Abstract
Background & objectives:
Leptospirosis, a spirochetal zoonosis, is underreported from the northern States of India. This study reports results of a 10-year retrospective sero-epidemiological survey of leptospirosis conducted in a tertiary care hospital in New Delhi, India.
Method:
A total of 1453 patients clinically suspected for leptospirosis were included and investigated initially by IgM ELISA. A proportion of these were subjected to culture, microscopic agglutination test (MAT) and polymerase chain reaction (PCR).
Results:
Of the 1453 patients, 391 (26.90%) were positive serologically by IgM ELISA. Seropositive and seronegative patients revealed no significant difference in clinical features and laboratory parameters. Amongst the IgM seropositive cases, culture for leptospires was positive in 5 of 192 (2.6%), MAT in 50 of 138 (36.23%), PCR from blood and urine in 10 of 115 (8.7%) and 10 of 38 (26.31%) cases, respectively. In Leptospira spp. positive patients co-infections with viral hepatitis E, malaria and dengue fever were diagnosed in 27 cases.
Interpretation & conclusions:
The overall seropositivity for leptospirosis was 26.9 per cent in our study. A decreasing trend in seropositivity was observed in recent years. Co-infections with malaria, dengue, hepatitis A and E were also seen. Since leptospirosis is a treatable disease, correct and rapid diagnosis may help in effective management of patients.
Keywords: Co-infection, Delhi, IgM ELISA, leptospirosis, MAT, PCR, sero-epidemiology
Leptospirosis, a spirochetal zoonosis caused by pathogenic members of the genus Leptospira, is an emerging global disease1. The disease is a diagnostic challenge owing to its protean manifestations and elusive features, which range from asymptomatic or mild cases to a severe fulminant disease presenting with a multitude of manifestations like jaundice, renal failure, pneumonia or haemorrhage and shock2. Leptospirosis is a disease amenable to antibiotic therapy, and if left untreated, may prove fatal. Hence there is a need for availability of rapid diagnostic modalities to initiate proper and timely management3.
The true incidence of leptospirosis in the northern region of India might be underestimated. Although endemic in the southern and western States of India, its true incidence in the northern States of the country remains underreported. This may be accounted by the fact that most of the infections are asymptomatic or mild hence often go unnoticed. Also, the lack of awareness of the disease amongst physicians has contributed to the paucity of data from this region. This may be explained by the scant data available on leptospirosis from Delhi4,5,6,7,8.
The present study is a sero-epidemiological retrospective study of leptospirosis in patients who were clinically suspected to have leptospirosis and were admitted or treated in the out patient department of Medicine, Gastroenterology, Neurology etc at the All India Institute of Medical Sciences (AIIMS), New Delhi, over a 10-years period. The aim was to find out the pattern of occurrence of leptospirosis in suspected patients, to analyze the clinical manifestations of proven leptospirosis cases, to detect leptospirosis by a battery of tools and to look for any existing co-infections with other common pathogens prevailing in the region.
Material & Methods
A total of 1453 patients clinically suspected to be suffering from leptospirosis who were either admitted or attended the out-patient department (OPD) of Medicine, Gastroenterology, Neurology, etc. the All India Institute of Medical Sciences (AIIMS), New Delhi, from April 2000 to March 2010, were evaluated in the Department of Microbiology by the following techniques - culture, serology, microscopic agglutination test (MAT) and PCR. The clinical data of a representative group of patients were also analyzed.
Inclusion criteria as laid down in the International Leptospirosis Society (ILS) guidelines were followed3. Patients who were suspected clinically of leptospirosis and presented with history of fever for more than seven days accompanied with any of the following manifestations i.e. severe headache, prostration, severe myalgia, conjunctival suffusion, uveitis, arthralgia, rash, hepato-splenomegaly, evidence of haemorrhage, renal failure, icterus, aseptic meningitis, acute respiratory distress syndrome (ARDS) and pulmonary haemorrhage were included in this study. Patients diagnosed with fulminant hepatitc failure (FHF, defined as development of hepatic encephalopathy within 4 wk of development of icterus) were also included in the study.
Blood samples (5 ml) were collected in plain vial during the acute phase and the serum was separated. The serum samples were stored at -20°C until further use. Whole blood sample and urine sample were collected in citrate vials and plain vials, respectively and were used immediately for culture, and a part of these used for DNA extraction for PCR and were stored at -20°C till further use.
Overall, 1453 samples were serologically evaluated for leptospirosis using IgM ELISA kit (Panbio, Australia) following the manufacturer's instructions. In case of any indeterminate/equivocal results, repeat blood sample was requested after one week and the test was repeated. Serum samples obtained from 158 healthy persons, including hospital staff and patient relatives who served as controls, were also analyzed for presence of anti-antibodies to Leptospira spp.
A total of 192 IgM ELISA positive blood and urine samples were subjected to culture for leptospires. Culture was performed by injecting one drop of blood and urine in separate tubes containing the Ellinghausen, McCullough, Johnson, and Harris (EMJH) medium supplemented with 3 per cent rabbit serum enriched with L-asparagin (3%), calcium chloride (1%), magnesium chloride (1%), pyruvate sodium (1%) and 0.1% agarose/agar (w/v). This culture medium (in house) was prepared in two formulations, one without antibiotics (non-selective) and the other with addition of 5-fluoruracil (300 mg/l) and nalidixic acid (20 mg/l), named as the selective medium. The medium, after inoculation, was incubated aerobically at 28°C for a maximum period of 6 wk. A drop from each culture medium was examined by dark field microscopy from second week onwards to a maximum period of up to six weeks.
Of the IgM ELISA positive samples (n=391), MAT was performed in 138 serum samples, whereas PCR was performed in 115 blood and 38 urine samples. MAT was performed using the following eight serovars - L.interrogans sensu lato (Australis, Autumnalis, Pomona, Sejroe, Tarassovi, Icterohaemorrhagica, Hebdomadis) and L.biflexa serovar Patoc, and an agglutination titre of 100 was considered reactive, where the end point was defined as the highest dilution of serum which agglutinated 50 per cent of the leptospires.
DNA was extracted from blood and urine samples by Qiagen DNA extraction Kit (Gmbh, Hilden, Germany). PCR was performed on blood and urine samples by the procedure described by Merien F et al9 with some modifications. Briefly, the oligonucleotide primers used were lep I, 5’ -GGCGGCGCGTCTITAAACATG - 3’; lep II, 5’ – TTCCCCCCATTGAGCAAGATT - 3’. Oligonucleotides lep I and lep II correspond to nucleotides 38 to 57 and 348 to 368 in the primary structure of the L. interrogans 16S gene, respectively. Amplification of DNA was performed in a total volume of 25 μl. The reaction mixture consisted of 1.5 mM MgCl2, 20 pmole each oligonucleotide primer, 200 μM each dATP, dTTP, dCTP, and dGTP, one unit of Taq DNA polymerase and 50-100 ng of template. The 331 base pair PCR product from a standard culture strain L. interrogans (L. australis) was cloned in the pGEM-T easy vector; recombinant plasmid was confirmed by sequencing. Recombinant plasmid was used as positive control. PCR was performed in a DNA thermal cycler (Applied Biosciences 1200, USA). The PCR cycle consisted of initial denaturation at 94°C for 2 min, followed by 35 cycles of denaturation at 94°C for 1 min, primer annealing at 56°C for 1.5 min and extension at 72°C for 1.5 min. A final extension at 72°C for 5 min for the completion of polymerization of every amplified fragment.
In this study we also looked for co-infections which share some or all clinical manifestations with leptospirosis. Those specifically looked for, included malaria, dengue/dengue haemorrhagic fever (DHF), hepatitis A and hepatitis E. Malaria screening included examination of peripheral blood smear with Giemsa staining, acridine-orange staining under UV light, immunochromatographic test to detect lactate dehydrogenase (LDH) for Plasmodium falciparum (Advantage MAL CARD, J. Mitra & Co. Pvt. Ltd, New Delhi, India) and the Quantitative Buffy Coat (QBC) assay (QBC Diagnostic Inc., Philipsburg, USA). Dengue virus, hepatitis A virus (HAV) and hepatitis E virus (HEV) infections were diagnosed serologically using commercial ELISA kits (Dengue Duo cassette, PanBio, France; Anti-HAV total, Diasorin; HEV IgM Beijing Wantai Biological Enterprise Ltd., China).
Clinical data of the patients were collected and analyzed using a structured questionnaire, which included the detailed clinical history and laboratory data from the hospital records.
Results
This study was conducted from April 2000 to March 2010 (10 years study period) and it was observed that the number of cases with suspected leptospirosis started to rise in the month of July and peaked in August. The number almost plateaued in the next two months and was finally declined after October. More than 50 per cent of the suspected as well as serologically diagnosed cases were registered during these four months (i.e. July to October).
The age of the patients with suspected leptospirosis ranged from 1-82 yr with 94 per cent of patients falling in the age group of 12-60 yr. Majority of the suspected patients were males 68 per cent as compared to 32 per cent females.
Of the 1453 patients who were clinically suspected of leptospirosis as per the ILS guidelines, 391 (26.90%) were positive serologically by IgM ELISA kit. Of the 158 healthy control, seven (4.45%) were positive for anti-leptospira antibodies by ELISA. On analyzing the clinical and demographic data of the 391 serologically positive patients it was observed that 226 cases (58%) were male and 42 per cent (165 of 391) were females. The age of the patients ranged from 6-80 yr with 94 per cent (367/391) of patients falling in the age group of 12-60 yr. Fifty nine per cent (230/391) patients were outdoor workers. History of exposure to animals and fresh-water could be elicited in 5.88 per cent (23/391) cases. A history of similar illness in the family could be elicited in 2 per cent (8/391) patients.
The common presenting features were fever in 377 (96.5%) followed by vomiting/nausea in 193 (49.4%), headache in 197 (50.5%), myalgia in 206 (52.8%), evidence of haemorrhage in 117 (29.8%), difficulty in breathing in 135 (34.4%), behavioural changes in 117 (29.8%), rash in 94 (24%), conjunctivitis in four (10%), arthralgia in 107 (27.5%), nuchal rigidity in 45 (11.5%) and sore throat in 68 (17.2%). In 211 (54%) cases, there was presumptive evidence of renal involvement with elevated blood urea and serum creatinine levels whereas 233 (59.7%) patients presented with jaundice and elevated liver enzymes (AST and ALT).
The clinical and demographic data of the 1062 patients suspected clinically of leptospirosis, but who were serologically negative were also analyzed (Table). It was observed that there was no significant difference in clinical features and laboratory parameters in the two (seropositive and seronegative) groups except for, lymphadenopathy, serum AST/ALT levels and serum creatinine levels, which were significantly higher in the seropositive group (Table).
Table.
Comparison of some clinical / laboratory features significantly different in the IgM seropositive and IgM eronegative groups

Of the 391 IgM antibody positive samples, 192 were subjected to blood and urine culture, 138 were subjected to MAT. Culture of blood and urine revealed positivity in five (2.6%). Of these 138 samples, MAT was positive in 50 (36.23%) samples. The predominant serovars observed were L. tarassovi (32 %) and L. australis (20%). All samples could not be tested by MAT due to technical limitations.
Amongst the IgM ELISA positive cases, PCR was performed on 115 blood and 38 urine samples. Ten samples each of blood (8.7%) and urine (26.31%) were positive by PCR. Of these, two cases were those in whom both blood and urine were positive for Leptospira spp. by PCR. Positivity of PCR in urine was found to be higher than that in blood.
Of the IgM ELISA negative cases, PCR was performed in selected blood and urine samples. PCR from blood was positive in 14 of 136 (10.29 %) cases, whereas PCR from urine was positive in 12 of 46 (26%) cases. Of these, three were positive for both blood and urine for leptospires by PCR. Again, positivity of PCR in urine was found to be better than that in blood.
Of the total cases (1,453) included in this study, overall 414 (391 ELISA positive and 23 PCR positive) were positive. The year-wise seropositivity in the initial three year was constant (30%) but gradually decreased to 10 per cent in the last four years of the study (Fig.). There was clustering of cases in the monsoon season. This increases the likelihood of co-infections by various other pathogens which co-circulate in the region during these months. The co-infections specifically looked for in this study were malaria, dengue/dengue hemorrhagic fever (DHF), hepatitis A and hepatitis E. The maximum number of co-infections of leptopsirosis was observed with hepatitis E (13 cases), followed by malaria (7 cases), dengue (5 cases). One patient had co-infections of dengue fever and hepatitis E, while another had co-infections malaria, dengue fever and hepatitis E.
Fig.
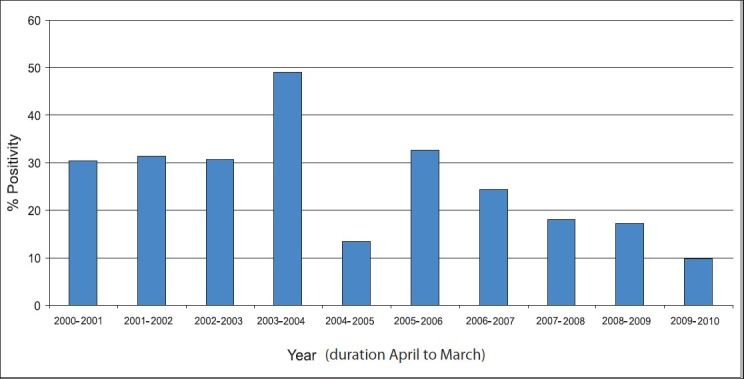
Year-wise distribution of cases.
Discussion
There is paucity of data on leptospirosis from Delhi. The first report of human leptospirosis from Delhi came in 19664. The authors found 3.22 per cent patients with pyrexia to be positive for leptospirosis. Following this study, apart from a few case reports5, there was no published data on leptospirosis from Delhi until 2002, when we reported a study from this region6. Of the 75 patients with suspected leptospirosis, 32 (42.6%) were positive by ELISA for Anti-leptospira IgM antibody. In a study done in the suburbs of East Delhi, 180 serum samples collected from febrile patients for the widal test (for enteric fever) were also tested for leptospira IgM antibodies (Panbio ELISA kit)7. Of these, 15 per cent were positive for leptospirosis. In another study, 10.7 per cent serum samples were found positive for leptospirosis by indirect hemeagglutination8.
Weil's disease is a term reserved for severe leptospirosis characterized by severe hepato-renal dysfunction associated with haemorrhagic diasthesis. The mortality rate varies from 5-15 per cent. Epidemics of leptospirosis are increasingly being reported from India, with most outbreaks being reported during rainy season6,8. Our study also revealed a seasonal peak from July to October.
The total seropositivity was 26.90 per cent in our study. We observed about 30 per cent seropositivity during 2000 to 2003 which decreased to less than 10 per cent in 2009-2010. The seroprevalence of leptospirosis in the Delhi region has been reported between 15 to 42.6 per cent4,5,6,7,8. The seroprevalence of leptospirosis in this study was in accordance with other studies especially those from the western and southern States of our country. In a study from Nagpur (Maharashtra), the prevalence of leptospirosis in patients being investigated for fever of unknown origin (FUO) was 32.73 per cent10. In another study from Chennai (Tamil Nadu), the year-wise prevalence of leptospirosis in 2004, 2005 and 2006 were 14.7, 24.9 and 32.3 per cent, respectively11.
In our study, the most common features were fever, vomiting, headache, myalgia, haemorrhagic manifestation and pulmonary features. There is need to investigate the patients in the microbiology laboratory to confirm the clinical suspicion of leptospirosis in order to institute treatment early and effectively.
Culture is technically demanding in leptospirosis, and the sensitivity reported is quite low as compared to PCR and serology. We also observed similar results of about 2.6 per cent in the selected patients in whom it was performed. The isolation of Leptospira spp. from the blood would be maximal during the early (leptospiremic) phase of the disease. As the disease progresses, the leptospires concentrate in the renal tubules and are excreted in the urine (leptospiuric phase). In this phase, urine rather than blood is the sample of choice to demonstrate and isolate the leptospires. A subset of the IgM seropositive cases were subjected to MAT and PCR for leptospires from blood and urine. The MAT positivity was 36.23 per cent in ELISA positive cases. PCR revealed more positivity in urine than in blood. This could be explained by the concentration of the Leptospira spp. in the kidney and excretion in urine.
A subset of the IgM seronegative cases were subjected to PCR for leptospires from blood and urine. This led to identification of an additional 26 cases which would have been missed if IgM ELISA alone was used to diagnose this disease. Again, PCR revealed more positivity in urine than in blood.
The reasons for high prevalence of leptospirosis in our study could be explained as follows: First, ours is a tertiary care centre and considering the protean manifestations of the disease, which in its milder forms can be highly non-specific, it is easy to comprehend that milder forms of the disease are unlikely to be admitted at our referral center. Second, in the severely affected cases, the patient may have succumbed to the illness at an early stage, even before the diagnosis had been established or leptospirosis considered as a possible diagnosis. Hence, the real magnitude of the problem may be higher than that reflected.
In this study, the patients were also assessed for co-existing infections like malaria, dengue, and enterically transmitted viral hepatitis as reported earlier12,13,14,15,16,17. This was a prudent step, since HEV and HAV both cause hepatitis, which is an important feature of icteric leptospirosis. Dengue haemorrhagic fever also presents with myriad features. Important ones include fever, haemorrhagic diathesis including thrombocytopenia, backache, pleural effusion, etc. Malaria, which is endemic in the tropics, including India, can also mimic many of the features of leptospirosis. Fever with chills and rigors, splenomegaly and in severe cases, haemorrhagic rash make this disease to be an important differential diagnosis for leptospirosis. Malaria by Plasmodium falciparum can lead to renal failure and mimic leptospirosis.
Twenty seven patients had one or more of those co-infections. One patient had co-infections with three organisms and another had co-infections with four organisms. This has been reported earlier also18,19.
Leptospirosis and malaria are amenable to antimicrobial and anti-malarial therapy, respectively. Dengue and HEV infection need intensive supportive care. Monitoring for bleeding diathesis i.e. platelet count in dengue, along with plasma volume maintenance are of prime importance. HEV usually resolves spontaneously, but can cause high morbidity and mortality during pregnancy. Hence, early diagnosis and management of these co-infections become imperative.
Since leptospirosis can be treated with beta-lactam antibiotics, correct and rapid diagnosis and differentiation from other diseases help in effective management of these patients. The knowledge of the local sero-epidemiology of this disease also helps the health authorities to undertake curative as well as preventive measures.
Acknowledgment
Authors acknowledge financial grant from Department of Biotechnology. We also acknowledge help of Drs Benu Dhawan, Shyam Tiwari, S.K. Kabra, Suryanaraynan and all clinicians who have sent patient's samples. Senior research fellowship to the third author (MMP) by Indian Council of Medical Research and technical assistance of Shri Pramod is also acknowledged.
References
- 1.Meslin FX. Global aspects of emerging and potential zoonoses: a WHO perspective. Emerg Infect Dis. 1997;3:223–8. doi: 10.3201/eid0302.970220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Speelman P, Hartskeerl R. Leptospirosis. In: Fauci, Braunwald, Kasper, editors. Harrison's Principles of Internal Medicine. 17th ed. McGrawHill; 2008. pp. 1048–51. [Google Scholar]
- 3.Singhal RL, Sood OP. Proceedings of the 3rd Round Table Conferences; 1998 Feb 23. New Delhi, India: Ranbaxy Science Foundation; 1998. Leptospirosis. [Google Scholar]
- 4.Joseph KM, Kalra SL. Leptospirosis in India. Indian J Med Res. 1966;54:611–4. [PubMed] [Google Scholar]
- 5.Chaudhry R. Leptospirosis presenting with hepatic encephalopathy: A case report. Indian Pract. 1999;52:423–5. [Google Scholar]
- 6.Chaudhry R, Premlatha MM, Mohanty S, Dhawan B, Singh KK, Dey AB. Emerging leptospirosis, north India. Emerg Infect Dis. 2002;8:1526–7. doi: 10.3201/eid0812.020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaur IR, Sachdeva R, Arora V, Talwar V. Preliminary survey of leptospirosis among febrile patients from urban slums of East Delhi. J Assoc Physicians India. 2003;51:249–51. [PubMed] [Google Scholar]
- 8.Gupta N, Rao RS, Bhalla P, Agarwal SK. Seroprevalence of leptospirosis in Delhi using indirect haemagglutination assay. Indian J Med Microbiol. 2004;22:134–5. [PubMed] [Google Scholar]
- 9.Mérien F, Amouriaux P, Perolat P, Saint Girons I. Polymerase chain reaction for the detection of Leptospira spp. in clinical samples. J Clin Microbiol. 1992;30:2219–24. doi: 10.1128/jcm.30.9.2219-2224.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Angnani R, Pathak AA, Mishra M. Prevalence of leptospirosis in various risk groups. Indian J Med Microbiol. 2003;21:271–3. [PubMed] [Google Scholar]
- 11.Sumathi G, Narayanan R, Shiv Kumar S. Leptospirosis Laboratory, Madras Medical College: Review of our experience (2004-2006) Indian J Med Microbiol. 2008;26:206–7. doi: 10.4103/0255-0857.40553. [DOI] [PubMed] [Google Scholar]
- 12.Sulzer AJ, Sulzer KR, Cantella RA, Colichon H, Latorre CR, Welch M. Study of coinciding foci of malaria and leptospirosis in the Peruvian Amazon area. Trans R Soc Trop Med Hyg. 1978;72:76–83. doi: 10.1016/0035-9203(78)90305-x. [DOI] [PubMed] [Google Scholar]
- 13.Wongsrichanalai C, Murray CK, Gray M, Miller RS, McDaniel P, Liao WJ, et al. Coinfection with malaria and leptospirosis. Am J Trop Med Hyg. 2003;68:583–5. doi: 10.4269/ajtmh.2003.68.583. [DOI] [PubMed] [Google Scholar]
- 14.Levett PN, Branch SL, Edwards CN. Detection of dengue infection in patients investigated for leptospirosis in Barbados. Am J Trop Med Hyg. 2000;62:112–4. doi: 10.4269/ajtmh.2000.62.112. [DOI] [PubMed] [Google Scholar]
- 15.Karande S, Gandhi D, Kulkarni M, Bharadwaj R, Pol S, Thakare J, et al. Concurrent outbreak of leptospirosis and dengue in Mumbai, India 2002. J Trop Pediatr. 2005;51:174–81. doi: 10.1093/tropej/fmh100. [DOI] [PubMed] [Google Scholar]
- 16.Flannery B, Pereira MM, Vellosa L de F, Carvalho C de C, De Codes LG, Orrico G de S, et al. Referral pattern of leptospirosis cases during a large urban epidemic of dengue. Am J Trop Med Hyg. 2001;65:657–63. doi: 10.4269/ajtmh.2001.65.657. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki A, Kumashiro R, Shirachi M, Kuroki M, Suzuku H, Tanikawa K, et al. Markedly prolonged jaundice from simultaneous infection with hepatitis E virus and leptospira. Kurume Med J. 2003;50:155–9. doi: 10.2739/kurumemedj.50.155. [DOI] [PubMed] [Google Scholar]
- 18.Chaudhry R, Pandey A, Das A, Broor S. Infection Potpourri: Are we watching? Indian J Pathol Microbiol. 2009;52:125. doi: 10.4103/0377-4929.44990. [DOI] [PubMed] [Google Scholar]
- 19.Behera B, Chaudhry R, Pandey A, Mohan A, Dar L, Premlatha MM, et al. Co-infections due to leptospira, dengue and hepatitis E: a diagnostic challenge. J Infect Dev Ctries. 2009;4:48–50. doi: 10.3855/jidc.535. [DOI] [PubMed] [Google Scholar]


