Abstract
Vitamin D is mainly derived from endogenous ultraviolet-B induced vitamin D synthesis in the skin, and the current high prevalence of vitamin D deficiency can, therefore, largely be attributed to lifestyle related low sunlight exposure. Regulation of bone and mineral metabolism is a classic vitamin D effect, but the identification of the vitamin D receptor (VDR) in almost all human cells suggests a role for vitamin D also in extra-skeletal diseases. Experimental studies demonstrated several antihypertensive and vascular protective effects of vitamin D, such as suppression of the renin angiotensin aldosterone system, beneficial modulation of classic cardiovascular risk factors, and anti-atherosclerotic properties including improvements of endothelial function. Additional neuroprotective actions of vitamin D have also been reported. In line with this, epidemiological studies have largely shown that vitamin D deficiency is an independent risk factor for arterial hypertension and strokes. Data from randomized controlled trials (RCTs) are, however, limited and less promising, with currently no confirmation that vitamin D reduces stroke incidence. Whereas some RCTs suggest that vitamin D supplementation might modestly reduce blood pressure, this has not been consistently observed in all studies. It is, therefore, premature to recommend vitamin D supplementation for the prevention and treatment of arterial hypertension and stroke. Nevertheless, the fact that patients with arterial hypertension and cerebrovascular disease are at a relatively high risk of vitamin D deficiency, and therewith associated musculoskeletal diseases can serve as a rationale for the evaluation, prevention and treatment of vitamin D deficiency in these patients.
Keywords: Arterial hypertension, blood pressure, cerebrovascular, epidemiology, stroke, vitamin D
Introduction
Vitamin D is currently of great public health interest, because vitamin D deficiency is common and is causally associated with musculoskeletal diseases1,2,3. Vitamin D supplementation is therefore, in many countries, recommended to prevent rickets in children and is also a standard treatment for patients with osteoporosis because vitamin D supplementation reduces fractures and falls3,4,5,6. These skeletal effects of vitamin D are closely related to the role of vitamin D in regulating bone and mineral metabolism, by ensuring physiologic calcium absorption in the gut and thus providing sufficient calcium for bone mineralization7,8. In addition, there has been an increasing interest over the last few years in the relationship of vitamin D with extra-skeletal diseases1,2,3,8. This increased interest was caused by the identification of vitamin D receptors (VDRs) in almost all human cells8. Several epidemiological studies have shown associations of vitamin D deficiency with a variety of chronic extra-skeletal diseases, including cardiovascular and renal diseases, cancer, autoimmune, neurological and infectious diseases1,8,9,10,11,12,13,14,15. It remains, however, unclear whether vitamin D deficiency is a consequence or may be the cause of these diseases. To address this issue with regard to vitamin D and its link to arterial hypertension and cerebrovascular disease, we aim to summarize the current knowledge on this topic in this review in which particular attention has been paid to the most recent developments. This review provides an overview of vitamin D physiology and vitamin D status classification, and summarizes data on the relationship of vitamin D with arterial hypertension and, in brief, also with other cardiovascular risk factors.
Vitamin D physiology
When vitamin D was discovered in 1922 by McCollum, it was termed “D” because it was the fourth known vitamin16. It is, however, nowadays clear that vitamin D and its metabolites should be rather classified as (pro-) hormones than as vitamins1,8. Vitamin D has a unique metabolism and its main source is endogenous vitamin D synthesis in the skin1,8,17. This non-enzymatic process requires the ultraviolet-B spectrum of sunlight, to induce the conversion of the precursor 7-dehydrocholesterol to vitamin D1,8. Approximately 80 to 90 per cent of vitamin D is derived by this sunlight induced vitamin D production in the skin, whereas nutrition usually contains only minor amounts of vitamin D1,8,18. Vitamin D rich foods are fatty fish or mushrooms, but some countries such as the US or Finland already perform food fortification with vitamin D, e.g. in milk or orange juice. To exert its biological effects, vitamin D has to be converted to its most active form, 1,25-dihydroxyvitamin D[1.25(OH)2D = calcitriol]8. This involves two hydroxylation steps with the first one taking place in the liver, where vitamin D is converted by the enzyme 25-hydroxylase to 25-hydroxyvitamin D [25(OH)D]. Then, 25(OH)D is transported in the bloodstream to the kidneys by its carrier vitamin D binding protein (DBP). Proximal tubule cells of the kidneys express the enzyme 1-alpha-hydroxylase that catalyzes the conversion of 25(OH)D to 1,25(OH)2D. Serum concentrations of 1,25(OH)2D are thus mainly determined by renal production of 1,25(OH)2D. This process is tightly controlled by parameters of mineral metabolism, which try to maintain calcium and phosphorus levels within physiological ranges1,8. In detail, parathyroid hormone (PTH) that is secreted by the parathyroid glands in response to low serum calcium levels, increases 1-alpha hydroxylation, whereas fibroblast growth factor-23 (FGF-23) or high phosphate levels decrease 1-alpha hydroxylation in the kidneys. For a long time it was believed that only the kidneys are able to synthesize significant amounts of 1,25(OH)2D8,19. However, over the last few decades it has been discovered that many extra-renal tissues are also able to synthesize 1,25(OH)2D on a local and intracellular level19. Regulation of extra-renal 1-alpha-hydroxylase is different compared to the kidneys, and is likewise significantly dependent on the availability of 25(OH)D19. Based on this, it can be assumed that local tissue levels of 1,25(OH)2D are determined by concentrations of circulating 25(OH)D levels19. This is one of the reasons why serum levels of 25(OH)D and not of 1,25(OH)2D are measured to assess and classify vitamin D status. In this context, it should also be underlined that serum concentrations of 25(OH)D are up to 1000 fold higher compared to 1,25(OH)2D. Affinity to the VDR is, however, much higher for 1,25(OH)2D, which is thus often called the active vitamin D. On a molecular level, 1,25(OH)2D binds to the almost ubiquitously expressed intracellular VDR, and after forming a heterodimer with the retinoid X receptor (RXR) this complex translocates to the nucleus8. There, the VDR/RXR interacts with several co-regulatory proteins and binds to specific regions on the DNA, the so called vitamin D responsive elements. This is followed by either up- or downregulation of gene expression, and in this way VDR activation leads to the regulation of approximately three per cent of the human genome, including a variety of genes that are also involved in blood pressure regulation, cerebrovascular and neurological functions8,20,21. Degradation of vitamin D metabolites is initiated by 24-hydroxlation and the resulting vitamin D metabolites [e.g. 1,24,25(OH)3D or 24,25(OH)2D] are subsequently converted to calcitroic acid, which is excreted via the urine8.
Vitamin D status classification
A general consensus exists that serum concentrations of 25(OH)D should be measured to assess vitamin D status, because levels of 25(OH)D are the best indicator of whole body vitamin D status1,2,3. Measurements of 1,25(OH)2D are not recommended to assess vitamin D status, but may be of value in particular situations such as inherited disorders of vitamin D metabolism, or clarification of hypercalcaemia with e.g. increased 1,25(OH)2D levels in sarcoidosis. Regarding cut-off levels for vitamin D deficiency and vitamin D intoxication, there is a debate ongoing2,3. Classically, the definition of vitamin D deficiency was based on the fact that below certain 25(OH)D levels there are adverse effects with regard to bone and mineral metabolism. These can be characterized by signs of rickets or osteomalacia and by an increase in PTH, as a consequence of low calcium levels due to reduced calcium absorption in the gut. It has been extensively discussed whether the lower limit of a normal vitamin D level is either 20 ng/ml (50 nmol/l) or 30 ng/ml (75 nmol/l) and there is still no final consensus on this controversy2,3. On the other hand, it is obvious that according to all published guidelines a 25(OH)D serum concentration below 20 ng/ml (50 nmol/l) should indeed be avoided by preventive or therapeutic strategies. Optimal 25(OH)D levels are also subject to ongoing discussions, but based on observational data on mortality and other multiple health outcomes, it can be concluded that optimal 25(OH)D levels range from approximately 30 to 40 ng/ml (75 to 100 nmol/l)22,23. Finally, also the cut-offs for the upper limit of normal and for vitamin D intoxication are not consistently reported in the literature. Sufficient epidemiological data on significant long-term health outcomes exist only for levels until approximately 50 ng/ml (125 nmol/l) and these levels can thus be considered as safe2,22. We also know that hypercalcaemia, which is the classic sign of vitamin D intoxication, does usually not occur below 25(OH)D levels of 100 ng/ml (250 nmol/l), except in specific circumstances such as granulomatous diseases (e.g. sarcoidosis or tuberculosis)1,24,25. Hence, there is a range in 25(OH)D concentrations from 50 to 100 ng/ml (125 to 250 nmol/l) for which we know that there is no risk for acute vitamin D intoxication, but we do not have sufficient long-term outcome data from epidemiological studies on these levels. On the other hand, several individuals and population groups have, presumably due to sunlight induced vitamin D synthesis in the skin, reported 25(OH)D levels >50 ng/ml (125 nmol/l)26. This knowledge gap for levels between 50 to 100 ng/ml (125 to 250 nmol/l) is, therefore, a dilemma and needs to be addressed in future studies. One practical solution would be not to put a stamp of vitamin D intoxication on individuals who reach 25(OH)D levels up to 100 ng/ml (250 nmol/l) solely with endogenous vitamin D synthesis and natural foods, but to reduce the vitamin D dose in patients on vitamin D supplements and with 25(OH)D levels exceeding 50 ng/ml (125 nmol/l).
The discussion on vitamin D status classification must however, also take into account that laboratory measurements of 25(OH)D are challenging, and that comparability of 25(OH)D levels between different laboratories and different assay methods is poor27,28,29,30. In 2004, Binkley et al30 showed that when samples were sent to laboratory A, 90 per cent were below an arbitrary threshold of 32 ng/ml (80 nmol/l), while only 17 per cent were below this cut-off in laboratory B. Laboratory methods have surely improved over the last few years, but some laboratory challenges remain such as the required extraction of 25(OH)D from its carrier DBP before measurement. In addition, it is doubtful whether all analytical procedures work properly by the high-throughput automated 25(OH)D assays. Standardization of 25(OH)D measurements is, therefore, an important aim for the future and has already led to the initiation of the Vitamin D Standardization Program (VDSP) by the NIH Office of Dietary Supplements (ODS), in collaboration with the CDC National Center for Environmental Health (NCEH), the National Institute of Standards and Technology (NIST) and the Ghent University29. Such approaches may, however, also cause new challenges because past results of vitamin D research might appear in a new light after standardization. Hence, we might have to reconsider the cut-offs for vitamin D status classification in the future, when taking into account that our current knowledge is mainly based on non-standardized assay methods, which might show a significant systematic bias in comparison to the gold standard mass spectrometry method.
Vitamin D and arterial hypertension
Possible mechanisms
Several mechanisms have been proposed on how vitamin D could be involved in blood pressure regulation and the pathophysiology of arterial hypertension, which is a major risk factor for stroke (Fig.). Vitamin D effects on the renin angiotensin aldosterone system (RAAS) have been extensively investigated by experimental studies31. VDR knockout mice exhibit an increased renin expression, arterial hypertension and myocardial hypertrophy8,31. Subsequent studies confirmed the molecular mechanisms by which VDR activation down-regulates renin expression, but it is not clear whether these significant effects observed in vitro are also of relevance in vivo, i.e. in a clinical setting8,31. High PTH levels, which are a hallmark of vitamin D deficiency, may also increase blood pressure32,33. PTH-receptors are expressed throughout the cardiovascular system and PTH infusions in healthy volunteers increase blood pressure32,33. Epidemiological studies have largely, but not consistently confirmed a positive correlation of PTH levels and blood pressure21,32. Apart from this, PTH is considered an independent risk factor for cardiovascular events, as well as for mortality8,34,35. Particular attention is paid on this topic in nephrology, because vitamin D is routinely used to treat secondary hyperparathyroidism in patients with chronic kidney diseases12,36. Several studies indicated that a complex interplay of vitamin D metabolism and vitamin D effects exists with kidney function12,36. Experimental data suggest that VDR activation may exert anti-proteinuric effects e.g. by protection against podocyte damage and induction of megalin expression, which is required for tubular reabsoprtion of albumin as well as DBP bound 25(OH)D12,36. Various other nephroprotective actions of vitamin D have also been described, as has been excellently reviewed elsewhere12,36. These partially involve anti-inflammatory actions of vitamin D by suppression of nuclear factor-κB (NF- κB)8,12,36. On the other hand, there are also data supporting the hypothesis that inflammation itself may adversely affect vitamin D metabolism. This is in line with a study showing an acute and significant decrease of 25(OH)D levels after knee surgery, which can be regarded as an inflammatory insult37. In addition, evidence exists suggesting that disturbed calcium homeostasis in the setting of vitamin D deficiency, may independently of PTH contribute to blood pressure regulation38. Calcium is involved in the regulation of peripheral vascular resistance by modulating contractility of vascular smooth muscle cells38. Moreover, it has also been shown that ionized extracellular calcium inhibits renin secretion in the juxtaglomerular cells of the kidneys38. With regard to hypertension and calcium it should also be acknowledged that a high sodium intake increases urinary calcium loss, and may in parallel also impact on vitamin D metabolism21,39. In this context, some experimental data suggest that a high salt diet may have adverse effects on vitamin D status and its metabolism by increased urinary loss of vitamin D metabolites21,39. Other mechanisms linking vitamin D and blood pressure may be related to direct vitamin D effects on the vasculature40,41. In experimental studies, VDR activation has been shown to exert a variety of anti-atherosclerotic effects40,41. These involve, amongst others, vitamin D induced decrease of endothelial adhesion molecules, increase of nitric oxide (NO) production and inhibition of macrophage to foam cell formation40,41,42,43. Data from clinical studies addressing these issues are, however, less clear, but there are at least some studies suggesting a relationship of vitamin D deficiency and endothelial dysfunction40,44,45. Taken together, several plausible pathophysiological mechanisms exist that may account for the link between vitamin D deficiency and arterial hypertension.
Fig.
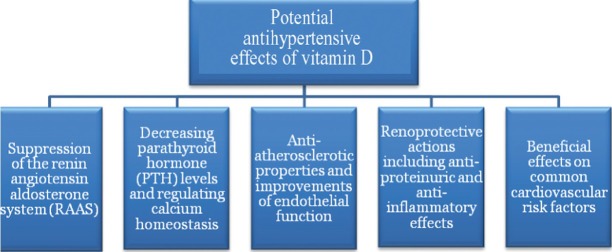
Potential antihypertensive effects of vitamin D.
Observational studies
Considering that sunlight exposure of the skin is required for endogenous vitamin D synthesis, the initial speculations on a possible relationship of vitamin D and arterial hypertension were based on the observation that the prevalence of arterial hypertension is low in sunny regions, but decreases with increasing distance from the equator46. In line with this, the majority of cross-sectional studies have reported an inverse association between 25(OH)D levels and both blood pressure levels, as well as the prevalence of arterial hypertension21,47,48. Prospective studies addressing this issue are less clear. Some studies report that low 25(OH)D levels predict increase of blood pressure or hypertension incidence, while others do not report on such significant associations21,47,48. Considering all data published until November 2010, Burgaz et al49 reported in their meta-analysis that 25(OH)D is inversely associated with hypertension. In summary, most but by far not all observational studies, support the notion that low 25(OH)D levels are associated with higher blood pressure or arterial hypertension.
Interventional studies
Randomized controlled trials (RCTs) that specifically address possible antihypertensive effects of vitamin D treatment are sparse. Krause et al50 evaluated in 18 hypertensive patients the effect of thrice weekly full body ultra violet B (UVB) radiation, which is known to induce vitamin D synthesis in the skin, compared to UVA radiation, which has usually no significant impact on vitamin D production. After 6 weeks, there was no significant change in blood pressure in the UVA group, but both systolic and diastolic blood pressure decreased significantly by 6 mm Hg in the UVB group. These results could, however, not be confirmed by Scragg et al51, who performed a RCT comparing UVB vs. UVA radiation in 119 vitamin D deficient men and women. After twelve weeks there was no significant blood pressure reduction in the UVB compared to the UVA group with a difference of -2.2 (95% CI: -7.8 to 3.3; P=0.42) mm Hg for systolic and -2.7 (-6.5 to 1.0; P=0.12) mm Hg for diastolic blood pressure. Considering that patients treated for hypertension were excluded from the study by Scragg et al51, the authors hypothesized that one possible explanation for these inconsistent results could be that vitamin D treatment might only be beneficial in hypertensive patients.
RCTs on vitamin D supplementation and blood pressure as a primary outcome have also produced mixed results, with some studies showing no significant result whereas others reporting antihypertensive vitamin D effects21,47,49,50,51,52,53,54,55,56,57. A few meta-analyses have already addressed this topic and have also either shown no or a marginally significant result supporting the notion that vitamin D lowers systolic blood pressure49,50,51,52. A recent study has, however, shown some interesting results that may help to further clarify this issue. This study was performed in 130 hypertensive patients in Denmark, who were randomly allocated to either placebo or 3000 IU vitamin D per day over 12 weeks during winter58. Using 24 h ambulatory blood pressure measurements, there was only a moderate trend for a reduction in systolic (-3 mm Hg; P=0.26) and diastolic (-1 mm Hg; P=0.18) blood pressure in the treatment compared to the placebo group58. In analyses restricted to patients with 25(OH)D levels below 32 ng/ml (80 nmol/l) there was a reduction in systolic blood pressure by -4 mm Hg (P=0.05) and in diastolic blood pressure by -3 mm Hg (P=0.01) in the vitamin D supplementation group58. Hence, these results along with some other RCTs showing no significant blood pressure effect of vitamin D in largely normotensive individuals, suggest that if antihypertensive effects of vitamin D are actually present, these may only be observed in groups with both low vitamin D levels and high blood pressure58,59.
Vitamin D and other cardiovascular risk factors
Beyond associations of vitamin D deficiency with arterial hypertension and the relationships to kidney dysfunction and inflammation, there is compelling evidence that low vitamin D levels are also associated with other classic cardiovascular risk factors8,9,34. In particular, obesity is closely associated with vitamin D deficiency, and it has been hypothesized that vitamin D deposition in the adipose tissue could be an explanation for this link9. In line with this, it has been shown that changes in body weight are accompanied by changes in 25(OH)D levels with increases of 25(OH)D as a consequence of losing body weight60. Besides obesity, there is also a significant association of 25(OH)D and physical activity61. While this association may probably be related to more sunlight exposure of physically active individuals, it is also apparent that the relationship of vitamin D status with obesity and physical activity produces significant confounding problems for observational studies. Also, there is accumulating evidence from experimental studies that VDR activation may improve insulin sensitivity and increase insulin secretion8,62. Observational studies have, by the majority, confirmed an association of vitamin D deficiency with type 2 diabetes and disturbed glucose metabolism. Data from RCTs have, however, largely failed to show beneficial vitamin D effects on glucose metabolism and it is thus still unclear whether vitamin D is relevant for the pathogenesis of type 2 diabetes62. Apart from this, there is also evidence that vitamin D could be relevant for autoimmunological diseases including type 1 diabetes63. This hypothesis is based on the fact that vitamin D is known to exert a variety of effects within the immune system63,64. Clinical studies have already shown that vitamin D supplementation increases regulatory T cells, which are considered to protect against autoimmunity64. It has also been observed that supplementing recommended vitamin D doses during childhood is associated with a significantly reduced incidence of type 1 diabetes65. Despite these promising data it must be underlined that further RCTs are still needed before drawing final conclusions on vitamin D and type 1 diabetes. Vitamin D deficiency has also been linked to dyslipidaemia and oxidative stress in observational studies66. With regard to lipids, existing trials did not show consistent and significant vitamin D effects on blood lipids warranting further studies to clarify this issue66.
Vitamin D and cerebrovascular disease
Observational studies on vitamin D and stroke
It is well established that low 25(OH)D levels are a risk factor for mortality in general populations, as well as in specific patient groups such as chronic kidney disease patients22,67,68. Data from prospective observational studies indicate that vitamin D deficiency is also an independent risk factor for stroke69,70. A meta-analysis of seven studies including 47,809 participants and 926 cerebrovascular events showed that when adjusting for cardiovascular risk factors the risk of cerebrovascular disease was significantly reduced in individuals with high 25(OH)D levels70. In detail, the relative risk (RR) in the highest versus the lowest 25(OH)D tertile was 0.60 (95% CI: 0.48 to 0.72). A further meta-analysis including 1214 stroke events reported similar results with a RR in the low vs. high 25(OH)D group of 1.52 (95% CI: 1.20-1.85)69. Moreover, in 7935 Japanese-American men derived from the Honolulu Heart Program, low dietary vitamin D intake was an independent predictor of strokes after 34 years of follow up, during which 960 stroke events were recorded71.
Interventional studies on vitamin D and stroke
While the observational data strongly support the notion that a compromised vitamin D status is a risk factor for stroke, there are only limited and less promising data from RCTs available20. In fact, none of the few available RCTs has shown that there are significant overall effects of vitamin D supplementation on stroke incidence20,72,73,74. These negative findings must, however, be interpreted with caution, because none of these studies was powered or designed to evaluate effects on stroke. In the largest RCT, the Women's Health Initiative (WHI), 36,282 postmenopausal women were randomly allocated to receive daily either 400 International Units (IU) vitamin D plus 1000 mg calcium or placebo over 7 years72. At the end of the study, the hazard ratio (HR) for fatal cerebrovascular events in the treatment versus the placebo group was 0.96 (95% CI: 0.82-1.10). Poor compliance and the relatively low vitamin D dose of 400 IU per day were widely discussed limitations of the WHI trial. In addition, the combined vitamin D plus calcium intake is also an important issue, since it has been shown that calcium supplements with and without vitamin D modestly increase the risk of cardiovascular events, in particular myocardial infarction75. When summarizing existing RCT data on vitamin D and stroke one can conclude that previous studies did not show significant effects on stroke incidence, which is presumably due to the above discussed limitations. We, therefore, cannot draw definite conclusions on this topic and have to wait for further RCT results in the future. In this context, several large RCTs are currently ongoing to evaluate vitamin D effects (without calcium) on major clinical outcomes, including stroke in older general populations76. Results of these RCTs can be expected in the years 2017 to 202076. While these trial results will significantly improve our knowledge on general vitamin D supplementation in older individuals, none of these RCTs is selecting individuals with overt vitamin D deficiency who are most likely to benefit from vitamin D76,77.
Vitamin D in stroke patients
Beyond being a risk factor for stroke, it has been shown in 386 stroke patients that 25(OH)D levels at hospital admission are associated with stroke severity, as well as with poor early functional outcomes78. Considering that the association of low 25(OH)D with poor outcome at discharge remained significant even after adjustments for confounders including stroke severity, the authors hypothesized that vitamin D might promote neuroplastic changes that may in turn improve clinical recovery78. Since 25(OH)D can cross the brain blood barrier and VDRs have been identified within the brain, it has been subsequently suggested that vitamin D may exert neuroprotective actions20,79. In a rat model of cerebral ischaemia, infarct size could be significantly reduced by 1,25(OH)2D treatment80. In support of these data, it was also shown that vitamin D deficiency in rats is associated with an increased infarct volume81. More detailed evaluations showed that vitamin D deficiency caused a dysregulation in the inflammatory response and decreased neuroprotective factors such as insulin growth factor-1 (IGF-1)81. In addition to these experimental data on neuroprotective actions of vitamin D, observational data suggest that vitamin D deficiency is associated with reduced cognitive function, which is an important issue for stroke patients13. RCTs are, therefore, needed to address a possible effect of vitamin D on cognition. Apart from this, it must be underlined that stroke patients are at high risk of musculoskeletal complications, including fractures and falls that are classically related to vitamin D deficiency. While meta-analyses of RCTs show a reduction of fractures and falls in older individuals with vitamin D supplementation, small studies exist that document these effects also in stroke patients3,4,82,83. Moreover, sunlight exposure in stroke patients is associated with improved bone mineral density and reduced fracture incidence84. Hence, beyond avoidance of immobilization, which may lead to calcium mobilisation from the bone with subsequent hypercalcaemia and increased bone turnover, compelling evidence exists that vitamin D supplementation can effectively prevent musculoskeletal complications in stroke patients20,82,83,84. Whether vitamin D supplementation may also improve cardiovascular risk, as suggested by a small study showing improved endothelial function after vitamin D supplementation in stroke patients, warrants further in-depth studies45.
Vitamin D treatment
Although evidence is currently insufficient to make general recommendations for vitamin D supplementation for the prevention and treatment of arterial hypertension and cerebrovascular disease, it must be underlined that vitamin D deficiency is definitely common among patients suffering from these pathologies. Deleterious consequences of vitamin D deficiency with regard to musculoskeletal health are, therefore, the main rationale for evaluation, prevention and treatment of vitamin D deficiency in patients with arterial hypertension and stroke. Whereas controversies are present in the literature on precise indications for vitamin D testing, i.e. measurements of 25(OH)D, a wide consensus exists that 25(OH)D levels below 20 ng/ml (50 nmol/l) are insufficient and should thus be prevented and treated1,2,3,11. Considering that vitamin D treatment is mainly justified due to beneficial effects on bone health, it seems reasonable to use vitamin D doses ranging from 800 to 2000 IU per day, because these doses have been shown to significantly reduce fractures4. It has been shown that daily, weekly or monthly vitamin D dosing regimens can equally raise 25(OH)D levels; but existing literature does not support the use of single doses exceeding approx. 100,000 IU vitamin D85,86. Compliance and effectiveness of vitamin D treatment can be evaluated by measuring 25(OH)D levels, but this should not be done earlier than three months after starting vitamin D supplementation, because reaching a steady state in 25(OH)D levels takes some time11. Whereas it is considered as the minimum goal to achieve 25(OH)D levels of at least 20 ng/ml (50 nmol/l), there is accumulating evidence that the optimal 25(OH)D levels may range from approximately 30 to 40 ng/ml (75 to 100 nmol/l)22,23. When choosing the vitamin D dose for a patient it should also be considered that the expected increase in 25(OH)D levels is inversely associated with body mass index (BMI)87,88. Obese patients may thus require a much higher vitamin D dose than lean individuals to achieve the same 25(OH)D levels. In this context it should be noted that according to the European Food Safety Authority (EFSA) and the Institute of Medicine (IOM) in the US, the safe tolerable upper intake level for vitamin D is 4000 IU per day in adults2,89. While most individuals will not require doses exceeding this level of 4000 IU per day, some individuals with morbid obesity or vitamin D absorption problems such as in inflammatory bowel disease may require higher dosages. For such specific patients with still insufficient 25(OH)D levels after supplementation with 4000 IU per day, doses up to 10,000 IU per day may be used. Such approaches are also supported by the Endocrine Society guidelines as well as by some other expert groups, who consider doses up to 10,000 IU per day as safe3,24. Another important issue is the fact that vitamin D is frequently given in combination with calcium. In line with the conclusions of a recent meta-analysis, it should be noted that concomitant calcium supplementation can reduce the compliance of vitamin D supplementation90. It must also be underlined that a significant part of our knowledge on beneficial vitamin D effects is based on studies using concomitant vitamin D plus calcium supplementation. While meta-analyses of RCTs have shown that vitamin D significantly reduces mortality compared to placebo, the impact of varying doses of concomitant calcium intake on these results remains unclear91,92. We, therefore, have to acknowledge that the possible interactions of calcium and vitamin D effects are still puzzling at the moment and it will be a challenge to address these issues in future91,92,93.
Conclusions
The majority of epidemiological studies have shown that vitamin D deficiency is a risk factor for arterial hypertension and cerebrovascular disease. Experimental studies support the notion that vitamin D exerts antihypertensive effects and has a beneficial impact on the overall cerebrovascular risk profile and on stroke outcome. Some small RCTs have already shown a modest blood pressure reduction by vitamin D treatment, but this has not been consistently observed in all studies. Published RCTs did not document a significant vitamin D effect on stroke incidence but these studies have not been properly designed to address this issue. While the promising data on potentially beneficial vitamin D effects on arterial hypertension and cerebrovascular disease need to be further addressed in large RCTs, it must also be pointed out that patients with arterial hypertension and stroke are at relatively high risk for vitamin D deficiency and therewith associated musculoskeletal diseases. This latter consideration can serve as a rationale for the evaluation, prevention and treatment of vitamin D deficiency in individuals with arterial hypertension and stroke.
References
- 1.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 2.Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53–8. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–30. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 4.Bischoff-Ferrari HA, Willett WC, Orav EJ, Lips P, Meunier PJ, Lyons RA, et al. A pooled analysis of vitamin D dose requirements for fracture prevention. N Engl J Med. 2012;367:40–9. doi: 10.1056/NEJMoa1109617. [DOI] [PubMed] [Google Scholar]
- 5.Jain V, Gupta N, Kalaivani M, Jain A, Sinha A, Agarwal R. Vitamin D deficiency in healthy breastfed term infants at 3 months & their mothers in India: seasonal variation & determinants. Indian J Med Res. 2011;133:267–73. [PMC free article] [PubMed] [Google Scholar]
- 6.Balasubramanian S. Vitamin D deficiency in breastfed infants & the need for routine vitamin D supplementation. Indian J Med Res. 2011;133:250–2. [PMC free article] [PubMed] [Google Scholar]
- 7.Lips P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocr Rev. 2001;22:477–501. doi: 10.1210/edrv.22.4.0437. [DOI] [PubMed] [Google Scholar]
- 8.Bouillon R, Carmeliet G, Verlinden L, van Etten E, Verstuyf A, Luderer HF, et al. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev. 2008;29:726–76. doi: 10.1210/er.2008-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muscogiuri G, Sorice GP, Ajjan R, Mezza T, Pilz S, Prioletta A, et al. Can vitamin D deficiency cause diabetes and cardiovascular diseases? Present evidence and future perspectives. Nutr Metab Cardiovasc Dis. 2012;22:81–7. doi: 10.1016/j.numecd.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Pilz S, Tomaschitz A, Drechsler C, Dekker JM, März W. Vitamin D deficiency and myocardial diseases. Mol Nutr Food Res. 2010;54:1103–13. doi: 10.1002/mnfr.200900474. [DOI] [PubMed] [Google Scholar]
- 11.Souberbielle JC, Body JJ, Lappe JM, Plebani M, Shoenfeld Y, Wang TJ, et al. Vitamin D and musculoskeletal health, cardiovascular disease, autoimmunity and cancer: Recommendations for clinical practice. Autoimmun Rev. 2010;9:709–15. doi: 10.1016/j.autrev.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 12.Mirković K, van den Born J, Navis G, de Borst MH. Vitamin D in chronic kidney disease: new potential for intervention. Curr Drug Targets. 2011;12:42–53. doi: 10.2174/138945011793591572. [DOI] [PubMed] [Google Scholar]
- 13.Soni M, Kos K, Lang IA, Jones K, Melzer D, Llewellyn DJ. Vitamin D and cognitive function. Scand J Clin Lab Invest. 2012;243(Suppl):79–82. doi: 10.3109/00365513.2012.681969. [DOI] [PubMed] [Google Scholar]
- 14.Hewison M. Vitamin D and immune function: autocrine, paracrine or endocrine? Scand J Clin Lab Invest. 2012;243(Suppl):92–102. doi: 10.3109/00365513.2012.682862. [DOI] [PubMed] [Google Scholar]
- 15.Pilz S, Tomaschitz A, Obermayer-Pietsch B, Dobnig H, Pieber TR. Epidemiology of vitamin D insufficiency and cancer mortality. Anticancer Res. 2009;29:3699–704. [PubMed] [Google Scholar]
- 16.Holick MF. McCollum Award Lecture, 1994: vitamin D--new horizons for the 21st century. Am J Clin Nutr. 1994;60:619–30. doi: 10.1093/ajcn/60.4.619. [DOI] [PubMed] [Google Scholar]
- 17.Kochupillai N. The physiology of vitamin D : current concepts. Indian J Med Res. 2008;127:256–62. [PubMed] [Google Scholar]
- 18.Macdonald HM. Contributions of sunlight and diet to vitamin D status. Calcif Tissue Int. 2013;92:163–76. doi: 10.1007/s00223-012-9634-1. [DOI] [PubMed] [Google Scholar]
- 19.Peterlik M, Cross HS. Vitamin D and calcium insufficiency-related chronic diseases: molecular and cellular pathophysiology. Eur J Clin Nutr. 2009;63:1377–86. doi: 10.1038/ejcn.2009.105. [DOI] [PubMed] [Google Scholar]
- 20.Pilz S, Tomaschitz A, Drechsler C, Zittermann A, Dekker JM, März W. Vitamin D supplementation: a promising approach for the prevention and treatment of strokes. Curr Drug Targets. 2011;12:88–96. doi: 10.2174/138945011793591563. [DOI] [PubMed] [Google Scholar]
- 21.Pilz S, Tomaschitz A, Ritz E, Pieber TR. Vitamin D status and arterial hypertension: a systematic review. Nat Rev Cardiol. 2009;6:621–30. doi: 10.1038/nrcardio.2009.135. [DOI] [PubMed] [Google Scholar]
- 22.Zittermann A, Iodice S, Pilz S, Grant WB, Bagnardi V, Gandini S. Vitamin D deficiency and mortality risk in the general population: a meta-analysis of prospective cohort studies. Am J Clin Nutr. 2012;95:91–100. doi: 10.3945/ajcn.111.014779. [DOI] [PubMed] [Google Scholar]
- 23.Bischoff-Ferrari HA. Optimal serum 25-hydroxyvitamin D levels for multiple health outcomes. Adv Exp Med Biol. 2008;624:55–71. doi: 10.1007/978-0-387-77574-6_5. [DOI] [PubMed] [Google Scholar]
- 24.Hathcock JN, Shao A, Vieth R, Heaney R. Risk assessment for vitamin D. Am J Clin Nutr. 2007;85:6–18. doi: 10.1093/ajcn/85.1.6. [DOI] [PubMed] [Google Scholar]
- 25.Davidson MB, Duran P, Lee ML, Friedman TC. High-dose vitamin D supplementation in people with prediabetes and hypovitaminosis D. Diabetes Care. 2013;36:260–6. doi: 10.2337/dc12-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luxwolda MF, Kuipers RS, Kema IP, Janneke Dijck-Brouwer DA, Muskiet FA. Traditionally living populations in East Africa have a mean serum 25-hydroxyvitamin D concentration of 115 nmol/l. Br J Nutr. 2012;108:1557–61. doi: 10.1017/S0007114511007161. [DOI] [PubMed] [Google Scholar]
- 27.Herrmann M. The measurement of 25-hydroxy vitamin D - an analytical challenge. Clin Chem Lab Med. 2012;50:1873–5. doi: 10.1515/cclm-2012-0526. [DOI] [PubMed] [Google Scholar]
- 28.Carter GD. Accuracy of 25-hydroxyvitamin D assays: confronting the issues. Curr Drug Targets. 2011;12:19–28. doi: 10.2174/138945011793591608. [DOI] [PubMed] [Google Scholar]
- 29.Sempos CT, Vesper HW, Phinney KW, Thienpont LM, Coates PM Vitamin D Standardization Program (VDSP) Vitamin D status as an international issue: national surveys and the problem of standardization. Scand J Clin Lab Invest. 2012;243(Suppl):32–40. doi: 10.3109/00365513.2012.681935. [DOI] [PubMed] [Google Scholar]
- 30.Binkley N, Krueger D, Cowgill CS, Plum L, Lake E, Hansen KE, et al. Assay variation confounds the diagnosis of hypovitaminosis D: a call for standardization. J Clin Endocrinol Metab. 2004;89:3152–7. doi: 10.1210/jc.2003-031979. [DOI] [PubMed] [Google Scholar]
- 31.Li YC. Molecular mechanism of vitamin D in the cardiovascular system. J Investig Med. 2011;59:868–71. doi: 10.231/JIM.0b013e31820ee448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fitzpatrick LA, Bilezikian JP, Silverberg SJ. Parathyroid hormone and the cardiovascular system. Curr Osteoporos Rep. 2008;6:77–83. doi: 10.1007/s11914-008-0014-8. [DOI] [PubMed] [Google Scholar]
- 33.Fliser D, Franek E, Fode P, Stefanski A, Schmitt CP, Lyons M, et al. Subacute infusion of physiological doses of parathyroid hormone raises blood pressure in humans. Nephrol Dial Transplant. 1997;12:933–8. doi: 10.1093/ndt/12.5.933. [DOI] [PubMed] [Google Scholar]
- 34.Pilz S, Tomaschitz A, März W, Drechsler C, Ritz E, Zittermann A, et al. Vitamin D, cardiovascular disease and mortality. Clin Endocrinol (Oxf) 2011;75:575–84. doi: 10.1111/j.1365-2265.2011.04147.x. [DOI] [PubMed] [Google Scholar]
- 35.Pilz S, Tomaschitz A, Drechsler C, Ritz E, Boehm BO, Grammer TB, et al. Parathyroid hormone level is associated with mortality and cardiovascular events in patients undergoing coronary angiography. Eur Heart J. 2010;31:1591–8. doi: 10.1093/eurheartj/ehq109. [DOI] [PubMed] [Google Scholar]
- 36.Dusso AS, Tokumoto M. Defective renal maintenance of the vitamin D endocrine system impairs vitamin D renoprotection: a downward spiral in kidney disease. Kidney Int. 2011;79:715–29. doi: 10.1038/ki.2010.543. [DOI] [PubMed] [Google Scholar]
- 37.Reid D, Toole BJ, Knox S, Talwar D, Harten J, O’Reilly DS, et al. The relation between acute changes in the systemic inflammatory response and plasma 25-hydroxyvitamin D concentrations after elective knee arthroplasty. Am J Clin Nutr. 2011;93:1006–11. doi: 10.3945/ajcn.110.008490. [DOI] [PubMed] [Google Scholar]
- 38.Guessous I, Bochud M, Bonny O, Burnier M. Calcium, vitamin D and cardiovascular disease. Kidney Blood Press Res. 2011;34:404–17. doi: 10.1159/000328332. [DOI] [PubMed] [Google Scholar]
- 39.Thierry-Palmer M, Tewolde TK, Emmett NL, Bayorh MA. High dietary salt does not significantly affect plasma 25-hydroxyvitamin D concentrations of Sprague Dawley rats. BMC Res Notes. 2010;3:332. doi: 10.1186/1756-0500-3-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brewer LC, Michos ED, Reis JP. Vitamin D in atherosclerosis, vascular disease, and endothelial function. Curr Drug Targets. 2011;12:54–60. doi: 10.2174/138945011793591617. [DOI] [PubMed] [Google Scholar]
- 41.Tare M, Emmett SJ, Coleman HA, Skordilis C, Eyles DW, Morley R, et al. Vitamin D insufficiency is associated with impaired vascular endothelial and smooth muscle function and hypertension in young rats. J Physiol. 2011;589:4777–86. doi: 10.1113/jphysiol.2011.214726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Molinari C, Uberti F, Grossini E, Vacca G, Carda S, Invernizzi M, et al. 1α,25-dihydroxycholecalciferol induces nitric oxide production in cultured endothelial cells. Cell Physiol Biochem. 2011;27:661–8. doi: 10.1159/000330075. [DOI] [PubMed] [Google Scholar]
- 43.Oh J, Weng S, Felton SK, Bhandare S, Riek A, Butler B, et al. 1,25(OH)2 vitamin D inhibits foam cell formation and suppresses macrophage cholesterol uptake in patients with type 2 diabetes mellitus. Circulation. 2009;120:687–98. doi: 10.1161/CIRCULATIONAHA.109.856070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harris RA, Pedersen-White J, Guo DH, Stallmann-Jorgensen IS, Keeton D, Huang Y, et al. Vitamin D3 supplementation for 16 weeks improves flow-mediated dilation in overweight African-American adults. Am J Hypertens. 2011;24:557–62. doi: 10.1038/ajh.2011.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Witham MD, Dove FJ, Sugden JA, Doney AS, Struthers AD. The effect of vitamin D replacement on markers of vascular health in stroke patients - a randomised controlled trial. Nutr Metab Cardiovasc Dis. 2012;22:864–70. doi: 10.1016/j.numecd.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 46.Rostand SG. Ultraviolet light may contribute to geographic and racial blood pressure differences. Hypertension. 1997;30:150–6. doi: 10.1161/01.hyp.30.2.150. [DOI] [PubMed] [Google Scholar]
- 47.Tamez H, Thadhani RI. Vitamin D and hypertension: an update and review. Curr Opin Nephrol Hypertens. 2012;21:492–9. doi: 10.1097/MNH.0b013e3283557bf0. [DOI] [PubMed] [Google Scholar]
- 48.Jorde R, Figenschau Y, Emaus N, Hutchinson M, Grimnes G. Serum 25-hydroxyvitamin D levels are strongly related to systolic blood pressure but do not predict future hypertension. Hypertension. 2010;55:792–8. doi: 10.1161/HYPERTENSIONAHA.109.143990. [DOI] [PubMed] [Google Scholar]
- 49.Burgaz A, Orsini N, Larsson SC, Wolk A. Blood 25-hydroxyvitamin D concentration and hypertension: a meta-analysis. J Hypertens. 2011;29:636–45. doi: 10.1097/HJH.0b013e32834320f9. [DOI] [PubMed] [Google Scholar]
- 50.Krause R, Bühring M, Hopfenmüller W, Holick MF, Sharma AM. Ultraviolet B and blood pressure. Lancet. 1998;352:709–10. doi: 10.1016/S0140-6736(05)60827-6. [DOI] [PubMed] [Google Scholar]
- 51.Scragg R, Wishart J, Stewart A, Ofanoa M, Kerse N, Dyall L, et al. No effect of ultraviolet radiation on blood pressure and other cardiovascular risk factors. J Hypertens. 2011;29:1749–56. doi: 10.1097/HJH.0b013e328349666d. [DOI] [PubMed] [Google Scholar]
- 52.Witham MD, Nadir MA, Struthers AD. Effect of vitamin D on blood pressure: a systematic review and meta-analysis. J Hypertens. 2009;27:1948–54. doi: 10.1097/HJH.0b013e32832f075b. [DOI] [PubMed] [Google Scholar]
- 53.Pittas AG, Chung M, Trikalinos T, Mitri J, Brendel M, Patel K, et al. Systematic review: vitamin D and cardiometabolic outcomes. Ann Intern Med. 2010;152:307–14. doi: 10.1059/0003-4819-152-5-201003020-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu SH, Ho SC, Zhong L. Effects of vitamin D supplementation on blood pressure. South Med J. 2010;103:729–37. doi: 10.1097/SMJ.0b013e3181e6d389. [DOI] [PubMed] [Google Scholar]
- 55.Elamin MB, Abu Elnour NO, Elamin KB, Fatourechi MM, Alkatib AA, Almandoz JP, et al. Vitamin D and cardiovascular outcomes: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96:1931–42. doi: 10.1210/jc.2011-0398. [DOI] [PubMed] [Google Scholar]
- 56.Margolis KL, Ray RM, Van Horn L, Manson JE, Allison MA, Black HR, et al. Effect of calcium and vitamin D supplementation on blood pressure: the Women's Health Initiative Randomized Trial. Hypertension. 2008;52:847–55. doi: 10.1161/HYPERTENSIONAHA.108.114991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pfeifer M, Begerow B, Minne HW, Nachtigall D, Hansen C. Effects of a short-term vitamin D(3) and calcium supplementation on blood pressure and parathyroid hormone levels in elderly women. J Clin Endocrinol Metab. 2001;86:1633–7. doi: 10.1210/jcem.86.4.7393. [DOI] [PubMed] [Google Scholar]
- 58.Larsen T, Mose FH, Bech JN, Hansen AB, Pedersen EB. Effect of cholecalciferol supplementation during winter months in patients with hypertension: a randomized, placebo-controlled trial. Am J Hypertens. 2012;25:1215–22. doi: 10.1038/ajh.2012.111. [DOI] [PubMed] [Google Scholar]
- 59.Wood AD, Secombes KR, Thies F, Aucott L, Black AJ, Mavroeidi A, et al. vitamin D3 supplementation has no effect on conventional cardiovascular risk factors: A parallel-group, double-blind, placebo-controlled RCT. J Clin Endocrinol Metab. 2012;97:3557–68. doi: 10.1210/jc.2012-2126. [DOI] [PubMed] [Google Scholar]
- 60.Rock CL, Emond JA, Flatt SW, Heath DD, Karanja N, Pakiz B, et al. Weight loss is associated with increased serum 25-hydroxyvitamin D in overweight or obese women. Obesity (Silver Spring) 2012;20:2296–301. doi: 10.1038/oby.2012.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wicherts IS, van Schoor NM, Boeke AJ, Visser M, Deeg DJ, Smit J, et al. Vitamin D status predicts physical performance and its decline in older persons. J Clin Endocrinol Metab. 2007;92:2058–65. doi: 10.1210/jc.2006-1525. [DOI] [PubMed] [Google Scholar]
- 62.Mitri J, Muraru MD, Pittas AG. Vitamin D and type 2 diabetes: a systematic review. Eur J Clin Nutr. 2011;65:1005–15. doi: 10.1038/ejcn.2011.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Badenhoop K, Kahles H, Penna-Martinez M. Vitamin D, immune tolerance, and prevention of type 1 diabetes. Curr Diab Rep. 2012;12:635–42. doi: 10.1007/s11892-012-0322-3. [DOI] [PubMed] [Google Scholar]
- 64.Bock G, Prietl B, Mader JK, Höller E, Wolf M, Pilz S, et al. The effect of vitamin D supplementation on peripheral regulatory T cells and β cell function in healthy humans: a randomized controlled trial. Diabetes Metab Res Rev. 2011;27:942–5. doi: 10.1002/dmrr.1276. [DOI] [PubMed] [Google Scholar]
- 65.Hyppönen E, Läärä E, Reunanen A, Järvelin MR, Virtanen SM. Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. Lancet. 2001;358:1500–3. doi: 10.1016/S0140-6736(01)06580-1. [DOI] [PubMed] [Google Scholar]
- 66.Zittermann A, Gummert JF, Börgermann J. The role of vitamin D in dyslipidemia and cardiovascular disease. Curr Pharm Des. 2011;17:933–42. doi: 10.2174/138161211795428786. [DOI] [PubMed] [Google Scholar]
- 67.Pilz S, Iodice S, Zittermann A, Grant WB, Gandini S. Vitamin D status and mortality risk in CKD: a meta-analysis of prospective studies. Am J Kidney Dis. 2011;58:374–82. doi: 10.1053/j.ajkd.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 68.Pilz S, Tomaschitz A, Friedl C, Amrein K, Drechsler C, Ritz E, et al. Vitamin D status and mortality in chronic kidney disease. Nephrol Dial Transplant. 2011;26:3603–9. doi: 10.1093/ndt/gfr076. [DOI] [PubMed] [Google Scholar]
- 69.Sun Q, Pan A, Hu FB, Manson JE, Rexrode KM. 25-hydroxyvitamin D levels and the risk of stroke: a prospective study and meta-analysis. Stroke. 2012;43:1470–7. doi: 10.1161/STROKEAHA.111.636910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chowdhury R, Stevens S, Ward H, Chowdhury S, Sajjad A, Franco OH. Circulating vitamin D, calcium and risk of cerebrovascular disease: a systematic review and meta-analysis. Eur J Epidemiol. 2012;27:581–91. doi: 10.1007/s10654-012-9729-z. [DOI] [PubMed] [Google Scholar]
- 71.Kojima G, Bell C, Abbott RD, Launer L, Chen R, Motonaga H, et al. Low dietary vitamin D predicts 34-year incident stroke: the Honolulu Heart Program. Stroke. 2012;43:2163–7. doi: 10.1161/STROKEAHA.112.651752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hsia J, Heiss G, Ren H, Allison M, Dolan NC, Greenland P, et al. Calcium/vitamin D supplementation and cardiovascular events. Circulation. 2007;115:846–54. doi: 10.1161/CIRCULATIONAHA.106.673491. [DOI] [PubMed] [Google Scholar]
- 73.Trivedi DP, Doll R, Khaw KT. Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: randomised double blind controlled trial. BMJ. 2003;326:469. doi: 10.1136/bmj.326.7387.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Avenell A, MacLennan GS, Jenkinson DJ, McPherson GC, McDonald AM, Pant PR, et al. Long-term follow-up for mortality and cancer in a randomized placebo-controlled trial of vitamin D(3) and/or calcium (RECORD trial) J Clin Endocrinol Metab. 2012;97:614–22. doi: 10.1210/jc.2011-1309. [DOI] [PubMed] [Google Scholar]
- 75.Bolland MJ, Grey A, Avenell A, Gamble GD, Reid IR. Calcium supplements with or without vitamin D and risk of cardiovascular events: reanalysis of the Women's Health Initiative limited access dataset and meta-analysis. BMJ. 2011;342:d2040. doi: 10.1136/bmj.d2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kupferschmidt K. Uncertain verdict as vitamin D goes on trial. Science. 2012;337:1476–8. doi: 10.1126/science.337.6101.1476. [DOI] [PubMed] [Google Scholar]
- 77.Pilz S, Rutters F, Dekker JM. Disease prevention: vitamin D trials. Science. 2012;338:883. doi: 10.1126/science.338.6109.883-c. [DOI] [PubMed] [Google Scholar]
- 78.Daubail B, Jacquin A, Guilland JC, Hervieu M, Osseby GV, Rouaud O, et al. Serum 25-hydroxyvitamin D predicts severity and prognosis in stroke patients. Eur J Neurol. 2013;20:57–61. doi: 10.1111/j.1468-1331.2012.03758.x. [DOI] [PubMed] [Google Scholar]
- 79.Buell JS, Dawson-Hughes B. Vitamin D and neurocognitive dysfunction: preventing “D” ecline? Mol Aspects Med. 2008;29:415–22. doi: 10.1016/j.mam.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang Y, Chiang YH, Su TP, Hayashi T, Morales M, Hoffer BJ, et al. Vitamin D(3) attenuates cortical infarction induced by middle cerebral arterial ligation in rats. Neuropharmacology. 2000;39:873–80. doi: 10.1016/s0028-3908(99)00255-5. [DOI] [PubMed] [Google Scholar]
- 81.Balden R, Selvamani A, Sohrabji F. Vitamin D deficiency exacerbates experimental stroke injury and dysregulates ischemia-induced inflammation in adult rats. Endocrinology. 2012;153:2420–35. doi: 10.1210/en.2011-1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sato Y, Iwamoto J, Kanoko T, Satoh K. Low-dose vitamin D prevents muscular atrophy and reduces falls and hip fractures in women after stroke: a randomized controlled trial. Cerebrovasc Dis. 2005;20:187–92. doi: 10.1159/000087203. [DOI] [PubMed] [Google Scholar]
- 83.Dhesi JK, Jackson SH, Bearne LM, Moniz C, Hurley MV, Swift CG, et al. Vitamin D supplementation improves neuromuscular function in older people who fall. Age Ageing. 2004;33:589–95. doi: 10.1093/ageing/afh209. [DOI] [PubMed] [Google Scholar]
- 84.Iwamoto J, Takeda T, Matsumoto H. Sunlight exposure is important for preventing hip fractures in patients with Alzheimer's disease, Parkinson's disease, or stroke. Acta Neurol Scand. 2012;125:279–84. doi: 10.1111/j.1600-0404.2011.01555.x. [DOI] [PubMed] [Google Scholar]
- 85.Ish-Shalom S, Segal E, Salganik T, Raz B, Bromberg IL, Vieth R. Comparison of daily, weekly, and monthly vitamin D3 in ethanol dosing protocols for two months in elderly hip fracture patients. J Clin Endocrinol Metab. 2008;93:3430–5. doi: 10.1210/jc.2008-0241. [DOI] [PubMed] [Google Scholar]
- 86.Sanders KM, Stuart AL, Williamson EJ, Simpson JA, Kotowicz MA, Young D, et al. Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. JAMA. 2010;303:1815–22. doi: 10.1001/jama.2010.594. [DOI] [PubMed] [Google Scholar]
- 87.van Groningen L, Opdenoordt S, van Sorge A, Telting D, Giesen A, de Boer H. Cholecalciferol loading dose guideline for vitamin D-deficient adults. Eur J Endocrinol. 2010;162:805–11. doi: 10.1530/EJE-09-0932. [DOI] [PubMed] [Google Scholar]
- 88.Gallagher JC, Sai A, Templin T, 2nd, Smith L. Dose response to vitamin D supplementation in postmenopausal women: a randomized trial. Ann Intern Med. 2012;156:425–37. doi: 10.7326/0003-4819-156-6-201203200-00005. [DOI] [PubMed] [Google Scholar]
- 89.EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific opinion on the tolerable upper intake level of vitamin D. EFSA J. 2012;10(2813):45. [Google Scholar]
- 90.Autier P, Gandini S, Mullie P. A systematic review: influence of vitamin D supplementation on serum 25-hydroxyvitamin D concentration. J Clin Endocrinol Metab. 2012;97:2606–13. doi: 10.1210/jc.2012-1238. [DOI] [PubMed] [Google Scholar]
- 91.Bjelakovic G, Gluud LL, Nikolova D, Whitfield K, Wetterslev J, Simonetti RG, et al. Vitamin D supplementation for prevention of mortality in adults. Cochrane Database Syst Rev. 2011;7:CD007470. doi: 10.1002/14651858.CD007470.pub2. [DOI] [PubMed] [Google Scholar]
- 92.Rejnmark L, Avenell A, Masud T, Anderson F, Meyer HE, Sanders KM, et al. Vitamin D with calcium reduces mortality: patient level pooled analysis of 70,528 patients from eight major vitamin D trials. J Clin Endocrinol Metab. 2012;97:2670–81. doi: 10.1210/jc.2011-3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lips P. Interaction between vitamin D and calcium. Scand J Clin Lab Invest. 2012;243(Suppl):60–4. doi: 10.3109/00365513.2012.681960. [DOI] [PubMed] [Google Scholar]


