Abstract
Background & Aims
Angiogenesis contributes to vascular remodeling during cirrhosis. In cirrhotic livers, cholangiocytes and myofibroblastic hepatic stellate cells (MF-HSC) produce Hedgehog (Hh) ligands. During embryogenesis Hh ligands are released from ligand-producing cells in microparticles and activate Hh signaling in endothelial cells. We studied whether adult liver cell-derived microparticles contain Hh ligands that alter hepatic sinusoidal endothelial cells (SEC).
Methods
MF-HSCs and cholangiocytes were exposed to platelet-derived growth factor (PDGF) to induce Hh ligands; microparticles were isolated from medium, analyzed by transmission electron microscopy (TEM) and immunoblots, and applied to Hh-reporter containing cells. Microparticles were also obtained from serum and bile of rats after bile duct ligation (BDL) or sham surgery and applied to normal primary liver SEC with or without cyclopamine, a Hh signaling inhibitor. Effects on SEC gene expression were evaluated by QRT-PCR and immunoblotting. Finally, Hh target gene expression and SEC activation markers were compared in primary SEC and in liver sections from healthy and BDL rats.
Results
PDGF-treated MF-HSC and cholangiocytes released exosome-enriched microparticles containing biologically active Hh ligands. BDL also increased release of Hh-containing exosome-enriched microparticles into plasma and bile. TEM and immunoblots revealed similarities among microparticles from all sources; all microparticles induced similar Hh-dependent changes in SEC gene expression. SEC from healthy livers did not express Hh target genes or activation markers, but both were up-regulated in SEC after BDL.
Conclusions
Hh-containing exosome-enriched microparticles released from liver cells alter hepatic SEC gene expression, suggesting a novel mechanism for cirrhotic vasculopathy.
Keywords: microparticles, exosomes, liver cirrhosis, hedgehog, sinusoidal endothelial cells, nitric oxide
Introduction
Tissue remodeling is a cardinal feature of cirrhosis. Within damaged livers themselves, remodeling results in fibrosis, parenchymal nodularity, and changes in the sinusoidal architecture that alter intrahepatic blood flow.1 Remodeling of the extrahepatic vasculature also occurs during cirrhosis, and together with the alterations in intrahepatic blood flow, plays a major role in both the pathogenesis and pathophysiology of portal hypertension.2
Cirrhosis-related changes in the vasculature result, at least in part, from angiogenesis, which is a critical component of successful wound healing responses in various injured adult tissues.2 Repair-related angiogenesis is thought to be regulated by vascular pericytes, such as hepatic stellate cells (HSC).3 Several key mediators of this process have been discovered, including platelet derived growth factor (PDGF)-BB, but the mechanisms involved are not yet fully understood. Recent studies demonstrated that PDGF-BB enhanced HSC-driven vascular tube formation,4 complementing evidence that paracrine signaling between pericytes and adjacent endothelial progenitor cells directs neovascularization in other tissues, and stimulating efforts to identify common pericyte-derived factors that may be involved.
Many factors, including morphogens of the Hedgehog (Hh) family, are capable of modulating the fate of endothelial cells. During development, the Hedgehog pathway is a key regulator of vasculogenesis and angiogenesis.5 Recent data obtained by studying repair responses in adult myocardium and skeletal muscle suggest that the Hh pathway may contribute to re-vascularization of wounded adult tissues.6, 7 There is growing evidence that the Hh pathway also becomes activated in myofibroblastic cells and immature ductular cells that accumulate in injured adult livers.8 However, whether or not Hh signaling has a role in modifying the phenotypes of endothelial cells during adult liver injury has not, to our knowledge, been examined.
Hh ligands function as paracrine mediators of growth and differentiation in Hhresponsive cells. As they mature within ligand-producing cells, Hh ligands become lipidmodified and glycosolated, eventually trafficking to cholesterol-rich domains within cell membranes.9, 10 Hh ligands are capable of initiating signaling in target cells that are remote from ligand producing cells. Such “long-range” signaling requires release of lipid-modified Hh ligands from ligand-producing cells. This process involves incorporation of Hh ligands within amphipathic micelles which facilitates the dispersal of Hh ligands within relatively aqueous extracellular spaces.10 However, the exact mechanisms regulating release of Hh ligands from ligand-producing cells remain poorly understood. Two observations are potentially pertinent to this issue. First, biologically active Hh ligands were demonstrated in membranous microparticles released from certain types of apoptotic T cells.11 Second, Hh ligand-like molecules were demonstrated in exosomes that were released during epidermal development in C.elegans.12 We reported that conditioned medium from liver myofibroblasts and cholangiocytes contains biologically active Hh ligands,13 but did not examine whether these ligands were localized within membranous particles.
Both microparticles and exosomes are membranous structures that contain numerous membrane-associated proteins. Proteomic analysis has demonstrated qualitative and quantitative differences in the protein content of both types of particles. Such variability reflects inherent differences in the various types of cells that are capable of generating them, as well as variations in the methods that have been used to obtain the particles from their cells of origin.14 Phosphatidyl serine is often present on the surface of microparticles, permitting them to be identified by their interaction with Annexin V.15 Exosomes, on the other hand, often express Annexin V protein. Expression of TSG101 is one characteristic that distinguishes exosomes from microparticles.16 Exosomes are small membranous particles that form within endosomes when the latter come into contact with different intracellular membranes, such as the Golgi and endoplasmic reticulum. Endosomes that harbor multiple exosomes have been dubbed “multivesicular bodies (MVB)”. Some MVB fuse with the plasma membrane, emptying their exosome cargo into the extracellular space. MVB-plasma membrane fusion is thought to be an energy-dependent, calcium-regulated process, but seemingly “spontaneous” (i.e., unregulated) release of exosomes has also been documented to occur.17
The two primary objectives of this study were to determine if the Hh ligands released from adult liver cells were physically associated with membranous particles, and if so, to demonstrate whether or not membrane-associated Hh ligands were capable of initiating Hh signals that modified the phenotype of liver sinusoidal endothelial cells. A secondary objective was to characterize the nature of such particles. With reference to the later, we focused on determining whether Hh ligands associated with exosomes because much is already known about cellular mechanisms that regulate the generation and release of exosomes. Thus, if Hh ligands were discovered to localize to exosomes released from adult mammalian cells, this finding would open novel areas of research with broad relevance to repair of adult tissues, including damaged livers. In particular, such data would suggest a novel mechanism for vascular remodeling during cirrhosis, whereby membranous Hh-containing particles released from myofibroblastic and/or ductular-type cells might stimulate angiogenesis in neighboring, and perhaps remote, endothelial cells.
Material and Methods
Animal Studies
Male Sprague-Dawley rats (16–20 weeks) were purchased from Harlan Sprague Dawley (Indianapolis, IN) and maintained in a temperature- and light-controlled facility. They were permitted ad libitum consumption of water and standard rodent food. All animal care and surgical procedures were approved by the Duke University Medical Center Institutional Animal Care and Use Committee as set forth in the “Guide for the Care and Use of Laboratory Animals” published by the National Institutes of Health.
BDL and sham surgery were performed according with previously published procedure.18
Sinusoidal Endothelial Cell Isolation and Cell Culture Experiments
Primary sinusoidal endothelial cells (SEC) were isolated as described by Liu et al.19 and were plated in DMEM (Sigma, St.Louis, MO), supplemented with 10%FBS and Penicillin/Streptomycin (GIBCO/BRL, Grand Island, NY) on collagen coated dishes, and used within 24h of isolation.
Other experiments were conducted with a myofibroblastic rat hepatic stellate cell line (MF-HSC 8B; obtained from M. Rojkind, GWU (Washington D.C.))20 and mouse cholangiocyte cell line (603B; cells were kindly provided by Yoshiyuki Ueno from Tohoku University, Sendai, Japan, and G. Gores from Mayo Clinic, Rochester, MN)21. MF-HSC were cultured in 10% serum-supplemented RPMI-1640 medium (GIBCO/BRL, Grand Island, NY), 10mM Hepes, Penicillin/Streptomycin. Cholangiocytes were maintained in 10% serum-supplemented DMEM, penicillin/streptomycin.
Histology, IHC and Electron Microscopy
Formalin-fixed, paraffin-embedded liver sections were stained with hematoxylin and eosin to assess general histology; Sirius red staining was used to evaluate liver fibrosis. Standard IHC was performed to localize expression of Hh target genes and other cell markers (Supplemental material).
All TEM procedures were performed by Duke Electron Microscopy Service, Department of Pathology.
Isolation and Purification of Microparticles
Cellular crude microparticles (MPs) were isolated following modified techniques for microparticle isolation previously published.14 Briefly, cells were grown on multiple 15cm plates until reaching 70% confluence, serum-starved for 16h, and then treated with PDGF-BB (20ng/ml) in the same serum free media. After 48hr, 0.5µg/ml of Actinomycin-D (Sigma-Aldrich, St Louis, MO) was added for an additional 24hr to induce MPs release. Media (~30ml) was collected and centrifuged twice at 2000g for 15min each time to remove dead cells and cellular debris. Supernatant was transferred to new tubes and centrifuged again at 50,000g for 45min. The pellet was then resuspended in Hanks Buffered Saline Solution (HBSS) and centrifuged again at 50,000g for 45min. MPs were recovered in 200ul of HBSS out of which 100ul was used for protein analysis and 100ul stored in 4°C. All assays with use of functional MPs were performed within 72hr post collection. Prior to use, MPs were carefully resuspended in HBSS with repeated pipetting and gentle vortex.
Sucrose gradient centrifugation was also used in some experiments to purify different fractions from the crude MPs preparations. Crude MPs were carefully resuspended in 2ml of 2.5M sucrose in HBSS and placed in the bottom of centrifuge tubes.22 After carefully overlaying 12ml of continuous 0.25–2.0M sucrose gradient on top, tubes were centrifuged at 100,000g for 15hr; fractions containing 2ml of sucrose and density floated membrane particles were collected from the top and assigned numbers from F1 to F7.
For the isolation of MPs from animals, blood (10–12ml) was collected into heparin containing tubes at the time of sacrifice, and then centrifuged at 2000g for 10min to produce platelet-poor-plasma (PPP). Bile (4–5ml/rat) was collected from BDL rats and centrifuged at 2000g for 10min. Next, samples of PPP and bile were centrifuged twice at 2000g and twice at 50,000g to obtain pure preparations of plasma and bile MPs.
RNA and Protein Analysis
Total RNA was extracted and analyzed as previously escribed.13 For protein analysis, the MPs pellet was digested with 50ul of standard RIPA buffer containing Protease Inhibitor Cocktail Tablets from Roche (Indianapolis, IN). Approximately 10ug of total protein was loaded on Tris-Glycine 4–20% gels. Following standard Western Blot procedure, proteins were analyzed for presence of Ihh, Shh, Gli2, Annexin V, TSG101, K19, and B-Actin. Note, B-Actin was not used as loading control because equal amounts of protein from different MPs preparations differed in their content of this protein. SuperSignal West Femto Maximum Sensitivity Substrate (Pierce Biotechnology) was used for low abundance proteins (Ihh, Shh, Gli2, TSG101, K19). Standard Pico-Sensitivity substrate was used for the more abundant Annexin V and B-Actin.
Statistical Analysis
Results are expressed as mean ±SD. Significance was established using the student’s t-test and analysis of variance when appropriate. Differences were considered significant when p<0.05.
Results
Activation of MF-HSC with PDGF-BB induces production of Hh-containing MPs
PDGF-BB enhances HSC-driven vascular tube formation.4 It also promotes accumulation of MF-HSC 23 and stimulates these cells to produce Hh ligands.24 To determine if PDGF-BB enhances incorporation of Hh ligands into MPs, we treated the clonally-derived rat MF-HSC line, HSC 8B,20 with vehicle or 20ng/ml of PDGF-BB for 48 h. According to protocols that others have used to generate MPs 11, these cultures were then exposed to Actinomycin-D (0.5ug/ml) to increase release of MPs.
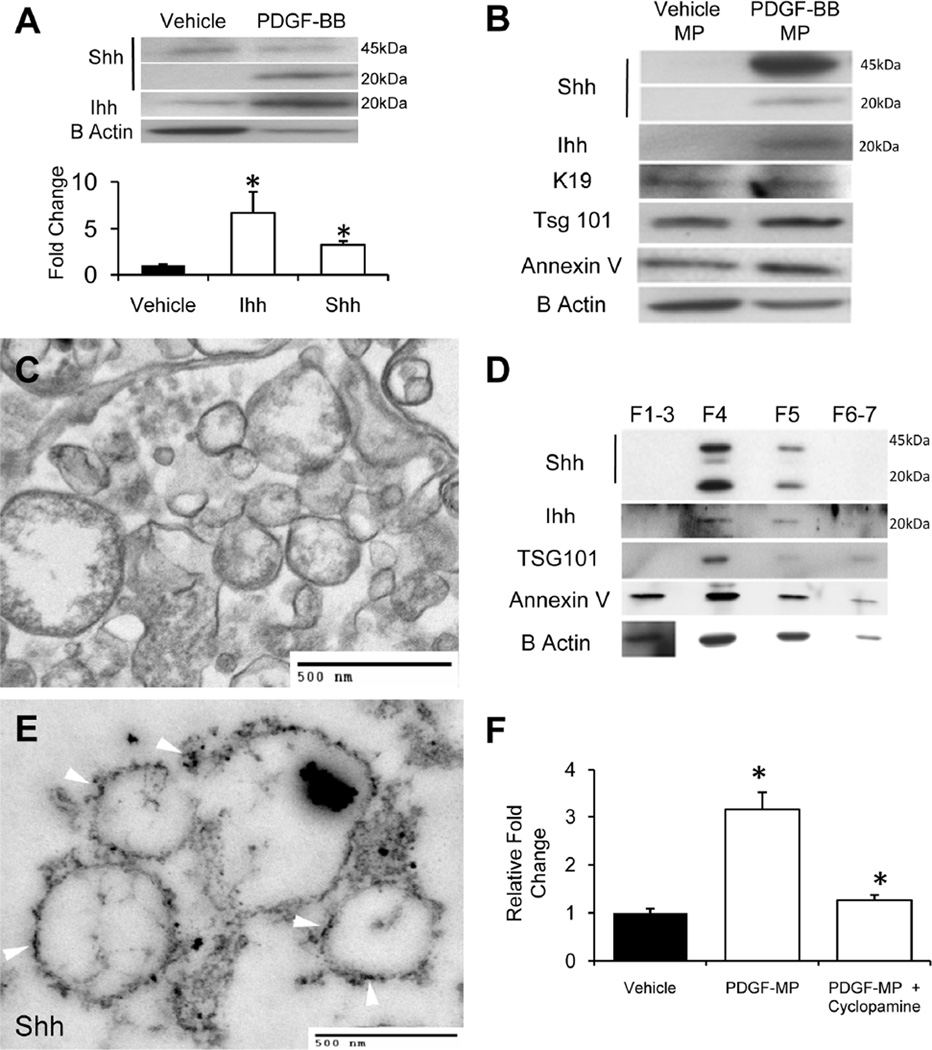
As expected, PDGF-BB treatment increased expression of both Shh and Ihh mRNAs by about 8 fold (p<0.005 vs. vehicle-treated cells). This was accompanied by a 4–6 fold increase in the cellular content of the mature, 20kD signaling-competent Hh ligands (Fig 1A). Crude preparations of MPs were then isolated from the conditioned medium of vehicle or PDGF-treated cells; 10ug of protein was loaded and analyzed by Western blot. MPs from PDGF-treated, but not vehicle-treated, cells contained Shh and Ihh (Fig 1B). In addition to mature, 20kD Shh and Ihh peptides, the former MPs were also enriched with full-length (45kD) Shh. In contrast, crude MPs from both vehicle- and PDGF-treated cells contained the exosomal marker, TSG101, and also expressed B-Actin, annexin V and keratin 19 (K19).
Figure 1. Activation of MF-HSC with PDGF-BB induces production of Hh-containing MPs.
(A) Western blot demonstrating full length 45kDa Shh and signaling 20kDa Shh peptide in cell protein from vehicle-treated and PDGF-BB-treated MF-HSC (10ug protein/lane); (B) Western blot of protein from microparticles (MPs) that were released into the culture medium by vehicle-treated and PDGF-BB-treated MF-HSC (10ug protein/lane). Note presence of Shh and Ihh signaling peptides (20kDa) in the MPs from PDGF-BB-treated cells and the expression of TSG101, an exosomal marker, in both lysates; (C) Transmission electron microscopy (TEM) image of crude MPs preparation from PDGF-treated MF-HSC; (D) Western blot of protein lysates from different fractions that were obtained purifying crude MPs isolated from PDGF-BB-treated MF-HSC-conditioned medium through a continuous (0.25–2.0%) sucrose density gradient. In order to obtain sufficient protein for immunoblot analysis, fractions 1–3 were pooled. Note strong co-localization of Shh, Ihh, and TSG101 in fraction 4; (E) TEM image of material in sucrose density gradient fraction 4 after incubation with primary anti-Shh antibody and gold-labeled secondary antibody. Note that immunogold labeled Shh particles decorate the surfaces of the membranous particles (arrows); (F) Gliresponsive luciferase reporter activity in target cells treated with MPs from vehicletreated or PDGF-BB-treated cultures. Cyclopamine was added to inhibit Hh pathway activity. All Results are mean +/−SD of triplicate experiments (*P<0.05;**P<0.005).
The array of markers in these crude MPs preparations suggested that our protocol might have generated a mixture of membranous particles. To examine this possibility, the crude MPs were evaluated by transmission electron microscopy (TEM). The latter verified that the crude MPs preparations derived from PDGF-activated MF-HSC were heterogeneous (Fig 1C). Therefore, this crude MPs preparation was subjected to continuous (0.25–2.5M) sucrose gradient centrifugation to identify the exosome containing fraction(s). Expression of the exosomal marker, TSG101, was detected almost exclusively in fraction 4. This fraction also contained the vast majority of the Shh and Ihh that was present in the crude mixture, whereas other fractions also expressed some annexin V and B-Actin (Fig 1D). Subsequent transmission EM analysis of fraction 4 that had been incubated with immunogold-labeled anti-Shh antibody demonstrated strong labeling (Fig 1E). Together, these results demonstrate that our crude MPs preparations contained exosomes and that most of the Hh ligands were localized within the exosomal fraction.
To determine if membrane-associated Hh ligands were biologically active, crude MPs from vehicle-treated or PDGF-treated MF-HSC were added to NIH3T3 cells that had been transfected with Gli-luciferase reporter constructs. Crude MPs from PDGF-treated MF-HSC tripled Hh transcriptional activity and this response was virtually abolished by cyclopamine, a highly specific inhibitor of Hh signaling (Fig 1F). In aggregate, our results support the concept that biologically active Shh and Ihh are contained in exosomes derived from activated, MF-HSC.
Activation of cholangiocytes with PDGF-BB induces production of Hh-containing MPs
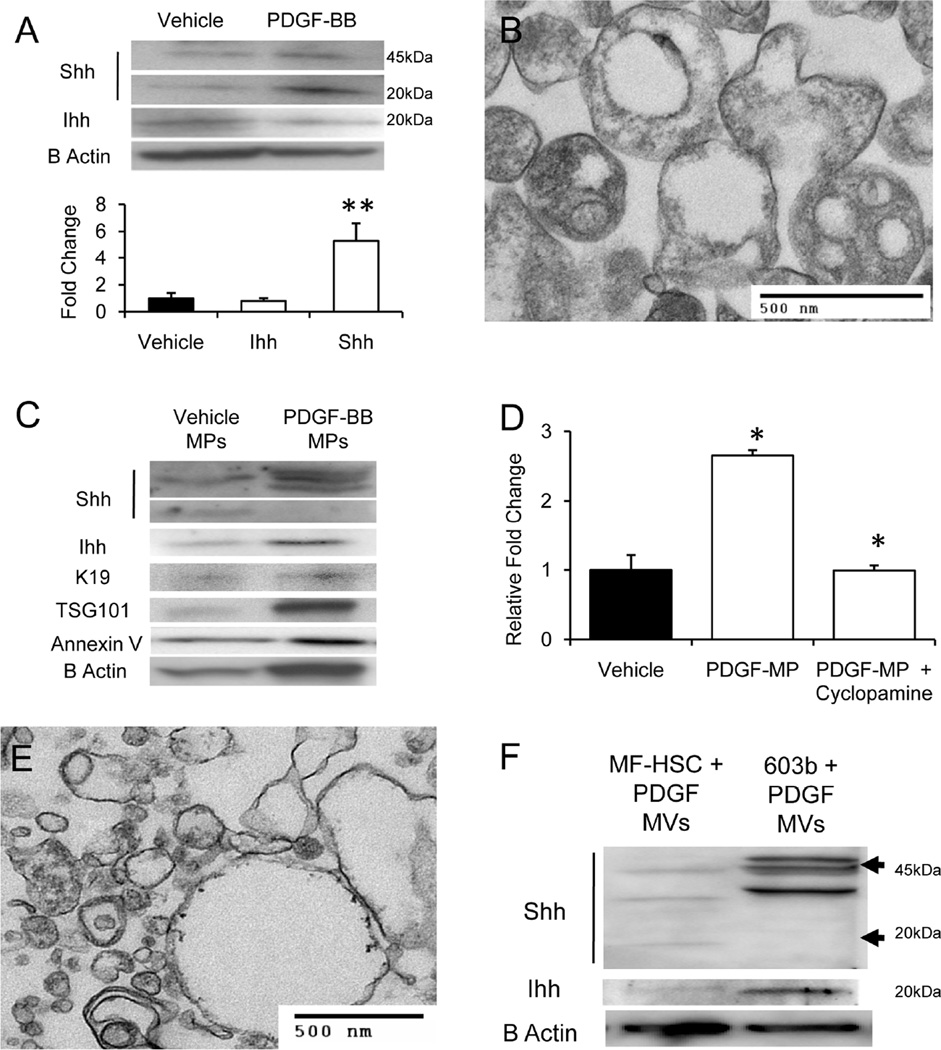
Because PDGF-BB has also been shown to induce cholangiocyte production of Shh,24 we treated a cultured cholangiocyte cell line (603B) with 20ng/ml of PDGF-BB to determine if this also enhanced generation of Hh-containing MPs. PDGF-BB treatment tripled mRNA expression of Shh (p<0.05 vs. vehicle treated cells), but had no effect on expression of Ihh mRNA. Western blot analysis of cell protein from vehicle and PDGF-treated cholangiocytes confirmed that PDGF treatment caused at least a 5 fold increase in the cellular content of mature, 20kDa Shh, but did not enhance accumulation of Ihh (Fig 2A). TEM analysis of crude MPs prepared from conditioned medium of PDGF-treated cholangiocytes demonstrated a mixture of membranous particles of comparable size (200–500nm) to those that were released from PDGF-treated MF-HSC (Fig 1C). Some of the cholangiocyte-derived particles resembled multivesicular bodies (MVB) that typically contain exosomes (Fig 2B). Western blot analysis of equal amounts of crude MPs protein extracts from vehicle-treated and PDGF-treated cholangiocytes confirmed differential expression of Hh ligands, with MPs from PDGF-treated cells expressing substantially more of several ~45kDa Shh-reactive bands, as well as 20kDa Ihh (Fig 2C). Compared to MPs from vehicle-treated cholangiocytes, MPs from PDGF-treated cholangiocytes increased Hh transcriptional activity in target cells harboring Gliluciferase reporter constructs, and this response was blocked by cyclopamine (Fig 2D). Together, these results suggest that activated cholangiocytes release membraneassociated Hh ligands that are biologically active and capable of triggering Hh signaling in other Hh-responsive cells.
Figure 2. Activation of cholangiocytes with PDGF-BB induces production of Hh-containing MPs.
(A) Western blot of cell protein from vehicle-treated and PDGF-BB- treated cholangiocytes (10ug protein/lane); (B) TEM image of MPs released from PDGF-treated cholangiocytes after Actinomycin-D treatment; (C) Western blot of the crude MPs preparations released into the culture medium after exposing vehicle-treated and PDGF-treated cholangiocytes to Actinomycin-D (10ug protein/lane); (D) Gliresponsive luciferase reporter activity in target cells treated with MPs that were released from vehicle-treated or PDGF-BB-treated cultures. Cyclopamine was added to inhibit Hh pathway activity; (E) TEM image of MPs released into the culture medium of PDGF-treated cholangiocytes in the absence of Actinomycin-D exposure; (F) Western blot of MPs released spontaneously (without Actinomycin-D) from PDGF-treated MF-HSC and cholangiocytes (10ug protein/lane). All Results are mean +/−SD of triplicate experiments (*P<0.05;**P<0.005)
To determine if apoptosis was required for release of crude MPs from liver cells, studies were repeated in the absence of Actinomycin-D. Although the yield of MPs was substantially reduced, TEM demonstrated striking similarities in the size and ultrastructural appearance of crude MPs from non-apoptotic, PDGF-treated cholangiocytes (Fig 2E) and the exosome-enriched, Shh-immunoreactive fraction 4 that had been purified from MF-HSC-derived crude MPs by sucrose gradient separation (Fig 1E). Because PDGF-treated MF-HSC also released MPs without actinomycin-D exposure (data not shown), Western blot analysis was done to determine if there were qualitative and/or quantitative differences in Shh and Ihh content in comparable amounts of protein (10ug/sample) extracted from crude MPs preparations generated from non-apoptotic MF-HSC and cholangiocytes (Fig 2F). Cholangiocyte-derived MPs contained greater amounts of 45kD Shh and 20kD Ihh, but less 20kD Shh, than MPs that were derived from non-apoptotic MF-HSC cultures.
Bile duct ligation (BDL) activates release of Hh morphogens in MPs
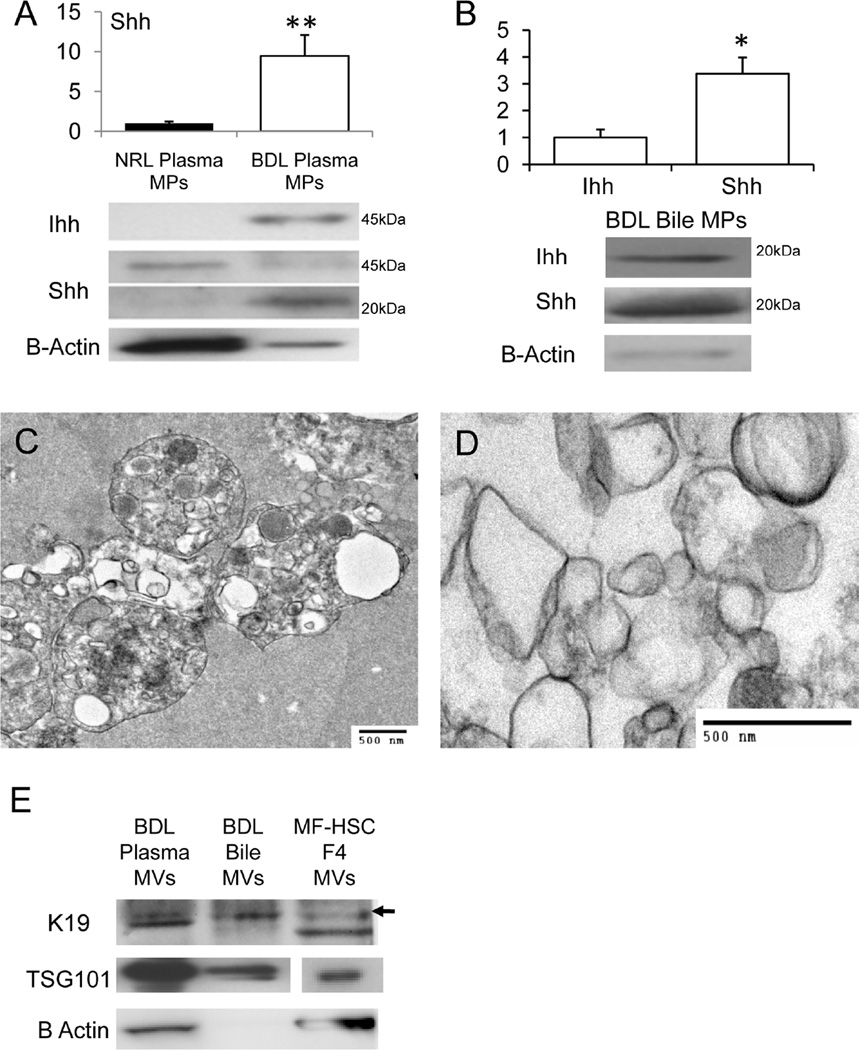
To determine whether Hh-containing MPs were also produced when MF-HSC and cholangiocytes became activated during liver injury, rats were subjected to BDL or sham surgery (Suppl. Data). Protein extracted from MPs that were purified from the plasma of BDL rats contained generally more Hh ligands and less B-Actin than an equal amount of protein from plasma MPs of sham-operated rats (Fig 3A). Compared to plasma MPs from sham-operated controls, plasma MPs from BDL rats harbored 5–10 times more of the 20kDa Shh. There were also qualitative differences in the Hh composition of plasma MPs from BDL and sham-operated rats. Control MPs contained no Ihh and only pre-processed (full-length, 45kDa) Shh, whereas MPs from BDL rats contained abundant Ihh and 20kDa Shh signaling peptide, but less full-length Shh. Biliary MPs from BDL rats contained both 20kDa Shh and 20kDa Ihh, and the content of Shh was about 3 fold greater than that of Ihh (Fig 3B). Thus, both plasma and biliary MPs from BDL rats were significantly enriched with mature, 20kDa Shh signaling peptide.
Figure 3. Bile Duct Ligation (BDL) activates release of Hh morphogens in MPs.
Western blot analysis of mature 20kDa Shh peptide in plasma MPs from sham-operated normal (NRL) and 14 days post-BDL rats (A); Western blot analysis of MPs fractions purified from bile of rats post-BDL (10ug protein/lane). Bile from NRL rats was not sufficient to isolate MPs and only bile from BDL rats was analyzed (B). Graphs demonstrate mean +/−SD results of 3 experiments. In each experiment, plasma or bile were pooled from at least 3 rats/group to obtain MPs (*P<0.05;**P<0.005); TEM images of plasma MPs (C) and biliary MPs (D) from BDL rats; Western blot demonstrating expression of K19, TSG101, and B-Actin in MPs that were isolated from the plasma and bile of BDL rats. Results are compared to expression of these proteins in an equivalent amount (10ug protein/lane) of sucrose density purified fraction 4 from PDGF-treated cultured MF-HSC (E).
Since crude MPs preparations from MF-HSC and cholangiocyte-conditioned medium were heterogeneous, we suspected that plasma and biliary MPs from BDL rats might also contain a mixture of different types of membranous particles. To address this issue, aliquots of each preparation were examined by TEM. Significant differences between the ultrastructural characteristics of crude plasma MPs (Fig 3C) and those of biliary MPs (Fig 3D) were demonstrated, with the features of biliary MPs (Fig 3D) more closely approximating those of the crude MPs preparations from cultured MF-HSC (Fig 1C) and cholangiocytes (Fig 2E). Western blot analysis demonstrated that both preparations of crude MPs contained TSG101 and the biliary K19 (Fig 3E). In these regards, the crude plasma MPs and biliary MPs isolated from BDL rats resembled the MPs obtained from the conditioned culture medium of two different types of liver cell lines (Fig 1B and 2C).
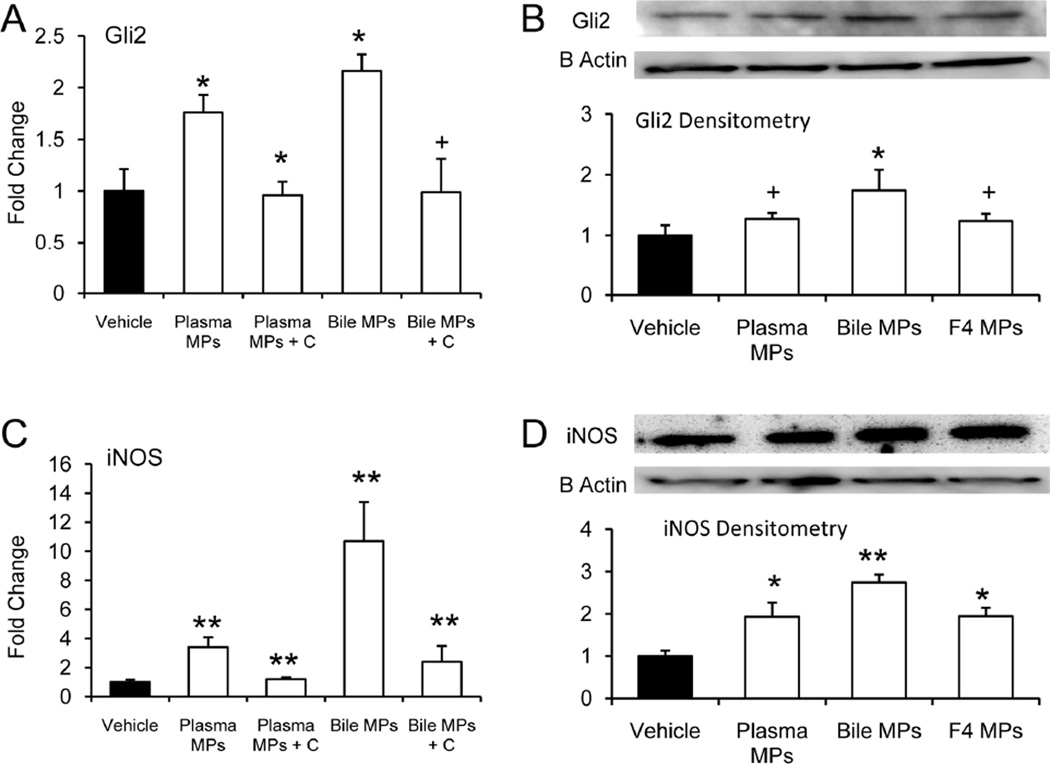
Because many types of endothelial cells are known to be Hh responsive 5, we tested the effects of Hh-containing MPs on primary hepatic sinusoidal endothelial cells (SEC). Like crude MPs derived from cultured liver cell lines (Figs 1F and 2D), plasma and biliary MPs from BDL rats activated Hh signaling in Hh-responsive target cells. SEC mRNA and protein expression of Gli2 increased about 2 fold when these cells were treated with either plasma MPs or bile MPs from BDL rats (Fig 4A–B). Similarly, plasma MPs and bile MPs from BDL rats significantly induced SEC expression of iNOS mRNA and protein (Fig 4C–D). Treatment with the Hh pathway inhibitor, cyclopamine, blocked these responses (Fig 4C–D). Also, the level of iNOS protein induction that was evoked by treating hepatic SEC with crude plasma MPs or bile MPs from BDL rats approximated the response that occurred when the cells were treated with the exosome-enriched fraction from MF-HSC-derived MPs. In aggregate, these findings show that MPs containing biologically active Hh ligands accumulate in plasma and bile after BDL, and raise the intriguing possibility that this process might contribute to tissue remodeling during chronic cholestatic liver injury.
Figure 4. Hh-containing plasma and biliary MPs regulate expression of activation markers by hepatic Sinusoidal Endothelial Cells (SEC).
Primary SEC from normal rats were treated with MPs that had been isolated from plasma or bile of rats that had undergone BDL. Cultures were done in the absence or presence of cyclopamine (C), a highly-specific Hh pathway inhibitor. RNA was isolated and expression of SEC activation markers was assessed by QRT-PCR (A,C). Studies were repeated and SEC protein was isolated and analyzed by Western blot (10ug protein/lane) (B,D). For comparison purposes, some SEC cultures were treated with the sucrose density gradient-purified fraction 4 of MPs that were derived from the culture medium of PDGF-treated MF-HSC cultures (F4 MPs). Results (mean +/−SD) of triplicate experiments are normalized to vehicle-treated SEC. (A–B) Gli2; (C–D) iNOS (+P=0.05;*P<0.05;**P<0.005).
Induction of Hh-target genes and increased expression of activation markers by hepatic SEC after BDL
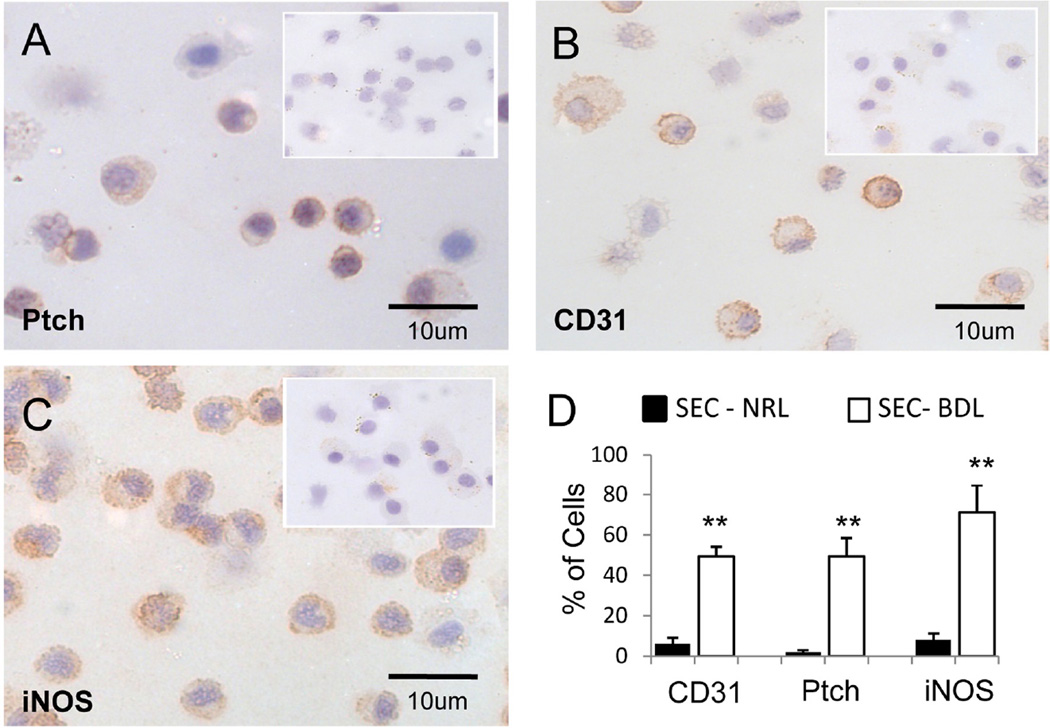
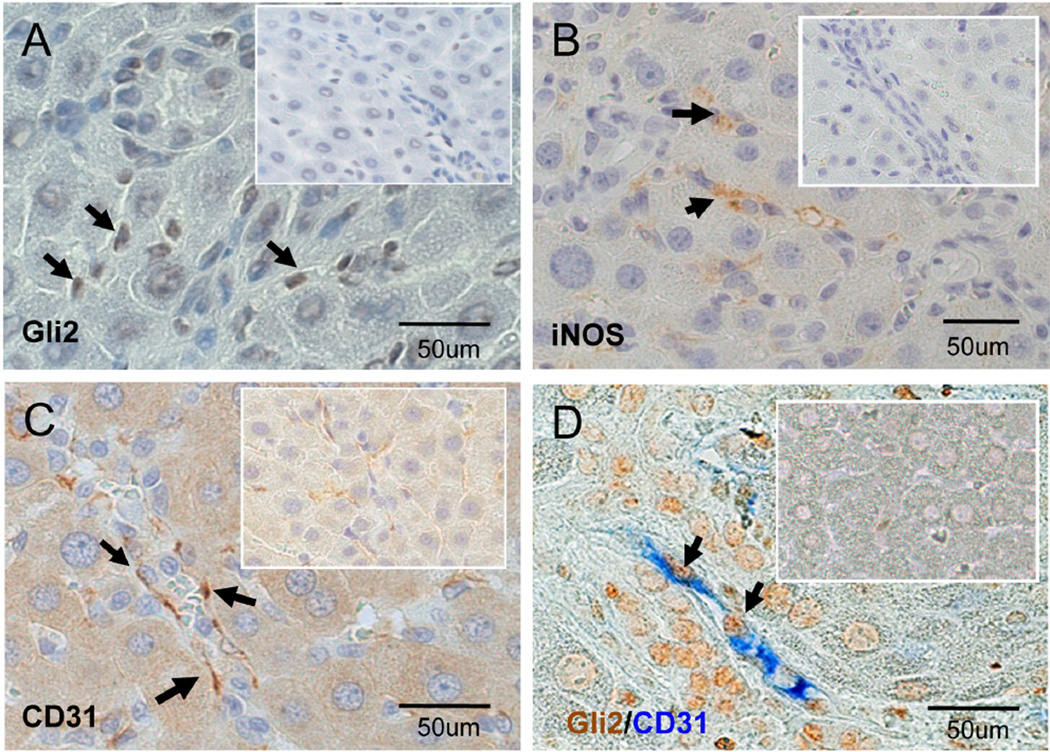
To begin to evaluate this hypothesis more directly, we isolated hepatic SEC from normal sham (NRL) and BDL rats. IHC was used to assess protein expression of Ptc, the cell surface receptor for Hh ligands, as well as CD31 (PECAM) and iNOS, two markers that are expressed negligibly in normal SEC but up-regulated in injury-activated SECs.25,26 BDL increased the proportion of SEC that expressed Ptc by 4–5 fold (Fig 5A,D), and had a similar effect on the proportion of SEC that expressed CD31 (Fig 5B,D) and iNOS (Fig 5C,D). Since primary SEC isolates may be contaminated with other types of cells, immunocytochemistry of sham-operated and BDL rats was also performed to localize Hh-responsive cells and marker gene expression in healthy and cholestatic livers. Cells expressing the Hh-target gene Gli2 were rarely demonstrated along sinusoids of healthy rats (Fig 6A, insert), but such cells were abundant following BDL (Fig 6A). Similarly, SEC activation markers, such as iNOS and CD31, were not evident in healthy livers (Fig 6B,C, inserts) but were easily demonstrated along sinusoids of cholestatic livers following BDL (Fig 6B,C). Double IHC for the Hh-target gene, Gli2, and CD31 demonstrated no double-positive sinusoidal cells in healthy livers (Fig 6D, insert), but many double-positive sinusoidal cells in BDL livers (Fig 6D), confirming that the changes that occur in hepatic sinusoids following BDL occur in Hhresponsive SEC. Thus, activation of Hh signaling occurs in SEC during cholestasis, and may play some role in the sinusoidal remodeling process that is triggered by BDL.
Figure 5. Differential expression of Hh ligands and activation markers in freshly isolated primary hepatic SEC from sham-operated and BDL rats.
SECs were isolated from sham-operated and BDL rats, and IHC was done to demonstrate Ptch (A), CD31 (B), iNOS (C). Inserts demonstrate results from sham rats. Large figures demonstrate findings in SEC from BDL rats. (D) Quantitative analysis of positivelystained cells in 10 (40x) fields of cytospins from each group (approximately 500cells were scored. (**P<0.005)
Figure 6. Activated hepatic SEC express Hh responsive in BDL livers.
Liver sections from sham-operated and BDL rats were stained for Gli2 (A), iNOS (B), and CD31(C). All markers were difficult to locate in sham animals (Insert), but were readily demonstrated in sinusoidal cells following BDL (arrows). Double immuno-staining colocalized expression of the Hh-target gene, Gli2 (brown), and the SEC activation marker, CD31 (blue) (D).
Discussion
The current work suggests a novel mechanism that may contribute to vascular remodeling during cirrhosis, namely Hh-mediated induction of angiogenic responses in endothelial cells. Our findings demonstrate that two of the major cell populations that accumulate in cirrhotic livers, MF-HSC and reactive cholangiocytes, release membranous microparticles (MPs) that contain active Shh and Ihh when treated with PDGF-BB. Earlier, we reported that hepatic expression of PDGF-BB, Shh, and Ihh increased significantly in rats that developed biliary fibrosis/cirrhosis after chronic BDL.8 Another group recently showed that PDGF-BB enhanced the ability of MF-HSC to promote vascular tube formation,4 suggesting that liver pericytes in cirrhotic livers might release factors that regulate angiogenesis via paracrine/endocrine routes. Here, we show that Hh-containing MPs increase in the plasma during chronic biliary fibrosis, and demonstrate that bile from BDL rats also contains MP-associated Hh ligands. Moreover, we present evidence that both plasma and biliary MPs activate Hh signaling in cultured primary SEC and demonstrate that this results in some of the gene expression changes that are known to occur as these cells undergo capillarization in vivo. The latter findings suggest that Hh-containing MPs might be released from Hh-producing liver cells that accumulate in fibrotic livers and promote remodeling of the hepatic sinusoids during cirrhosis. This concept is further supported by novel evidence that the Hh pathway becomes activated in SEC during biliary fibrosis in BDL rats, and data demonstrating that the Hh-reactive SEC in these fibrotic livers exhibit increased expression of the same activation/capillarization markers that were up-regulated when primary SEC from healthy rats were treated with Hh-containing MPs in culture.
Biologically active Shh and Ihh peptides undergo post-translational lipid modifications prior to their release from Hh ligand-producing cells.27 Data in developing embryos and cell culture systems indicate that the lipid-modified Hh ligands must be incorporated within micelles in order to initiate Hh-signaling in remote Hh-target cells.28 However, not much is known about the mechanisms that mediate this process. A recent study in C.elegans suggested that Hh ligands might enter the endolysosomal signaling pathway and be released within exosomes.12 Whether or not similar mechanisms are involved in the release of Hh ligands from mammalian cells is, to our knowledge, unknown. Proteomic analysis of crude membrane microparticles that were generated by inducing apoptosis in cultured T lymphocytes demonstrated Shh.11 However, those MPs preparations were not analyzed to determine whether or not they contained exosomes, or if Hh ligands in the preparation localized with exosomal markers. Thus, our demonstration that exosome-enriched membranous microparticles released from liver myofibroblasts and cholangiocytes contain biologically-active, mature Shh and Ihh peptides provides the first evidence that mechanisms controlling the dispersal of these potent morphogens might be conserved across species. Further research will be required to investigate this possibility and determine its relevance for liver cell biology.
At this point, the precise origin(s) of the exosome-associated Hh ligands within the membrane MPs that we prepared from the plasma and bile of BDL rats is also uncertain. Several lines of evidence support the concept that at least some of these factors were derived from the injured livers. Most notable is the fact that chronic liver injury significantly increased the abundance of Hh ligand-containing MPs in plasma and bile. Also, certain ultrastructural characteristics and types of proteins (e.g., TSG101, K19, Shh, Ihh) that were contained within the MPs that we isolated from plasma and bile of BDL rats were similar to those of the MPs that we prepared from liver cell-conditioned culture medium. Indeed, the concept that injured livers release factors that contribute to the extra-hepatic manifestations of cirrhosis is not novel. Several other groups have already reported that the egress of liver-derived soluble factors plays a key role in the pathogenesis of cirrhosis-related vasculopathy.29, 30 In addition, Jaroszewicz et.al recently reported significant increases in plasma VEGF and sVEGF-R1 in cirrhotic patients.31 Given strong evidence for increased VEGF expression by SEC in cirrhosis,32 the latter observation also supports the concept that vasoregulatory factors, including Hh ligands and Hh-induced mediators such as NO, may escape from cirrhotic livers. This might be particularly important because NO mediates many of the systemic vascular responses that accompany cirrhosis,2 and earlier work with Hh-containing T cell-derived MPs 11, as well as the present studies with Hh-containing, liver cell-derived MPs, demonstrate that both types of MPs induce robust endothelial cell synthesis of NO.
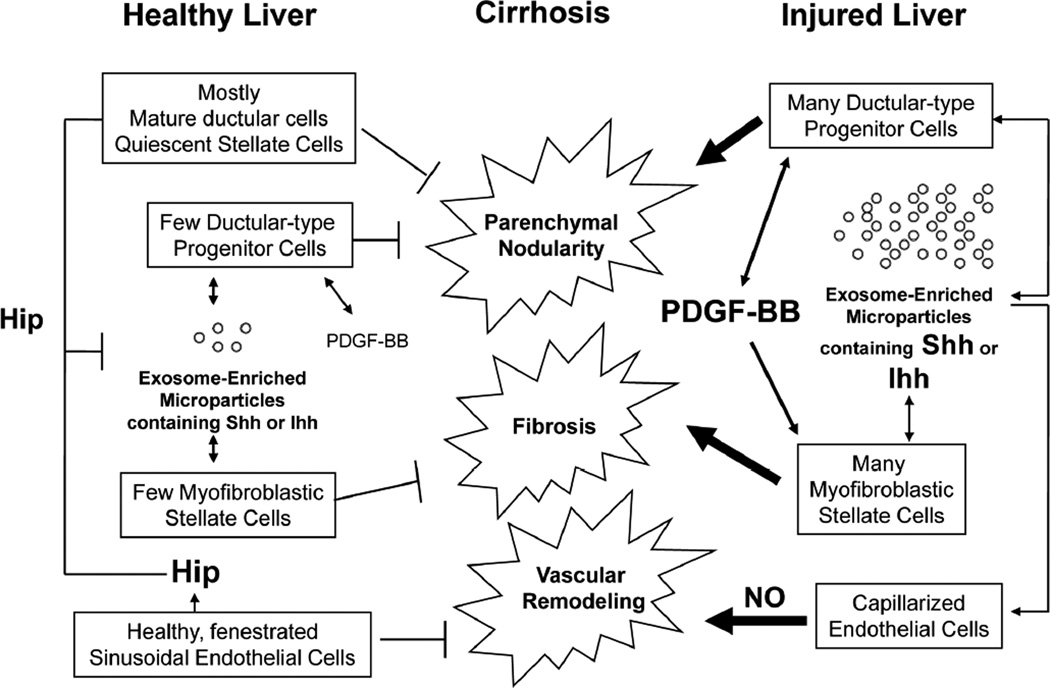
Finally, although the concept that MPs carrying Hh ligands might play a role in the pathogenesis of portal hypertensive vasculopathy is novel, several lines of evidence support the potential physiologic relevance of Hh signaling in regulating adult liver endothelial cell function. First, hepatic SEC in healthy adult livers express Hh interacting protein (Hip), a potent Hh ligand antagonist.33 Second, Hip declines dramatically after BDL,13 a type of liver injury that is accompanied by relatively rapid vascular remodeling both within the liver and extrahepatic tissues, such as the lung.34 Third, biliary injury induces expression of Hh ligands by hepatic myofibroblasts and cholangiocytes. In turn, the Hh pathway regulates the activation/accumulation of these cells during BDL-related cholestasis.13 The current studies extend this knowledge by demonstrating that activated MF-HSC and ductular cells release MPs that contain biologically active forms of Shh and Ihh, and showing that these factors evoke some of the changes in SEC gene expression that occur during injury-related capillarization of hepatic sinusoids. Together, these findings suggest that hepatic SEC cells may also become targets for Hh-regulation during chronic biliary injury. Moreover, since MPs provide a convenient mechanism for dispersing Hh ligands to distant sites and MPs containing Hh ligands appear in peripheral blood and bile during chronic cholestasis, liver-derived MPs may mediate some of the changes in the extra-hepatic microvasculature that occur during cirrhosis (Figure 7). If validated by further research, these results broaden the role of the Hh pathway in adult liver repair, and also suggest novel diagnostic markers and therapeutic targets in progressive forms of chronic liver damage that may cause portal hypertension.
Figure 7. Hedgehog-mediated alterations in liver SEC during cirrhosis.
Healthy livers contain large numbers of ductular cells, quiescent hepatic stellate cells and fenestrated SEC. The latter two cell types express Hedgehog interacting protein (Hip), which antagonizes the actions of the small amounts of Shh or Ihh that are released from rare immature ductular-type progenitor-cells and MF-HSCs that exist in uninjured livers. Liver injury represses Hip and activates ductular-type progenitor cells to produce PDGF-BB. PDGF-BB stimulates the accumulation of MF-HSCs, and induces production of Hh ligands by both MF-HSCs and ductular-type progenitor cells. Hh ligands promote proliferation and inhibit apoptosis of these cell types, expanding their numbers. This contributes to liver fibrosis and parenchymal nodularity. The MF-HSCs and ductulartype progenitor cells also release increased number of membranous exosome-enriched microparticles that contain Hh ligands. These interact with neighboring SEC and induce Hh-dependent changes in sinusoidal gene expression that result in capillarization and the release of vasoactive factors, such as nitric oxide (NO), that promote vascular remodeling. Therefore, Hh pathway activation during liver injury contributes to tissue remodeling that occurs in cirrhosis.
ACKNOWLEDGEMENTS
This work was supported in part by NIH 5RO1-AA010154
ABBREVIATIONS
- MPs
Microparticles
- Hh
Hedgehog
- Shh
Sonic Hedgehog
- Ihh
Indian Hedgehog
- MF-HSC
Myofibroblastic hepatic stellate cells
- SEC
Sinusoidal endothelial cells
- PDGF
Platelet Derived Growth Factor
- BDL
Bile duct ligation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No conflicts of interest exist.
References
- 1.Parsons CJ, Takashima M, Rippe RA. Molecular mechanisms of hepatic fibrogenesis. J Gastroenterol.Hepatol. 2007;22(Suppl.1):S79–S84. doi: 10.1111/j.1440-1746.2006.04659.x. [DOI] [PubMed] [Google Scholar]
- 2.Iwakiri Y, Groszmann RJ. Vascular endothelial dysfunction in cirrhosis. J.Hepatol. 2007;46:927–934. doi: 10.1016/j.jhep.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 3.Moreira RK. Hepatic stellate cells and liver fibrosis. Arch Pathol Lab Med. 2007;131:1728–1734. doi: 10.5858/2007-131-1728-HSCALF. [DOI] [PubMed] [Google Scholar]
- 4.Semela D, Das A, Langer D, et al. PDGF signaling through ephrin-b2 regulates hepatic vascular structure and function. Gastroenterology. 2008;135:671–679. doi: 10.1053/j.gastro.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vokes SA, Yatskievych TA, Heimark RL, et al. Hedgehog signaling is essential for endothelial tube formation during vasculogenesis. Development. 2004;131:4371–4380. doi: 10.1242/dev.01304. [DOI] [PubMed] [Google Scholar]
- 6.Straface G, Aprahamian T, Flex A, et al. Sonic Hedgehog Regulates Angiogenesis and Myogenesis During Post-Natal Skeletal Muscle Regeneration. J.Cell.Mol.Med. 2008 doi: 10.1111/j.1582-4934.2008.00440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asai J, Takenaka H, Kusano KF, et al. Topical sonic hedgehog gene therapy accelerates wound healing in diabetes by enhancing endothelial progenitor cellmediated microvascular remodeling. Circulation. 2006;113:2413–2424. doi: 10.1161/CIRCULATIONAHA.105.603167. [DOI] [PubMed] [Google Scholar]
- 8.Omenetti A, Popov Y, Jung Y, et al. The Hedgehog Pathway Regulates Remodeling Responses to Biliary Obstruction in Rats. Gut. 2008 doi: 10.1136/gut.2008.148619. [DOI] [PubMed] [Google Scholar]
- 9.Omenetti A, Diehl AM. The adventures of sonic hedgehog in development and repair. Am.J.Physiol.Gastrointest.Liver.Physiol. 2008;294:G595–G598. doi: 10.1152/ajpgi.00543.2007. [DOI] [PubMed] [Google Scholar]
- 10.Callejo A, Torroja C, Quijada L, et al. Hedgehog lipid modifications are required for Hedgehog stabilization in the extracellular matrix. Development. 2006;133:471–483. doi: 10.1242/dev.02217. [DOI] [PubMed] [Google Scholar]
- 11.Agouni A, Mostefai HA, Porro C, et al. Shh carried by microparticles corrects endothelial injury through nitric oxide release. FASEB.J. 2007;21:2735–2741. doi: 10.1096/fj.07-8079com. [DOI] [PubMed] [Google Scholar]
- 12.Liegeois S, Benedetto A, Garnier JM, et al. The V0-ATPase mediates apical secretion of exosomes containing Hedgehog-related proteins in C.elegans. J.Cell.Biol. 2006;173:949–961. doi: 10.1083/jcb.200511072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Omenetti A, Yang L, Li YX, et al. Hedgehog-mediated mesenchymal-epithelial interactions modulate hepatic response to bile duct ligation. Lab Invest. 2007;87:499–514. doi: 10.1038/labinvest.3700537. [DOI] [PubMed] [Google Scholar]
- 14.Jy W, Horstman LL, Jimenez JJ, et al. Measuring circulating cell-derived microparticles. J.Thromb.Haemost. 2004;2:1842–1851. doi: 10.1111/j.1538-7836.2004.00936.x. [DOI] [PubMed] [Google Scholar]
- 15.Piccin A, Murphy WG. Circulating microparticles: pathophysiology and clinical implications. Blood.Rev. 2007;21:157–171. doi: 10.1016/j.blre.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Thery C, Boussac M, Veron P, et al. Proteomic analysis of dendritic cell-derived exosomes. J.Immunol. 2001;166:7309–7318. doi: 10.4049/jimmunol.166.12.7309. [DOI] [PubMed] [Google Scholar]
- 17.Denzer K, Kleijmeer MJ, Heijnen HF, et al. Exosome: from internal vesicle of the multivesicular body to intercellular signaling device. J.Cell.Sci. 2000;113 Pt 19:3365–3374. doi: 10.1242/jcs.113.19.3365. [DOI] [PubMed] [Google Scholar]
- 18.Boigk G, Stroedter L, Herbst H, et al. Silymarin retards collagen accumulation in early and advanced biliary fibrosis secondary to complete bile duct obliteration in rats. Hepatology. 1997;26:643–649. doi: 10.1002/hep.510260316. [DOI] [PubMed] [Google Scholar]
- 19.Liu S, Premont RT, Kontos CD, et al. Endothelin-1 activates endothelial cell nitric-oxide synthase via heterotrimeric G-protein betagamma subunit signaling to protein jinase B/Akt. J.Biol.Chem. 2003;278:49929–49935. doi: 10.1074/jbc.M306930200. [DOI] [PubMed] [Google Scholar]
- 20.Greenwel P, Schwartz M, Rosas M, et al. Characterization of fat-storing cell lines derived from normal and CCl4-cirrhotic livers. Lab.Invest. 1991;65:644–653. [PubMed] [Google Scholar]
- 21.Yahagi K, Ishii M, Kobayashi K, et al. Primary culture of cholangiocytes from normal mouse liver. In.Vitro.Cell.Dev.Biol.Anim. 1998;34:512–514. doi: 10.1007/s11626-998-0106-x. [DOI] [PubMed] [Google Scholar]
- 22.Raposo G, Nijman HW, Stoorvogel W, et al. B lymphocytes secrete antigenpresenting vesicles. J.Exp.Med. 1996;183:1161–1172. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Minicis S, Seki E, Uchinami H, et al. Gene expression profiles during hepatic stellate cell activation in culture and in vivo. Gastroenterology. 2007;132:1937–1946. doi: 10.1053/j.gastro.2007.02.033. [DOI] [PubMed] [Google Scholar]
- 24.Yang L, Wang Y, Mao H, et al. Sonic hedgehog is an autocrine viability factor for myofibroblastic hepatic stellate cells. J.Hepatol. 2008;48:98–106. doi: 10.1016/j.jhep.2007.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeLeve LD, Wang X, Hu L, et al. Rat liver sinusoidal endothelial cell phenotype is maintained by paracrine and autocrine regulation. Am.J.Physiol.Gastrointest.Liver Physiol. 2004;287:G757–G763. doi: 10.1152/ajpgi.00017.2004. [DOI] [PubMed] [Google Scholar]
- 26.Rockey DC, Chung JJ. Regulation of inducible nitric oxide synthase and nitric oxide during hepatic injury and fibrogenesis. Am.J.Physiol. 1997;273:G124–G130. doi: 10.1152/ajpgi.1997.273.1.G124. [DOI] [PubMed] [Google Scholar]
- 27.Mann RK, Beachy PA. Novel lipid modifications of secreted protein signals. Annu.Rev Biochem. 2004;73:891–923. doi: 10.1146/annurev.biochem.73.011303.073933. [DOI] [PubMed] [Google Scholar]
- 28.Callejo A, Culi J, Guerrero I. Patched, the receptor of Hedgehog, is a lipoprotein receptor. Proc.Natl.Acad.Sci. 2008;105:912–917. doi: 10.1073/pnas.0705603105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fallon MB, Abrams GA. Pulmonary dysfunction in chronic liver disease. Hepatology. 2000;32:859–865. doi: 10.1053/jhep.2000.7519. [DOI] [PubMed] [Google Scholar]
- 30.Lo Iacono O, Rincon D, Hernando A, et al. Serum levels of soluble vascular cell adhesion molecule are related to hyperdynamic circulation in patients with liver cirrhosis. Liver.Int. 2008 doi: 10.1111/j.1478-3231.2008.01763.x. [DOI] [PubMed] [Google Scholar]
- 31.Jaroszewicz J, Januszkiewicz M, Flisiak R, et al. Circulating vascular endothelial growth factor and its soluble receptors in patients with liver cirrhosis. Cytokine. 2008 doi: 10.1016/j.cyto.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 32.Deleve LD, Wang X, Guo Y. SEC prevent rat stellate cell activation and promote reversion to quiescence. Hepatology. 2008 doi: 10.1002/hep.22351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olsen CL, Hsu PP, Glienke J, et al. Hedgehog-interacting protein is highly expressed in endothelial cells but down-regulated during angiogenesis and in several human tumors. BMC.Cancer. 2004;4:43. doi: 10.1186/1471-2407-4-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bosch J. Vascular deterioration in cirrhosis: the big picture. J.Clin.Gastroenterol. 2007;41:S247–S253. doi: 10.1097/MCG.0b013e3181572357. [DOI] [PubMed] [Google Scholar]