Abstract
Background & objectives:
Tumour necrosis factor-alpha (TNF-α)- 308 promoter gene polymorphism has been shown to be associated with several autoimmune disorders and infections such as tuberculosis. There is no study on TNF-α gene polymorphism in Takayasu's arteritis (TA) till date. We aimed to study this polymorphism in TA, a granulomatous vasculitis, probably triggered by Mycobacterium tuberculosis.
Methods:
TNF-α - 308 gene polymorphism was studied in 34 patients with TA and 39 healthy controls recruited from Christian Medical College, India. PCR was done followed by enzyme digestion. G and A polymorphisms were analysed. Occurrence of alleles in the disease group was compared with controls as well as with historical controls.
Results:
GG allele was most frequent in TA and in controls. GA allele was detected in four controls but only in one patient who was the oldest in the study group. AA polymorphism was detected in one control but not in TA. When compared with controls from other populations, it was found that our allelic frequency was similar to that in Japan as well as from USA with mixed population. However, predominantly Caucasian population studied from Netherlands, Germany and England, where TA is rare, had a higher frequency of A allele as compared to our controls.
Interpretation & conclusions:
Our preliminary results indicated that G allele at TNF-α - 308 was more common in TA patients and controls similar to that in other Indian as well as Japanese population. Compared to the western population, A allele was relatively less common in our study subjects.
Keywords: Giant cell arteritis, India, polymorphism, Takayasu's arteritis, TNF-α -308
Takayasu arteritis (TA) also known as pulseless disease, is an idiopathic chronic inflammatory disease of aorta and its branches leading to stenosis, occlusion and aneurysmal dilatation. In majority, the disease starts around 3rd and 4th decade of life. TA predominantly affects women with a female:male ratio of 5:11. It is most commonly seen in India, Japan, South East Asia and Mexico. Incidence in Indian population has not been calculated. It is believed to be similar to Japan where the reported incidence is nearly 150 per million per year. In western Europe and North America, it is only 0.2 to 2.6 per million2.
Tumour necrosis factor (TNF) is a cytokine with pleomorphic actions. TNF-α is pivotal in host defense against infections and has a major role in autoimmune diseases as well. It is also a crucial cytokine for granuloma formation. The level of TNF-α varies from individual to individual and is genetically determined3. The gene for TNF-α is located within the major histocompatibility complex (MHC) region on chromosome 6p21.3 which is a highly polymorphic region. There are many biallelic single nucleotide polymorphisms (SNPs) in and around the TNF-α gene. One such G/A polymorphism is located upstream of gene at -308 and is known to influence TNF-α levels. As compared with the - TNF-α -308G allele, A allele has higher transcriptional activity4,5.
TNF-α - 308 promoter gene polymorphism has been reported to be associated with several autoimmune disorders including systemic lupus erythematosus, rheumatoid arthritis and infections such as tuberculosis6. There was no study on TNF-α gene polymorphism in TA during the manuscript writing. During the manuscript review period, we came across one study reporting no TNF-α promoter polymorphism in TA amongst Han Chinese populatin7. We, therefore, carried out this pilot study to analyse TNF-α -308 polymorphism in TA which is a granulomatous vasculitis with an autoimmune basis, probably triggered by Mycobacterium tuberculosis8.
Material & Methods
Sample collection: Consecutive patients with TA fulfilling American College of Rheumatology (ACR) 1990 criteria were recruited from outpatient and inpatient services of Clinical Immunology & Rheumatology and Cardiology Departments of Christian Medical College, Vellore, India, between October 2009 and May 2010. Matched healthy controls were also reported from amongst the hospital staff. After obtaining informed consent, 2 ml of peripheral blood was collected and stored at -20 °C till DNA was extracted. Study protocol was approved by the Institutional Review Board of Christian Medical College, Vellore.
DNA extraction: DNA was extracted using commercially available DNA extraction kit (QIAGEN) and stored at -20°C. Genotyping for TNF-α polymorphism was done for the isolated DNA for the polymorphic sites -308 by polymerase chain reaction (PCR) using thermal cycler (Applied Biosystems Gene Amp PCR System 9700, USA). Polymorphisms were analysed using restriction fragment length polymorphism (RFLP) method and primers specific to sites -308 was used. PCR was carried out in 25 μl reactions as previously described2. Forward primer 5’AGG CAA TAG GTT TTG AGG GCC AT 3’ and reverse primer 5’TCC TCC CTG CTC CGA TTC 3’ (Sigma, USA) were used to amplify a 107 bp fragment and then digested using Nco1 restriction enzyme (New England Biolabs) and incubated at 37°C for 12 h. The analysis for the restriction fragments obtained (87 and 20 bp for -308G and 107 bp for -308A) was done on 2 per cent agarose gel. Each batch of samples was accompanied by positive and negative controls.
Statistical analysis: Frequencies of the genotypes between the study and the healthy groups were compared using Fisher's exact test. Results were also compared with historical controls from various populations across the globe.
Results & Discussion
A total of 33 patients with TA (28 female, 5 male) and 39 healthy controls were included in the study. The median age of onset of disease group was 27 yr (range 10-62 yr). Four (12.12 %) patients had disease onset at >50 yr. The median age of the controls was 25 yr (range 21-42). Female to male ratio were 28:5 for the patients and 29:10 for the control group respectively. The most common angiographic type was Type V that was seen in 18 patients (53%). Types I, III, II and IV were seen in 6, 5, 3 and 1 patient, respectively.
PCR for the TNF-α gene gave a 107-bp fragment (Fig. a). Following enzyme digestion of the PCR product, three results were obtained. A complete NcoI cut for homozygous TNF-α (-308G/G), resulting in two fragments of 87 and 20 base pairs; a partial cut for heterozygous TNF-α (-308G/A), resulting in three fragments of 107, 87 and 20 base pairs; and an uncut homozygous TNF-α (-308A/A), resulting in a 107 base pair fragment (Fig. b).
Fig.
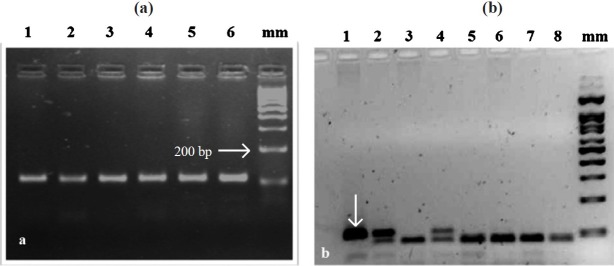
(a) Agarose gel (2%) electrophoresis showing PCR products of TNF-α gene promoter region after amplification. mm -100 bp marker; Lanes 5 1 to 6-107 bp PCR product. (b). Agarose gel (2%) electrophoresis depicting restriction digetion of TNF-α(-308 polymorphism) with Ncol enzyme Lane 1-undigested PCR product; Lanes 2 and 4-GA allele; 3, 5, 6, 7, 8- GG allele;mm -100 bp marker.
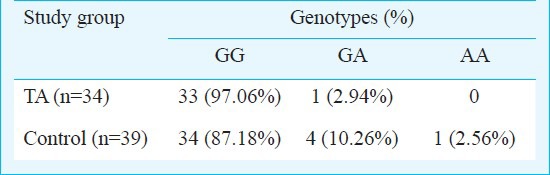
Genotype distribution is given in Table I. The difference in frequency of genotype distribution of the polymorphism between patients and controls was not statistically significant. However, no AA allele was detected in the disease group. GA allele was detected in the oldest patient with TA.
Table I.
Genotype distribution of alleles of TNF-α polymorphism TA patients and controls
Allelic frequencies of this polymorphism in the control group were compared with that of different populations using Fisher's exact test. G allele was significantly more in our controls as compared to population of England (P=0.008), Netherlands (P=0.002), Germany (P=0.014) but not significant when compared to the population of USA and Japan (Table II).
Table II.
Allele frequencies of TNF-α polymorphism (-308) in different populations
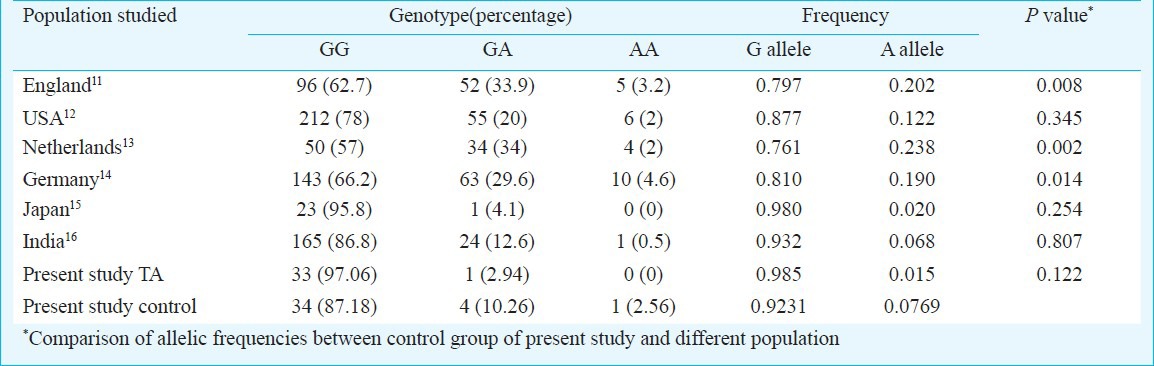
The allele frequencies of TNF-α polymorphism (-308) of cases and controls were not statistically different. The allelic frequency was found to be similar to that of an earlier study on Indian population9. It is noteworthy that in Japan and India where TA is commonly seen, the G allele frequency is higher than that of the other countries. In England, Netherlands and Germany, G allele frequency is relatively less and A allele frequency relatively high as compared to India and Japan (Table II). There was no difference when our controls were compared with controls from USA; this apparent discrepancy may be because 40 per cent of their controls were of non-Caucasian ancestry. Therefore, perhaps G allele frequency closely mirrors the distribution of TA. It is well known fact that geographic distribution of Giant Cell Arteritis (GCA) is in sharp contrast to that of TA10. GCA is least common in Japanese and Asian Indians and is predominantly seen in the Scandinavian nations and North European countries10.
It has been shown in earlier studies that TNF-α -308 G allele is associated with lesser TNF-α production as compared to A allele4,5. Does GG allele predispose to TA and does AA allele protect from developing TA? Considering the fact that TNF-α is reportedly involved in pathogenesis of TA16,17, it may seem surprising that lesser levels of TNF-α production are associated with the disease.
This seemingly paradoxical finding needs to be explained in the light of existent literature on pathogenetic mechanism in TA. Low levels of TNF-α and TNF-α blockade predispose to tuberculosis, which is implicated in pathogenesis of TA8. Is it possible that those with GG allele are less competent than those with the GA or AA allele in mounting an immune response against tuberculosis and eliminating it from the body. This could result in persistence of tuberculous antigen predisposing individuals with GG allele to autoimmunity. Could this ultimately lead to TA in presence of correct mix of other genetic and environmental factors?
Also, elderly age group is usually susceptible to GCA, the western counterpart of TA. GCA shares similarities in pathophysiology with TA and it is likely that these similarities are more common in the late-onset TA. In our study GA allele was present in one of the four late onset TA patients. This patient was the oldest in the study group. Does -308A allele protect from developing large vessel vasculitis in early life? Looking for this polymorphism in late onset TA and GCA may throw more light in this regard. Our study is limited by a small sample size. However, this is only a preliminary study and larger studies are required to determine the strength of association between TNF-α gene polymorphism, especially in late onset TA and GCA.
In conclusion, G allele at TNF-α - 308 was more common in TA patients and controls similar to that in other Indian as well as Japanese population. Compared to the western population, A allele was relatively less common in our population.
Acknowledgment
This study was financially supported by Fluid Research Grants from the Christian Medical College, Vellore, India. Authors thank Dr Ripal Shah for help in manuscript preparation.
References
- 1.Jain S, Sharma N, Singh S, Bali HK, Kumar L, Sharma BK. Takayasu arteritis in children and young Indians. Int J Cardiol. 2000;75:S153–7. doi: 10.1016/s0167-5273(00)00180-7. [DOI] [PubMed] [Google Scholar]
- 2.González-Gay MA, García-Porrúa C. Epidemiology of the vasculitides. Rheum Dis Clin North Am. 2001;27:729–49. doi: 10.1016/s0889-857x(05)70232-5. [DOI] [PubMed] [Google Scholar]
- 3.Wilson AG, di Giovine FS, Blakemore AI, Duff GW. Single base polymorphism in the human tumour necrosis factor alpha (TNF alpha) gene detectable by NcoI restriction of PCR product. Hum Mol Genet. 1992;1:353. doi: 10.1093/hmg/1.5.353. [DOI] [PubMed] [Google Scholar]
- 4.Braun N, Michel U, Ernst BP, Metzner R, Bitsch A, Weber F, et al. Gene polymorphism at position -308 of the tumor necrosis- factor-alpha (TNF-alpha) in multiple sclerosis and it's influence on the regulation of TNF-alpha production. Neurosci Lett. 1996;215:75–8. [PubMed] [Google Scholar]
- 5.Wilson AG, Symons JA, McDowell TL, Mc Devitt HO, Duff GW. Effects of a polymorphism in the human tumor necrosis factor alpha promoter on transcriptional activation. Proc Natl Acad Sci USA. 1997;94:3195–9. doi: 10.1073/pnas.94.7.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hajeer AH, Hutchinson IV. TNF-alpha gene polymorphism: clinical and biological implications. Microsc Res Tech. 2000;50:216–28. doi: 10.1002/1097-0029(20000801)50:3<216::AID-JEMT5>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 7.Lv N, Dang A, Zhu X, Liu Y, Liu Y, Zheng D, Hui R, Liu G. The role of tumour necrosis factor-a promoter genetic variation in Takayasu arteritis susceptibility and medical treatment. J Rheumatol. 2011;38:2602–7. doi: 10.3899/jrheum.110231. [DOI] [PubMed] [Google Scholar]
- 8.Aggarwal A, Chag M, Sinha N, Naik S. Takayasu's arteritis: role of Mycobacterium tuberculosis and its 65 kDa heat shock protein. Int J Cardiol. 1996;55:49–55. doi: 10.1016/0167-5273(96)02660-5. [DOI] [PubMed] [Google Scholar]
- 9.Gupta V, Sehajpal PK. Rapid detection of single nucleotide (-308) polymorphism in TNF-a promoter using ARMS–PCR. Curr Sci. 2003;85:1521–3. [Google Scholar]
- 10.Pereira LS, Yoon MK, Hwang TN, Hong JE, Ray K, Porco T, et al. Giant cell arteritis in Asians: a comparative study. Br J Ophthalmol. 2011;95:214–6. doi: 10.1136/bjo.2009.177220. [DOI] [PubMed] [Google Scholar]
- 11.Li Kam Wa TC, Mansur AH, Britton J, Williams G, Pavord I, Richards K, et al. Association between -308 tumor necrosis factor promoter polymorphism and bronchial hyperreactivity in asthma. Clin Exp Allergy. 1999;29:1204–8. doi: 10.1046/j.1365-2222.1999.00638.x. [DOI] [PubMed] [Google Scholar]
- 12.Witte JS, Palmer LJ, O’Connor RD, Hopkins PJ, Hall JM. Relation between tumour necrosis factor polymorphism TNFalpha-308 and risk of asthma. Eur J Hum Genet. 2002;10:82–5. doi: 10.1038/sj.ejhg.5200746. [DOI] [PubMed] [Google Scholar]
- 13.Westendorp RG, Langermans JA, Huizinga TW, Elouali AH, Verweij CL, Boomsma DI, et al. Genetic influence on cytokine production and fatal meningococcal disease. Lancet. 1997;349:170–3. doi: 10.1016/s0140-6736(96)06413-6. [DOI] [PubMed] [Google Scholar]
- 14.Schaaf BM, Seitzer U, Pravica V, Aries SP, Zabel P. Tumor necrosis factor-alpha -308 promoter gene polymorphism and increased tumor necrosis factor serum bioactivity in farmer's lung patients. Am J Respir Crit Care Med. 2001;163:379–82. doi: 10.1164/ajrccm.163.2.2002062. [DOI] [PubMed] [Google Scholar]
- 15.Shinohara Y, Ezura Y, Iwasaki H, Nakazawa I, Ishida R, Nakajima T, et al. Three TNF alpha single nucleotide polymorphisms in the Japanese population. Ann Hum Biol. 2002;29:579–83. doi: 10.1080/03014460110116444. [DOI] [PubMed] [Google Scholar]
- 16.Tripathy NK, Chauhan SK, Nityanand S. Cytokine mRNA repertoire of peripheral blood mononuclear cells in Takayasu's arteritis. Clin Exp Immunol. 2004;138:369–74. doi: 10.1111/j.1365-2249.2004.02613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park MC, Lee SW, Park YB, Lee SK. Serum cytokine profiles and their correlations with disease activity in Takayasu's arteritis. Rheumatology (Oxford) 2006;45:545–8. doi: 10.1093/rheumatology/kei266. [DOI] [PubMed] [Google Scholar]


