Abstract
The serum of some patients with insulin-resistant “diabetes” contains antibodies that bind to and block the cell membrane receptors for insulin. In this report, we have characterized the effects of the antireceptor antibodies on the interaction of 125I-insulin with its receptor on the human lymphoblastoid cell line IM-9. Up to 95% of specific insulin binding can be inhibited by pretreatment of the cells with these immunoglobulins. The onset of the inhibitory effect is time- and temperature-dependent, and the effect is reversed extremely slowly if the cells are suspended in a large excess of antibody-free buffer. These features of antibody binding can be easily distinguished from those for insulin binding to its receptor. The inhibitory effect of the antibodies can be reversed by exposure of the cells to conditions known to elute surface immunoglobulins.
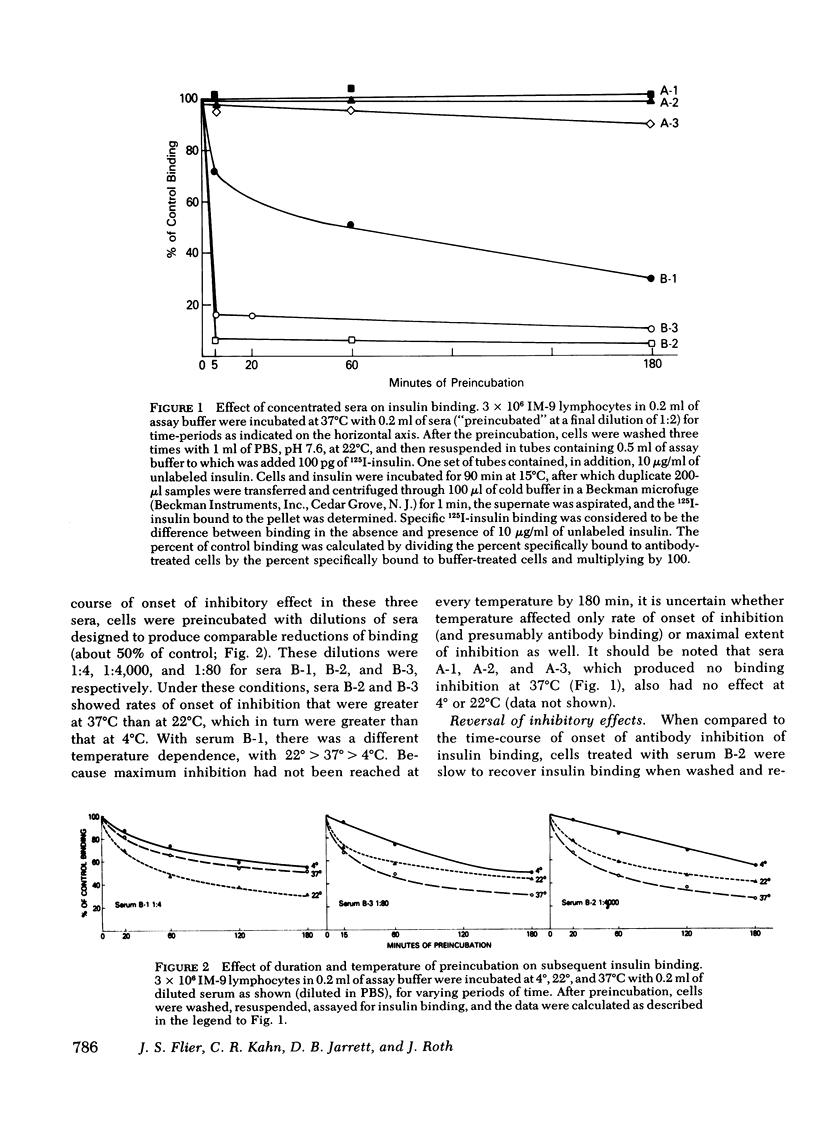
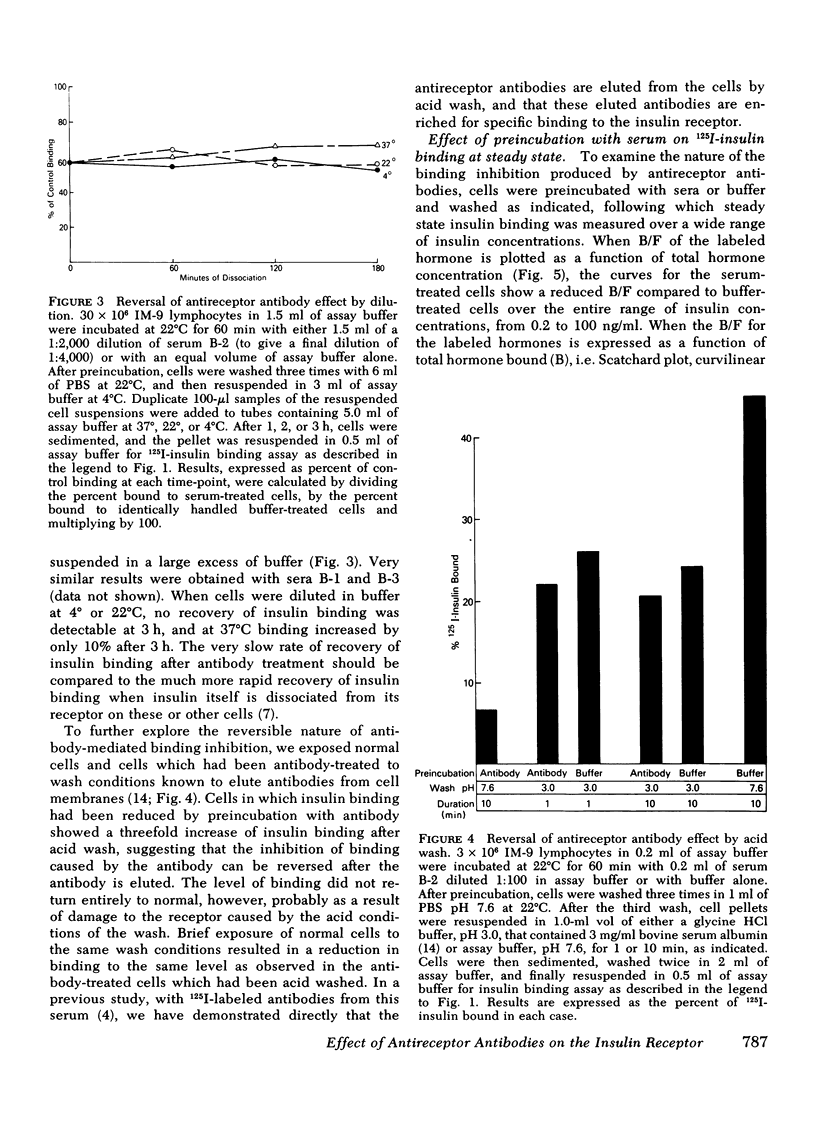
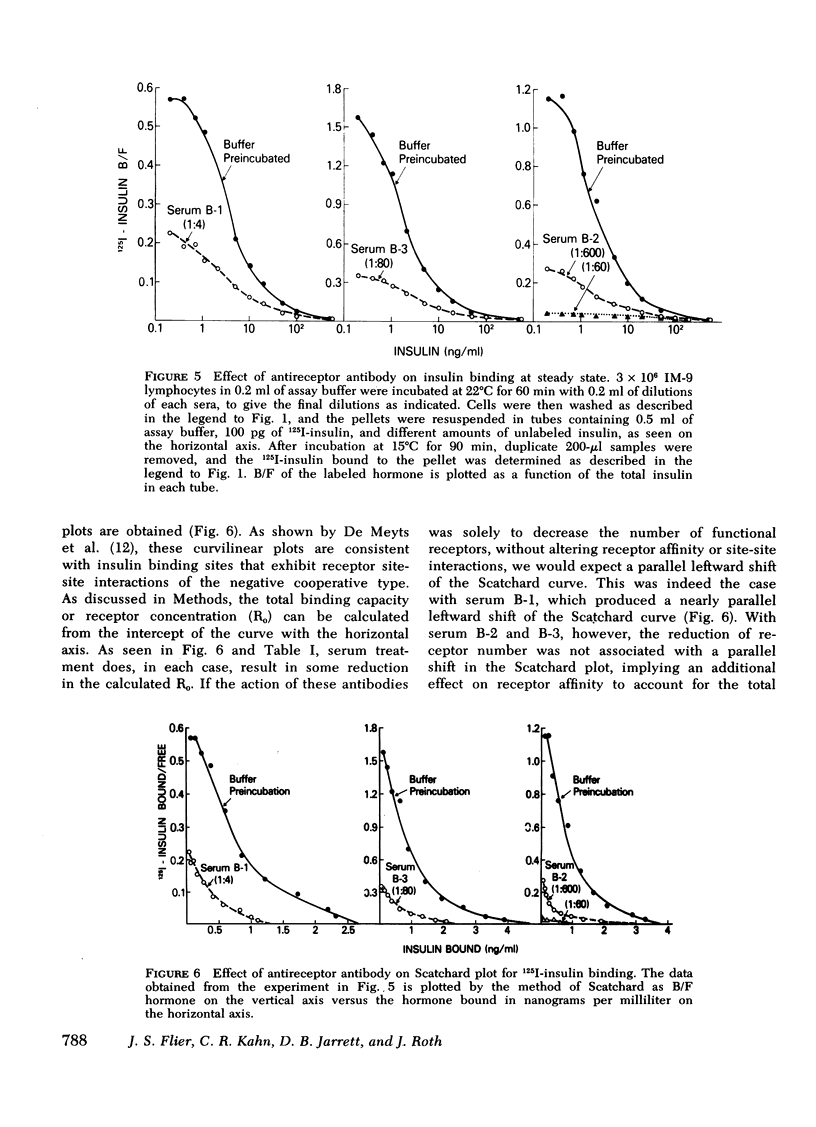
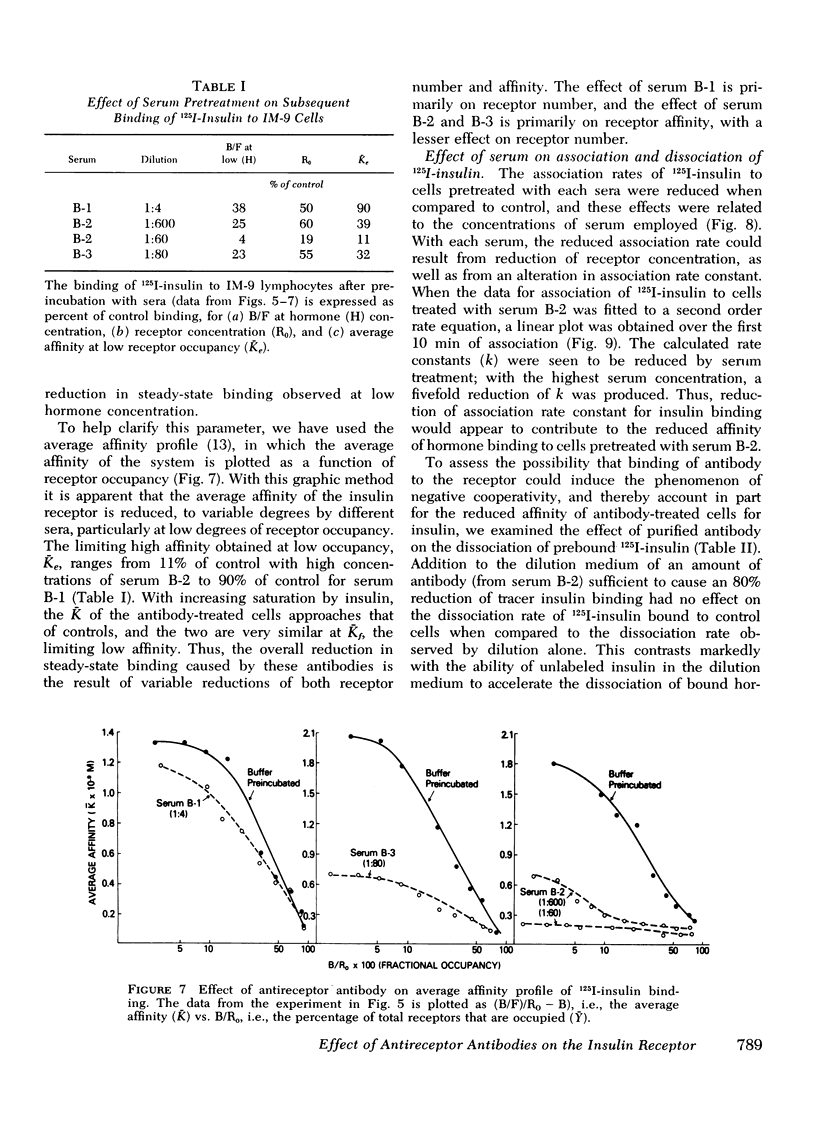
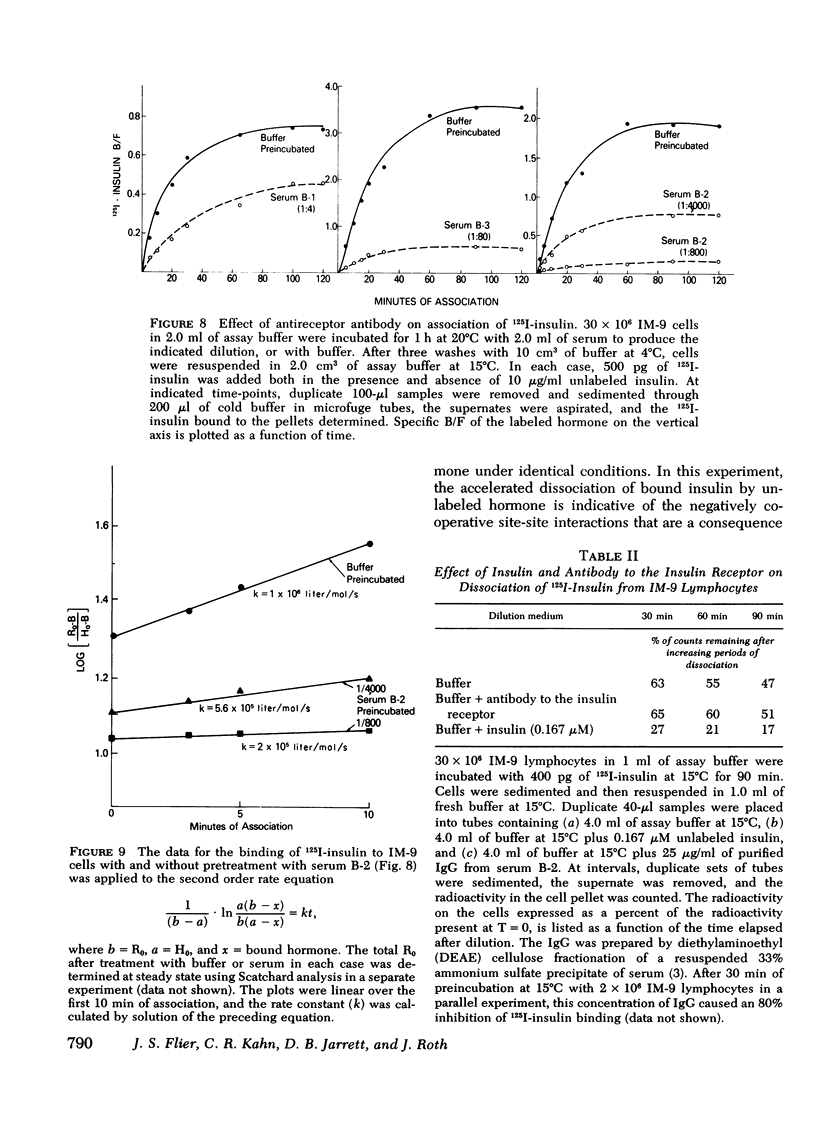
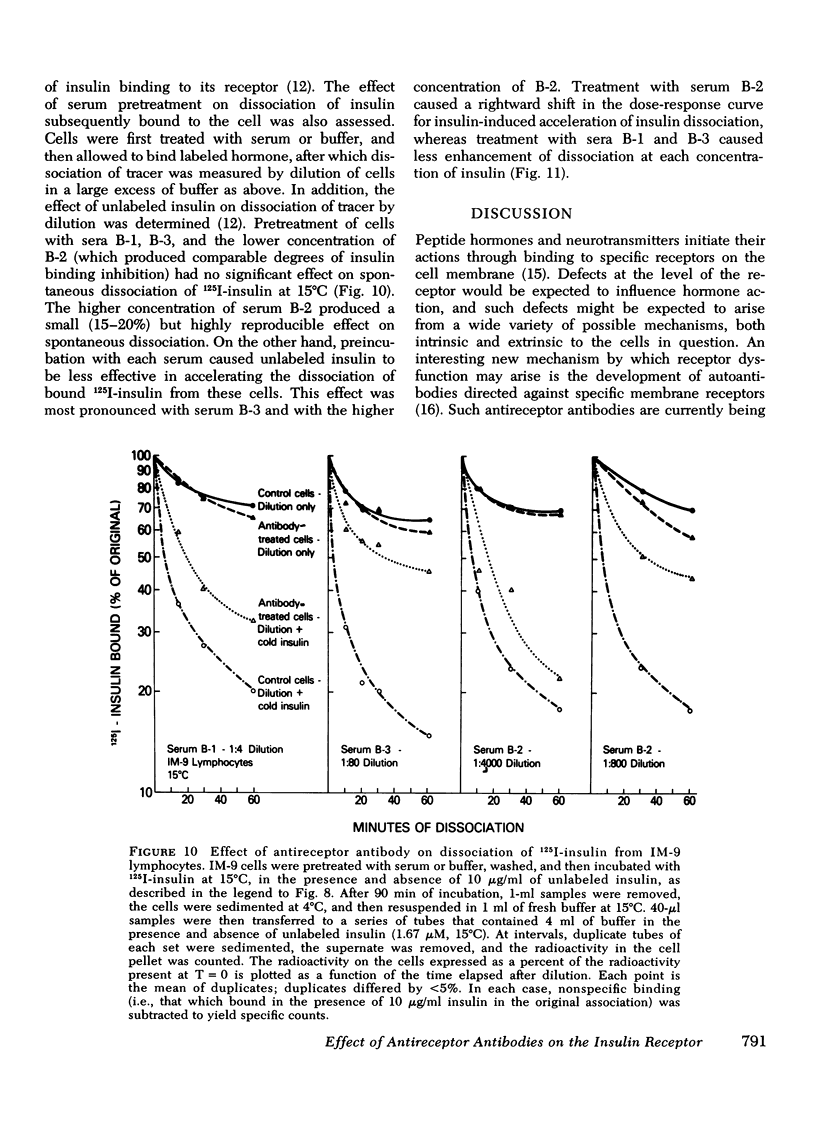
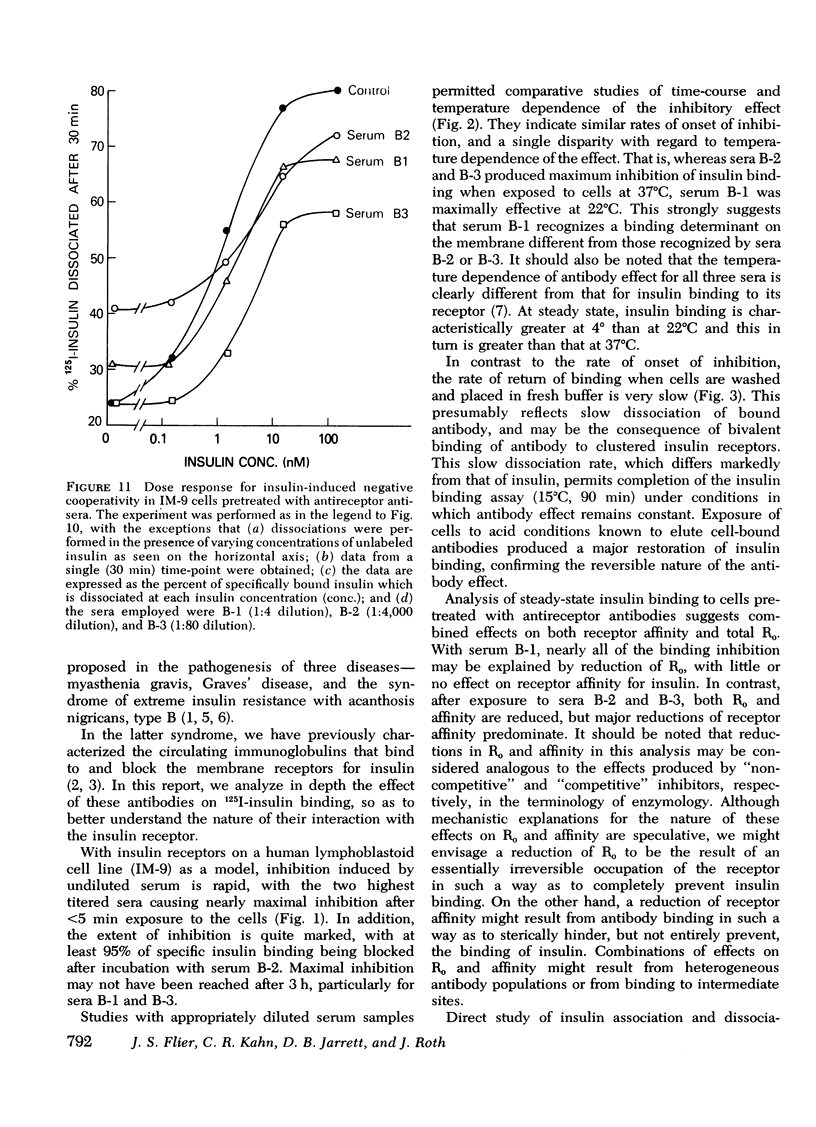
The three antireceptor sera studied appear to alter the insulin-receptor interaction in different ways. Two antisera markedly reduce receptor affinity through combined effects on the insulin association and dissociation rates, and, additionally, have smaller effects on available receptor number. A third antiserum primarily affects available receptor number and has little effect on receptor affinity. All three antisera inhibit the capacity of insulin to promote negatively cooperative site-site interactions among insulin receptors. The data suggest that these autoantibodies to the insulin receptor bind to different determinants on the receptor and may therefore be useful as unique probes of insulin receptor structure and function.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carnegie P. R., Mackay I. R. Vulnerability of cell-surface receptors to autoimmune reactions. Lancet. 1975 Oct 11;2(7937):684–687. doi: 10.1016/s0140-6736(75)90779-5. [DOI] [PubMed] [Google Scholar]
- DeMeyts P., Bainco A. R., Roth J. Site-site interactions among insulin receptors. Characterization of the negative cooperativity. J Biol Chem. 1976 Apr 10;251(7):1877–1888. [PubMed] [Google Scholar]
- Flier J. S., Kahn C. R., Roth J., Bar R. S. Antibodies that impair insulin receptor binding in an unusual diabetic syndrome with severe insulin resistance. Science. 1975 Oct 3;190(4209):63–65. doi: 10.1126/science.170678. [DOI] [PubMed] [Google Scholar]
- Freychet P., Roth J., Neville D. M., Jr Monoiodoinsulin: demonstration of its biological activity and binding to fat cells and liver membranes. Biochem Biophys Res Commun. 1971 Apr 16;43(2):400–408. doi: 10.1016/0006-291x(71)90767-4. [DOI] [PubMed] [Google Scholar]
- Gavin J. R., 3rd, Gorden P., Roth J., Archer J. A., Buell D. N. Characteristics of the human lymphocyte insulin receptor. J Biol Chem. 1973 Mar 25;248(6):2202–2207. [PubMed] [Google Scholar]
- Ginsberg B. H., Kahn C. R., Roth J., De Meyts P. Insulin-induced dissociation of its receptor into subunits: possible molecular concomitant of negative cooperativity. Biochem Biophys Res Commun. 1976 Dec 20;73(4):1068–1074. doi: 10.1016/0006-291x(76)90232-1. [DOI] [PubMed] [Google Scholar]
- Gliemann J., Gammeltoft S. The biological activity and the binding affinity of modified insulins determined on isolated rat fat cells. Diabetologia. 1974 Apr;10(2):105–113. doi: 10.1007/BF01219665. [DOI] [PubMed] [Google Scholar]
- Ishizaka T., Ishizaka K. Mechanisms of passive sensitization. IV. Dissociation of IgE molecules from basophil receptors at acid pH. J Immunol. 1974 Mar;112(3):1078–1084. [PubMed] [Google Scholar]
- Jarrett D. B., Roth J., Kahn C. R., Flier J. S. Direct method for detection and characterization of cell surface receptors for insulin by means of 125I-labeled autoantibodies against the insulin receptor. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4115–4119. doi: 10.1073/pnas.73.11.4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn C. R., Flier J. S., Bar R. S., Archer J. A., Gorden P., Martin M. M., Roth J. The syndromes of insulin resistance and acanthosis nigricans. Insulin-receptor disorders in man. N Engl J Med. 1976 Apr 1;294(14):739–745. doi: 10.1056/NEJM197604012941401. [DOI] [PubMed] [Google Scholar]
- Kahn C. R., Freychet P., Roth J., Neville D. M., Jr Quantitative aspects of the insulin-receptor interaction in liver plasma membranes. J Biol Chem. 1974 Apr 10;249(7):2249–2257. [PubMed] [Google Scholar]
- Kahn C. R. Membrane receptors for hormones and neurotransmitters. J Cell Biol. 1976 Aug;70(2 Pt 1):261–286. doi: 10.1083/jcb.70.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstrom J. M., Seybold M. E., Lennon V. A., Whittingham S., Duane D. D. Antibody to acetylcholine receptor in myasthenia gravis. Prevalence, clinical correlates, and diagnostic value. Neurology. 1976 Nov;26(11):1054–1059. doi: 10.1212/wnl.26.11.1054. [DOI] [PubMed] [Google Scholar]
- Smith B. R., Hall R. Thyroid-stimulating immunoglobulins in Graves' disease. Lancet. 1974 Aug 24;2(7878):427–431. doi: 10.1016/s0140-6736(74)91815-7. [DOI] [PubMed] [Google Scholar]


