Abstract
Rheumatic fever (RF) and rheumatic heart disease (RHD) continue to be a major health hazard in most developing countries as well as sporadically in developed economies. Despite reservations about the utility, echocardiographic and Doppler (E&D) studies have identified a massive burden of RHD suggesting the inadequacy of the Jones’ criteria updated by the American Heart Association in 1992. Subclinical carditis has been recognized by E&D in patients with acute RF without clinical carditis as well as by follow up of RHD patients presenting as isolated chorea or those without clinical evidence of carditis. Over the years, the medical management of RF has not changed. Paediatric and juvenile mitral stenosis (MS), upto the age of 12 and 20 yr respectively, severe enough to require operative treatement was documented. These negate the belief that patients of RHD become symptomatic ≥20 years after RF as well as the fact that congestive cardiac failure in childhood indicates active carditis and RF. Non-surgical balloon mitral valvotomy for MS has been initiated. Mitral and/or aortic valve replacement during active RF in patients not responding to medical treatment has been found to be life saving as well as confirming that congestive heart failure in acute RF is due to an acute haemodynamic overload. Pathogenesis as well as susceptibility to RF continue to be elusive. Prevention of RF morbidity depends on secondary prophylaxis which cannot reduce the burden of diseases. Primary prophylaxis is not feasible in the absence of a suitable vaccine. Attempts to design an antistreptococcal vaccine utilizing the M-protein has not succeeded in the last 40 years. Besides pathogenesis many other questions remain unanswered.
Keywords: Antistreptococcal vaccine, heart disease, myocarditis, rheumatic fever, rheumatic heart disease, streptococcal infections, subclinical carditis
Rheumatic heart disease (RHD) follows rheumatic fever (RF), as a non-suppurative manifestation of group A beta haemolytic streptococcal (GAS) pharyngitis. RF is widely accepted as an immunological disorder following GAS infection. Although the burden has come down in developed countries, RHD continues to be a prominent cause of morbidity and mortality in developing countries of the world. This review highlights the changes that have occurred in the area of RF and RHD in the last 50 years.
Historical perspective
About 100 years back RF/RHD was believed to be a disease of “temperate climate”. In 1835, Malcomson observed that rheumatism was prevalent among sepoys1 and in 1870 Moore2 reported numerous cases of rheumatism in Rajasthan. Rogers3 indicated absence of RF in India as except one possible case he did not find RHD in 4800 postmortem records in 37 years in Calcutta (Kolkata) inspite of 25 cases of mitral stenosis which he labelled as non rheumatic. Megaw4 reported RHD from plains of India but felt that it was less common than seen in colder climates. Clark5 reported absence of haemolytic streptococcal infections and low prevalence of RF/RHD in tropics. Keats6 did not find a single case of RHD in 600 autopsies in Amritsar. Drury7 found mitral valve disease in 62 per cent and mitral stenosis in 10 per cent in an analysis of 319 clinically diagnosed cases of heart disease admitted to the Medical College Hospital in Calcutta (Kolkata). Basu8 found 8.3 per cent cases of rheumatic carditis and pericarditis in 446 patients of acquired heart disease. Hughes and Yusuf9 referred to mitral stenosis in an article on heart disease in Punjab9. Hodge reported on rheumatism and indicated that it was not rare in India.
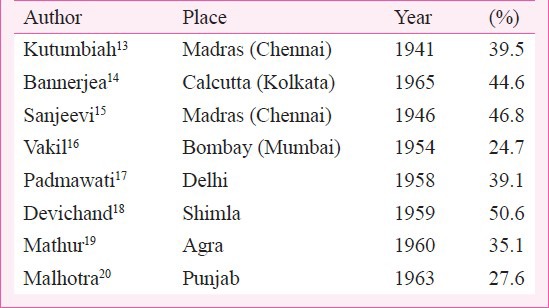
The first clinical evidence of RF came from Punjab by Wig in 193511 and on rheumatism in childhood and adolescence by Kutumbiah in 194012. This was followed by a large number of hospital-based surveys for the relatively “new” disease accounting for 20 to 50 per cent admissions in hospitals in various parts of the country (Table I). With the results, rheumatic fever was labelled as severe or malignant in India with multivalve involvement and congestive cardiac failure even in the initial attack of RF21. Roy delineated the features of RF and compared with features seen in Boston (USA)22. The presence of RF/RHD was not only established but also considered to be the commonest heart disease in the country by mid 1950s.
Table I.
Percentage of RHD patients in hospital admission
Burden of the disease in India
The information regarding the burden of disease comes from hospital data, population based studies and school surveys. Hospital based data between 1945 and 1963 indicated that anywhere from 20 to 50 per cent hospital admissions for cardiac patients were for RHD (Table I). Since the hospital-based data do not represent the population of the region, there is a bias towards the worst affected and those seeking admission for procedures. Additional bias may be introduced through changes in the population served by the hospital over many years. With increasing marginalization of the poorer sections of the society some hospitals may no longer be serving those who are worst affected with RHD. Perhaps the most important source of bias is in the preference of the admitting units. With emergence of the epidemic of coronary artery disease (CAD), hospital admissions are largely represented by CAD patients in most hospitals.
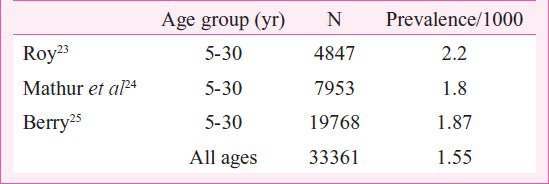
Population based surveys for prevalence are very few and scattered. (Table II). In a study in rural Haryana prevalence of RHD was found to be 2.2/1000 in 5 to 30 year old subjects23. Mathur in a study of the urban population of Agra found RHD in 1.8/1000 in the same age group24. Berry25 studied the urban population of Chandigarh and found RHD in 1.23/1000 male and 2.07/1000 in the female population of all age groups. A recent Indian Council of Medical Research (ICMR) study (between 2000 and 2010) in 10 different, mostly urban, locations of the country found the prevalence to range from 0.2 to 1.1/1000 for RHD and 0.0007 to 0.2 /1000 for RF26. The data were based on registration of all cases in one million population by approaching hospitals, private practitioners and extensive advertising for establishing a registry of all known cases. The recent registry data suggests decline but registries are able to collect about 50 to 70 per cent cases. Hence, overall decline is debatable.
Table II.
Prevalence of RHD in population surveys
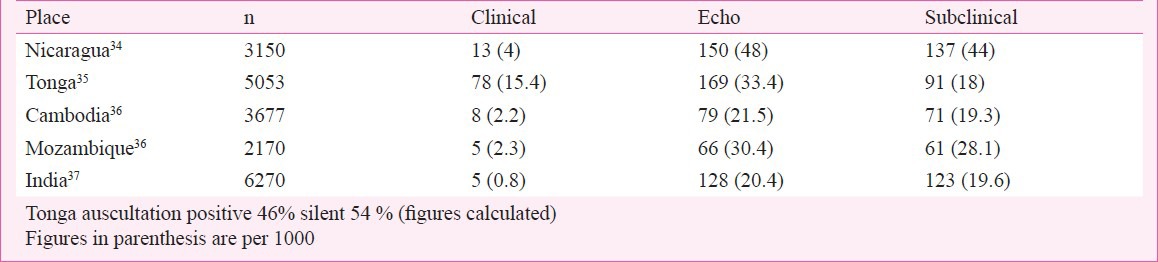
ICMR has conducted three school-based surveys in children 5 to 14 yr in age over a 40-year period between 1970 and 2010. The first survey from 1972 to 1975 was in schools at Agra, Alleppy, Bombay (Mumbai), Delhi and Hyderabad. The second from 1984 to 1987 included schools at Delhi, Varanasi and Vellore. The third study included children from 10 centres in the country located at Shimla, Jammu, Chandigarh, Jodhpur, Indore, Kochi, Wayanad, Mumbai, Vellore and Dibrugarh. It has a wider coverage but not of the whole country. In the first study (1972-1975), 1,33,000 children were evaluated and the prevalence of RHD varied from 0.8 to 11/1000, overall 5.3/1000. The second study (1984-1987) included 53,786 children and the prevalence ranged from 1.0 to 5.6/1000 overall 2.9/1000. The third and the largest study included 1,76,904 school children with a prevalence varying from 0.13 to 1.5/1000 (overall 0.9/1000) in the 5 to 14 yr age range26. The data suggest a progressive decline in RHD from 5.3 to 2.9 to below 1.0/1000 between 1970 to 2010. In the last study echocardiographic evaluation was performed in all children clinically diagnosed to have a heart murmur and children with congenital heart disease could be excluded. In a study on 1,18,212 school children 4-18 yr in age a heart murmur was found in 389 normal children. Echo evaluation identified 61 children with RHD giving a prevalence of 0.5/1000 children in Uttar Pradesh27. Studies from Punjab, Gujarat, Rajasthan, Uttar Pradesh and Tamil Nadu have found the prevalence to range from 0.67 to 4.54/1000 children (Table III). The figures are variable but suggest a decline in the prevalence of RHD over time, however, whether they identify a real decline in prevalence is a difficult question to answer. At the same time, addition of echocardiographic RHD surveys of normal children have introduced a new dimension to the assessment of disease burden. Most available echocardiographic evaluation studies for the presence of RHD in school children suggest more than 10 to 20 times higher prevalence of clinically “silent” RHD (Table IV). The reliability or acceptability of the prevalence data based on clinical evaluation alone is not known with certainty. Further, echocardiographic diagnosis has fallacies and follow up studies of the clinically silent or subclinical (SC) RHD are required to establish the significance of disease identified through echocardiography alone. Is this “subclinical” valve disease really silent RHD? A long-term follow up of the patients is required to establish the natural history of disease identified through echocardiography alone.
Table III.
Prevalence of rheumatic heart disease (RHD) in school surveys
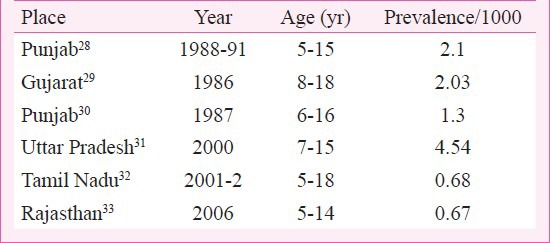
Table IV.
Prevalence of subclinical carditis in echo studies of school children
An analysis of the shortcomings associated with the estimation of the prevalence of RF/RHD in our country has been discussed earlier38. The overall prevalence estimated to be about 1.5-2/1000 in all age groups, in India (total population about 1.3 billion) suggests that there are about 2.0 to 2.5 million patients of RHD in the country.
Global and Asian burden of RF/RHD
The burden of RF/ RHD has been described in detail by Carapetis and colleagues39,40. Excluding developed economies, the global burden of RHD in the 5 to 14 yr old children was estimated to be 0.8 - 5.7/1000 with a median of 1.3/1000. The estimated number of children would be about 2.4 million (Table V). Subsequent data from studies in Asia suggested that the number of children with RHD in Asia could be between 1.96 to 2.21 million. The findings were extrapolated to include all ages and estimated that globally there were 15.6 - 19.6 million patients. In the study of Asian countries the burden of RHD was estimated to be 10.8 - 15.9 million patients. The estimates of RHD in Asian countries indicate that the global burden is significantly higher than the earlier estimates for children as well as all age groups.
Table V.
Global and Asian magnitude of RF/RHD (Excluding developed economies)
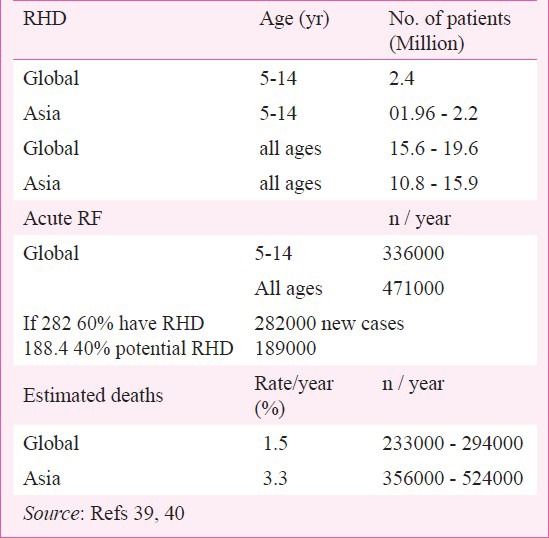
On the basis of 1.5 per cent mortality per year, global deaths from RHD were estimated to be 233,000 - 294,000/year. The mortality in Asian countries was calculated on the basis of a study showing 3.3 per cent per year mortality41. As such the mortality in Asia accounts for 356,000 to 524,000 deaths/year suggesting that the global mortality must be higher.
For acute RF a global estimate in the 5-14 yr age group suggested 336000 new cases per year. Extrapolating to all ages it indicated that about 471,000/ year get RF. Calculating on the basis of 60 per cent patients of RF getting carditis, about 282,000 new cases of RF are added each year with the remaining 40 per cent or 189,000/year having a potential for subclinical RHD (Table V). A review of the incidence of acute RF, in population based studies in the world has estimated that the overall incidence varies from 5 to 51/100,000 population with a mean of 19/100, 00042.
These estimates do not take into account subclinical carditis identified on the basis of echocardiography and Doppler studies in surveys of school children (Table IV). Although the exact significance of the subclinical carditis in terms of morbidity has not been established, it cannot be disregarded. Studies suggest that subclinical RHD can progress to clinical RHD. At the same time it is well known that recurrences of RHD have mimetic features and the subclinical disease may become a clinically obvious disease in recurrences in the absence of secondary prophylaxis43. The magnitude of subclinical carditis, 10 to 20 times higher than manifest RHD, indicates the difficulties regarding secondary prophylaxis. Secondary prophylaxis is ethically mandatory in this age group. Can we identify children with subclinical carditis and not put them on secondary prophylaxis ?
Diagnosis of RF
The criteria for the diagnosis enunciated by Dr T. Duckett Jones’ have been modified, revised and updated by the American Heart Association (AHA)44. The diagnostic criteria consist of major manifestations carditis, arthritis, subcutaneous nodules, erythema marginatum and chorea. Rheumatic carditis resulting in a more or less permanent damage to the heart is the main virulent manifestation of RF.
The minor manifestations consist of fever, arthralgia, elevated sedimentation rate, C-reactive protein (CRP) and prolonged PR interval in the electrocardiogram. Presence of two major or one major and two minor manifestations with an evidence for recent GAS infection (essential criterion) indicate acute RF. Evidence for recent GAS infection can be in the form of a positive throat culture, elevated anti-streptococcal, antibodies or presence of features for recent scarlet fever, rare in our country.
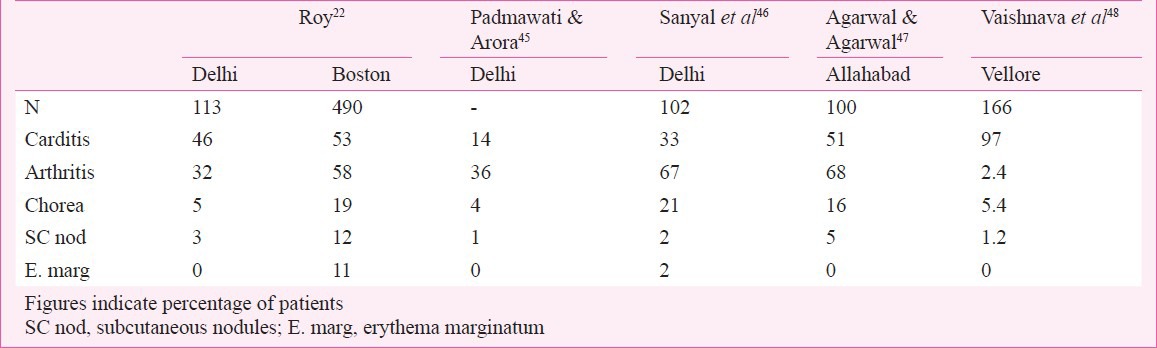
The components of major, minor and essential criteria for the diagnosis remain more or less as before in AHA guidelines44. The updated criteria emphasize the value of indolent carditis and chorea to be accepted as evidence of RF and have removed previous RF or presence of RHD as a minor manifestation to simplify the diagnosis of first attack of RF. In the presence of previous history of RF/RHD one major or more than one minor criterion is acceptable for the diagnosis of recurrent RF. Additionally echocardiogram based diagnosis of carditis has been questioned in the absence of clinical findings to indicate cardiac involvement. The clinical manifestations of RF, except for minor differences in frequency, is the same all over the world. In our country erythema marginatum is not recognized possibly because of the darker skin complexion (Table VI)22,45,46,47,48.
Table VI.
Major manifestations of acute RF
It needs to be emphasized that the diagnostic criteria are guidelines that help in the identification of RF. However, physicians have a right to make a diagnosis of RF on the basis of clinical judgment even if the updated criteria are not satisfied. This may be due to (i) absence of history suggestive of RF in almost 50 per cent patients of RHD, and (ii) identification of subclinical carditis by echocardiographic studies, indicating inadequacy of clinical diagnosis.
Identification of RF
RHD can occur only after a patient has had RF. Evaluation of data indicates that about 65 per cent patients get clinically recognizable RHD following RF. In the global estimate a conservative figure of 60 per cent carditis has been used for calculating the burden of RHD39. This suggests that at least 40 per cent patients who have had RF could be potentially patients of subclinical carditis. On the basis of Utah study, 27 per cent patients had subclinical carditis49. Hence, the actual estimated burden could be much more than the actual burden. Secondly, most prevalence figures indicate that the prevalence of RF in surveys is about one tenth or even less than that of RHD (0.1/1000 vs 1/1000)26. The inference could be that the diagnosis of RF is being missed more often than desirable or acceptable. Less than half of all RHD patients give history of past RF. Unfortunately, diagnosis of past RF is not possible unless patients give history of arthritis, arthralgia, chorea or have established RHD. Hence, retrospective diagnosis or identification of past RF is missed or not available in almost 50 per cent patients with RHD.
Follow up of patients with pure chorea without RHD or of patients who have had RF but no clinical evidence of carditis indicates that RHD can develop over a period of time. Bland50 in a 20 year follow up of patients with isolated chorea found 23 per cent patients without clinical carditis to develop RHD predominantly mitral valve obstruction (MS). Similarly, Aron et al51 in a 30-year follow up of 50 patients of pure chorea ended up with RHD, predominantly MS, in 34 per cent patients. Roy et al52 drew attention towards the onset of symptomatic severe MS below the age of 20 yr and designated it as juvenile MS in India. In our evaluation of children below 12 yr of age who have been operated for MS 57 per cent gave history consistent with RF, haemodynamic studies in 29 and operation in 35 of the 42 patients indicated moderately severe to severe MS requiring operative treatment below the age of 12 yr53. The assessment of the exact time of RF and the interval between RF and onset of symptoms of MS could be fallacious since it was dependent entirely on the past history of arthritis and arthralgia. Nine patients became symptomatic, within a year and five within two years, all below 12 yr in age. The youngest patient was six years old at the time of operation without a history suggestive of RF53. In a subsequent evaluation of 125 children below of the age of 12 yr with isolated mitral stenosis, past history of rheumatic fever was available in 54 (51%)54.
These studies indicate that MS can occur very quickly following RF. Secondly 43-50 per cent developed significant mitral obstruction without a history to suggest RF indicating that acute RF is not being recognized, possibly because RF is occurring with subclinical carditis but without arthritis, arthralgia, subcutaneous nodules, and chorea. Missing a clinical diagnosis of acute RF in children less than 12 yr of age is most disturbing since the time available to forget the manifestation of acute RF is very short if we accept that RF must have occurred beyond the age of 2 to 3 yr in most.
Presence of subclinical carditis diagnosed using echocardiography in surveys of 5-14 yr old children when combined with the findings of the children with MS suggests that RF is being missed more often than desirable. Some patients have only one clinical manifestation - fever with valvulitis during the episode of RF and the diagnosis of RF is being missed since the carditis is asymptomatic, mild and not associated with any murmurs (subclinical). The findings suggest that the diagnosis of RF based on the 1992 guidelines of AHA are necessary but insufficient in identifying RF in a fairly large number of patients44.
Diagnostic tests in RF/RHD
The diagnostic tests can be considered as those meant for (i) diagnosis of RF, (ii) presence of active vs. inactive RF in recurrences, and (iii) identification of carditis and valve damage in RHD.
Diagnosis of RF:
-
(1)
The diagnosis of RF is dependent on some laboratory tests included as minor criteria and consist of the following:
-
(i)Acute phase reactants (leukocytosis, elevated sedimentation rate and presence of C reactive protein CRP).
-
(ii)Prolonged PR interval in the electrocardiogram.
-
(i)
-
(2)
The diagnosis requires presence of essential criteria in the form of evidence for recent GAS infection and consists of:
-
(i)elevated antistreptococcal antibodies,
-
(ii)positive throat culture for GAS, and
-
(iii)evidence for recent scarlet fever- rare in India.
-
(i)
Elevated erythrocyte sedimentation rate (ESR) is a nonspecific evidence for an active disease. It is elevated in acute RF but can be normal if the patient has congestive failure and can be high in the presence of anaemia. Normal CRP is against the diagnosis of active RF. Prolonged PR interval can be seen in the electrocardiograms in patients with active RF. Prolonged PR interval is a non specific finding and does not indicate the presence of myocarditis. Elevated antistreptococcal antibodies identify recent streptococcal infection. A fair amount of confusion exists about the exact level of antibodies to be considered as high. Generally antistreptolysin O (ASLO) is the commonest antibody measured. It appears in about 7 to 10 days and peaks in 2 to 3 wk55. It is considered high if the figure is more than the baseline value present in the community. In endemic areas the baseline ASLO could be 250 Todd units or more whereas in non-endemic areas it could be as low as 50 Todd units. Increasing titres indicate recent GAS infection. Using two antibody titres, that is, ASLO combined with deoxyribonuclease B titres increases the specificity of diagnosis to 90 per cent56.
Presence of GAS in throat culture with low values of ASLO suggests a carrier state. As such a positive throat culture for GAS cannot be taken as recent infection unless the antibody titres are elevated.
Presence of active vs. inactive RF in recurrences: Two investigations have been tried to assess the presence or absence of active RF in patients with recurrences besides ESR, CRP and evidence for recent GAS infection.
(i) Induced subcutaneous nodules (SCN): Massell et al57 tried inducing SCN by injecting five dl autologous blood drawn from a vein and injecting over the olecranon process of one elbow and saline in the other elbow. Frictional pressure was applied to the injected sites. Appearance of a SCN in 5 to 10 days was accepted as indicating active RF. Vasan modified this test and used concentrated leukocyte injection instead of whole blood with 86 per cent sensitivity and 94 per cent specifically to identify active RF. The test offers the advantage of being cheap and easily available everywhere. The potential utility of the test lies in identifying active RF. However, additional validation studies are perhaps needed.
(ii) Myocardial biopsy: A study of myocardial histology to identify active vs. inactive RF was utilized in patients of RF59. Myocardial biopsies were performed in 89 patients of active RF and chronic RHD to identify active carditis Myocardial biopsies failed to improve on clinically assessed presence of active RF. Myocardial biopsy was felt to be insensitive for identifying presence of active carditis58.
Rheumatic carditis and valve damage
The virulence of RF is related to its capacity to cause cardiac damage. Clinically carditis has been reported by several investigators in the initial attack in India (Table VI)22,45,46,47,48. Rheumatic carditis has been considered to be a pancarditis causing pericardial, myocardial and endocardial disease.
Pericarditis occurs in about 15 per cent cases. It is identified by the presence of a pericardial friction rub and may be associated with precordial chest pain. It can be evanescent and may appear for a brief period. An echocardiogram can identify the effusion, which never results in tamponade and subsides without any sequelae.
Studies strongly suggest that RF does not cause myocarditis. Absence of myocarditis has been documented by (i) absence of increase in markers of myocardial damage - MB fraction of creatinine kinase (CK-MB), troponin I & T and myoglobin60,61,62, (ii) normal left ventricular systolic function and myocardial contractility by echocardiographic studies62,63, (iii) radionuclide studies using technetium pyrophosphate scanning and indiumIII labelled anticardiac myocin Fab (FAB) do not indicate presence of myocardial damage64,65, (iv) myocardial biopsy studies have not been able to identify the presence of myocarditis59, (v) normalization of heart size and disappearance of congestive failure following surgical mitral and/or aortic valve replacement in patients deteriorating in spite of aggressive medical management66, (vi) histopathology of the left ventricular myocardium showing absence of myocardial or inter-myocardial connective tissue damage67 and (vii) immunopathology of Ashoff nodule (AN), the diagnostic marker of rheumatic pathology, being derived from mesenchymal cells, complete absence of cells of myocardial origin in AN and absence of actin, myosin and desmin (of myocardial origin) in the AN indicating that AN is not of myocardial origin68. Hence, congestive cardiac failure in acute RF is due to an acute volume overload from mitral and/or aortic regurgitation but not due to myocarditis per se.
Rheumatic endocarditis represented by cardiac valve involvement results in the only permanent damage from RF. Pathological evaluation of the valves of patients dying from acute RF indicates microscopic disease in each of the four cardiac valves69. However, clinically mitral valve involvement occurs in 90 to 95 per cent of whom in 20 to 25 per cent, it is associated with aortic valve disease as well. Clinically isolated aortic valve involvement has been reported in less than five to eight per cent cases70,71. Tricuspid valve involvement in acute RF is uncommon and the pulmonary valve involvement very rare. Tricuspid valve disease has been found to occur in up to 30 to 50 per cent in necropsy studies72. Since pericarditis subsides without sequelae and myocarditis does not occur, the morbidity and mortality of RF is determined by valvulitis the cause of permanent cardiac damage in RF.
In an evaluation of patients with RF the final diagnosis regarding the presence or absence of carditis is determined by clinical findings related to the mitral and aortic valve disease. Clinically carditis has been found in anywhere between 14 to 97 per cent patients (Table VI). The commonest clinical finding is the presence of mitral regurgitation with or without aortic regurgitation.
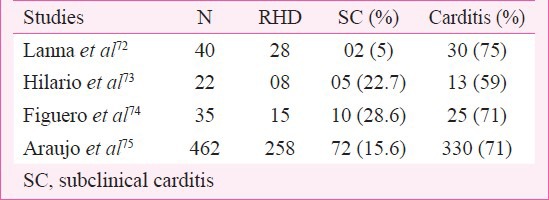
More recent studies on patients with acute RF utilizing echocardiography have brought out the shortcomings of auscultation in identifying valve disease which does not result in haemodynamic abnormalities consisting of regurgitant systolic (mitral) or diastolic (aortic) murmurs. This has resulted in the identification of sub-clinical carditis (SC). Data for SC are now available from a number of studies. However, follow up data are insufficient. Subclinical carditis has been assessed by echocardiographic and Doppler (E&D) studies in (i) patients with acute RF, (ii) follow up of patients with past RF who were clinically judged not to have carditis (NHD), and (iii) evaluation of normal children. SC has been found in 5 to 29 per cent patients of acute RF (Table VII)73,74,75,76 by E&D studies. In the Utah epidemic of acute RF clinically identifiable carditis was present in 64 per cent whereas SC diagnosed by E&D was found in 27 per cent resulting in an overall prevalence of 91 per cent49. Although the American Heart Association (AHA) has not accepted E&D studies for the identification of carditis, many clinicians feel that E&D studies are necessary for the care of patients with acute RF since SC cannot be disregarded. Follow up data on patients of acute RF with SC are unsatisfactory. Available follow up E&D studies indicate that the SC can worsen to become clinically obvious RHD, improve or remain unchanged. Lanna et al73 reported an 8-year follow up on 40 patients of RF. Initially they found two patients with SC but after eight years six lost clinical evidence of mitral regurgitation resulting in eight patients (20%) having SC. Araujo et al76 followed 462 patients for 2 to 23 yr (mean 13.6 yr). Initial evaluation indicated that 258 (56%) had carditis and 204 without clinical evidence for heart disease. E&D studies identified 72 (16%) with SC. At the end of follow up (13.6 yr) the number with clinical carditis went up to 298 (65 from 56%)76. The E&D studies thus indicate that patients identified as SC could improve and lose features of SC or become worse and develop clinical carditis (RHD).
Table VII.
Frequency of subclinical (SC) carditis in acute RF
A review of SC involving more than 1700 patients found overall prevalence to be 16.8 per cent77. WHO criteria for the identification of SC by E&D were satisfied by 10 studies which gave a prevalence of 18.1 per cent. Of the 99 patients whose follow up of up to 2 years was available, 48 per cent showed improvement and 52 per cent either no change or became worse indicating variable course of SC77.
In one of the largest study of 1000 patients of acute RF with a 100 per cent 20 year follow up, 154 (15%) patients developed RHD out of 347 (35%) patients initially diagnosed as NHD (labeled as potential RHD), indicating the presence of SC in retrospect78. In the same study 32 (20%) of the 157 children with pure chorea (NHD) developed RHD, predominantly mitral stenosis in 20 years identifying the presence of SC. Bland and Jones78 in their study made two crucial statements while detailing the delayed appearance of RHD. Both statements are sharp clinical judgments (in 1951) in the absence of investigative facilities for identification of carditis.
“It may be that minimally scarred valves (initially silent as far as physical signs are concerned) provide a locus for ------------------- deformed and stenotic orifice”78.
“In an occasional instance a blowing diastolic murmur (slight aortic regurgitation) of grade 1to 2 intensity has been observed to disappear. We suspect that minimal scarring persists in spite of the absence of murmurs or enlargement. Postmortem examination in one instance following accidental death supports this suspicion, as well as the insidious appearance of mitral stenosis in a few patients 10 to 20 years later78”.
A large amount of data is now accumulating and identifying SC by E&D studies of normal children (Table IV). The exact significance of SC identified in apparently normal school children needs to be established. The criteria for identifying SC by E&D studies need careful definition. Presently WHO and World Heart Federation (WHF) guidelines are available79,80. WHO has used only Doppler based guidelines identifying the presence and severity of valve regurgitation. WHF has used changes in valve morphology as well as Doppler estimation of valve regurgitation. At least one study has already indicated that WHO guidelines are insufficient81. Logically diagnosis of SC should be based on changes of valve damage as well as the haemodynamic consequences of valve damage. We believe that morphological changes indicating valve damage should be considered more important and essential rather than the presence of valve regurgitation alone, since it is the rheumatic valve damage which is responsible for the haemodynamic changes in RHD.
At present we need to (i) establish E&D guidelines for identification of SC, (ii) identify the magnitude of SC in apparently “healthy” children, and (iii) follow up studies of SC in apparently healthy children to decide the line of management. If follow up studies indicate that SC can deteriorate to RHD in the absence of secondary prophylaxis those identified as having SC will have to be put on secondary prophylaxis. In the absence of long term follow up it is desirable to evaluate adults 20 to 35 yr in age to find out the prevalence of SC. This should help in defining the course of SC to some extent. The study from Nicaragua of 376 adults identifying 23/1000 with SC is useful but too small for any conclusion34.
Pathogenesis of RF
It is well established that RF causes permanent damage only to the cardiac valves. Clinically the mitral aortic, tricuspid and pulmonary valves are involved in order of frequency. Mitral valve involvement is the commonest and the pulmonary valve involvement is rare. However, pathological evaluation of valves from patients dying of acute RF indicates that microscopic involvement of tricuspid and pulmonary valves occurs in almost 100 per cent cases69. The cardiac valve damage is the basic reason why RF needs to be controlled to reduce the morbidity and mortality related to RF.
Cardiac valves are derived from the ventricular myocardium by a process of undermining. The valves are composed of a central core of connective tissue covered on both sides by endothelium. The central core of connective tissue is derived from the ventricular myocardium - muscle and inter-myocardial connective tissue. Histopathology indicates absence of myocardial and connective tissue damage in carditis due to acute RF. Immuno-histopathology excludes myocardial damage in RF. Hence, the site of damage in the valves derived from the ventricular myocardium has to be the valve endothelium82. Endothelium per se consists of two components – the endothelial cells and the basement membrane to which the cells are attached. By exclusion the findings suggest that the valve damage is related to the valve endothelium- the endothelial cells, the basement membrane and the substance binding these together82.
It is well established that RF follows GAS infection of the tonsillopharynx and does not follow skin infection. Mesothelium and endothelium are derived from mesenchymal cells. Mesothelial cells cover tonsillopharyngeal region whereas ectodermal cells, which are completely different in composition from mesothelial cells, cover skin. Why should RF follow pharyngeal infection but not dermal infection? Is it because the GAS infection affecting the pharyngeal mesothelial cells sensitizes the cells in a way which later manifests as endothelial cell damage of the valve tissue and mesothelial cell damage elsewhere (arthritis, etc.) later on ?
Pathogenesis of RF is not known. Research to elucidate the pathogenesis has been directed almost exclusively toward myocarditis and myosin for the last more than 60 years without any breakthrough83,84. An alternative approach with endothelium as the target of rheumatic damage as well as guidelines for further research have been suggested in the hope that these may help in identifying the GAS antigen (s) responsible for RF82.
Acute RF: duration of disease
A combination of some clinical manifestations and laboratory tests put together by Jones, revised and modified from time to time by the AHA, identify the syndrome of RF. Elevated ESR and CRP are nonspecific and identify the presence of an active inflammatory disease. Elevated anti-streptococcal antibodies indicate streptococcal infection. Thus there is no specific investigation, which is diagnostic for RF. Of the clinical manifestations arthritis, erythema marginatum and carditis suggest acute and active RF. Subcutaneous nodules and chorea are late manifestations and indicate past RF not active RF.
The inference that RF lasts 10 to 12 wk in about 80 per cent patients was dependent on the elevated ESR and CRP. As of today we do not know the duration of active rheumatic “process” per se. Arthritis suggests active RF, however, if the rheumatic process is active why should arthritis subside without treatment? The central nervous system (CNS) damage occurs with acute RF. At the time patients present with chorea the ESR and CRP may be normal indicating absence of an active disease process. Is the rheumatic inflammation active or inactive at the time a patient presents as chorea? Hence high or normal ESR and CRP do not identify active or inactive rheumatic process. Why should chorea occur three to six months after the CNS damage that occurs during acute RF? What is the duration of active rheumatic inflammation in RF? The damage resulting from active rheumatic process has to be separated from the residual effect of the damage caused by the rheumatic disease. The duration of the disease in acute glomerulonephritis, the other non-suppurative manifestation of GAS infection, is less than seven to ten days85. Majority of patients recover within that time. Urinalysis continues to show microscopic haematuria for several months in spite of clinical recovery. Unfortunately there are no investigations, which can identify active rheumatic disease process itself.
Management
There has been no significant change in the management of acute RF in the last 50 years. Patients need penicillin to eradicate GAS present in throat. Anti inflammatory agents - aspirin or steroids - are used to control rheumatic activity. Aspirin or steroids do not cure RF. These suppress the inflammatory response which lasts for about 12 wk in more than 80 per cent patients. Hence, the standard dose of aspirin (90-120 mg/kg/day) is given for ten weeks and tapered in the next two weeks. The dose of prednisone 60 mg/day above 20 kg and 40 mg /day below 20 kg in weight is given for three weeks and tapered in the next nine weeks. The standard 12 week course can be reduced to four to eight weeks depending on the patient's response. Patients without carditis can have weekly follow up of ESR and CRP. If they normalize, the course can be reduced to a shorter period. Aspirin is preferred over steroids as long as the carditis is mild and the patient is not in congestive failure. However, with severe carditis and congestive failure steroid is the drug of choice because of the more potent suppressive effect.
Non-steroidal anti-inflammatory drugs (NSAIDs) have not been systematically utilized to establish their usefulness. Immunosuppressive agents like azathioprine and cyclosporine A have also been considered for acute rheumatic fever. Despite of the concerns of side effects, toxicity and late onset of lymphomas with the use of these immunosuppressive it is possible to argue that a short course of 6 to 8 wk may result in a greater benefit than harm. However, most ethics committees will hesitate to permit systematic testing of these agents.
It is now well accepted that rheumatic endocarditis involving heart valves is the main cause of morbidity and mortality in RF. Surgical management consisting of mitral and /or aortic valve replacement in patients whose congestive failure cannot be controlled by aggressive medical treatment during acute RF, is life saving. It the congestive failure cannot be controlled with maximal medical therapy and the patient is deteriorating due to mitral regurgitation, mitral valve replacement during active RF is indicated. In spite of clinical evidence for active RF, the heart size returns to normal and congestive failure disappears, confirming that rheumatic myocarditis plays little or no role in the mortality of RF66.
Management of chorea: It has a self limiting course, hence parents need reassurance. The children could be treated with sedatives like phenobarbitone 30 mg thrice daily. chlorpromazine, valium, diphendydramine or promethazine can be used as sedatives. Haloperidol 5 to 10 mg twice daily has been used effectively. Although aspirin and steroids are not supposed to have a place in the treatment of chorea, some patients have shown dramatic response to steroids, if they do not show adequate response to sedatives86,87. Since, long term follow up of chorea patients have identified subclinical carditis in 20 to 30 per cent patients, penicillin prophylaxis is essential and should be continued on a long term basis50,51.
Rheumatic heart disease: Surgical management of valve disease was the standard approach till balloon mitral valvotomy was introduced in 1985. Mitral stenosis could be corrected surgically either by closed valvotomy, open commissurotomy or by valve replacement if the valve was calcified. Balloon valvotomy provides results as good as surgical valvotomy and has become the treatment of choice in spite of being more expensive. For mitral regurgitation the choice of treatment would be valve repair especially in younger patients to avoid long-term anti-coagulant therapy. Most patients with mitral or aortic valve regurgitation end up with valve replacement. Hence, although surgical help is very useful it is expensive and requires prolonged care with anticoagulant therapy with the associated complications of valve thrombosis and systemic embolic disasters especially in the low-income population of the country. Over a long follow up period relatively few patients remain free of events.
Balloon mitral valvotomy has been utilized in the paediatric patients below 12 yr in age with acceptable results. It has been extended to patients of mitral stenosis with acute RF, without additional risk and acceptable results. In the presence of acute RF restenosis rate was, however, 40 per cent compared to 10 per cent in those without active RF88,89.
Prevention of RF and RHD
A disease which follows a bacterial infection should, theoretically, be preventable if the organism does not become resistant to antibiotics. GAS have remained sensitive to penicillin and should have been eradicated. Unfortunately despite a decline in prevalence, RF continues to occur in socioeconomically disadvantaged populations and even developed countries have witnessed resurgences in localized areas49,90. Steps in the development of RF consist of GAS pharyngitis, which should be symptomatic enough to require medical attention, throat culture to confirm the diagnosis and ensuring that the course of penicillin treatment has been completed. The last epidemic of RF in Utah area in USA occurred in well to do middle class families, absence of overcrowding and with access to good medical care. The findings of the epidemic indicated that the preceding pharyngitis was asymptomatic in 78 per cent, 18 per cent obtained medical help and the 10 day course of oral penicillin was not completed by patients49,90.
Prevalence of RF/RHD has been attributed to overcrowding and unhygienic living related to low socio-economic status. Unhygienic living results in persistent GAS in the environment. Since GAS spreads by droplet dissemination, overcrowding causes cross infection from person to person. Low socio-economic status may undermine nutrition and seriously limit access to medical treatment. Poor nutritional status is believed to contribute to a decreased immune response. The result is not only endemic RF but also a more severe or virulent disease (Fig. 1).
Fig. 1.
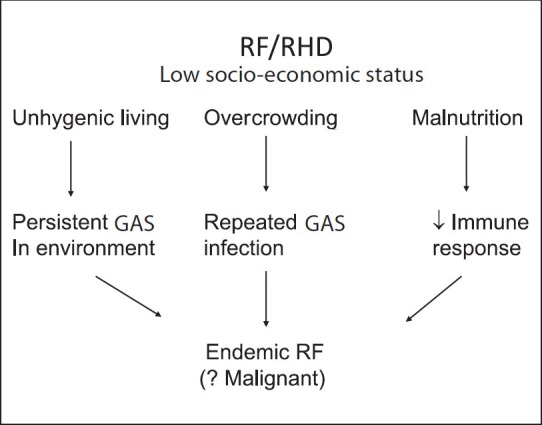
Relationship of low socio-economic status to rheumatic fever (RF).
It is possible at the same time that the initial attack of RF is mild and results in mild carditis, which remains subclinical, undiagnosed, and as such the patient does not get prophylaxis to prevent recurrences. In low socio-economic settings, recurrences causing further cardiac damage result in symptomatic RHD with multivalve involvement and congestive failure identified as the first attack of severe (malignant) RF (Fig. 2) The high prevalence of subclinical carditis found by echocardiographic studies suggests that the initial attack of RF is probably relatively mild and in the absence of secondary prophylaxis it is the recurrences, which are responsible for patients presenting with severe disease being labelled as the first attack of RF.
Fig. 2.
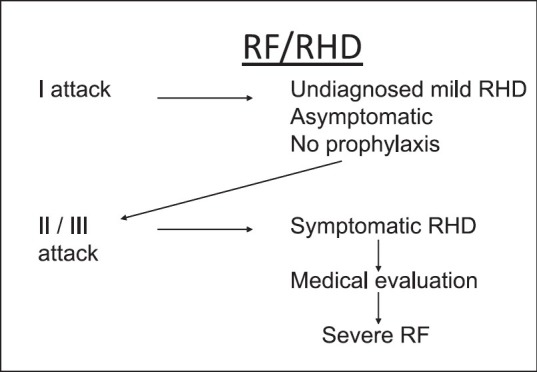
Possible mechanism to explain why RF appears to be severe in the initial attack.
The strategies for prevention consist of primordial prevention, primary prevention and secondary prevention.
Primordial prevention: It requires, preventing the development of ‘risk factors’ in the community to prevent the disease in the population and thus protect individuals. Requirements for primordial prevention in relation with RF and RHD consist of (i) improvement in socio-economic status, (ii) prevention of overcrowding, (iii) improving nutritional status, (iv) availability of prompt medical care, and (v) public education regarding the risk of RF from sore throat specially below the age of 15 yr.
Improvement in nutrition improves immune response and the capacity of individuals to resist and fight infection. Public education is the most important component for primordial prevention. Unless parents know that a sore throat can cause RF and RHD, it is most unlikely to be seen by a physician and treated. Improvement in socio-economic status and preventing overcrowding cannot be relied upon to reduce the burden of RHD.
Primary prevention: Primary prevention is theoretically feasible but practically extremely difficult to achieve. Primary prevention requires identification of (GAS) sore throat and use of penicillin to eradicate the streptococci. The requirement for primary prevention consist of (i) public awareness regarding danger of RF from sore throat (ii) identification of sore throat as being due to GAS infection, and (iii) use of injectable penicillin to cure the infection.
It is important to know that oral penicillin may not be effective in preventing RF. RF occurred in 15 to 48 per cent children given oral penicillin for 10 days in an earlier epidemic in USA90. Compliance of a 10 day oral treatment even in educated families is not certain. It is, therefore, essential that injectable penicillin is used to prevent RF. The recommended dose of penicillin is 400,000 units of procaine penicillin twice daily for ten days. Although recommended, one injection of 1.2 mega units of benzathine penicillin may not be enough to eradicate GAS infection especially in endemic areas91. Since GAS infection spreads through droplets from person to person, eradication of GAS even in one individual will reduce the total burden of the organism in the community. The inability to utilize primary prevention at the community level is due to the large number of sore throats requiring treatment to prevent a single episode of RF.
Community level management requires a sledgehammer approach, that is, each sore throat must be treated. At present, bacteriological facilities required to identify GAS sore throat at the community level for the whole country, would be expensive, do not exist and are not likely in the near future. Hence, each sore throat will need to be treated to reduce the cost. Treating each sore throat is logistically not feasible. Anywhere from 3 to 20 per cent of sore throats can be due to GAS infection, the rest being viral infections, which do not require treatment. About 0.3 per cent of streptococcal sore throats result in RF92. Recent data suggest that almost 90 per cent of those who get RF develop RHD49. Hence if 10,000 sore throats were treated by the sledgehammer approach, anywhere between 300-2000 GAS sore throats would be treated (assuming 3 to 20% are due to GAS). This would result in preventing RF in one to six children (0.3% GAS throats cause RF), and RHD in either five or six children. Therefore, 10,000 sore throats need to be treated to prevent RHD in five to six children. A community level primary prevention by sledge-hammer approach is not feasible.
Another problem in sledgehammer approach is the identification of sore throat and its treatment. The data from resurgence of RF in USA indicate that as much as 78 per cent of GAS sore throats may be asymptomatic, ten day oral penicillin treatment was not followed by well educated families and 48 per cent of those given oral penicillin developed RF49,90. Unless a sore throat is symptomatic, it would not be treated and could still result in RF. Even an individual patient cannot be protected if the preceding sore throat is asymptomatic. This makes primary prevention based on the diagnosis of GAS sore throat and use of oral penicillin inadequate to reduce the burden of RHD in the country. The amount of penicillin required for sledgehammer approach is not available in the country.
As of today there are no markers that can be used to identify susceptibility to RF. Studies on HLA and the B-lymphocyte antigen, D8/17 have not given results which can be used to identify the susceptible people in the population to practice primary prevention92.
Primary prevention is possible if an anti-streptococcal vaccine becomes available.
Secondary prevention: Secondary prevention requires identification of those with RF or RHD and maintenance of a registry. Once identified, the patient needs injections of benzathine penicillin, given once in two to three weeks, depending on age, body size and muscle mass. Benzathine penicillin is painful, may result in fever and very rarely in anaphylactic reactions. Most physicians are very reluctant to give penicillin injections. The necessity of penicillin prophylaxis is due to the fact that RF has a tendency for recurrences in those who have had RF in the past. Each new attack causes further damage to the valve tissue making the disease worse than before43. Secondary prevention can reduce the damage of recurrences but cannot prevent the initial damage. Further, secondary prevention cannot reduce the burden of RHD in the community. Secondary prevention has been found to help in disappearance of clinical findings of RHD. However, disappearance of murmurs does not indicate that the heart disease has disappeared. Recurrence of RF results not only in appearance of murmurs but also the valve damage is worse than before.
Anti-streptococcal vaccine
Availability of a vaccine, which could prevent streptococcal infection, is essential for primary prevention of RF. It is at present not available. GAS infection results in suppurative and non-suppurative manifestations. Suppurative diseases like toxic shock syndrome and necrotizing fasciitis could be lethal. The two non-suppurative manifestations are acute RF and acute glomerulonephritis (GN). Several GAS protein and polysaccharide components have been considered in developing a vaccine. Most work has been in relation with the M- protein, considered to be the virulence factor of the GAS83,84. Other components of GAS being tried for preparing a vaccine are GAS C5a peptidase, a major surface virulence factor; fibronectin binding protein sfb1, and the chimeric peptide J8 from the conserved region of the M- protein93.
M- protein has been found to be strain-specific, that is, each strain has its specific characteristics and protective against only that particular strain. Since more than 250 different strains have been identified, it is essential that the vaccine must be polyvalent, that is, incorporate all the strains present in the community94. GAS has a strong tendency for mutation and the vaccine may not be effective if the infection is due to an organism, which has mutated after being incorporated in the vaccine95. Virulent GAS infection causing death from toxic shock syndrome is now known not to express M-protein96. Hence, M-protein cannot be the chief virulence factor of GAS. Vaccine based on M-protein is unlikely to succeed because:
On the basis of emm typing of M-protein more than 250 strains of GAS can cause infection and provide only strain-specific immunity. Hence, the anti GAS vaccine has to include all the strains in the community94.
Heterogeneous distribution of strains varies from place to place and keeps changing even within a closed community in a short period. Vaccine based on M-protein made in Delhi may not be effective in Chandigarh, Chennai or Mumbai or even in Delhi after a few months97.
GAS mutation alters the emm gene sequence of the M-protein. Mutation can occur in a few weeks. The vaccine may not be effective against infection by a strain that has mutated after being incorporated in the vaccine even in a very short time95.
GAS infection has resulted in lethal toxic shock syndrome without expressing M-protein, electron microscopy failing to identify M-protein fibrils on the surface of the organism and the isolate failing to resist phagocytosis, suggesting the absence of “functional M-protein”96.
M-protein has been excluded as the antigen responsible for acute GN the other non suppurative manifestation of GAS infection85.
The surface M-protein of GAS was designated as the virulence factor of GAS. The similarity in the structure of M-protein and the human tropomyosin has resulted in accepting, without proof, that it is responsible for RF. There is no evidence to indicate that M-protein is the antigen responsible for RF. There is no information regarding the role M-protein plays in the suppurative diseases due to GAS infection.
Toxic shock syndrome occurred in the absence of functional M-protein and the paediatric nephrologists have excluded M-protein as being responsible for acute GN. Therefore, there is evidence that at least two, one suppurative and one non-suppurative of the various GAS related manifestations are unlikely to be prevented by a vaccine based on M-protein The vaccine if and when available is expected to prevent GAS infection. This will prevent GAS infection related diseases - necrotizing fasciitis, toxic shock syndrome, acute glomerulonephritis, rheumatic fever, pyoderma and septic arthritis, etc.
Unfortunately in spite of extensive evaluation and efforts it has not been possible to formulate a M-protein based vaccine in the last 40 years. We need to consider as to why it is necessary to start from RF and its relation with GAS M-protein to make a vaccine. Why are we not looking for the virulent epitope of GAS from infections causing septic shock, pyoderma or septic arthritis for its suitability for anti GAS vaccine, since the interest is in preventing GAS infection? It is well known that GAS infection precedes rheumatic fever but which epitope of GAS results in rheumatic fever is not known. The interest is in preventing GAS infection by a suitable vaccine, which should prevent all GAS infection related manifestations. Prevention of rheumatic fever, pyoderma or glomerulonephritis will be specific benefits of such a vaccine.
The answer to the question “Is it possible to prevent rheumatic fever”? has to be “No”, for primary prevention at the community level. Primary prevention will have to wait till a safe and effective GAS vaccine becomes available.
In our country the health of the child generally remains a priority responsibility of the parents even when the child becomes an adult. Hence, prevention of RF and RHD is possible to a large extent if we can provide the message, in local languages, to the population (parents) that sore throats should not be neglected; that sore throats should be shown to a doctor for treatment to prevent RF and RHD. Radio and television are available for reaching each corner of the country and should be utilized for this purpose. If education can be made compulsory till the age of 15 yr, school health education and school health care facilities can be utilized to control RF.
RF and RHD continue to be an undesirable burden. RF occurring at a young age results in morbidity as well as mortality in adolescents and young adults, and also becomes one of the major causes of loss of the most productive years of life in our country. With the identification of subclinical carditis in normal children, the total burden of RHD is much higher than that estimated in various studies. Although the disease (RF) follows a bacterial (GAS) infection, the pathogenesis has not been worked out in more than 60 years. Duration of the disease, specific medical treatment to control or prevent cardiac damage and primary prevention of RF continue to be elusive. Primary prevention has to depend on designing a vaccine to prevent GAS infection related suppurative as well as non-suppurative disease manifestations.
References
- 1.Kutumbiah P. Rheumatic fever and rheumatic heart disease in India; review of 25 years of study and progress. Indian J Pediatr. 1958;25:240–5. [PubMed] [Google Scholar]
- 2.Moore WJ. A month's practice in a small Indian dispensary. Indian Med Gaz. 1870;5:207–11. [PMC free article] [PubMed] [Google Scholar]
- 3.Rogers L. Gleanings from the Calcutta post mortem records III- Diseases of the circulatory system. Indian Med Gaz. 1910;45:84–90. [PMC free article] [PubMed] [Google Scholar]
- 4.Megaw JWD. Note on the causation of diseases of the heart and aorta in Europeans in India. Indian Med Gaz. 1910;45:81–4. [PMC free article] [PubMed] [Google Scholar]
- 5.Clark JT. The geographical distribution of rheumatic fever. J Trop Med Hyg. 1930;33:249–58. [Google Scholar]
- 6.Keates HC. Rheumatic fever and the tropics. Br Med J. 1932;ii:900. [Google Scholar]
- 7.Drury FJ. Circulatory disease in India. Indian Med Gaz. 1910;45:41–5. [PMC free article] [PubMed] [Google Scholar]
- 8.Basu UP. Preliminary observations on acquired diseases of the heart and aorta as met with in Bengal. Indian Med Gaz. 1925;60:307–10. [PMC free article] [PubMed] [Google Scholar]
- 9.Hughes TA, Yusuf M. Heart disease in the Punjab with special reference to mitral stenosis. Indian Med Gaz. 1930;65:483. [Google Scholar]
- 10.Hodge EHV. Rheumatism in India. Indian Med Gaz. 1932;67:241–4. [PMC free article] [PubMed] [Google Scholar]
- 11.Wig KL. Clinical evidence of rheumatic fever in the Punjab. Indian Med Gaz. 1935;70:260–3. [PMC free article] [PubMed] [Google Scholar]
- 12.Kutumbiah P. A study of the lesions in rheumatic heart disease in South India. Indian J Med Res. 1940;27:631–41. [Google Scholar]
- 13.Kutumbiah P. Rheumatism in childhood and adolescence. Part 1. Indian J Pediatr. 1941;8:65–86. [Google Scholar]
- 14.Banerjea JC. Incidence of rheumatic heart disease in India. Indian Heart J. 1965;17:201–2. [PubMed] [Google Scholar]
- 15.Sanjeevi KS. Heart Disease in south India. Proceedings of Association of Physician India. 1946 Quoted by ref. 17. [Google Scholar]
- 16.Vakil RJ. Heart disease in India. Am Heart J. 1954;48:439–48. doi: 10.1016/0002-8703(54)90031-9. [DOI] [PubMed] [Google Scholar]
- 17.Padmavati S. A five year survey of heart disease in Delhi (1951-1955) Indian Heart J. 1958;10:33–40. [Google Scholar]
- 18.Devichand Etiology and incidence of heart disease in India, with special reference to acquired valvular lesions. Indian Heart J. 1959;11:117–9. [Google Scholar]
- 19.Mathur KS. Problem of heart disease in India. Am J Cardiol. 1960;5:60–5. [Google Scholar]
- 20.Malhotra RP, Gupta SP. Rheumatic heart disease in Punjab with special emphasis on clinical patterns that differ from those reported already. Indian Heart J. 1963;15:107–13. [Google Scholar]
- 21.Padmavati S. Epidemiology of cardiovascular disease in India. I. Rheumatic heart disease. Circulation. 1962;25:703–10. doi: 10.1161/01.cir.25.4.703. [DOI] [PubMed] [Google Scholar]
- 22.Roy SB. The diagnosis of rheumatic fever. J Indian Med Assoc. 1960;35:344–6. [PubMed] [Google Scholar]
- 23.Roy SB. Prevalence of rheumatic fever and rheumatic heart disease in Ballabhgarh. Annual Report, Indian Council of Medical Research. 1968-1969:52. [Google Scholar]
- 24.Mathur KS, Banerji SC, Nigam DK, Prasad R. Rheumatic heart disease and rheumatic fever-prevalence in a village community of Bichpuri Block Agra. J Assoc Physicians India. 1971;19:151–6. [PubMed] [Google Scholar]
- 25.Berry JN. Prevalence survey of chronic rheumatic heart disease and rheumatic fever in Northern India. Br Heart J. 1972;34:134–49. doi: 10.1136/hrt.34.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shah B, Sharma M, Kumar R, Brahmadathan KN, Abraham VJ, Tandon R. Rheumatic heart disease: Progress and challenges in India. Indian J Pediatr. 2013;80(Suppl 1):77–86. doi: 10.1007/s12098-012-0853-2. [DOI] [PubMed] [Google Scholar]
- 27.Misra M, Mittal M, Singh R, Verma A, Rai R, Chandra G, et al. Prevalence of rheumatic heart disease in school-going children of Eastern Uttar Pradesh, Indian. Heart J. 2007;59:42–3. [PubMed] [Google Scholar]
- 28.Grover A, Dhawan A, Iyengar SD, Anand IS, Wahi PL, Ganguly NK. Epidemiology of rheumatic fever and rheumatic heart disease in a rural community in northern India. Bull World Health Organ. 1993;71:59–66. [PMC free article] [PubMed] [Google Scholar]
- 29.Patel DC, Patel NI, Patel JD, Patel SD. Rheumatic fever and rheumatic heart disease in school children of Anand. J Assoc Physicians India. 1986;34:837–9. [PubMed] [Google Scholar]
- 30.Avasthi G, Singh D, Singh C, Aggarwal SP, Bidwai PS, Avasthi R. Prevalence survey of rheumatic fever (RF) and rheumatic heart disease (RHD) in urban & rural school children in Ludhiana. Indian Heart J. 1987;39:26–8. [PubMed] [Google Scholar]
- 31.Lalchandani A, Kumar HR, Alam SM, Dwivedi RN, Krishna G, Srivastava JP, et al. Prevalence of rheumatic fever and rheumatic heart disease in rural and urban school children of district Kanpur (UP) Indian Heart J. 2000;52:672. [Google Scholar]
- 32.Jose VJ, Gomathi M. Declining prevalence of rheumatic heart disease in rural school children in India: 2001-2002. Indian Heart J. 2003;55:158–60. [PubMed] [Google Scholar]
- 33.Periwal KL, Gupta BK, Panwar RB, Khatri PC, Raja S, Gupta R. Prevalence of rheumatic heart disease in school children in Bikaner: an echocardiographic study. J Assoc Physicians India. 2006;54:279–82. [PubMed] [Google Scholar]
- 34.Paar JA, Berrios NM, Rose JD, Cáceres M, Peña R, Pérez W, et al. Prevalence of rheumatic heart disease in children and young adults in Nicaragua. Am J Cardiol. 2010;105:1809–14. doi: 10.1016/j.amjcard.2010.01.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carapetis JR, Hardy M, Fakakovikaetau T, Taib R, Wilkinson L, Penny DJ, et al. Evaluation of a screening protocol using auscultation and portable echocardiography to detect asymptomatic rheumatic heart disease in Tongan school children. Nat Clin Pract Cardiovascu Med. 2008;5:411–7. doi: 10.1038/ncpcardio1185. [DOI] [PubMed] [Google Scholar]
- 36.Marijon E, Ou P, Celermajer DS, Ferreira B, Mocumbi AO, Jani D, et al. Prevalence of rheumatic heart disease detected by echocardiographic screening. N Eng J Med. 2007;357:470–6. doi: 10.1056/NEJMoa065085. [DOI] [PubMed] [Google Scholar]
- 37.Saxena A, Ramakrishnan S, Roy A, Sethi S, Krishnan A, Misra P, et al. Prevalence and outcome of subclinical rheumatic heart disease in India. The RHEUMATIC (Rheumatic Heart Echo Utilisation and Monitoring Actuarial Tends in Indian Children) Study. Heart. 2011;97:2018–22. doi: 10.1136/heartjnl-2011-300792. [DOI] [PubMed] [Google Scholar]
- 38.Kumar RK, Paul M, Francis FT. RHD in India: are we ready to shift from secondary prophylaxis to vaccinating high-risk children? Curr Sci. 2009;97:397–404. [Google Scholar]
- 39.Carapetis JR, Steer AC, Mulholland EK, Weber M. The global burden of group A streptococcal disease. Lancet Infect Dis. 2005;5:685–94. doi: 10.1016/S1473-3099(05)70267-X. [DOI] [PubMed] [Google Scholar]
- 40.Carapetis JR. Rheumatic heart disease in Asia. Circulation. 2008;118:2748–53. doi: 10.1161/CIRCULATIONAHA.108.774307. [DOI] [PubMed] [Google Scholar]
- 41.Kumar R, Raizada A, Aggarwal AK, Ganguly NK. A community-based rheumatic fever rheumatic heart disease cohort: twelve-year experience. Indian Heart J. 2002;54:54–8. [PubMed] [Google Scholar]
- 42.Tibazarwa KB, Volmink JA, Mayosi BM. Incidence of acute rheumatic fever in the world: a systematic review of population-based studies. Heart. 2008;94:1534–40. doi: 10.1136/hrt.2007.141309. [DOI] [PubMed] [Google Scholar]
- 43.Feinstein AR, Spagnuolo M. Mimetic features of rheumatic-fever recurrences. N Engl J Med. 1960;262:533–40. doi: 10.1056/NEJM196003172621101. [DOI] [PubMed] [Google Scholar]
- 44.Guidelines for the diagnosis of rheumatic fever. Jone criteria. 1992 update. Special Writing Group of the Committee on Rheumatic fever, Endocarditis and Kawasaki disease of the Council on Cardiovascular Disease in the young of the American Heart Association. JAMA. 1992;268:2069–73. [No authors listed] [PubMed] [Google Scholar]
- 45.Padmawati S, Arora R. Profile of rheumatic fever and rheumatic heart disease in India. In: Ahuja MMS, editor. Progress in clinical medicine in India. 2nd series. New Delhi: Arnold Heinemann; 1978. p. 219. [Google Scholar]
- 46.Sanyal SK, Thapar MK, Ahmed SH, Hooja V, Tewari P. The initial attack of acute rheumatic fever during childhood in North India; a prospective study of the clinical profile. Circulation. 1974;49:7–12. doi: 10.1161/01.cir.49.1.7. [DOI] [PubMed] [Google Scholar]
- 47.Agarwal BL, Agrawal R. Rheumatic fever. Clinical profile of the initial attack in India. Bull World Health Organ. 1986;64:573–8. [PMC free article] [PubMed] [Google Scholar]
- 48.Vaishnava S, Webb JK, Cherian J. Juvenile rheumatism in South India. A clinical study of 166 cases. Indian J Child Health. 1960;9:290–9. [Google Scholar]
- 49.Veasy LG. Lessons learned from the resurgence of rheumatic fever in the United States. In: Narula J, Virmani R, Reddy KS, Tandon R, editors. Rheumatic fever. Washington DC: American Registry of Pathology. Armed Forces Institute of Pathology; 1999. pp. 69–78. [Google Scholar]
- 50.Bland EF. Chorea as a manifestation of rheumatic fever: a long-term perspective. Trans Am Clin Climatol Assoc. 1961;73:209–13. [PMC free article] [PubMed] [Google Scholar]
- 51.Aron AM, Freeman JM, Carter S. The natural history of sydenham's chorea. Review of the literature and long-term evaluation with emphasis on cardiac sequelae. Am J Med. 1965;38:83–95. doi: 10.1016/0002-9343(65)90162-2. [DOI] [PubMed] [Google Scholar]
- 52.Roy SB, Bhatia ML, Lazaro EJ, Ramalingaswami V. Juvenile mitral stenosis in India. Lancet. 1963;ii:1193–95. doi: 10.1016/s0140-6736(63)92922-2. [DOI] [PubMed] [Google Scholar]
- 53.Tandon R, Potti S, Mathur VS, Roy SB. Critical rheumatic mitral stenosis in children. Indian Pediatr. 1972;9:171–3. [PubMed] [Google Scholar]
- 54.Shrivastava S, Tandon R. Severity of rheumatic mitral stenosis in children. Int J Cardiol. 1991;30:163–7. doi: 10.1016/0167-5273(91)90091-3. [DOI] [PubMed] [Google Scholar]
- 55.Strollerman GH. Clinical microbiology of group A streptococci. In Narule. :89. [Google Scholar]
- 56.Kawakita S, Takeuchi T, Inoue J, Onishi T, Uemura Y. Infection of group A streptococcus and antibody response to extracellular antigens. Jpn Circ J. 1981;45:1384–90. doi: 10.1253/jcj.45.1384. [DOI] [PubMed] [Google Scholar]
- 57.Massell BE, Mote JR, Jones TD. The artificial induction of subcutaneous nodules in patients with rheumatic fever. J Clin Invest. 1937;16:125–8. doi: 10.1172/JCI100830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tandon R. Induced subcutaneous nodules in the diagnosis of acute rheumatic fever. Indian Heart J. 2005;57:767–8. [PubMed] [Google Scholar]
- 59.Narula J, Chopra P, Talwar KK, Reddy KS, Vasan RS, Tandon R, et al. Does endomyocardial biopsy aid in the diagnosis of active rheumatic carditis? Circulation. 1993;88:2198–205. doi: 10.1161/01.cir.88.5.2198. [DOI] [PubMed] [Google Scholar]
- 60.Oran B, Coban H, Karaaslan S, Atabek E, Gurbilek M, Erkul I. Serum cardiac troponin-I in active rheumatic carditis. Indian J Pediatr. 2001;68:943–4. doi: 10.1007/BF02722592. [DOI] [PubMed] [Google Scholar]
- 61.Gupta M, Lent RW, Kaplan EL, Zabriskie JB. Serum cardiac troponin-I in acute rheumatic fever. Am J Cardiol. 2002;89:779–82. doi: 10.1016/s0002-9149(01)02358-x. [DOI] [PubMed] [Google Scholar]
- 62.Kamblock J, Payot L, Iung B, Costes P, Gillet T, Le Goanvic C, et al. Does rheumatic myocarditis really exist? Systematic study with echocardiography and cardiac troponin-I blood levels. Eur Heart J. 2003;24:855–62. doi: 10.1016/s0195-668x(02)00825-4. [DOI] [PubMed] [Google Scholar]
- 63.Vasan RS, Shrivastava S, Vijayakumar M, Narang R, Lister BC, Narula J. Echocardiographic, evaluation of patients with acute rheumatic fever and rheumatic carditis. Circulation. 1996;94:73–82. doi: 10.1161/01.cir.94.1.73. [DOI] [PubMed] [Google Scholar]
- 64.Radhakrishnan S, Reddy KS, Malhotra A, Bhatia ML. New Delhi: Thesis submitted to All India Institute of Medical Sciences; 1986. Myocardial imaging with technitium 99m stannous pyrophosphate in acute rheumatic fever. [Google Scholar]
- 65.Narula J, Reddy KS, Khaw BA. Can indium III antimyosin scintigraphy complement Jones’ criteria for the diagnosis of active rheumatic carditis? In: Khaw BA, Narula J, Strauss HW, editors. Monoclonal antibodies in cardiovascular diseases. Philadelphia: Lea and Febiger; 1994. pp. 109–17. [Google Scholar]
- 66.Kinsley RH, Girdwood RW, Milner S. Surgical treatment during the acute phase of rheumatic carditis. In: Nyhus LM, editor. Surgery annual. Vol. 13. New York: Appleton-Century-Crofts; 1981. pp. 299–323. [PubMed] [Google Scholar]
- 67.Virmani R, Farb A, Burke AP, Narula J. Pathology of acute rheumatic carditis. In: Narula J, Virmani R, Reddy KS, Tandon R, editors. Rheumatic fever. Washington DC: American Registrty of Pathology, Armed Forces Institute of Pathology; 1999. pp. 217–34. [Google Scholar]
- 68.Gulizia JM, McManus BM. Immunopathologic studies of Rheumatic fever. In: Narula J, Virmani R, Reddy KS, Tandon R, editors. Rheumatic fever. Washington DC: American Registry Pathology, Armed Forces Institute of Pathology; 1999. pp. 235–44. [Google Scholar]
- 69.Gross L, Friedberg CK. Lesions of the cardiac valves in rheumatic fever. Am J Pathol. 1936;12:855–910. [PMC free article] [PubMed] [Google Scholar]
- 70.Markowitz M, Gordis L. Philadelphia: W.B. Saunders; 1972. Rheumatic fever; pp. 62–70. [Google Scholar]
- 71.Stollerman GH. New York: Grune and Stratton; 1975. Rheumatic fever and streptococcal infections; pp. 152–64. [Google Scholar]
- 72.Kinare SG. Chronic rheumatic valvular heart disease. Ann Indian Acad Med Sci. 1972;8:47–51. [Google Scholar]
- 73.Lanna CC, Tonelli E, Barros MV, Goulart EM, Mota CC. Subclinical rheumatic valvitis: a long-term follow-up. Cardiol Young. 2003;13:431–8. [PubMed] [Google Scholar]
- 74.Hilário MO, Andrade JL, Gasparian AB, Carvalho AC, Andrade CT, Len CA. The value of echocardiography in the diagnosis and followup of rheumatic carditis in children and adolescents: a 2 year prospective study. J Rheumatol. 2000;27:1082–6. [PubMed] [Google Scholar]
- 75.Figueroa FE, Fernández MS, Valdés P, Wilson C, Lanas F, Carrión F, et al. Prospective comparison of clinical and echocardiographic diagnosis of rheumatic carditis: long term follow up of patients with subclinical disease. Heart. 2001;85:407–10. doi: 10.1136/heart.85.4.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Araújo FD, Goulart EM, Meira ZM. Prognostic value of clinical and Doppler echocardiographic findings in children and adolescents with significant rheumatic valvular disease. Ann Pediatr Cardiol. 2012;5:120–6. doi: 10.4103/0974-2069.99610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tubridy-Clark M, Carapetis JR. Subclinical carditis in rheumatic fever: a systematic review. Int J Cardiol. 2007;119:54–8. doi: 10.1016/j.ijcard.2006.07.046. [DOI] [PubMed] [Google Scholar]
- 78.Bland EF, Jones TD. Rheumatic fever and rheumatic heart disease: a twenty year report on 1000 patients followed since childhood. Circulation. 1951;4:836–43. doi: 10.1161/01.cir.4.6.836. [DOI] [PubMed] [Google Scholar]
- 79.Geneva 29 Oct-1 Nov 2001. Geneva, Switzerland: WHO; 2004. WHO. Rheumatic fever and rheumatic heart disease: report of a WHO expert consultation. [Google Scholar]
- 80.Reményi BO, Wilson N, Steer A, Ferreira B, Kado J, Kumar K, et al. World Heart Federation criteria for echocardiographic diagnosis of rheumatic heart disease-an evidence-based guideline. Nat Rev Cardiol. 2012;9:297–309. doi: 10.1038/nrcardio.2012.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Marijon E, Celermajer DS, Tafflet M, El-Haou S, Jani DN, Ferreira B, et al. Rheumatic heart disease screening by echocardiography: the inadequacy of World Health Organization criteria for optimizing the diagnosis of subclinical disease. Circulation. 2009;120:663–8. doi: 10.1161/CIRCULATIONAHA.109.849190. [DOI] [PubMed] [Google Scholar]
- 82.Tandon R. Rheumatic fever pathogenesis: Approach in research needs change. Ann Pediatr Cardiol. 2012;5:169–78. doi: 10.4103/0974-2069.99621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Krisher K, Cunningham MW. Myosin, a link between streptococci and heart. Science. 1985;227:413–5. doi: 10.1126/science.2578225. [DOI] [PubMed] [Google Scholar]
- 84.Ellis NM, Kurahara DK, Vohra H, Mascaro-Blanco A, Erdem G, Adderson EE, et al. Priming the immune system for heart disease: a perspective on group A streptococci. J Infect Dis. 2010;202:1059–67. doi: 10.1086/656214. [DOI] [PubMed] [Google Scholar]
- 85.Batsford SR, Mezzano S, Mihatsch M, Schiltz E, Rodriguez-Iturbe B. Is the nephritogenic antigen in post streptococcal glomerulonephritis pyrogenic exotoxin B (SPE-B) or GAPDH? Kidney Int. 2005;68:1120–9. doi: 10.1111/j.1523-1755.2005.00504.x. [DOI] [PubMed] [Google Scholar]
- 86.Walker KG, Wilmshurst JM. An update on the treatment of Sydenham's chorea: the evidence for established and evolving interventions. Ther Adv Neurol Discord. 2010;3:301–9. doi: 10.1177/1756285610382063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kalra U. Sydenham's chorea (Rheumatic chorea) In: Ghai OP, Paul VK, Bagga A, editors. Ghai Essential Pediatrics. 7th ed. New Delhi: CBS Publication; 2009. pp. 556–7. [Google Scholar]
- 88.Kothari SS, Ramakrishnan S, Kumar CK, Juneja R, Yadav R. Intermediate-term results of percutaneous transvenous mitral commissurotomy in children less than 12 years of age. Catheter Cardiovasc Interv. 2005;64:487–90. doi: 10.1002/ccd.20317. [DOI] [PubMed] [Google Scholar]
- 89.Kothari SS, Ramakrishnan S, Juneja R, Yadav R. Percutaneous transvenous mitral commissurotomy in patients with severe mitral stenosis and acute rheumatic fever. Pediatr Cardiol. 2006;27:347–50. doi: 10.1007/s00246-005-1255-2. [DOI] [PubMed] [Google Scholar]
- 90.Veasy LG, Wiedmeier SE, Orsmond GS, Ruttenberg HD, Boucek MM, Roth SJ, et al. Resurgence of acute rheumatic fever in the intermountain area of the United States. N Engl J Med. 1987;316:421–7. doi: 10.1056/NEJM198702193160801. [DOI] [PubMed] [Google Scholar]
- 91.Dajani A, Taubert K, Ferrieri P, Peter U, Shulman S. Treatment of acute streptococcal pharyngitis and prevention of rheumative fever: a statement for health professionals. Committee on Rheumatic fever, Endocarditis and Kawasaki Disease of the Council on Cardiovascular disease in the young, the American Heart Association. Pediatrics. 1995;96:758–64. [PubMed] [Google Scholar]
- 92.Krishna Kumar R, Rammohan R, Narula J, Kaplan EL. Epidemiology of streptocoecal pharyngitis, rheumatic fever and rheumatic heart disease. In: Narula J, Virmani R, Reddy KS, Tandon R, editors. Rheumatic fever. Washington DC: American Registry of Pathology. Armed Forces Institute of Pathology; 1999. pp. 41–68. [Google Scholar]
- 93.McMillan DJ, Davies MR, Browning CL, Good MF, Sriprakash KS. Prospecting for new group A streptococcal vaccine candidates. Indian J Med Res. 2004;119(Suppl):121–5. [PubMed] [Google Scholar]
- 94.Smeesters PR, McMillan DJ, Sriprakash KS. The streptococcal M Protein: a highly versatile molecule. Trends Microbiol. 2010;18:275–82. doi: 10.1016/j.tim.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 95.Kaplan EL, Wotton JT, Johnson DR. Dynamic epidemiology of group A streptococcus serotypes associated with pharyngitis. Lancet. 2001;358:1334–7. doi: 10.1016/S0140-6736(01)06415-7. [DOI] [PubMed] [Google Scholar]
- 96.Bennett-Wood V, Selvaraj G, Goodfellow A, Martin-D, Sriprakash KS, Robins-Browne R, et al. Presented at the XV Lancefield International Symposium on Streptococci and Streptococcal Diseases. Goa, India: 2002. Oct, Highly virulent group A streptococci that appear not to express M protein. Abstract 05.6. [Google Scholar]
- 97.Sharma M, Shah B, Dhaliwal RS, Kumar R, Brahmadathan KN, Vohra H, et al. Heterogeneity of community based pediatric GAS isolates from India: Challenges to the multivalent vaccine approach. In: Sriprakash KS, editor. International Congress Series 1289. “Streptococci: New insights into an old enemy”. Netherlands: Elsevier Publications; 2006. pp. 49–53. [Google Scholar]


