving daily intraperotoneal administration of DSCG or vehicle alone. Aortic arch lesion Mac-3-positive macrophage content (A), CD4-positive T-cell content (B), MHC class II-positive area (C). Representative images for panels A and C were shown to the left. D. Aortic lesion (including arch, brachiocephalic artery, left common carotid artery, and left subclavian artery) total numbers of HRP-avidin-positive MCs (left) and percentage of activated MCs (right). E. Aortic arch (including adventitia and intima) total numbers of HRP-avidin-positive MCs (left) and percentage of activated MCs (middle). Representative figures on the right showed typical inactive and active MCs from HRP-avidin-stained aortic atherosclerotic lesion sections. The number of mice per group was indicated in each bar.
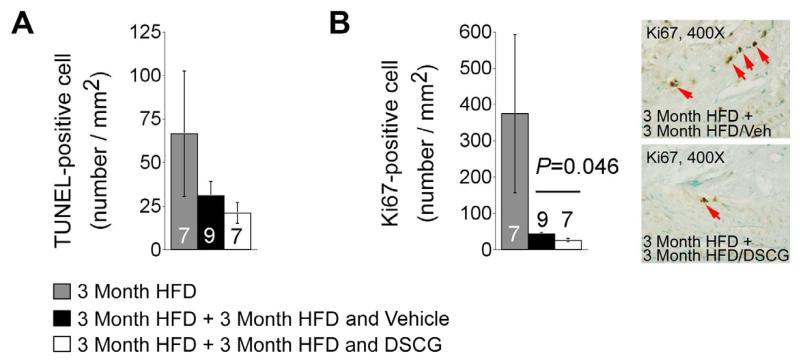
Three months of DSCG treatment did not affect significantly TUNEL-positive apoptotic cell numbers in the aortic arch intima (Figure 4A) in Ldlr−/− mice. This observation may explain insignificant differences of lesion SMC content (data not shown) and media sizes (Figure 2C) at this time point. However, DSCG did reduce lesion Ki67-positive proliferating cells (Figure 4B), supporting a role of MCs in regulating inflammatory (e.g. CD8+ T cells) or vascular cell (e.g. endothelial cells) proliferation [31, 32].
Figure 4.
Cell apoptosis and proliferation in atherosclerotic lesions from aortic arches from Ldlr−/− mice that consumed 3 months of an atherogenic diet followed by another 3 months of the same diet with and without treatment with DSCG. A. TUNEL-positive cell numbers per mm2 in aortic arch. B. Ki67-posiive cell numbers per mm2 in aortic arch atherosclerotic lesions. Representative images were shown to the right. The number of mice per group was indicated in each bar.
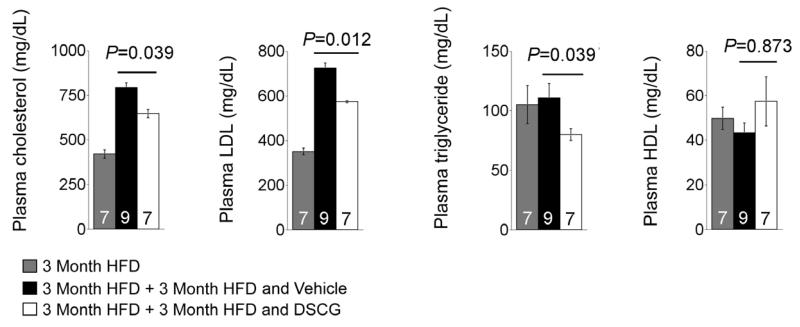
In perivascular collar placement-induced carotid artery intima thickness in Apoe−/− and Ldlr−/− mice, absence of MCs reduced plasma total cholesterol, LDL, or triglyceride and phospholipid levels [23, 24]. Compared with those consumed 3 months of an atherogenic diet, Ldlr−/− mice demonstrated significant increases of plasma total cholesterol and LDL levels and reduction of HDL levels after mice consumed an additional 3 months of an atherogenic diet, although HDL reduction did not achieve statistical significance (Figure 5). Three months of DSCG treatment, although mice were remained on the same atherogenic diet, significantly reduced plasma total cholesterol, LDL, and triglyderide levels. DSCG also increased plasma HDL levels above the levels of before DSCG treatment, despite no statistical significance (Figure 5).
Figure 5.
Plasma levels of total cholesterol, LDL, triglyceride, and HDL from Ldlr−/− mice that consumed 3 months of an atherogenic diet followed by another 3 months of the same diet with and without treatment with DSCG. The number of mice per group was indicated in each bar.
4. Discussion
This study provided an important evidence that MC stabilization with cromolyn, an anti-allergy medicine with more than 40 years of history in the clinic application [33], can effectively attenuate aortic arch intima atherosclerotic lesion areas and reduce plasma cholesterol levels (including total cholesterol, LDL, and triglyceride) in atherosclerosis-prone Ldlr−/− mice. In the same experimental mice with pre-established atherosclerosis, cromolyn can suppress significantly lipid deposition in the thoracic-abdominal aortas, reduce lesion inflammatory cell infiltration and cell proliferation in the aortic arches, and blunt diet-induced plasma cholesterol (including total cholesterol, LDL, and triglyceride) increase.
Previous studies showed that MC activation with DNP, substance P, or complement C5α increased carotid artery perivascular collar placement-induced and vein graft-induced carotid artery intima thickness [25-27] whereas MC stabilization with cromolyn reduced perivascular collar placement-induced and vein graft-induced carotid artery intima thickness [25, 27]. Avidin binds strongly to the heparin-containing serglycin proteoglycans in the MC secretory granules. Thus, HRP-conjugated avidin allowed us to detect MCs in longuitudinal sections of aortic arches [30]. As we anticipated, there were no differences in total numbers of MCs from the arch-plus-branches section (containing aortic arch, brachiocephalic artery, left common carotid artery, and left subclavian artery) or aortic arch only from Ldlr−/− mice received three months of DSCG or vehicle treatments. However, MC degranulation (activation) from aortic section or arch was significantly suppressed by DSCG treatment (Figure 3D/3E), which may explain reduced aortic arch macrophage and T-cell infiltrations, providing another evidence that activated MC might induce infiltration of other inflammatory cells to the site of inflammation [34]. Our observation agrees with prior findings from perivascular collar placement-induced carotid artery intima thickness models [25, 27] that MC stabilization may serve as a new therapeutic approach of atherosclerosis. However, several observations remain unexplained and merit further investigation.
In mice receiving 6 months of DSCG treatment, we observed smaller atherosclerotic lesion intima area and lower lesion grade in aortic arch than in those received no treatment, but such differences did not reach statistical significance (Figure 1A and 1B). Such insignificance could be due to our relative small sample size (n = 8~11). Furthermore, daily intraperitoneal injection might have stressed to the mice and caused intraperitoneal inflammation. A different way of DSCG administration, such as gavage dosing or nebulization may reduce intraperitoneal injection-associated stress or inflammation. In pre-established atherosclerotic Ldlr−/− mice that had already consumed 3 months of an atherogenic diet, 3 months of DSCG treatment only reduced atherosclerotic lesions in the thoracic-abdominal aortas (Figure 2A), but did not affect those in the aortic arch (Figure 2B). Several studies have shown that atherosclerotic lesion development is more advanced in the aortic sinus, followed by aortic arch and then thoracic-abdominal aorta in both Apoe−/− and Ldlr−/− mice [35-37]. These staged developments of atherosclerosis may explain our observation of significant suppression of atherosclerosis by DSCG in the thoracic-abdominal aorta (Figure 2A) but not in aortic arch (Figure 2B). Nevertheless, we were still able to detect MC stabilization-associated beneficial effects in aortic arch, including reduced inflammatory cell (macrophages and T cells) infiltration, MHC class II expression, and lesion cell proliferation and improved plasma cholesterol metabolism. Sustained high percentages of activated MCs in aortic arch, brachiocephalic artery, left common carotid artery, left subclavian artery (Figure 3D/3E), and possibly in other sites of the aortic tree at both 3-month and 6-month time points in Ldlr−/− mice suggest a role of MCs in recruiting macrophages and T cells to atherosclerotic lesions from early to late stages [34]. We detected comparable numbers of aortic arch macrophage contents between the two time points, but much higher numbers of CD4+ T cells at an early time point than a later time point (Figure 3A/3B). Aortic lesion cell proliferation was also higher at earlier time point than that from the later time point (Figure 4B). We and others showed early recruitment of T cells in studies using this experimental atherosclerosis model [29, 38-40]. These observations may suggest an important role of T cells in inflammation and immunity at early stages of atherogenesis [41], such as blood-borne leukocyte recruitment [42].
In conclusion, this study proved an important role of MCs in experimental atherosclerosis in Ldlr−/− mice. Pharmacological stabilization of MCs with MC inhibitors, such as DSCG (cromolyn), ketotifen fumarate (Zaditor®), and nedocromil sodium (Tilade®) may attenuate atherosclerosis progression, reduce lesion inflammation, and improve blood cholesterol metabolism, thereby having therapeutic potential in treating patients or animals with atherosclerosis and associated complications.
Highlights.
We demonstrated that in atherosclerosis-prone low-density lipoprotein receptor knockout (Ldlr−/−) mice, mast cell activation expedited atherosclerosis and mast cell stabilization attenuated atherosclerosis in aortic arches.
In Ldlr−/− mice with pre-established atherosclerosis, 3 months treatment with a mast cell inhibitor crymolyn reduced atherosclerosis in the thoracic-abdominal aortas, but did not affect the aortic arches.
In diet-induced atherosclerosis in Ldlr−/− mice, mast cell stabilization with cromolyn reduced plasma total cholesterol, LDL, and triglyceride and increased plasma high-density lipoproteins (HDL).
Acknowledgements
The authors thank Jiusong Sun, Wendy Yu, and Eugenia Shvartz for technical assistance.
Funding
This study is supported by grants from the National Institutes of Health (HL60942, HL81090, HL88547) (GPS); and by an Established Investigator Award (0840118N) from the American Heart Association (GPS). Sara Sjöberg is sponsored by the Swedish research council (K2010-78PK-21625-01-2).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
JW performed all mouse atherosclerosis studies, including DSCG administration. SS, VT, BS, and HC helped with aortic arch embedding, sectioning, immunostaining, and calculations. MY helped with mouse compound administration. GKS helped the immunohistology, data analysis, and manuscript revision. GPS designed the study, analyzed the data, and wrote the manuscript.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Constantinides P. Mast cells and susceptibility to experimental atherosclerosis. Science. 1953;117:505–506. doi: 10.1126/science.117.3045.505. [DOI] [PubMed] [Google Scholar]
- 2.Cairns A. Constantinides P. Mast cells in human atherosclerosis. Science. 1954;120:31–32. doi: 10.1126/science.120.3105.31. [DOI] [PubMed] [Google Scholar]
- 3.Lindstedt KA, Kovanen PT. Mast cells in vulnerable coronary plaques: potential mechanisms linking mast cell activation to plaque erosion and rupture. Curr Opin Lipidol. 2004;15:567–573. doi: 10.1097/00041433-200410000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Kovanen PT. Mast cells in atherogenesis: actions and reactions. Curr Atheroscler Rep. 2009;11:214–219. doi: 10.1007/s11883-009-0033-7. [DOI] [PubMed] [Google Scholar]
- 5.Xu JM, Shi GP. Emerging role of mast cells and macrophages in cardiovascular and metabolic diseases. Endocr Rev. 2012;33:71–108. doi: 10.1210/er.2011-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He A, Shi GP. Mast cell chymase and tryptase as targets for cardiovascular and metabolic diseases. Curr Pharm Des. 2012 doi: 10.2174/1381612811319060012. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang J, Alcaide P, Liu L, et al. Regulation of endothelial cell adhesion molecule expression by mast cells, macrophages, and neutrophils. PLoS One. 2011;6:e14525. doi: 10.1371/journal.pone.0014525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun J, Sukhova GK, Wolters PJ, et al. Mast cells promote atherosclerosis by releasing proinflammatory cytokines. Nat Med. 2007;13:719–724. doi: 10.1038/nm1601. [DOI] [PubMed] [Google Scholar]
- 9.Cheng XW, Shi GP, Kuzuya M, Sasaki T, Okumura K, Murohara T. Role for cysteine protease cathepsins in heart disease: focus on biology and mechanisms with clinical implication. Circulation. 2012;125:1551–1562. doi: 10.1161/CIRCULATIONAHA.111.066712. [DOI] [PubMed] [Google Scholar]
- 10.Sjöberg S, Shi GP. Cysteine Protease Cathepsins in Atherosclerosis and Abdominal Aortic Aneurysm. Clin Rev Bone Miner Metab. 2011;9:138–147. doi: 10.1007/s12018-011-9098-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun J, Zhang J, Lindholt JS, et al. Critical role of mast cell chymase in mouse abdominal aortic aneurysm formation. Circulation. 2009;120:973–982. doi: 10.1161/CIRCULATIONAHA.109.849679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang J, Sun J, Lindholt JS, et al. Mast cell tryptase deficiency attenuates mouse abdominal aortic aneurysm formation. Circ Res. 2011;108:1316–1327. doi: 10.1161/CIRCRESAHA.111.243758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.den Dekker WK, Tempel D, Bot I, et al. Mast cells induce vascular smooth muscle cell apoptosis via a toll-like receptor 4 activation pathway. Arterioscler Thromb Vasc Biol. 2012;32:1960–1969. doi: 10.1161/ATVBAHA.112.250605. [DOI] [PubMed] [Google Scholar]
- 14.Mäyränpää MI, Heikkilä HM, Lindstedt KA, Walls AF, Kovanen PT. Desquamation of human coronary artery endothelium by human mast cell proteases: implications for plaque erosion. Coron Artery Dis. 2006;17:611–621. doi: 10.1097/01.mca.0000224420.67304.4d. [DOI] [PubMed] [Google Scholar]
- 15.Judström I, Jukkola H, Metso J, Jauhiainen M, Kovanen PT, Lee-Rueckert M. Mast cell-dependent proteolytic modification of HDL particles during anaphylactic shock in the mouse reduces their ability to induce cholesterol efflux from macrophage foam cells ex vivo. Atherosclerosis. 2010;208:148–154. doi: 10.1016/j.atherosclerosis.2009.07.027. [DOI] [PubMed] [Google Scholar]
- 16.Lee M, Calabresi L, Chiesa G, Franceschini G, Kovanen PT. Mast cell chymase degrades apoE and apoA-II in apoA-I-knockout mouse plasma and reduces its ability to promote cellular cholesterol efflux. Arterioscler Thromb Vasc Biol. 2002;22:1475–1481. doi: 10.1161/01.atv.0000029782.84357.68. [DOI] [PubMed] [Google Scholar]
- 17.Yeong P, Ning Y, Xu Y, Li X, Yin L. Tryptase promotes human monocyte-derived macrophage foam cell formation by suppressing LXRalpha activation. Biochim Biophys Acta. 2010;1801:567–576. doi: 10.1016/j.bbalip.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 18.Lee M, Kovanen PT, Tedeschi G, Oungre E, Franceschini G, Calabresi L. Apolipoprotein composition and particle size affect HDL degradation by chymase: effect on cellular cholesterol efflux. J Lipid Res. 2003;44:539–546. doi: 10.1194/jlr.M200420-JLR200. [DOI] [PubMed] [Google Scholar]
- 19.Favari E, Lee M, Calabresi L, Franceschini G, Zimetti F, Bernini F, Kovanen PT. Depletion of pre-beta-high density lipoprotein by human chymase impairs ATP-binding cassette transporter A1- but not scavenger receptor class B type I-mediated lipid efflux to high density lipoprotein. J Biol Chem. 2004;279:9930–9936. doi: 10.1074/jbc.M312476200. [DOI] [PubMed] [Google Scholar]
- 20.Barish GD, Evans RM. PPARs and LXRs: atherosclerosis goes nuclear. Trends Endocrinol Metab. 2004;15:158–165. doi: 10.1016/j.tem.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 21.Joseph SB, McKilligin E, Pei L, et al. Synthetic LXR ligand inhibits the development of atherosclerosis in mice. Proc Natl Acad Sci U S A. 2002;99:7604–7609. doi: 10.1073/pnas.112059299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bot I, Bot M, van Heiningen SH, et al. Mast cell chymase inhibition reduces atherosclerotic plaque progression and improves plaque stability in ApoE−/−mice. Cardiovasc Res. 2011;89:244–252. doi: 10.1093/cvr/cvq260. [DOI] [PubMed] [Google Scholar]
- 23.Smith DD, Tan X, Raveendran VV, Tawfik O, Stechschulte DJ, Dileepan KN. Mast cell deficiency attenuates progression of atherosclerosis and hepatic steatosis in apolipoprotein E-null mice. Am J Physiol Heart Circ Physiol. 2012;302:H2612–H2621. doi: 10.1152/ajpheart.00879.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heikkilä HM, Trosien J, Metso J, et al. Mast cells promote atherosclerosis by inducing both an atherogenic lipid profile and vascular inflammation. J Cell Biochem. 2010;109:615–623. doi: 10.1002/jcb.22443. [DOI] [PubMed] [Google Scholar]
- 25.Bot I, de Jager SC, Zernecke A, et al. Perivascular mast cells promote atherogenesis and induce plaque destabilization in apolipoprotein E-deficient mice. Circulation. 2007;115:2516–2525. doi: 10.1161/CIRCULATIONAHA.106.660472. [DOI] [PubMed] [Google Scholar]
- 26.Bot I, de Jager SC, Bot M, et al. The neuropeptide substance P mediates adventitial mast cell activation and induces intraplaque hemorrhage in advanced atherosclerosis. Circ Res. 2010;106:89–92. doi: 10.1161/CIRCRESAHA.109.204875. [DOI] [PubMed] [Google Scholar]
- 27.de Vries MR, Wezel A, Schepers A, et al. Complement factor C5a as mast cell activator mediates vascular remodelling in vein graft disease. Cardiovasc Res. 2013;97:311–320. doi: 10.1093/cvr/cvs312. [DOI] [PubMed] [Google Scholar]
- 28.Mach F, Schönbeck U, Sukhova GK, Atkinson E, Libby P. Reduction of atherosclerosis in mice by inhibition of CD40 signalling. Nature. 1998;394:200–203. doi: 10.1038/28204. [DOI] [PubMed] [Google Scholar]
- 29.Sukhova GK, Zhang Y, Pan JH, et al. Deficiency of cathepsin S reduces atherosclerosis in LDL receptor-deficient mice. J Clin Invest. 2003;111:897–906. doi: 10.1172/JCI14915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thakurdas SM, Melicoff E, Sansores-Garcia L, et al. The mast cell-restricted tryptase mMCP-6 has a critical immunoprotective role in bacterial infections. J Biol Chem. 2007;282:20809–20815. doi: 10.1074/jbc.M611842200. [DOI] [PubMed] [Google Scholar]
- 31.Stelekati E, Bahri R, D’Orlando O, et al. Mast cell-mediated antigen presentation regulates CD8+ T cell effector functions. Immunity. 2009;31:665–676. doi: 10.1016/j.immuni.2009.08.022. [DOI] [PubMed] [Google Scholar]
- 32.Compton SJ, Cairns JA, Holgate ST, Walls AF. The role of mast cell tryptase in regulating endothelial cell proliferation, cytokine release, and adhesion molecule expression: tryptase induces expression of mRNA for IL-1 beta and IL-8 and stimulates the selective release of IL-8 from human umbilical vein endothelial cells. J Immunol. 1998;161:1939–1946. [PubMed] [Google Scholar]
- 33.Streumer J. Treatment of asthma with DSCG in juveniles during and after hospitalization. Respiration. 1970;27(Suppl):363–368. [PubMed] [Google Scholar]
- 34.Liu J, Divoux A, Sun J, et al. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat Med. 2009;15:940–945. doi: 10.1038/nm.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choy K, Beck K, Png FY, et al. Processes involved in the site-specific effect of probucol on atherosclerosis in apolipoprotein E gene knockout mice. Arterioscler Thromb Vasc Biol. 2005;25:1684–1690. doi: 10.1161/01.ATV.0000174125.89058.b6. [DOI] [PubMed] [Google Scholar]
- 36.Lewis P, Stefanovic N, Pete J, et al. Lack of the antioxidant enzyme glutathione peroxidase-1 accelerates atherosclerosis in diabetic apolipoprotein E-deficient mice. Circulation. 2007;115:2178–2187. doi: 10.1161/CIRCULATIONAHA.106.664250. [DOI] [PubMed] [Google Scholar]
- 37.Niwa T, Wada H, Ohashi H, et al. Interferon-gamma produced by bone marrow-derived cells attenuates atherosclerotic lesion formation in LDLR-deficient mice. J Atheroscler Thromb. 2004;11:79–87. doi: 10.5551/jat.11.79. [DOI] [PubMed] [Google Scholar]
- 38.Kitamoto S, Sukhova GK, Sun J, et al. Cathepsin L deficiency reduces diet-induced atherosclerosis in low-density lipoprotein receptor-knockout mice. Circulation. 2007;115:2065–2075. doi: 10.1161/CIRCULATIONAHA.107.688523. [DOI] [PubMed] [Google Scholar]
- 39.Gotsman I, Grabie N, Gupta R, et al. Impaired regulatory T-cell response and enhanced atherosclerosis in the absence of inducible costimulatory molecule. Circulation. 2006;114:2047–2055. doi: 10.1161/CIRCULATIONAHA.106.633263. [DOI] [PubMed] [Google Scholar]
- 40.Gotsman I, Grabie N, Dacosta R, Sukhova G, Sharpe A, Lichtman AH. Proatherogenic immune responses are regulated by the PD-1/PD-L pathway in mice. J Clin Invest. 2007;117:2974–2982. doi: 10.1172/JCI31344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hansson GK. Immune mechanisms in atherosclerosis. Arterioscler Thromb Vasc Biol. 2001;21:1876–1890. doi: 10.1161/hq1201.100220. [DOI] [PubMed] [Google Scholar]
- 42.Nishimura S, Manabe I, Nagasaki M, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15:914–920. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]