Abstract
Less supervision by the executive system after disruption of the right prefrontal cortex leads to increased risk taking in gambling because superficially attractive—but risky—choices are not suppressed. Similarly, people might gamble more in multitask situations than in single-task situations because concurrent executive processes usually interfere with each other. In the study reported here, we used a novel monetary decision-making paradigm to investigate whether multitasking could reduce rather than increase risk taking in gambling. We found that performing a task that induced cautious motor responding reduced gambling in a multitask situation (Experiment 1). We then found that a short period of inhibitory training lessened risk taking in gambling at least 2 hr later (Experiments 2 and 3). Our findings indicate that proactive motor control strongly affects monetary risk taking in gambling. The link between control systems at different cognitive levels might be exploited to develop new methods for rehabilitation of addiction and impulse-control disorders.
Keywords: gambling, impulse control, executive functions, stop signal, training, response inhibition, self-control
Flexible behavior and decision making require an executive control system, which oversees subordinate processes and intervenes when outcomes become suboptimal (Monsell & Driver, 2000). Impairments in executive control lead to maladaptive behavior because irrelevant motor actions are not inhibited. Similarly, less supervision by the executive system leads to impaired decision making because distracting information and suboptimal choices are not suppressed. Studies in the clinical and neuroscience domains suggest that executive control of the motor system may share mechanisms with high-level decision making. For instance, the latency of stopping motor responses is prolonged in pathological gamblers (e.g., Goudriaan, Oosterlaan, de Beurs, & Van Den Brink, 2006; but see Lipszyc & Schachar, 2010). Similar response-inhibition deficits have been observed in other impulse-control disorders, such as ADHD (Chamberlain & Sahakian, 2007; Nigg, 2001), or in individuals with substance dependence (Bechara, Noel, & Crone, 2006; de Wit, 2009).
Cognitive neuroscience studies suggest that brain areas associated with inhibiting motor output also regulate risk-taking behavior by suppressing superficially attractive but risky choices (Cohen & Lieberman, 2010; Knoch et al., 2006). For example, Knoch et al. (2006) used transcranial magnetic stimulation to show that temporarily disrupting the right dorsolateral prefrontal cortex (DLPFC)—which is important for executive control of motor actions (Bogacz, Wagenmakers, Forstmann, & Nieuwenhuis, 2010; Garavan, Ross, & Stein, 1999; Ivanoff, Branning, & Marois, 2008)—led to increased risk taking in gambling. On the basis of such findings, researchers have proposed that efficient control of impulses and urges in different domains relies on overlapping inhibitory mechanisms that allow people to suppress thoughts, actions, and decisions that are inappropriate, suboptimal, or potentially harmful (Chambers, Garavan, & Bellgrove, 2009; Crews & Boettiger, 2009; Goudriaan, Oosterlaan, de Beurs, & Van den Brink, 2004). However, direct support for such claims is scarce and mostly limited to correlational findings.
Therefore, we sought to uncover direct evidence that inhibitory motor control shares mechanisms with decision making when gambling by examining how these processes interact in multitask situations. Intuitively, people assume that the brain isolates decision making in different domains, with problems at higher cognitive levels (e.g., “should I take a day off or go to work?”) solved independently of problems at motor levels (e.g., “should I reach for that hot saucepan?”). However, behavioral scientists have shown that making multiple decisions simultaneously typically leads to impoverished behavior (Marois & Ivanoff, 2005; Pashler & Johnston, 1998). For example, using a cell phone in the car hinders driving performance (Strayer & Johnston, 2001). Similarly, monetary decision making might become less regulated in multitask situations because concurrent executive processes usually interfere with each other. Therefore, people might place riskier bets when gambling in multitask situations than in single-task situations because the executive system would be less able to suppress the tendency to pick higher and more appealing—yet also more risky—amounts.
But are the effects of multitasking on gambling and decision making necessarily detrimental? In the study reported here, we asked whether executive control in a concurrent task might actually reduce rather than increase risk taking in gambling. Specifically, we examined whether instructing participants to occasionally stop a motor response while they made monetary decisions encouraged them to place safer bets. Evidence of such a transfer of control between cognitive domains would have two important implications. First, it would provide direct support for the hypothesis that there is an overlap in executive mechanisms that regulate motor responses and decision making. Second, it could open new avenues for the treatment of psychiatric disorders that are linked to impaired inhibitory control, such as ADHD, substance abuse, and pathological gambling.
We examined the interaction between motor control and gambling using a novel behavioral task (Fig. 1). Participants were asked to gamble on one of six monetary amounts; however, participants were informed that the higher the amount, the less probable a win. Thus, selecting higher amounts constituted a more risky bet, whereas selecting lower amounts constituted a safer bet. Risk taking in our task consisted of preferring relatively higher amounts that carried a higher probability of losing (and in case of the most risky options, a negative expected value)1 over lower amounts that carried a lower probability of losing (Boyer, 2006); this is the same behavior that pathological gamblers engage in when, for example, wagering on horse races.
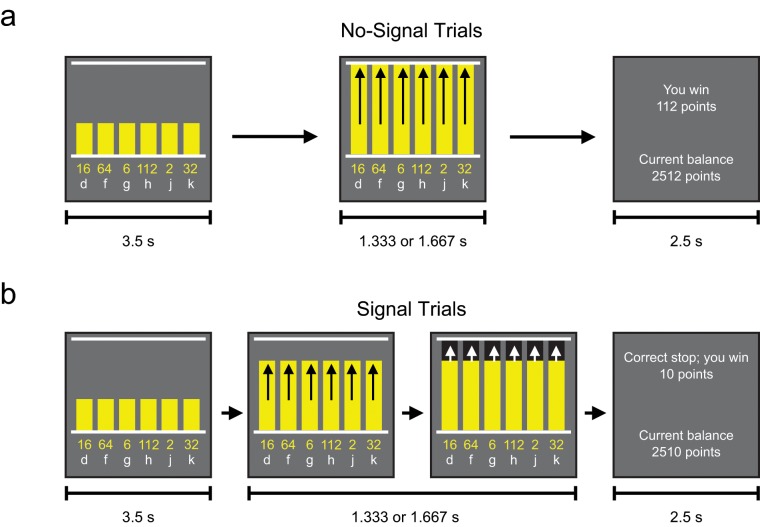
Fig. 1.
Example sequences for (a) no-signal trials and (b) signal trials in Experiment 1. In both types of trials, participants were presented with a display showing six numbers that indicated the number of points that could be won on that trial. These numbers were aligned above the letter keys to which they were assigned. The bars started rising after a 3.5-s delay and stopped at the top line after 1.333 s or 1.667 s (arrows are for illustrative purposes only). On no-signal trials, participants had to choose a number before the end of the trial but not sooner than 0.25 s before the bars reached the top line. On signal trials, the top of the bars turned black before the top line was reached. On these trials, participants either had to refrain from responding (stop group) or make an extra response by pressing the space bar after choosing a number (double-response group). On no-signal trials, participants received the chosen number of points if they won, and they forfeited half the chosen number of points if they lost; on signal trials, subjects won or lost a fixed amount (see the Method section for details). At the end of each trial, participants were told how much they had won or lost and what their current balance was.
In Experiment 1, participants performed the gambling task throughout the session. In some blocks (dual-task blocks), they also undertook a secondary task. The nature of the secondary task depended on the condition participants were assigned to. In the double-response condition, the secondary task required participants to occasionally execute an additional response when an extra signal occurred. Research has shown that monitoring for occasional signals and preparing additional responses increase dual-task demands (Verbruggen & Logan, 2009c). If executive processes at different levels of control interfere with each other, then participants should place riskier bets in dual-task blocks than in single-task blocks (in which participants had to perform only the gambling task) because the executive system would be less able to suppress the tendency to choose the riskier amounts (Cohen & Lieberman, 2010; Knoch et al., 2006).
In the stop condition, participants tried to stop themselves from making a choice when a signal occurred. Three accounts offered opposing predictions regarding participants’ choice behavior in the stop condition. According to the interference account, cross-task interference in multitask situations should cause participants to place riskier bets in dual-task blocks than in single-task blocks. By contrast, the transfer account holds that occasionally stopping motor responses should induce a general state of cautiousness that may propagate across cognitive domains. When preparing to stop, people make proactive adjustments and become more cautious in executing motor responses (Aron, 2011; Verbruggen & Logan, 2009c). If there is an overlap between mechanisms that regulate motor cautiousness and mechanisms that control gambling behavior, then cautiousness may transfer between domains; consequently, preparation for stopping motor responses might encourage risk-aversive behavior. Finally, according to the independence account, the cognitive processes involved in motor inhibition and gambling are mechanistically distinct; therefore, performing a double-response or stop task should not influence monetary decision making.
In Experiment 1, we found support for the transfer account. We therefore conducted two additional experiments in which participants completed a stop task or a double-response task prior to the gambling task, to test whether motor inhibition training leads to more cautious gambling behavior later in time.
Experiment 1
Method
Participants
Forty-four adults participated for monetary compensation (£6 per hr, plus money won in the gambling task). Table 1 shows participant characteristics and amounts won. Participants were divided equally between the double-response and stop groups. The groups were matched for gender and age. There were no group differences in impulsivity (assessed using the 11th version of the Barratt Impulsiveness Scale; Patton, Stanford, & Barratt, 1995) or general risk- seeking behavior (assessed using the Stimulating-Instrumental Risk Inventory; Zaleskiewicz, 2001). All experiments were approved by the research ethics committee of the Cardiff University School of Psychology.
Table 1.
Descriptive Statistics for Experiments 1 Through 3
| Variable | Experiment 1 (N = 44) | Experiment 2 (N = 81) | Experiment 3 (N = 54) |
|---|---|---|---|
| Gender | 52% female, 48% male | 63% female, 37% male | 69% female, 31% male |
| Mean age | 24.0 years (range = 18–40 years) | 23.6 years (range = 18–41 years) | 21.3 years (range = 18–33 years) |
| Mean winnings | £0.5 (range = £0–1.9) | £1.5 (range = £0–4.2) | £1.5 (range = £0–4.2) |
| Mean BIS-11 score | 62 (SD = 21) | 65 (SD = 9.8) | 65 (SD = 8.9) |
| Mean SIRI score | 39 (SD = 6.3) | 37 (SD = 6.6) | 39 (SD = 7.3) |
Note: The range of possible scores on the 11th version of the Barratt Impulsiveness Scale (BIS-11; Patton, Stanford, & Barratt, 1995) is 30 to 125; higher scores indicate more impulsive behavior. On the Stimulating-Instrumental Risk Inventory (SIRI; Zaleskiewicz, 2001), scores of 45 and below indicate a tendency toward avoiding taking risks.
Procedure
Stimuli were presented on a 19-in. LCD monitor against a gray background. The task was run using the Psychophysics Toolbox (Version 3; Brainard, 1997). On each trial, six vertical bars of equal height were arrayed left to right above a horizontal line; there was a second horizontal line at the top of the screen (Fig. 1). Each bar was associated with a different monetary amount and a specific response key (the “d,” “f,” “g,” “h,” “j,” or “k” key of a keyboard). Subjects were instructed to select one of the amounts by pressing the corresponding key, and they were informed that the probability of winning decreased as the amount increased. Rather than simply presenting the amounts from lowest to highest, we varied the order from trial to trial to prevent choice from being driven by spatial-attention or response-bias effects (which might occur, for example, if higher amounts were consistently presented on the right of the screen).
At the start of each trial in the single-task blocks (Fig. 1a), there was a 3.5-s delay, and then the bars started rising together. All bars reached the top line simultaneously after 1.33 s on low-bar trials (in which the distance between the bottom and top lines was approximately 7.5 cm) or after 1.67 s on high-bar trials (in which the distance between the bottom and top lines was approximately 9 cm). We manipulated bar height to test for effects of choice latency (see Supplementary Analyses Experiment 1 in the Supplemental Material available online). Trials ended 0.25 s after the bars reached the top line. The long time intervals (average = 5 s) and the initial phase in which the bars did not rise ensured that there was minimal time pressure to make a decision. Participants had to make a response before the end of the trial but not sooner than 0.25 s before the bars reached the top line. We used the moving bars and the response-window restrictions to ensure that stop signals could be presented at an optimal moment (see also Coxon, Stinear, & Byblow, 2007). Feedback was presented at the end of each trial to indicate how much participants had won or lost and what their current balance was. The feedback screen was replaced by a blank screen after 2.5 s, and the following trial started after a further 0.5 s.
In dual-task blocks, the procedure was the same as in single-task blocks on two of every three trials; however, on one of every three trials (signal trials), the top of the rising bars turned black just before reaching the top line (see Fig. 1b). On signal trials in the double-response group, participants had to press the space bar of the keyboard with either thumb after they had indicated their monetary choice. They had to press the space bar within 0.25 s after the bars reached the top line. On signal trials in the stop group, participants tried to refrain from making any response on the keyboard. In both groups, the moment of signal presentation was dynamically adjusted using a tracking procedure, which ensured that each individual would succeed in making the double response (double-response group) or withhold the response (stop group) on approximately 50% of the signal trials. Initially, the bars turned black 0.266 s before the top line was reached. When participants successfully stopped their response or pressed the space bar in time, this delay was decreased by 0.033 s on the following trial, which made it harder to successfully stop or execute the double response. When participants failed to stop or execute the double-response in time, the delay was increased by 0.033 s. At the beginning of each block, a cue (“NO-SIGNAL BLOCK” or “SIGNAL BLOCK”) was presented in the center of the screen.
On each trial in both single- and dual-task blocks, participants could win or lose points. The exact amount depended on the stakes (low, medium, or high). The numbers of points participants could win in the low-stakes condition were 112 (pwin = .15), 64 (pwin = .27), 32 (pwin = .39), 16 (pwin = .51), 6 (pwin = .63), or 2 (pwin = .75). When participants lost, they lost half of the amount they gambled. A random number generator determined whether a participant won or lost. On each trial, a number between 0 and 1 was selected; when the generated number was smaller than the probability of winning the chosen amount, participants won; otherwise, they lost. Amounts decreased exponentially to make the higher amounts more attractive. Without revealing the exact probabilities, we informed participants at the beginning of the experiment that the probability of winning was lower for higher amounts. Because we could not infer which response participants were planning to execute on successful stop-signal trials, the number of points won or lost on all signal trials was fixed. Participants won 10 points on successful signal trials and lost 10 points on unsuccessful signal trials in both the stop and double-response groups. Thus, on double-response trials, participants always won or lost 10 points, regardless of their choice. Similarly, on unsuccessful stop trials, participants always lost 10 points, regardless of the amount they indicated with their incorrectly executed choice response.
In the medium-stakes condition, all amounts were two times greater than in the low-stakes condition; in the high-stakes condition, amounts were four times greater than in the low-stakes condition. We manipulated the stakes for several reasons: to increase selection demands, to encourage processing of the different amounts on each trial, and to encourage participants to consider the relative risk or benefit of each amount. The three stakes occurred in random order with equal probability, and participants were not explicitly informed whether the trial featured low, medium, or high stakes. Each participant’s starting balance was 2,500 points. At the end of the experiment, the total amount won was converted to money (1,000 points = £1).
The experiment started with a short practice phase that consisted of a single-task block and a dual-task block. Practice trials were conducted following the same procedure used for test trials. The balance of points won or lost was reset after this practice phase. The experimental phase consisted of four single-task blocks and four dual-task blocks of 36 trials each. There was a short break between each block, and single- and dual-task blocks alternated.
Betting scores
For each participant, we calculated a betting score by taking the average of all his or her choices (range = 1–6). Choice 1 corresponded to the smallest amount, which had the highest probability of winning (hence was the safest bet). Choice 6 was the highest amount, which had the lowest probability of winning (hence was the most risky bet). Consequently, a higher average betting score indicated that participants preferred riskier bets with a lower probability of winning.
Results and discussion
Approximately 50% of responses on signal trials were correct, which confirmed the effectiveness of the tracking procedures (failed double responses = 46%, failed stops = 47%). Thus, there was no consistent difference in success rates on signal trials between the groups (F < 1). Even though we did not use separate tracking procedures for each stake, additional analyses showed that the percentage of failed signal trials was similar for each stake (low stakes = 47%, medium stakes = 46%, high stakes = 49%; p > .39). This was true for both groups (i.e., the interaction between stake and group was not significant, p > .18).
To test the effect of multitasking on gambling, we compared betting scores between dual-task and single-task blocks. We concentrated specifically on no-signal trials. This allowed us to isolate the behavioral effects of monitoring for extra signals and preparing to either make a double response or to stop the first response (in dual-task blocks), compared with conditions without such demands (single-task blocks).
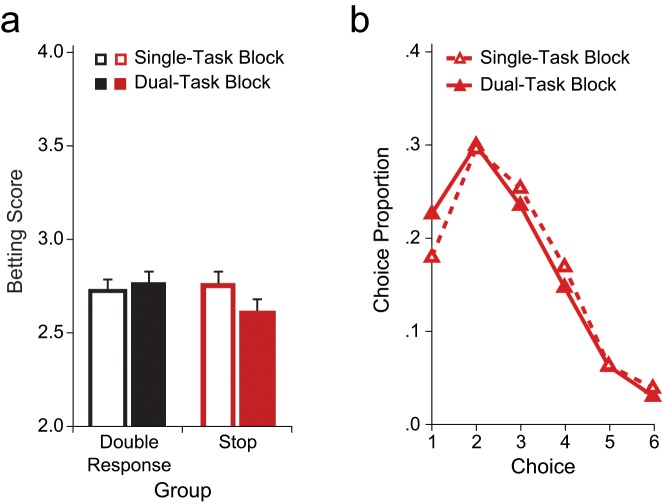
We analyzed betting scores using a mixed analysis of variance with block type (single task vs. dual task) and stake (low, medium, high) as within-subjects factors and group (double response vs. stop) as a between-subjects factor (see Table 2). Results showed that participants in the double-response group tended to place more risky bets in dual-task blocks (mean betting score = 2.77) than in single-task blocks (mean betting score = 2.72; Fig. 2a), but this effect failed to reach significance. More important, however, participants in the stop group showed the opposite result: They not only became more cautious when making their choices (as indexed by longer choice latencies; see Supplementary Analyses Experiment 1 in the Supplemental Material), but also placed overall safer bets in dual-task blocks (mean betting score = 2.62) than in single-task blocks (mean betting score = 2.77), F(1, 23) = 4.7, p = .04, ηp2 = .19.
Table 2.
Results of the Analysis of Variance for Experiment 1
| Factor | df | F | p |
|---|---|---|---|
| Group | 1, 46 | 0.05 | .83 |
| Block type | 1, 46 | 1.15 | .29 |
| Stake | 2, 92 | 135.3 | .001 |
| Group × Block Type | 1, 46 | 4.29 | .04 |
| Group × Stake | 2, 92 | 1.14 | .33 |
| Block Type × Stake | 2, 92 | 0.94 | .40 |
| Group × Block Type × Stake | 2, 92 | 0.38 | .69 |
Note: Significant results are presented in boldface (p < .05).
Fig. 2.
Results of Experiment 1. Mean betting scores for no-signal trials (a) are shown as a function of group and block type. Error bars show the standard error of the difference between dual-task and single-task blocks. The distribution of choices made by the stop group in the two block types is shown in (b). Choice 1 was the safest bet; Choice 6 was the riskiest bet.
The effect of block type in the stop group shows that preparing to stop motor responses encourages cautious monetary decisions and that this cautiousness counteracts and reverses the detrimental effects usually associated with multitasking. Thus, multitasking does not necessarily lead to increased risk taking in gambling; concurrent executive processes can make people generally risk aversive when these processes regulate cautiousness at a motor level.2 This conclusion was supported by a significant two-way Block Type × Group interaction (p < .05). There was also a main effect of stake, which indicated that betting scores were lower when stakes were high (p < .001; mean betting scores: high stakes = 2.29, medium stakes = 2.68, low stakes = 3.18). No other effects reached significance (Table 2).
Additional analyses of specific choices showed that participants in the stop group tended to select the most risky bets (Choice 6) less often in dual-task blocks than in single-task blocks (Fig. 2b).3 Furthermore, for the dual-task blocks in the stop group, there was a preference for Choice 1 (the safest option), which had a lower expected value than did Choices 3 through 5.4 Thus, it appears that participants in the stop group became overly cautious in dual-task blocks, as taking a certain amount of risk was rewarded in our gambling task. Further analyses also showed that the difference in betting scores between block types in the stop condition was not caused by differences in choice latencies, effects of probability learning, estimation of the expected value of the choice options, block order, increased variability, or priming of participants to focus more on either wins or losses (see Analyses of Average Standard Deviation and Supplementary Analyses Experiment 1 in the Supplemental Material). Finally, a closer inspection of the distribution of keys selected by participants demonstrated that in all conditions, participants took amounts into account when they made their choice (see Table E4 in the Supplemental Material).
Experiments 2 and 3
Experiment 1 demonstrated that simultaneously regulating motor performance and making monetary decisions does not necessarily lead to increased risk taking in gambling. On the contrary, preparing to withhold a motor response encourages a cautious executive control state that generalizes to seemingly unrelated monetary decisions. Next, we asked whether motor cautiousness would also influence monetary gambling when these processes were separated in time. A recent study showed that performing an inhibition task in which participants had to ignore words led to depletion of executive control resources; this caused more risk-taking behavior in a subsequent gambling task (Freeman & Muraven, 2010). This finding seems at odds with the results of Experiment 1. However, we propose that in our gambling task, proactive motor slowing—which is prominent in the stop task but not necessarily in other inhibition tasks—counteracted any depletion effects and encouraged risk-aversive monetary decision making.
In Experiments 2 and 3, we tested whether this transfer of cautiousness would still be present when the gambling task followed the stop task. Both experiments consisted of two phases: a training phase, which did not involve gambling, and a test phase, in which participants chose among different amounts they could win. The test phase did not involve an additional task; therefore, all test blocks were identical to the single-task blocks from Experiment 1. The only differences between Experiments 2 and 3 were that Experiment 2 included a control group that immediately started with the gambling task, and the test phase in Experiment 3 was conducted 2 hr after the training phase was completed.
Method
Participants
One hundred thirty-five adults participated for monetary compensation (£6 per hr, plus money won in the gambling task). Table 1 shows participant characteristics and amounts won. In each experiment, participants were divided equally into groups matched for gender and age. Participants were assessed for impulsivity and general risk-taking behavior as in Experiment 1, and there were no group differences in either of these factors.
Procedure
In addition to the double-response and stop groups, Experiment 2 included a control group, which started immediately with the gambling task. The double-response and stop groups started with a training phase in which the primary task was to identify a go stimulus (square vs. diamond) as rapidly and accurately as possible (Fig. 3). On no-signal trials, participants saw a central fixation point for 0.75 s, after which either an open square or an open diamond surrounded the fixation point for 1.5 s. Participants responded with their left or right hands, respectively (“c” or “m” on a keyboard) to identify the stimulus as either a square or a diamond.
Fig. 3.
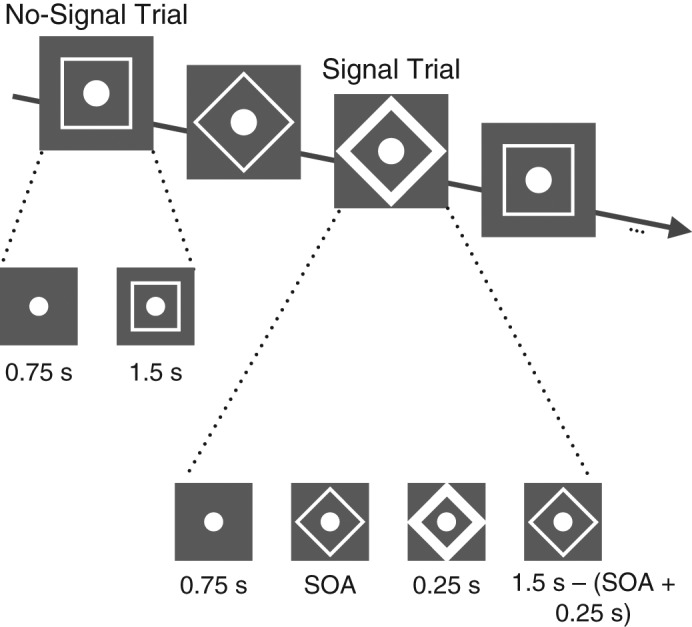
Example trial sequence from the training phase of Experiments 2 and 3. On no-signal trials, participants saw a central fixation point, after which either an open square or an open diamond surrounded the fixation point. Participants had to hit either “c” or “m” on the keyboard to indicate “square” or “diamond.” Signal trials began in the same way, but the shape around the fixation point turned bold after a variable stimulus onset asynchrony (SOA), which was initially set at 0.25 s and subsequently adjusted using a tracking procedure (see the Method section for details). The boldface was removed from the shape after 0.25 s, and the nonboldface shape remained on-screen for the remainder of the trial. Participants tried to either withhold a response (stop group) or generate an extra response by pressing an alternate key (double-response group).
Signal trials (25% of all training trials) began in the same way as no-signal trials, but the outline of the diamond or square shape turned bold after a variable stimulus onset asynchrony (SOA). On these trials, participants in the double-response group had to press the space bar as quickly as possible with either thumb after they pressed “c” or “m”; participants in the stop group were instructed to refrain from responding. The SOA between the go stimulus (the shape) and the signal was initially set at 0.25 s. In the stop group, the SOA was continuously adjusted according to a tracking procedure so that participants would be able to stop on approximately 50% of trials (Verbruggen & Logan, 2009b). When participants made a response, the SOA decreased by 0.05 s on the following trial (signal-respond trial); when participants successfully stopped, the SOA increased by 0.05 s on the following trial (signal-inhibit trial).
In the double-response group, we simulated a tracking procedure to produce a similar range of SOAs as in the stop group (see Verbruggen & Logan, 2009c, for a similar procedure). In the simulation, we used an estimate of the reaction time to the stop signal (0.225 s). This value was based on findings of our previous studies (e.g., Verbruggen & Logan, 2009a). When the latency of the first response on a double-response trial was shorter than the SOA plus 0.225 s, the SOA decreased by 0.05 s on the following trial (viz., signal-respond trial); when the latency of the first response on a double-response trial was longer than the SOA plus 0.225 s, the SOA increased by 0.05 s on the following trial (viz., signal-inhibit trial).
The training phase of Experiment 2 consisted of 10 blocks of 72 trials each (~30 min in total), with a short break between each block. No points were awarded in the training phase. Participants took a 2-min break after the training phase, then began the test phase, which consisted of 7 blocks of 12 trials each. The control group started immediately with the test phase. During the test phase, participants completed the same gambling task as in the single-task blocks of Experiment 1 (thus, all trials were no-signal trials in the test phase). The training phase of Experiment 3 consisted of 15 blocks of 56 trials each (~35 min in total). The test phase followed the training phase after 2 hr. During this 2-hr delay, participants were free to leave the lab but were asked to report what they had done after they returned for the test phase. There were 14 blocks in the test phase of Experiment 3.
Results and discussion
For each experiment, we analyzed betting scores using a mixed analysis of variance with block (Experiment 2: Test Block 1–7; Experiment 3: Test Block 1–14) and stake (low, medium, high) as within-subjects factors and group (double-response, stop, or control) as the between-subjects factor (Table 3). Because there were not enough observations for a full factorial analysis, we performed separate tests for block and stake.
Table 3.
Results of the Analyses of Variance for the Test Phases of Experiments 2 and 3
| Experiment 2 | Experiment 3 | |||||
|---|---|---|---|---|---|---|
| Factor | df | F | p | df | F | p |
| Group | 2, 78 | 6.44 | .003 | 1, 52 | 4.89 | .03 |
| Block | 6, 468 | 8.17 | .001 | 13, 676 | 6.42 | .001 |
| Stake | 2, 156 | 41.26 | .001 | 2, 104 | 13.97 | .001 |
| Group × Block | 12, 468 | 0.66 | .79 | 13, 676 | 0.51 | .92 |
| Group × Stake | 4, 156 | 0.63 | .64 | 2, 104 | 0.69 | .51 |
Note: Significant results are presented in boldface (p < .05).
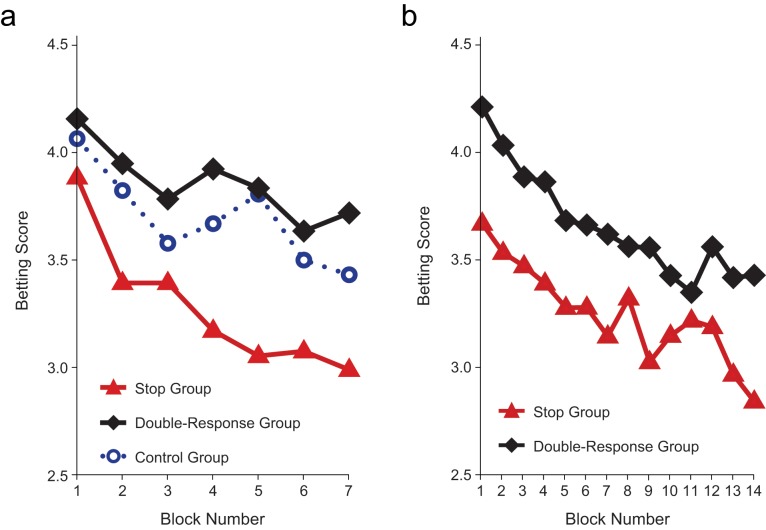
Results of Experiment 2 revealed a reliable aftereffect of executive control training on gambling behavior (Fig. 4). Participants in the stop group took 10% to 15% less monetary risk than did participants in the double-response group, F(1, 52) = 6.1, p = .02, ηp2 = .11, and the control group, which did not receive any training, F(1, 52) = 10.8, p = .002, ηp2 = .17. This finding demonstrates that motor cautiousness in the stop task transferred to monetary decision making, even when the stop and gambling tasks did not overlap in time. There was a numerical trend for higher betting scores in the double-response group than in the control group, which would be consistent with a depletion account; however, the difference was not significant (p = .29).
Fig. 4.
Mean betting score as a function of group and block in (a) Experiment 2 and (b) Experiment 3.
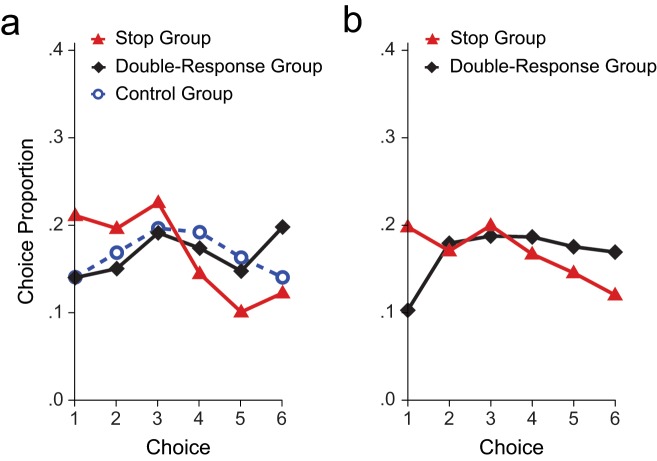
Thus, the cognitive characteristics of the training phase were crucial: Cautiousness transferred from the training phase to the test phase only when the training phase involved stopping and not when it involved executing a second response. This conclusion was supported by a significant main effect of group (p = .003; Table 3). A closer inspection of choice proportions (Fig. 5) showed that there were significant differences between the control group and the stop group for Choice 5, p < .001, and Choice 4, p < .029. When we collapsed across Choices 1 through 3, the difference between the control and stop groups was marginally significant (p = .05). There were differences between the double-response group and the stop group for Choice 6, p < .027, and Choice 5, p < .016. When we collapsed across Choices 1 through 3, the difference between double-response and stop group was also significant (p < .022).
Fig. 5.
Results of (a) Experiment 2 and (b) Experiment 3: distribution of choices in each of the groups. Choice 1 was the safest bet; Choice 6 was the riskiest bet.
In Experiment 3, we tested whether the transfer of cautiousness was still present when the delay between the training phase and the test phase was increased. Participants again performed either the double-response task or the stop task in the training phase, but the test phase was now undertaken 2 hr later. Consistent with Experiment 2, results of Experiment 3 showed that participants in the stop group took 10% to 15% less risk than did participants in the double-response group (Fig. 4). The main effect of group was again significant (p = .03; Table 3). As Figure 5 shows, participants in the stop group selected Choice 1 more often than participants in the double-response group did, p < .011. When we collapsed across Choices 5 and 6, the frequency of making this choice differed significantly between the stop and double-response groups, p < .05.
Taken together, the results of Experiments 2 and 3 show that cautiousness at a motor level influences gambling behavior, even when motor training and monetary decision making occur at least 2 hr apart. The effects in the test blocks did not correlate with the outcome of the stop process in the training phase and were not caused by differences in choice latencies (see Supplementary Analyses Experiments 2–3 in the Supplemental Material). Furthermore, analyses of choice proportions showed that after stop training, participants specifically avoided the two most risky bets (Fig. 5). On the basis of these findings, we propose that the requirement to occasionally stop a motor response can train people to become cautious and less impulsive when they make monetary decisions. This increased cautiousness might overcome the previously observed effect of depleting the executive control system (Freeman & Muraven, 2010).
A recent study showed that participants who were instructed to be cautious in a stop-signal task (similar to the one used here in Experiments 2 and 3) consumed less food in a subsequent test phase than participants who were instructed to respond as quickly and impulsively as possible (Guerrieri, Nederkoorn, Schrooten, Martijn, & Jansen, 2009). Unfortunately, the lack of an appropriate control condition in this previous study obscures the underlying basis of this effect, which could have arisen due to increased cautiousness, increased impulsivity, or a combination of both (Guerrieri et al., 2009). Nevertheless, these findings are consistent with our observation that engaging in an inhibitory motor task can boost behavioral caution and reduce impulsivity.
Future work should further explore how stopping-induced cautiousness and reduced motor impulsivity can transfer to various clinically relevant behaviors, including cigarette smoking and consumption of food and alcohol (see also Friese, Hofmann, & Wiers, 2011). Mechanisms that regulate stopping and motor cautiousness might also overlap with mechanisms that govern the choice between a small, immediate reward compared with a larger but delayed reward (Kim & Lee, 2011; but see also Dalley, Everitt, & Robbins, 2011). If there is indeed such an overlap, we would predict that stop training should bias intertemporal choice toward larger delayed rewards.
General Discussion
A convergence of evidence shows that decision making depends on two information streams: automatic processes that are associative and often emotionally driven, and reasoning processes that are rule governed and rational (for a review, see Evans, 2008). Suppression of the former in favor of the latter requires executive control processes. In the study reported here, we focused on how executive processes regulate decision making when people gamble. When gambling, most people realize that the odds are against them; in economic terms, they often know that the expected value of high-risk gambles is negative. As such, research on gambling can reveal important information about how people regulate choice when they are presented with superficially attractive but risky options.
We found that situational factors have a substantial impact on the executive control of decision making in a gambling task. In Experiment 1, motor cautiousness reduced risky betting in a novel gambling task, thus showing that concurrent executive processes need not interfere detrimentally. Instead, control in the motor domain can transfer to other decision-making domains, in this case monetary gambling. Furthermore, we found that training people, even briefly, in controlling their own motor actions can induce cautious and risk-aversive decision making for at least 2 hr afterward (Experiments 2 and 3). In these experiments, occasional motor inhibition reduced monetary risk taking by approximately 10% to 15%. This effect size is comparable to those found in previous studies that have manipulated risk taking using brain-stimulation methods. For instance, Knoch et al. (2006) found a 15% increase in risk taking in the Cambridge Gambling Task after transcranial magnetic stimulation of the right DLPFC; in contrast, Fecteau et al. (2007) found a 10% decrease in risk taking using the Balloon Analogue Risk Task following prefrontal transcranial direct current stimulation. Combined with evidence that transcranial direct current stimulation can potentiate learning (Nitsche et al., 2008), these findings suggest that brain stimulation could augment the training effects we have found.
We propose that increased motor cautiousness, which is a prominent feature of the stop task, reduced risk-taking behavior when making monetary decisions. Future studies should examine whether similar effects can be obtained through alternative methods of inducing motor caution, for example, by instructing people to favor accuracy over speed in a standard choice task. Many studies have shown that participants are more cautious when they are instructed to respond as accurately as possible, and we have proposed previously that strategy adjustments in the speed-accuracy paradigm resemble those in the stop-signal paradigm (Verbruggen & Logan, 2009c).
From a theoretical perspective, our results suggest that executive processes at motor domains share mechanisms with monetary decision making and gambling. Recent cognitive neuroscience studies have shown that frontal brain areas involved in action monitoring and response inhibition might also be involved in monetary decision making in gambling tasks (Clark, 2010; Knoch et al., 2006). Similarly, studies have shown that the latency of stopping a motor response is prolonged in pathological gamblers (e.g., Goudriaan et al., 2006). However, such correlational findings are difficult to interpret definitively. Instead, our results show that motor control can causally modulate risk taking in monetary gambling. This functional overlap suggests that inhibitory motor control and gambling share executive resources, which opens new avenues for the investigation of cognitive and neural mechanisms of executive control at different processing levels.
More generally, our results show that exerting executive control over actions and decisions can be practiced (see also Friese et al., 2011; Muraven, 2010). These findings have potential clinical relevance because impairments in executive control, and particularly stopping, have been linked to the development of several impulse-control disorders, including ADHD, substance abuse, and pathological gambling (Chambers et al., 2009; Verbruggen & Logan, 2008). Furthermore, recovery from addiction requires inhibition of repetitive addictive behavior (Crews & Boettiger, 2009). Consistent with the idea that response inhibition is critical for recovery, the findings of Goudriaan, Oosterlaan, De Beurs, and Van Den Brink (2008) showed that motor disinhibition was a strong predictor of relapse in gamblers. Similarly, motor-inhibition efficiency predicted the treatment outcome in people with eating disorders (Nederkoorn, Jansen, Mulkens, & Jansen, 2007). Therefore, the link we found in the present study between proactive motor control and monetary risk taking in gambling suggests promising new avenues for clinical therapy that target motor inhibition.
Acknowledgments
We thank Jessica Wildgoose, Solveige Stonkutė, Emma Smith, and Jemma Sedgmond for assistance with data collection, and Adam Aron, Aureliu Lavric, Andrew Lawrence, Ian McLaren, Fraser Milton, Stephen Monsell, Mark Stokes, Nick Chater, and the anonymous reviewers for helpful comments.
See Amounts, Probabilities of Winning, and Expected Values in the Supplemental Material available online for more information about expected values.
We replicated this finding in a pilot experiment (N = 40), in which the procedure was the same as in Experiment 1, except that we used fixed signal delays. In this experiment, there was a significant Group × Block Type × Stake interaction (p = .008). Separate comparisons showed that in the stop group, the dual-task effect corresponded to a reliable decrease in betting scores for low stakes, F(1, 19) = 8.0, p = .01, and medium stakes, F(1, 19) = 6.0, p = .02; there was also a strong trend for high stakes, F(1, 19) = 3.9, p = .06. In the double-response group, the dual-task effect instead led to increased betting when stakes were low, F(1, 19) = 5.2, p = .03; there was no reliable dual-task effect for medium and high stakes (both ps > .12). Thus, multitasking tended to increase betting in the double-response group but decrease betting in the stop group. However, because the probability of stopping was lower than the probability of responding in time on a double-response signal trial, we sought to replicate this finding in Experiment 1, in which the moment of signal presentation was dynamically adjusted in both groups.
In the stop group of the pilot experiment, we found that the probability of making Choice 1 was higher (p < .029) and the probability of making Choice 5 was lower (p < .0027) in the dual-task blocks than in the single-task blocks. In addition, the probability of making Choice 6 tended to differ between block types, p < .056. When we combined the data of Experiment 1 and the pilot experiment to increase power (N = 44), we found that the probability of making Choice 1 in dual-task blocks (.270) was significantly higher than the probability of making Choice 1 in single-task blocks (.215), p = .003. By contrast, the probability of making Choice 6 was reliably lower in dual-task blocks (.048) than in single-task blocks (.062); p = .0097; a similar difference was observed for the probability of making Choice 5 in dual-task blocks (.081) and in single-task blocks (.097; p = .028). None of the other differences reached significance (all ps > .11)
We manipulated expected values to ensure that there were two choices with a negative expected value, three options with positive expected values but different probabilities of winning, and one option with a high probability of winning but a positive expected value closer to zero (see Amounts, Probabilities of Winning, and Expected Values in the Supplemental Material).
Footnotes
Declaration of Conflicting Interests: The authors declared that they had no conflicts of interest with respect to their authorship or the publication of this article.
Funding: This work was supported by a David Phillips Fellowship from the Biotechnology and Biological Sciences Research Council to C. D. C., a Research Foundation – Flanders Grant to F. V., a Wales Institute of Cognitive Neuroscience Grant to C. D. C., and an Economic and Social Research Council Grant (ES/J00815X/1) to F. V. and C. D. C.
Supplemental Material: Additional supporting information may be found at http://pss.sagepub.com/content/by/supplemental-data
References
- Aron A. R. (2011). From reactive to proactive and selective control: Developing a richer model for stopping inappropriate responses. Biological Psychiatry, 69, e55–e68. 10.1016/j .biopsych.2010.07.024 10.1016/j.biopsych.2010.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A., Noel X., Crone E. A. (2006). Loss of willpower: Abnormal neural mechanisms of impulse control and decision making in addiction. In Wiers R. W., Stacy A. W. (Eds.), Handbook of implicit cognition and addiction (pp. 215–232). Thousand Oaks, CA: Sage [Google Scholar]
- Bogacz R., Wagenmakers E. J., Forstmann B. U., Nieuwenhuis S. (2010). The neural basis of the speed-accuracy tradeoff. Trends in Neurosciences, 33, 10–16. 10.1016/j.tins.2009.09.002 10.1016/j.tins.2009.09.002 [DOI] [PubMed] [Google Scholar]
- Boyer T. W. (2006). The development of risk-taking: A multi- perspective review. Developmental Review, 26, 291–345. 10.1016/j.dr.2006.05.002 10.1016/j.dr.2006.05.002 [DOI] [Google Scholar]
- Brainard D. H. (1997). The psychophysics toolbox. Spatial Vision, 10, 433–436 [PubMed] [Google Scholar]
- Chamberlain S. R., Sahakian B. J. (2007). The neuropsychiatry of impulsivity. Current Opinion in Psychiatry, 20, 255–261 [DOI] [PubMed] [Google Scholar]
- Chambers C. D., Garavan H., Bellgrove M. A. (2009). Insights into the neural basis of response inhibition from cognitive and clinical neuroscience. Neuroscience & Biobehavioral Reviews, 33, 631–646. 10.1016/j.neubiorev.2008.08.016 10.1016/j.neubiorev.2008.08.016 [DOI] [PubMed] [Google Scholar]
- Clark L. (2010). Decision-making during gambling: An integration of cognitive and psychobiological approaches. Philosophical Transactions of the Royal Society B: Biological Sciences, 365, 319–330. 10.1098/rstb.2009.0147 10.1098/rstb.2009.0147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. R., Lieberman M. D. (2010). The common neural basis of exerting self-control in multiple domains. In Hassin R., Ochsner K., Trope Y. (Eds.), Self control in society, mind, and brain (pp. 141–162). New York, NY: Oxford University Press [Google Scholar]
- Coxon J. P., Stinear C. M., Byblow W. D. (2007). Selective inhibition of movement. Journal of Neurophysiology, 97, 2480–2489. 10.1152/jn.01284.2006 10.1152/jn.01284.2006 [DOI] [PubMed] [Google Scholar]
- Crews F. T., Boettiger C. A. (2009). Impulsivity, frontal lobes and risk for addiction. Pharmacology, Biochemistry, and Behavior, 93, 237–247. 10.1016/j.pbb.2009.04.018 10.1016/j.pbb.2009.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley J. W., Everitt B. J., Robbins T. W. (2011). Impulsivity, compulsivity, and top-down cognitive control. Neuron, 69, 680–694. 10.1016/j.neuron.2011.01.020 10.1016/j.neuron.2011.01.020 [DOI] [PubMed] [Google Scholar]
- de Wit H. (2009). Impulsivity as a determinant and consequence of drug use: A review of underlying processes. Addiction Biology, 14, 22–31. 10.1111/j.1369-1600.2008.00129.x 10.1111/j.1369-1600.2008.00129.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J. S. (2008). Dual-processing accounts of reasoning, judgment, and social cognition. Annual Review of Psychology, 59, 255–278. 10.1146/annurev.psych.59.103006.093629 10.1146/annurev.psych.59.103006.093629 [DOI] [PubMed] [Google Scholar]
- Fecteau S., Knoch D., Fregni F., Sultani N., Boggio P., Pascual-Leone A. (2007). Diminishing risk-taking behavior by modulating activity in the prefrontal cortex: A direct current stimulation study. Journal of Neuroscience, 27, 12500–12505. 10.1523/jneurosci.3283-07.2007 10.1523/jneurosci.3283-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman N., Muraven M. (2010). Self-control depletion leads to increased risk taking. Social Psychological and Personality Science, 1, 175–181. 10.1177/1948550609360421 10.1177/1948550609360421 [DOI] [Google Scholar]
- Friese M., Hofmann W., Wiers R. W. (2011). On taming horses and strengthening riders: Recent developments in research on interventions to improve self-control in health behaviors. Self and Identity, 10, 336–351 [Google Scholar]
- Garavan H., Ross T. J., Stein E. A. (1999). Right hemispheric dominance of inhibitory control: An event-related functional MRI study. Proceedings of the National Academy of Sciences, USA, 96, 8301–8306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudriaan A. E., Oosterlaan J., de Beurs E., Van den Brink W. (2004). Pathological gambling: A comprehensive review of biobehavioral findings. Neuroscience & Biobehavioral Reviews, 28, 123–141. 10.1016/j.neubiorev.2004.03.001 10.1016/j.neubiorev.2004.03.001 [DOI] [PubMed] [Google Scholar]
- Goudriaan A. E., Oosterlaan J., de Beurs E., Van Den Brink W. (2006). Neurocognitive functions in pathological gambling: A comparison with alcohol dependence, Tourette syndrome and normal controls. Addiction, 101, 534–547. 10.1111/j.1360-0443.2006.01380.x 10.1111/j.1360-0443.2006.01380.x [DOI] [PubMed] [Google Scholar]
- Goudriaan A. E., Oosterlaan J., De Beurs E., Van Den Brink W. (2008). The role of self-reported impulsivity and reward sensitivity versus neurocognitive measures of disinhibition and decision- making in the prediction of relapse in pathological gamblers. Psychological Medicine, 38, 41–50. 10.1017/S0033291707000694 10.1017/S0033291707000694 [DOI] [PubMed] [Google Scholar]
- Guerrieri R., Nederkoorn C., Schrooten M., Martijn C., Jansen A. (2009). Inducing impulsivity leads high and low restrained eaters into overeating, whereas current dieters stick to their diet. Appetite, 53, 93–100. 10.1016/j.appet.2009.05.013 10.1016/j.appet.2009.05.013 [DOI] [PubMed] [Google Scholar]
- Ivanoff J., Branning P., Marois R. (2008). fMRI evidence for a dual process account of the speed-accuracy tradeoff in decision-making. PLoS One, 3(7), e2635 Retrieved from http://www.plosone.org/article/info:doi%2F10.1371%2Fjournal.pone.0002635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Lee D. (2011). Prefrontal cortex and impulsive decision making. Biological Psychiatry, 69, 1140–1146. 10.1016/j .biopsych.2010.07.005 10.1016/j.biopsych.2010.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoch D., Gianotti L. R. R., Pascual-Leone A., Treyer V., Regard M., Hohmann M., Brugger P. (2006). Disruption of right prefrontal cortex by low-frequency repetitive transcranial magnetic stimulation induces risk-taking behavior. The Journal of Neuroscience, 26, 6469–6472. 10.1523/jneurosci.0804-06.2006 10.1523/jneurosci.0804-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipszyc J., Schachar R. (2010). Inhibitory control and psychopathology: A meta-analysis of studies using the stop signal task. Journal of the International Neuropsychological Society, 16, 1–13. 10.1017/S1355617710000895 10.1017/S1355617710000895 [DOI] [PubMed] [Google Scholar]
- Marois R., Ivanoff J. (2005). Capacity limits of information processing in the brain. Trends in Cognitive Sciences, 9, 296–305. 10.1016/j.tics.2005.04.010 10.1016/j.tics.2005.04.010 [DOI] [PubMed] [Google Scholar]
- Monsell S., Driver J. (2000). Banishing the control homunculus. In Monsell S., Driver J. (Eds.), Control of cognitive processes: Attention and performance XVIII (pp. 3–32). Cambridge, MA: MIT Press [Google Scholar]
- Muraven M. (2010). Building self-control strength: Practicing self-control leads to improved self-control performance. Journal of Experimental Social Psychology, 46, 465–468. 10.1016/j .jesp.2009.12.011 10.1016/j.jesp.2009.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nederkoorn C., Jansen E., Mulkens S., Jansen A. (2007). Impulsivity predicts treatment outcome in obese children. Behaviour Research and Therapy, 45, 1071–1075. 10.1016/j.brat.2006 .05.009 10.1016/j.brat.2006.05.009 [DOI] [PubMed] [Google Scholar]
- Nigg J. T. (2001). Is ADHD a disinhibitory disorder? Psychological Bulletin, 127, 571–598 [DOI] [PubMed] [Google Scholar]
- Nitsche M. A., Cohen L. G., Wassermann E. M., Priori A., Lang N., Antal A., . . . Pascual-Leone A. (2008). Transcranial direct current stimulation: State of the art 2008. Brain Stimulation, 1, 206–223. 10.1016/j.brs.2008.06.004 10.1016/j.brs.2008.06.004 [DOI] [PubMed] [Google Scholar]
- Pashler H. E., Johnston J. C. (1998). Attentional limitations in dual-task performance. In Pashler H. E. (Ed.), Attention (pp. 155–180). Hove, England: Psychology Press [Google Scholar]
- Patton J. H., Stanford M. S., Barratt E. S. (1995). Factor structure of the Barratt impulsiveness scale. Journal of Clinical Psychology, 51, 768–774 [DOI] [PubMed] [Google Scholar]
- Strayer D. L., Johnston W. A. (2001). Driven to distraction: Dual-task studies of simulated driving and conversing on a cellular telephone. Psychological Science, 12, 462–466 [DOI] [PubMed] [Google Scholar]
- Verbruggen F., Logan G. D. (2008). Response inhibition in the stop-signal paradigm. Trends in Cognitive Sciences, 12, 418–424. 10.1016/j.tics.2008.07.005 10.1016/j.tics.2008.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen F., Logan G. D. (2009a). Automaticity of cognitive control: Goal priming in response-inhibition paradigms. Journal of Experimental Psychology: Learning, Memory, and Cognition, 35, 1381–1388. 10.1037/a0016645 10.1037/a0016645 [DOI] [PubMed] [Google Scholar]
- Verbruggen F., Logan G. D. (2009b). Models of response inhibition in the stop-signal and stop-change paradigms. Neuroscience & Biobehavioral Reviews, 33, 647–661. 10.1016/j.neubio rev.2008.08.014 10.1016/j.neubiorev.2008.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen F., Logan G. D. (2009c). Proactive adjustments of response strategies in the stop-signal paradigm. Journal of Experimental Psychology: Human Perception and Performance, 35, 835–854. 10.1037/a0012726 10.1037/a0012726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaleskiewicz T. (2001). Beyond risk seeking and risk aversion: Personality and the dual nature of economic risk taking. European Journal of Personality, 15, S105–S122 [Google Scholar]