Abstract
Background
The pain and autonomic symptoms of cluster headache (CH) result from activation of the trigeminal parasympathetic reflex, mediated through the sphenopalatine ganglion (SPG). We investigated the safety and efficacy of on-demand SPG stimulation for chronic CH (CCH).
Methods
A multicenter, multiple CH attack study of an implantable on-demand SPG neurostimulator was conducted in patients suffering from refractory CCH. Each CH attack was randomly treated with full, sub-perception, or sham stimulation. Pain relief at 15 minutes following SPG stimulation and device- or procedure-related serious adverse events (SAEs) were evaluated.
Findings
Thirty-two patients were enrolled and 28 completed the randomized experimental period. Pain relief was achieved in 67.1% of full stimulation-treated attacks compared to 7.4% of sham-treated and 7.3% of sub-perception-treated attacks (p < 0.0001). Nineteen of 28 (68%) patients experienced a clinically significant improvement: seven (25%) achieved pain relief in ≥50% of treated attacks, 10 (36%), a ≥50% reduction in attack frequency, and two (7%), both. Five SAEs occurred and most patients (81%) experienced transient, mild/moderate loss of sensation within distinct maxillary nerve regions; 65% of events resolved within three months.
Interpretation
On-demand SPG stimulation using the ATI Neurostimulation System is an effective novel therapy for CCH sufferers, with dual beneficial effects, acute pain relief and observed attack prevention, and has an acceptable safety profile compared to similar surgical procedures.
Keywords: Cluster headache, sphenopalatine ganglion, neurostimulation, randomized controlled trial
Introduction
Cluster headache (CH) is one of the most painful primary headache disorders. It is characterized by daily or almost daily attacks of unilateral excruciating periorbital pain associated with ipsilateral cranial autonomic symptoms, typically lasting between 15 and 180 minutes if untreated. While in the episodic form, bouts of CH attacks are separated by headache-free intervals; chronic cluster headache (CCH) is characterized by attacks occurring at least one year without remission or with remissions lasting less than one month (1).
CH belongs to a group of neurovascular headaches. Evidence, including limited human studies, indicates that CH pathophysiology could involve a cross-talk between trigeminal inputs and the cranial parasympathetic outflow from the superior salivary nucleus that is understood to be mediated primarily through the sphenopalatine ganglion (SPG) (2–4). The SPG is a large extracranial parasympathetic ganglion located in the pterygopalatine fossa (PPF). Post-ganglionic parasympathetic fibers from the SPG innervate facial structures and the cerebral and meningeal blood vessels (5,6). When activated, these fibers release neurotransmitters and vasodilators that activate sensory trigeminal fibers causing further activation of the trigeminal pain pathway, which, in turn, causes further parasympathetic outflow, referred to as the trigeminal-autonomic reflex (7).
The most effective treatments for CH attacks are injectable sumatriptan and oxygen inhalation (8,9). The former is contraindicated in patients with cardiovascular disease; the latter is hampered by impracticability in everyday life, while neither decreases attack frequency. Preventive drug therapies for CH include several substances (10,11), but their use may be limited by intolerance or contraindications, and evidence of efficacy in CCH is poor. Moreover, 10–20% of patients are not effectively treated by, or become resistant to, these therapies. Given the excruciating pain of this syndrome, alternative treatments are warranted.
Since 1908, when Sluder (12) performed the first pharmacological SPG block by applying a 20% cocaine solution in its vicinity, various interventions have targeted the SPG, including alcohol injection within the PPF, transnasal injection of lidocaine and other agents (13,14), pulsed radiofrequency ablations (15), and radiofrequency lesions (16). Success rates vary from 46% to 85%, but benefits are transient (17).
Neurostimulation-based therapies have been investigated for the treatment of refractory CCH patients, including hypothalamic deep brain stimulation (DBS) and occipital nerve stimulation (ONS) (18). The pioneering hypothalamic DBS work by Leone et al. (19) was followed by electrode implantation in 64 refractory CCH patients worldwide with an overall favorable response rate reported to be 70% (18). All of the DBS studies, however, were open studies with the notable exception of a study in 11 CCH patients that found no difference between sham and active DBS during the randomized phase (20). Unfortunately, DBS is associated with significant surgical risks including death (21). ONS was studied in 91 CCH patients worldwide with a reported 67% of patients experiencing at least a 50% reduction in attack frequency (22). However, all of the ONS studies were open, limited in size, and did not include a concurrent sham control. In addition, ONS is associated with a high frequency of lead migration, infection, battery depletion, and lead breakage with the consequence of repeated operations (23,24).
Recently, researchers have investigated the utility of SPG stimulation in CH. Ansarinia et al. published a proof of concept study on the response of CH patients to acute electrical stimulation of the SPG (25). In six patients, effective abolition was reported in 11/18 spontaneous or induced CH attacks; partial (>50% reduction in pain score) response was reported in an additional three headaches.
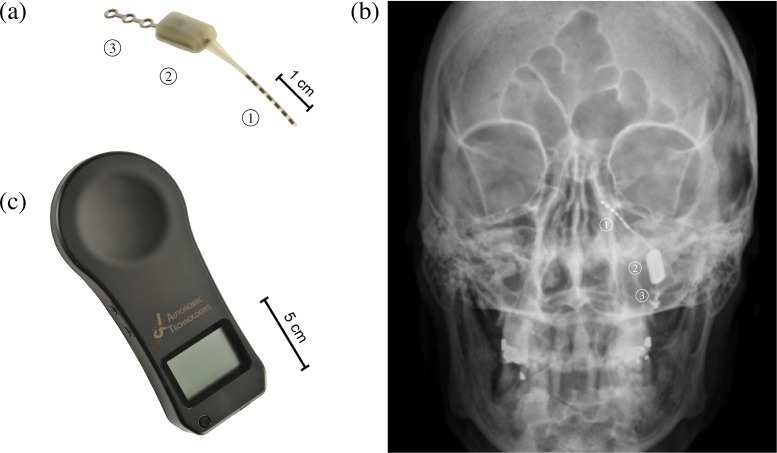
Based on these pathophysiological and therapeutic data, we aimed to conduct a prospective, randomized, blinded, multicenter study to test the efficacy and safety of acute electrical stimulation of the SPG using the Autonomic Technologies, Inc. (ATI) Neurostimulation System (Figure 1).
Figure 1.
(a) Autonomic Technologies, Inc. (ATI) Neurostimulator, a miniaturized implantable device with an integral lead ① containing six electrodes. The lead extends from the neurostimulator body ② to the sphenopalatine ganglion located within the pterygopalatine fossa. The fixation plate ③ is anchored to the zygomatic process of the maxilla. The ATI Neurostimulator is available in four lengths. (b) Image of the ATI Neurostimulator implanted within the facial anatomy. (c) ATI Remote Controller. A handheld device used by the patient to activate and control the implanted neurostimulator. The remote controller is also used by the physician to program the neurostimulator.
Methods
Patient selection
Patients who provided written informed consent and fulfilled the inclusion and exclusion criteria (Table 1) were invited to participate. They were required to maintain the type and dosage of preventive headache medications from one month prior to study enrollment through the completion of the experimental period. Thirty-two patients in six European clinical sites participated in the study. Study participation at all sites was approved by the appropriate national, regional and/or institutional study review boards. The study is registered at ClinicalTrials.gov (NCT01255813).
Table 1.
Selected inclusion and exclusion criteria.
| Selected inclusion criteria |
| 1. Age from 18 to 65 years old. |
| 2. Patient has been diagnosed with CCH according to the 2004 IHS criteria 3.1.2. |
| 3. Patient reported a minimum of four CHs/week. |
| 4. Patient reported dissatisfaction with current headache treatments. |
| 5. Patient was able to distinguish cluster headaches from other headaches. |
| Selected exclusion criteria |
| 1. Patient had a change in type or dosage of preventive headache medications within one month of enrollment. |
| 2. Women of childbearing age who were pregnant, nursing, or not using contraception. |
| 3. Patient had undergone facial surgery in the area of the pterygopalatine fossa or zygomaticomaxillary buttress ipsilateral to the planned implant site within the last four months. |
| 4. Patient had been treated with radiation to the facial region within the last six months. |
| 5. Patient had been diagnosed with any major infectious processes including osteomyelitis or primary or secondary malignancies of the face that were active or required treatment in the past six months. |
| 6. Patient had undergone lesional radiofrequency ablation of the ipsilateral SPG, had undergone a block of the ipsilateral SPG, or had undergone botulinium toxin injections of the head and/or neck in the last three months. |
| 7. Patient had another significant pain problem that might confound the study assessments in the opinion of the investigator. |
CCH: chronic cluster headache; IHS: International Headache Society; SPG: sphenopalatine ganglion.
SPG neurostimulator implantation procedure
The ATI SPG Neurostimulator was implanted under general anesthesia using a minimally invasive, trans-oral, gingival buccal technique. Prior to implant each subject received a parasinus computed tomography (CT) scan to aid in the surgical planning. The SPG neurostimulator (Figure 1(a)) was implanted so that the stimulating electrodes on the integral lead were positioned within the PPF proximate to the SPG, with the body of the SPG neurostimulator positioned on the lateral-posterior maxilla medial to the zygoma and anchored to the zygomatic process of the maxilla using the integral fixation plate (Figure 1(b)). The position of the SPG neurostimulator was verified with an X-ray immediately after implantation, and, if needed, at later time points.
Pre-implant baseline, post-implant stabilization, and therapy titration periods
As shown in Table 2, the pre-implant baseline period was a retrospective four-week period during which the baseline CH attack frequency, Headache Impact Test (HIT-6) score, and quality of life as evaluated using the Short Form Health Survey (SF-36v2) were established. Post-implant, patients entered a healing period that provided time for CH attack frequency and clinical characteristics to stabilize after surgery. Patients then underwent a therapy titration period during which stimulation parameters were adjusted bi-weekly as necessary; upon identification of efficacious parameters, or if efficacious parameters could not be identified and the neurostimulator lead positioning was considered correct, patients were moved into the experimental period. If the neurostimulator lead positioning was determined to be incorrect, a lead revision procedure was considered. Electrical stimulation parameters were adjusted according to provoked paresthesias in the root of the nose and/or treatment effect during an attack. The maximum amplitude was programmed to be slightly higher than the amplitude that provoked discomfort in each patient. Using the remote controller, the patient could apply stimulation and control the amplitude up to the highest level programmed by the clinician.
Table 2.
Pathway CH-1 study phases.
|
Pathway CH-1 Trial | ||||
|---|---|---|---|---|
| Pre-implant baseline | Post-implant stabilization | Therapy titration | Experimental | Open label |
| Implant of ATI Neurostimulator | ||||
| Four weeks | Three weeks minimum | Up to six weeks | Three weeks minimum or shortest period until 30 attacks treated or eight weeks maximum | Through one year post-implant |
| Allow for establishment of baseline attack frequency | Recovery from implantation | Use of stimulation and adjustment of selected electrodes and stimulation parameters for optimal therapy | Use treatment for each attack. The ATI Neurostimulation System randomizes each treatment to: a. Full stimulation b. Sub-perception stimulation c. Sham stimulation | Continued data collection out to one year post-implant, with patients receiving full stimulation therapy at each use |
ATI: Autonomic Technologies, Inc.
Experimental period
A categorical pain scale (CPS) was used to rate CH attacks according to the following five levels: 0—none, 1—mild, 2—moderate, 3—severe, 4—very severe (26). In this study, pain score was recorded using a custom electronic headache diary included in the ATI Remote Controller (Figure 1(c)) prior to each use and after the start of stimulation (15, 30, 60, and 90 minutes). At 15 and 90 minutes, acute medication use was collected.
Patients were instructed to use the remote controller to treat, during the experimental period, CH attacks that were at least of moderate pain intensity (CPS of 2 or higher), to apply stimulation for 15 minutes, and to use acute medications only if needed after 15 minutes of stimulation. Patients were in the experimental period until 30 CH attacks were treated or, if attack frequency was not high enough, for a maximum of eight weeks.
The study employed a random insertion of placebo (27) that used three stimulation doses randomly applied when treatment was initiated by the patient for a CH attack treated during the experimental period: full stimulation (i.e. customized stimulation parameters established during the therapy titration period), sub-perception stimulation, and sham stimulation. The sub-perception amplitude was programmed to 85% of the lowest amplitude that provoked a sensory perception in the patient, similar to the method used by Eddicks et al. (28). Patients were instructed that they would receive three different stimulation doses, one of which would be no stimulation and two of which they might or might not perceive. During the experimental period, stimulation doses were delivered randomly (1:1:1) using pre-specified, randomization sequences that were programmed into the remote controller. The remote controller applied the next stimulation dose, as determined by the randomization sequence, to each CH attack allowing for concealed allocation of therapy on a headache-by-headache basis that kept patients, investigators and the sponsor blinded to the stimulation dose being applied to each CH attack. During the experimental period, all programmed stimulation parameters were not to be changed, except for the sub-perception amplitude as necessary to maintain the level of stimulation to 85% that of the perception amplitude.
Endpoints
Attack treatment
Response to attack treatment was defined as pain relief if pain changed from 2, 3, or 4 on the CPS to 0 or 1 and pain freedom if pain reached 0 on the CPS (26). The primary efficacy endpoint was pain relief at 15 minutes following the start of stimulation.
Secondary endpoints
Secondary endpoints included pain freedom at 15 minutes following the start of stimulation, pain relief and pain freedom at 30, 60, and 90 minutes after initiating stimulation, and reduction in acute medication. All attack treatment endpoints were compared between full and sham stimulations. Pain relief at 15 minutes was also compared between sub-perception and sham stimulation.
The Pathway CH-1 study was designed to show an acute response to electrical stimulation of the SPG. However, many patients had a reduction in attack frequency with repeated SPG stimulation. The protocol was therefore amended and attack prevention was added as a secondary observational endpoint, comparing the frequency of CH attacks at the end of the experimental period to baseline frequency. As CH frequency was already being collected as part of the study, the protocol amendment did not alter study conduct.
To better assess the global therapeutic effect of SPG stimulation, a responder analysis was performed. An acute responder was defined as a patient who treated at least five attacks, with at least three treated with full-stimulation during the experimental period and achieving pain relief at 15 minutes in at least 50% of the full-stimulation-treated attacks. A frequency responder was a patient who had at least a 50% reduction in attack frequency during the experimental period relative to baseline without increasing or changing the type or dose of preventive medications. A therapeutic responder was defined as a patient who had an acute response, a frequency response, or both.
Disability and quality of life measures
Changes in headache disability were assessed with HIT-6 (29,30) and quality of life was assessed using physical (PCS) and mental (MCS) component summary scores of SF-36v2 (31). Changes were assessed by comparing scores obtained at the end of the experimental period with baseline values.
Safety
The primary safety endpoint, device- or procedure-related serious adverse events (SAEs), was assessed through tabulation of all device- or procedure-related SAEs from the implantation procedure through the end of the experimental period. All patients who underwent attempted implantation were included in the analysis for the primary safety endpoint.
Data analysis
Pain relief and pain freedom were evaluated at the end of the experimental period using a logistic generalized estimating equation (GEE) model that was fit to the data to take into account repeated measures within patients. In this study GEE was used to account for the potential correlation of multiple headaches treated within the same patient. Thus the GEE model estimates the correlation within subjects and adjusts for it in the analysis of the outcome. A compound symmetric correlation structure was used. Pain relief and pain freedom estimates were obtained from the model for each of the three stimulation doses; data are presented as least square means (LSM) representing the proportion of doses achieving success and their 95% confidence intervals. All patients who received therapy during the experimental period, treated CH attacks with an initial CPS rating of moderate or greater, and provided responses to the 15-minute pain score and acute medication responses were included in the analysis.
The proportion of CH attacks treated with acute medication within 90 minutes of initiating stimulation was compared, using a similar GEE model, across all three doses.
All patients who completed the experimental period, regardless of whether they treated any attacks during the experimental period, were included in the analysis of CH attack frequency reduction. Reduction in attack frequency during the experimental period was calculated relative to baseline frequency and the result analyzed with the non-parametric Wilcoxon rank sum test. The latter test was also used to monitor changes from baseline to the end of the experimental period in disability and quality of life using the HIT-6 and SF-36v2 questionnaires.
Role of funding source
The Pathway CH-1 study was funded by ATI, manufacturer of the SPG neurostimulator. JS was the principal investigator and chair of the study steering committee (SC), also composed of AM and ML-M. ATI together with the SC designed the study and JS, AM, RHJ, JML, CG, and ML-M enrolled patients and conducted the trial. Data were collected at each site using paper clinical report forms and centrally via the electronic remote controller. All investigators had unrestricted access to the data and all authors analyzed and interpreted the study results. The final manuscript was written by JS and AM after amendments by RHJ. All authors read and approved the final manuscript.
Results
Demographic characteristics
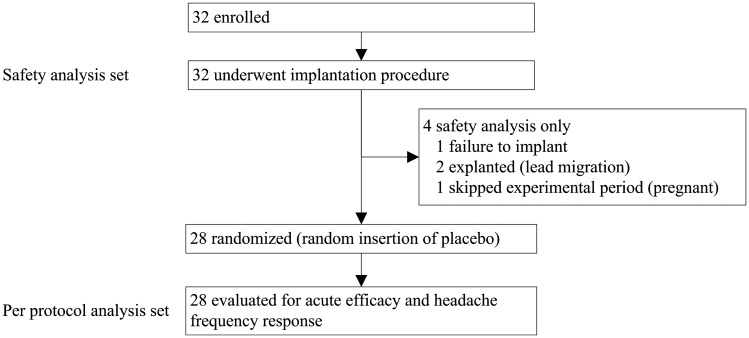
Patients included in the Pathway CH-1 study were representative of the CCH population described in the literature (32): predominantly male population (84%), mean age of 45 years (range: 20–63), average of 19.2 CH attacks per week or 2.7 per day (Table 3). The overall patient disposition is shown in Figure 2. Efficacy data are presented for 28 patients who received treatment in the experimental period, and safety data are presented for all 32 patients who underwent the implantation procedure.
Table 3.
Patient headache history — Four patients out of 32 reported a range of CH attacks per week, the minimum number of CH attacks was used for each of these patients.
| Headache history (N = 32 patients) | Frequency/average (range) | Percentage of patients | |
|---|---|---|---|
| Baseline CH attacks/week: | 19.2 (4–70) | ||
| CH attack laterality: | Left dominant | 15 | 47% |
| Percentage of CH attacks with associated cranial autonomic symptoms: | 100% | 19 | 59% |
| >75% | 4 | 13% | |
| 50–75% | 6 | 19% | |
| <50% | 3 | 9% | |
| CH attack-related associated and/or autonomic symptoms: | Lacrimation | 30 | 94% |
| Conjunctival injection | 29 | 91% | |
| Rhinorrhea | 26 | 81% | |
| Sense of restlessness | 24 | 75% | |
| Nasal congestion | 22 | 69% | |
| Ptosis | 22 | 69% | |
| Photophobia | 18 | 56% | |
| Phonophobia | 16 | 50% | |
CH: cluster headache.
Figure 2.
Pathway CH-1 patient disposition.
Attack treatment
A total of 566 CH attacks were treated. The average number of CH attacks treated per patient was 20.2 ± 24.5 (range: 0–86) during the experimental period. The primary efficacy endpoint, pain relief, was achieved in 67.1% of full stimulation-treated attacks at 15 minutes compared to 7.4% of sham stimulation-treated attacks (p < 0.0001). Pain freedom by 15 minutes was achieved in 34.1% of attacks with full stimulation, as compared to 1.5% with sham stimulation (p < 0.0001) (Table 4). Pain relief response per patient is shown in Table 5 for full stimulation and sham stimulation only; data from sub-perception stimulation are not shown.
Table 4.
Pain relief and freedom at 15 minutes after initiating therapy. Data are presented for least squared mean (LSM) estimate from the GEE model. The LSM has been back-transformed from the logit scale to the probability scale and represents the estimate of probability of pain relief.
| Full stimulation |
Sub-perception stimulation |
Sham stimulation |
||||
|---|---|---|---|---|---|---|
| Relief | Freedom | Relief | Freedom | Relief | Freedom | |
| Probability of pain Relief/freedom (GEE LSM) | 67.1% | 34.1% | 7.3% | 1.6% | 7.4% | 1.5% |
| 95% CI (GEE LSM) | 50.2–80.5% | 18.6–54.1% | 4.0–13.2% | 0.5–5.1% | 3.9–13.7% | 0.5–4.9% |
| p value compared to sham (GEE LSM) | <0.0001 | <0.0001 | 0.96 | 0·97 | — | — |
GEE: generalized estimating equation; CI: confidence interval.
Table 5.
Per patient pain relief and pain freedom at 15 minutes following the start of full, sub-perception or sham SPG stimulation during the experimental period. Not evaluable – patients who experienced a significant reduction in CH attack frequency and did not treat any CH attacks with the respective stimulation during the experimental period. Seventy-five CH attacks were excluded based on initial pain intensity of less than 2 on the CPS, and 13 CH attacks from nine patients were excluded because of missing 15-minute pain or medication usage response. A worst-case data imputation was performed for the 13 excluded attacks whereby full and sub-perception stimulation-treated attacks were counted as failures, and sham stimulation-treated attacks were counted as successes; the study conclusions were unaffected.
| Patient number | Full stimulation |
Sub-perception stimulation |
Sham stimulation |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Total number treated | number (%) with relief (including freedom) | Number (%)with freedom | Total number treated | Number (%) with relief (including freedom) | Number (%) with freedom | Total number treated | Number (%) with relief (including freedom) | Number (%) with freedom | |
| 1 | 30 | 20 (67%) | 0 (0%) | 25 | 2 (8%) | 0 (0%) | 30 | 3 (10%) | 0 (0%) |
| 2 | 30 | 28 (93%) | 7 (23%) | 29 | 0 (0%) | 0 (0%) | 27 | 0 (0%) | 0 (0%) |
| 3 | 17 | 14 (82%) | 14 (82%) | 15 | 0 (0%) | 0 (0%) | 15 | 1 (7%) | 0 (0%) |
| 4 | 17 | 15 (88%) | 10 (59%) | 19 | 3 (16%) | 0 (0%) | 21 | 1 (5%) | 1 (5%) |
| 5 | 14 | 14 (100%) | 13 (93%) | 13 | 1 (8%) | 0 (0%) | 13 | 0 (0%) | 0 (0%) |
| 6 | 13 | 7 (54%) | 1 (8%) | 12 | 1 (8%) | 1 (8%) | 11 | 1 (9%) | 0 (0%) |
| 7 | 11 | 0 (0%) | 0 (0%) | 13 | 0 (0%) | 0 (0%) | 13 | 0 (0%) | 0 (0%) |
| 8 | 10 | 4 (40%) | 0 (0%) | 9 | 2 (22%) | 0 (0%) | 12 | 3 (25%) | 0 (0%) |
| 9 | 10 | 1 (10%) | 1 (10%) | 10 | 1 (10%) | 0 (0%) | 10 | 0 (0%) | 0 (0%) |
| 10 | 7 | 7 (100%) | 7 (100%) | 6 | 0 (0%) | 0 (0%) | 5 | 0 (0%) | 0 (0%) |
| 11 | 7 | 6 (86%) | 5 (71%) | 8 | 0 (0%) | 0 (0%) | 7 | 0 (0%) | 0 (0%) |
| 12 | 6 | 0 (0%) | 0 (0%) | 6 | 0 (0%) | 0 (0%) | 4 | 0 (0%) | 0 (0%) |
| 13 | 4 | 1 (25%) | 1 (25%) | 6 | 0 (0%) | 0 (0%) | 4 | 0 (0%) | 0 (0%) |
| 14 | 3 | 2 (67%) | 1 (33%) | 2 | 0 (0%) | 0 (0%) | 2 | 0 (0%) | 0 (0%) |
| 15 | 2 | 2 (100%) | 2 (100%) | 1 | 1 (100%) | 1 (100%) | 1 | 0 (0%) | 0 (0%) |
| 16 | 2 | 1 (50%) | 1 (50%) | 0 | Not evaluable | 1 | 1 (100%) | 0 (0%) | |
| 17 | 2 | 2 (100%) | 0 (0%) | 2 | 0 (0%) | 0 (0%) | 2 | 2 (100%) | 1 (50%) |
| 18 | 1 | 1 (100%) | 1 (100%) | 2 | 2 (100%) | 0 (0%) | 3 | 2 (67%) | 0 (0%) |
| 19 | 1 | 1 (100%) | 1 (100%) | 0 | Not evaluable | 1 | 1 (100%) | 1 (100%) | |
| 20 | 1 | 0 (0%) | 0 (0%) | 1 | 0 (0%) | 0 (0%) | 2 | 0 (0%) | 0 (0%) |
| 21 | 1 | 0 (0%) | 0 (0%) | 0 | Not evaluable | 0 | Not evaluable | ||
| 22 | 1 | 1 (100%) | 0 (0%) | 1 | 0 (0%) | 0 (0%) | 4 | 0 (0%) | 0 (0%) |
| 23 | 0 | Not evaluable | 1 | 0 (0%) | 0 (0%) | 0 | Not evaluable | ||
| 24 | 0 | Not evaluable | 1 | 0 (0%) | 0 (0%) | 3 | 0 (0%) | 0 (0%) | |
| 25 | 0 | Not evaluable | 0 | Not evaluable | 0 | Not evaluable | |||
| 26 | 0 | Not evaluable | 0 | Not evaluable | 0 | Not evaluable | |||
| 27 | 0 | Not evaluable | 1 | 1 (100%) | 1 (100%) | 1 | 0 (0%) | 0 (0%) | |
| 28 | 0 | Not evaluable | 1 | 0 (0%) | 0 (0%) | 0 | Not evaluable | ||
| TOTAL | 190 | 127 | 65 | 184 | 14 | 3 | 192 | 15 | 3 |
SPG: sphenopalatine ganglion; CH: cluster headache; CPS: categorical pain scale.
Secondary endpoints
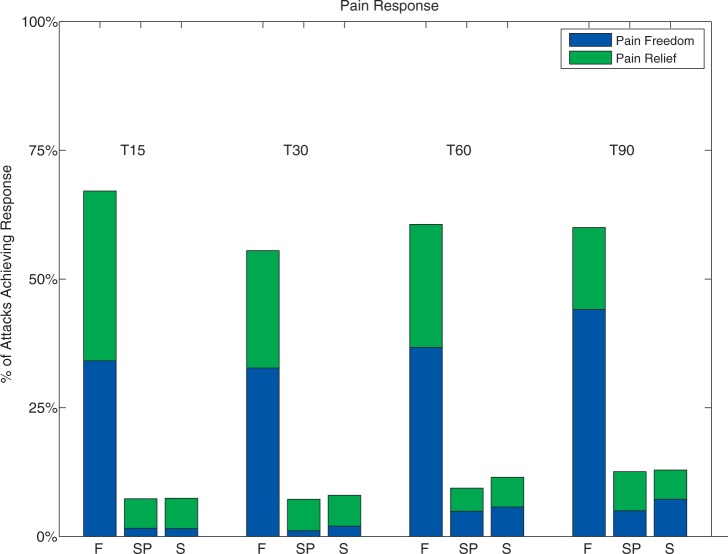
For CH attacks treated with full stimulation, pain relief was achieved in 55.5%, 60.6%, and 60.0% at 30, 60, and 90 minutes, respectively, compared to 8.0%, 11.5%, and 12.9% at 30, 60, and 90 minutes, for sham stimulation (p < 0.0001 at all time points). Figure 3 represents the GEE LSM data for pain relief and pain freedom at all time points. Pain relief at 15 minutes using sub-perception stimulation (7.3%) was not significantly different from sham stimulation (7.4%) (p = 0.96). Acute rescue medications were used in 31.0% of CH attacks treated with full stimulation compared to 77.4% of CH attacks treated with sham stimulation (p < 0.0001) and 78.4% with sub-perception stimulation (p < 0.0001 when compared to full stimulation, p < 0.68 when compared to sham stimulation).
Figure 3.
Pain relief and freedom at 15, 30, 60 and 90 minutes following initiation of full, sub-perception, and sham SPG stimulation (F, SP, and S, respectively). Stimulation was used at initial pain scores of moderate (Categorical Pain Score (CPS) = 2), severe (CPS = 3), and very severe (CPS = 4). SPG: sphenopalatine ganglion.
For the 28 subjects who participated in the experimental period, mean stimulation frequency was 120.4 ± 15.5 Hz (range: 80–180) and mean pulse width 389.7 ± 75.4 μs (range: 244–480). Mean intensity was 1.6 ± 0.8 mA (range: 0.6–3.9) during full stimulation, contrasting with 0.5 ± 0.3 mA (range: 0.1–1.4) during sub-perception stimulation.
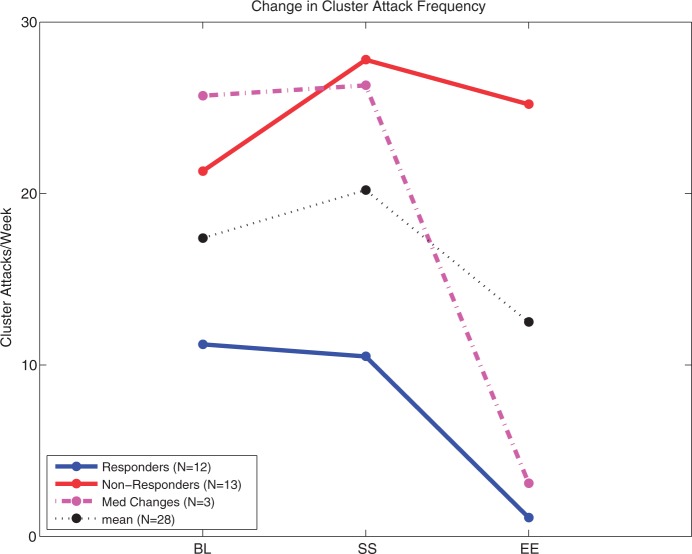
Mean CH attack frequency was 17.4 attacks/week (range: 4–70) during baseline, compared to 12.5 (range: 0–96) during the experimental period (p = 0.005) for the 28 patients who completed the experimental period. Overall, 12 out of 28 patients (43%) were frequency responders with an average reduction of 88% (Figure 4). Three additional patients experienced a frequency reduction but were conservatively not counted as frequency responders because of increases in dose or addition of new preventive medications, including prednisone, verapamil, and quetiapine that could have contributed to the CH frequency reduction.
Figure 4.
Change in cluster headache (CH) attack frequency (average attacks per week) shown at pre-implant baseline (BL), start of stimulation (SS), and end of experimental period (EE). Twelve frequency responders (Responders) had an average reduction of 88% at EE compared to BL. Three patients (Med Changes) who had a frequency response of >50%, but changed preventive medication dose or type, including prednisone, verapamil, and quetiapine, were excluded as responders. Thirteen were frequency non-responders (Non-responders). Overall, mean CH attack frequency was reduced by 4.9 CH attacks/week, or 31% (p = 0.005).
Due to the decrease in attack frequency, 14 of the 28 patients (50%) did not treat a sufficient number of attacks in the experimental period to be analyzed for acute response; they were analyzed only for frequency response. Nine of these 14 patients were frequency responders, i.e. their frequency reduction during the experimental period relative to baseline reached at least 50%.
Of the remaining 14 patients who treated at least five attacks among which at least three with full stimulation during the experimental period, nine were acute responders (64% of the 14 eligible patients or 32% overall).
In total, 19 patients out of 28 (68%) experienced an acute response, a frequency response, or both: n = 7 (25%), n = 10 (36%), and n = 2 (7%), respectively.
Headache disability and quality of life
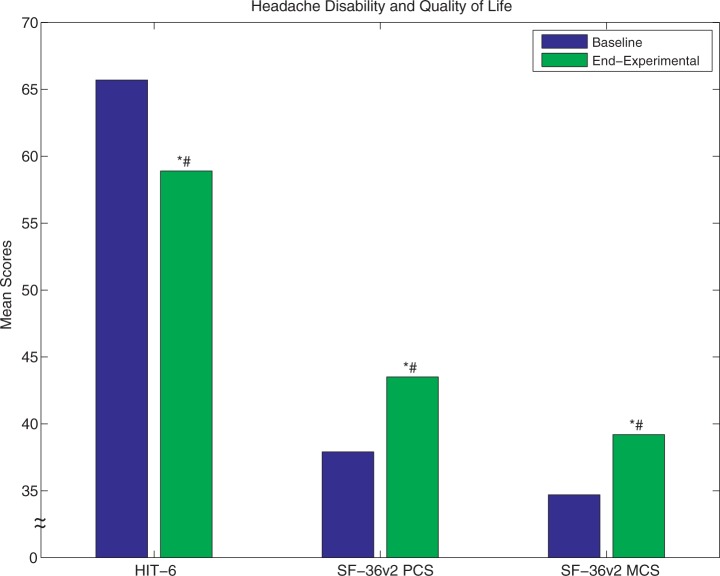
SPG stimulation resulted in a statistically and clinically significant reduction in headache disability. The HIT-6 score difference between the experimental period and pre-implant baseline was −6.8 ± 10.2 (p = 0.002) (Figure 5). Eighteen patients (64%) improved by more than the mean difference on the HIT-6 scale considered clinically significant (−2.3 units) (33).
Figure 5.
Change in headache disability (HIT-6) and quality of life (SF-36v2 Physical Component Summary (PCS) and Mental Component Summary (MCS) scores) from baseline to end of experimental period. Improvements both in headache disability (decrease in HIT-6 score) and in quality of life (increase in SF-36v2 score) were statistically significant (HIT-6: p = 0.002, SF-36v2 PCS: p = 0.005, SF-36v2 MCS p = 0.02, indicated by “*”, Wilcoxon Rank Sum). Improvements in headache disability and quality of life were also clinically significant as compared to the Mean Clinically Significant Difference (HIT-6 MCSD = –2.3, SF-36v2 MCSD = 4.0, indicated by “#”).
Quality of life, as evaluated by the SF-36v2, improved in 21 out of 28 patients (75%): 21% (n = 6) in physical score (PCS), 29% (n = 8) in mental score (MCS), and 25% (n = 7) in both (Figure 5). The differences between the experimental period and pre-implant baseline scores for both PCS and MCS components of the SF-36v2 were greater than or within the clinically significant difference range of three to five points (34).
Primary safety endpoint
Across all 32 patients, five device- or procedure-related SAEs occurred. Three SPG neurostimulator lead revisions and two SPG neurostimulator explant procedures were classified as SAEs. Two of the three revision procedures were due to misplacement of the lead within the PPF during the original implantation. The third revision procedure was due to lead placement within the maxillary sinus. In this patient, the SPG neurostimulator was explanted in an outpatient procedure under local anesthetics prior to completing the second implantation.
Two other patients had SAEs that resulted in SPG neurostimulator explant. In one early implanted patient, within hours of completing the implant procedure, the SPG neurostimulator lead migrated superiorly and laterally, near the region of the maxillary nerve as confirmed by CT imaging. Since the SPG neurostimulator was not explanted immediately, the patient reported dysesthesias in the facial and oral territory of the maxillary nerve, which improved after explant but had not resolved completely at the time of this report. In the second patient, an incorrectly sized SPG neurostimulator lead migrated within two weeks post-operatively, confirmed by X-ray imaging, and was explanted under general anesthesia with no further AEs.
Cranial nerve exams were performed to proactively identify potential AEs. Sensory disturbance occurred in 81% of patients (35% of peri-operative AEs) with localized loss of sensation in distinct distributions of maxillary nerve being the most common (21 of 32 events, occurring in 19 of 32 patients). Fifteen events in 15 patients resolved with an average resolution time of 97 days (range: 31–259 days). Six events had not resolved at the time of this report, but were reported to be mild or moderate.
Two patients suffered from infections, one at the incision site and another in the ipsilateral maxillary sinus. Both infections resolved with antibiotic therapy, and neither required SPG neurostimulator explant. Two patients reported mild paresis of the muscles around the nasolabial fold. Two patients had maxillary sinus puncture during the initial implantation or lead revision procedure, with no adverse clinical sequelae. A detailed list of the AEs is provided in Table 6.
Table 6.
Reported adverse events. A significant portion of the reported adverse events (AE) occurred during the first 30 days post-implant, and were associated with the surgical procedure.
| Adverse event | Peri-operative (within 30 days of implant procedure) |
Late-onset (>30 days after implant procedure) |
||||
|---|---|---|---|---|---|---|
| # AEs (% of total) | # Patients | # Patients resolved (%) (days (avg; range)) | # AEs | # Patients | # Patients resolved (%) (days (avg; range)) | |
| Sensory disturbances (includes localized loss of sensation, hypoesthesia, paresthesia, dysesthesia, allodynia) | 32 (35%) | 26 (81%) | 15 (58%) (82; 12–259) | 6 (17%) | 5 (16%) | 3 (60%) (27; 1–45) |
| Pain (face, cheek, gum, temporal mandibular joint, nose, incision site, or periorbital) | 15 (16%) | 12 (38%) | 12 (100%) (51; 0–231) | 6 (17%) | 6 (19%) | 3 (50%) (81; 19–121) |
| Tooth pain/sensitivity | 5 (5%) | 5 (16%) | 4 (80%) (100; 54–161) | 1 (3%) | 1 (3%) | 1 (100%) (34) |
| Swelling | 8 (9%) | 7 (22%) | 6 (86%) (88; 13–220) | — | — | — |
| Swelling and pain (post-operative) | 3 (3%) | 3 (9%) | 3 (100%) (26; 3–69) | — | — | — |
| Trismus | 5 (5%) | 5 (16%) | 4 (80%) (39; 13–71) | — | — | — |
| Headache | 4 (4%) | 3 (9%) | 3 (100%) (86; 0–258) | 3 (8%) | 3 (9%) | 1 (33%) (42) |
| Dry eye (xerophthalmia) | 3 (3%) | 3 (9%) | 1 (33%) (49) | 1 (3%) | 1 (3%) | — |
| Hematoma | 3 (3%) | 3 (9%) | 3 (100%) (28; 5–56) | — | — | — |
| Paresis | 2 (2%) | 2 (6%) | 1 (50%) (178) | — | — | — |
| Infection | 2 (2%) | 2 (6%) | 2 (100%) (34; 14–54) | 1 (3%) | 1 (3%) | 1 (100%) (18) |
| Reduced autonomic symptoms (tearing, noseblock) during cluster attacks | 1 (1%) | 1 (3%) | 1 (100%) (40) | — | — | — |
| Epistaxis | 1 (1%) | 1 (3%) | 1 (100%) (1) | — | — | — |
| Facial asymmetry | 1 (1%) | 1 (3%) | 1 (100%) (178) | 2 (6%) | 2 (6%) | — |
| Tearing | 1 (1%) | 1 (3%) | 1 (100%) (39) | — | — | — |
| Vomiting (day of surgery) | 1 (1%) | 1 (3%) | 1 (100%) (0) | — | — | — |
| Tenderness in cheek | 1 (1%) | 1 (3%) | — | — | — | — |
| Bites tongue | 1 (1%) | 1 (3%) | 1 (100%) (74) | — | — | — |
| Failure to implant | 1 (1%) | 1 (3%) | 1 (100%) (0) | — | — | — |
| Explant / lead revision | — | — | — | 5 (14%) | 5 (16%) | 5 (100%) (1; 0–2) |
| Lead migration, resulting in explant | 1 (1%) | 1 (3%) | 1 (100%) (27) | — | — | — |
| Maxillary sinus puncture | 1 (1%) | 1 (1%) | 1 (100%) (33) | — | — | — |
| Conjunctivitis | — | — | — | 2 (6%) | 1 (3%) | 1 (100%) (19; 16–22) |
| Itching | — | — | — | 1 (3%) | 1 (3%) | 1 (100%) (102) |
| Dry nose | — | — | — | 1 (3%) | 1 (3%) | 1 (100%) (134) |
| Dry skin | — | — | — | 1 (3%) | 1 (3%) | 1 (100%) (49) |
| Taste alterations | — | — | — | 1 (3%) | 1 (3%) | 1 (100%) (149) |
| Sensation of implant | — | — | — | 1 (3%) | 1 (3%) | — |
| Depressed gag reflex | — | — | — | 1 (3%) | 1 (3%) | — |
| TMJ | — | — | — | 1 (3%) | 1 (3%) | — |
| Increase in static electricity | — | — | — | 1 (3%) | 1 (3%) | 1 (100%) (105) |
| Sensation in infratemporal fossa | — | — | — | 1 (3%) | 1 (3%) | 1 (100%) (66) |
| Total # AEs | 92 | 32 | 36 | 32 | ||
Discussion
In the Pathway CH-1 study, 68% of the 32 enrolled CCH patients benefited from electrical stimulation of the SPG. Other implantable devices have reported comparable responder rates solely for preventive treatment of refractory CCH, but none in a large, sham-controlled study. The Pathway CH-1 study showed two positive effects that may be independent: significant pain relief from acute stimulation and an observed significant reduction in attack frequency. The clinical and pathophysiological implications of these two effects will be considered.
The pain relief and pain freedom rates of 67.1% and 34.1% for full stimulation-treated CH attacks at 15 minutes are close to those reported for injectable sumatriptan: 74% and 46%, respectively (26), although the two trials are not completely comparable. In the sumatriptan study only 17 out of 39 included patients had CCH, and these patients were not defined as refractory to preventive medical treatment and in addition, the sumatriptan study was a single-attack study, compared to the multiple-attack study presented. An important advantage of electrical stimulation of the SPG is that it may be applied without daily limitations and cardiovascular contraindications as opposed to parenteral sumatriptan, which is restricted for safety reasons to only two daily uses and is contraindicated in patients with vascular disease and/or uncontrolled arterial hypertension (35). After injectable sumatriptan, oxygen inhalation is the most effective treatment of acute CH attacks, providing benefit in up to 78% of attacks (8). However, in practice the effect of oxygen may take time and be transient (36). In this study, a significant effect of SPG stimulation occurred within 15 minutes and it was sustained, as suggested by the small difference (−7.1%) in response rates between the 15-minute and 90-minute time points. Oxygen treatment can be difficult to prescribe in certain countries and, since high flow rates (i.e. gas cylinder) are warranted, it can be difficult to use by patients who are socially and professionally active.
Patients were generally compliant with instructions to treat CH attacks for 15 minutes and applied electrical stimulation to the SPG on average for 14 minutes when using full stimulation. Stimulation duration did not vary dramatically between the three different stimulation doses, indicating the robustness of the random insertion of the placebo study design. During the titration period, full stimulation was used to optimize the stimulation parameters, and most patients experienced sensation from stimulation during this period. Stimulation-provoked paresthesias coupled with pain response limited the amount of blinding that could be achieved in the study, a limitation that was overcome by utilizing two non-perceived stimulation doses and one perceived stimulation dose, applied in random order with concealed allocation, which is understood to be important in producing study results that accurately predict magnitude of clinical effect (37).
Although this study was designed and powered to test the acute effects on spontaneous CH attacks, a rather dramatic reduction of attack frequency was observed in some patients after repetitive attack stimulation. The reduction in attack frequency affected the number of CH attacks treated during the experimental period, and thus the number of attacks treated across patients was variable. While this phenomenon was a limitation of the study design, it was anticipated and addressed with the use of the logistic GEE for data analysis. Overall 43% of patients (12/28) experienced an attack frequency reduction of ≥50% from baseline, although all patients had been suffering from the chronic form of CH for many years and had over time tried a number of preventive drugs without lasting benefit. The observed frequency reduction appeared strongly associated with the start of electrical stimulation to the SPG and not with effects from the surgical procedure, after which mean attack frequency increased (mean frequency at baseline 17.4 ± 14.5 /week vs. 20.2 ± 22.0 at the start of stimulation) (Figure 4). A preventive effect is not completely unexpected considering the reports of various injections into the PPF, including alcohol and steroids (13,14). The frequency reduction varies between patients and does not seem to depend on the number or duration of SPG stimulation attempts. In some patients, a short duration of stimulation resulted in sustained frequency reduction, whereas in others repeated stimulation that provided acute pain relief did not result in an attack frequency reduction. The possible influences of a placebo or regression to the mean effect cannot be excluded when considering the significant reduction in attack frequency observed. The apparent preventive effects of SPG stimulation, which may be clinically important, warrant further investigation.
As the treatment response varied between patients, we compared the clinical phenotype between acute responders, frequency responders and subjects who had both an acute and a frequency response. No clinical characteristic was found to be associated with response or type of response.
In addition to the significant findings of attack pain relief and CH attack frequency reduction, most patients had a clinically and statistically significant improvement in headache disability and quality of life with SPG stimulation. This result contrasts with an ONS study in CCH patients in which attack frequency and quality of life scores improved, but not significantly (38).
Oral maxillofacial surgeries are inherently associated with standard peri-operative AEs, including pain, swelling, hematoma, seroma, and sensory disturbances in the vast majority of patients (39,40). AEs associated with the surgical procedure in this study, including sensory disturbance localized to distinct areas innervated by branches of the maxillary nerve, are similar in number, severity, and duration to those observed in other procedures (40,41). The Pathway CH-1 incidence of lead migration and lead misplacements was 6.3% (two of 32) and 12.5% (four of 32), respectively, and the incidence of reoperation was 18.8% (six of 32), an average of 1.19 procedures per patient. A review of ONS and DBS literature indicates a lead migration incidence of 15.4% or more and the DBS literature reports lead misplacement incidences of 8.5%, with a total of 473 surgical procedures for 372 patients (average 1.27 procedures per patient) (22,24,42,43). The Pathway CH-1 study was the first surgical experience with the SPG neurostimulator across multiple surgical specialties. Over the course of the study, significant surgical experience was gained that led to improvements in surgical instrumentation and techniques as well as improved pre-operative planning and surgical targeting. Consequently, it is to be anticipated that surgery-related side effects will be less common than in the current sample.
Cardinal features of CH attacks are the prominent autonomic symptoms. They result from increased cranial parasympathetic outflow that is thought to activate trigeminal afferents via the trigeminovascular system (2). Electrical stimulation of the SPG may physiologically block the parasympathetic efferents, thereby turning off the efferent arm of the trigeminal-autonomic reflex. The stimulation frequency is likely to be important for this effect. The average stimulation frequency used in this study was 120 Hz, more than double the frequency found to result in pain relief and pain freedom in previous acute studies (25). This high-frequency stimulation is thought to act by causing depletion of stored neurotransmitter over time from the efferent parasympathetic fibers (44). Low-frequency stimulation of the SPG causes dural plasma extravasation in rats (45) and cortical blood flow peaks with stimulation at about 10 Hz (46). Even frequencies as high as 60 Hz were able to activate the parasympathetic arm of the trigeminal-autonomic reflex, but the resulting effects on cortical flow were transient and peaked quickly after stimulation onset (46), suggesting that stimulation at high frequencies such as 120 Hz may inhibit parasympathetic outflow or its effects (44,47). From a pathophysiological perspective, the positive results of this study confirm that the cranial parasympathetic system plays a crucial role in the occurrence as well as recurrence of CH attacks.
Electrical stimulation in the region of the SPG produces paresthesias in areas innervated by sensory fibers from the maxillary nerve that pass through the SPG en route to peripheral targets including the nasopharynx, soft palate, nasal cavity, and palatal gingiva (16,48). In this study, programming of stimulation parameters was guided in most patients using the location of these provoked paresthesias. However, in one patient, paresthesias were not able to be provoked, and instead stimulation parameter adjustments were made during a spontaneous CH attack judging from the obtained therapeutic response.
The observed CH attack frequency reduction in our study is likely to be attributed to direct electrical stimulation of the SPG, indicating that acute stimulations may have prolonged clinical effects. Thus, the question of how stimulation of a peripheral autonomic ganglion may lead to a reduction of a centrally mediated disorder (49) is of major interest. One possibility would be a repetitive depletion and hence exhaustion of parasympathetic neurotransmitters. Another could be a modulation of central structures via a parasympathetico-trigeminal feedback mechanism, which would be comparable to the activation of areas involved in descending pain control that are related to treatment efficacy after prolonged ONS in CCH patients (50). The mode of action may be different between acute and preventive effects of SPG stimulation and is further underlined by the absence of an obligatory association between the two effects in individual patients, although this effect may be underestimated, as many frequency responders could not be evaluated for acute response. Whatever the mode of action may be, the reduction in CH attacks was clinically meaningful and merits further investigation.
Conclusions
The multicenter European Pathway CH-1 study is the largest randomized, controlled neurostimulation study performed in CCH and confirms that patient-controlled electrical stimulation of the SPG is an effective treatment option for CCH sufferers with an acceptable safety profile. The efficacy data indicate that acute electrical stimulation of the SPG provides significant attack pain relief and in many cases pain freedom compared to sham stimulation. Adverse events associated with the ATI surgical procedure are similar to other trans-oral, gingival buccal surgical procedures. Electrical stimulation of the SPG was also observed to be associated with a significant and clinically meaningful reduction in CH attack frequency in some patients. The preventive effect is important in CCH and warrants further investigation. Overall, SPG stimulation significantly improves quality of life in these very disabled patients.
Clinical implications
This randomized, sham-controlled trial shows for the first time that stimulation of the ipsilateral sphenopalatine ganglion (SPG) with a remotely controlled neurostimulator implanted in the pterygopalatine fossa is highly effective in aborting attacks in patients suffering from chronic cluster headache (CCH).
Repeated SPG neurostimulation may decrease the frequency of CH attacks, but this clinically important preventive effect warrants further investigation.
The Autonomic Technologies, Inc. SPG Neurostimulator is well tolerated. Adverse events are similar to those reported in other oro-facial, surgical procedures. The most frequent adverse events are transient sensory disturbances in the territory of the maxillary nerve (V2).
This study supports the pivotal role of the SPG in CH pathophysiology and provides evidence that SPG neurostimulation is a feasible and possibly more effective alternative to available drug treatments in CCH patients.
Acknowledgements
The authors are grateful to their patients who agreed to pioneer for this novel neurostimulation treatment. We also would like to acknowledge the seminal contribution of all investigators, including our neurology and surgical colleagues who participated in patients’ follow-up and who implanted the SPG neurostimulator, in particular Alain Wilmont, Sandrine Machiels, Delphine Magis, Søren Hillerup, Jørgen Rostgaard, Mads Barløse, Tim Jürgens, Philip Pohlenz, Jan Klatt, Marco Blessmann, Alex Assaf, Denys Fontaine, Charles Savoldelli, Anna Garcia, Miguel Puché, Mariano Marqués, Kasja Rabe, Oliver Müller, Thomas Hoffmann, and Goetz Lehnerdt.
Funding
The Pathway CH-1 study was funded by ATI, manufacturer of the SPG Neurostimulator. JS was the principal investigator and chair of the study steering committee (SC), also composed of AM, and ML-M. ATI together with the SC designed the study and JS, AM, RHJ, JML, CG, and ML-M enrolled patients and conducted the trial. Data were collected at each site using paper clinical report forms and centrally via the electronic remote controller. All investigators had unrestricted access to the data and all authors analyzed and interpreted the study results. The final manuscript was written by JS and AM after amendments by RHJ. All authors read and approved the final manuscript.
Conflicts of interest
JS, RHJ, AM, JML, ML-M, and CG have been consultants for ATI since 2010 or 2011 and have received payment for services not related to the conduct of the Pathway CH-1 study. JS, ML-M, and AM are the steering committee members for the Pathway CH-1 trial. AG and AC are employees of ATI. All costs associated with patient care during the Pathway CH-1 trial were reimbursed to all enrolling institutions.
References
- 1.ICHD-II The International Classification of Headache Disorders: 2nd edition. Cephalalgia 2004; 24Suppl 19–160 [DOI] [PubMed] [Google Scholar]
- 2.Goadsby PJ. Pathophysiology of cluster headache: A trigeminal autonomic cephalgia. Lancet Neurol 2002; 1: 251–257 [DOI] [PubMed] [Google Scholar]
- 3.Goadsby PJ, Lipton RB, Ferrari MD. Migraine—current understanding and treatment. N Engl J Med 2002; 346: 257–270 [DOI] [PubMed] [Google Scholar]
- 4.Goadsby PJ, Edvinsson L. Human in vivo evidence for trigeminovascular activation in cluster headache. Neuropeptide changes and effects of acute attacks therapies. Brain 1994; 117(Pt 3): 427–434 [DOI] [PubMed] [Google Scholar]
- 5.Nozaki K, Moskowitz MA, Maynard KI, et al. Possible origins and distribution of immunoreactive nitric oxide synthase-containing nerve fibers in cerebral arteries. J Cereb Blood Flow Metab 1993; 13: 70–79 [DOI] [PubMed] [Google Scholar]
- 6.Ruskell GL. Orbital passage of pterygopalatine ganglion efferents to paranasal sinuses and nasal mucosa in man. Cells Tissues Organs 2003; 175: 223–228 [DOI] [PubMed] [Google Scholar]
- 7.May A, Goadsby PJ. The trigeminovascular system in humans: Pathophysiologic implications for primary headache syndromes of the neural influences on the cerebral circulation. J Cereb Blood Flow Metab 1999; 19: 115–127 [DOI] [PubMed] [Google Scholar]
- 8.Cohen AS, Burns B, Goadsby PJ. High-flow oxygen for treatment of cluster headache: A randomized trial. JAMA 2009; 302: 2451–2457 [DOI] [PubMed] [Google Scholar]
- 9.Ekbom K, Monstad I, Prusinski A, et al. Subcutaneous sumatriptan in the acute treatment of cluster headache: A dose comparison study. The Sumatriptan Cluster Headache Study Group. Acta Neurol Scand 1993; 88: 63–69 [DOI] [PubMed] [Google Scholar]
- 10.Evers S, Afra J, Frese A, et al. Gilhus NE, Barnes MP, Brainin M. Cluster headache and other trigemino-autonomic cephalgias. European handbook of neurological management 2nd edn, 2011, Oxford, UK: Blackwell Publishing Ltd [Google Scholar]
- 11.May A. Cluster headache: Pathogenesis, diagnosis, and management. Lancet 2005; 366: 843–855 [DOI] [PubMed] [Google Scholar]
- 12. Sluder G. The role of the sphenopalatine (or Meckle's) ganglion in nasal headaches. New York Med J 1908: 989–990.
- 13.Devoghel JC. Cluster headache and sphenopalatine block. Acta Anaesthesiol Belg 1981; 32: 101–107 [PubMed] [Google Scholar]
- 14.Felisati G, Arnone F, Lozza P, et al. Sphenopalatine endoscopic ganglion block: A revision of a traditional technique for cluster headache. Laryngoscope 2006; 116: 1447–1450 [DOI] [PubMed] [Google Scholar]
- 15.Chua NH, Vissers KC, Wilder-Smith OH. Quantitative sensory testing may predict response to sphenopalatine ganglion pulsed radiofrequency treatment in cluster headaches: A case series. Pain Pract 2011; 11: 439–445 [DOI] [PubMed] [Google Scholar]
- 16.Narouze S, Kapural L, Casanova J, et al. Sphenopalatine ganglion radiofrequency ablation for the management of chronic cluster headache. Headache 2009; 49: 571–577 [DOI] [PubMed] [Google Scholar]
- 17.Levin M. Nerve blocks in the treatment of headache. Neurotherapeutics 2010; 7: 197–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Magis D, Jensen R, Schoenen J. Neurostimulation therapies for primary headache disorders: Present and future. Curr Opin Neurol 2012; 25: 269–276 [DOI] [PubMed] [Google Scholar]
- 19.Leone M, Franzini A, Bussone G. Stereotactic stimulation of posterior hypothalamic gray matter in a patient with intractable cluster headache. N Engl J Med 2001; 345: 1428–1429 [DOI] [PubMed] [Google Scholar]
- 20.Fontaine D, Lazorthes Y, Mertens P, et al. Safety and efficacy of deep brain stimulation in refractory cluster headache: A randomized placebo-controlled double-blind trial followed by a 1-year open extension. J Headache Pain 2010; 11: 23–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schoenen J, Di Clemente L, Vandenheede M, et al. Hypothalamic stimulation in chronic cluster headache: A pilot study of efficacy and mode of action. Brain 2005; 128: 940–947 [DOI] [PubMed] [Google Scholar]
- 22.Magis D, Schoenen J. Advances and challenges in neurostimulation for headaches. Lancet Neurol 2012; 11: 708–719 [DOI] [PubMed] [Google Scholar]
- 23.Burns B, Watkins L, Goadsby PJ. Treatment of intractable chronic cluster headache by occipital nerve stimulation in 14 patients. Neurology 2009; 72: 341–345 [DOI] [PubMed] [Google Scholar]
- 24.Fontaine D, Christophe Sol J, Raoul S, et al. Treatment of refractory chronic cluster headache by chronic occipital nerve stimulation. Cephalalgia 2011; 31: 1101–1105 [DOI] [PubMed] [Google Scholar]
- 25.Ansarinia M, Rezai A, Tepper SJ, et al. Electrical stimulation of sphenopalatine ganglion for acute treatment of cluster headaches. Headache 2010; 50: 1164–1174 [DOI] [PubMed] [Google Scholar]
- 26.Ekbom K, Waldenlind E, Levi R. Treatment of acute cluster headache with sumatriptan. The Sumatriptan Cluster Headache Study Group. N Engl J Med 1991; 325: 322–326 [DOI] [PubMed] [Google Scholar]
- 27.Lipton RB, Bigal ME, Stewart WF. Clinical trials of acute treatments for migraine including multiple attack studies of pain, disability, and health-related quality of life. Neurology 2005; 65: S50–S58 [DOI] [PubMed] [Google Scholar]
- 28.Eddicks S, Maier-Hauff K, Schenk M, et al. Thoracic spinal cord stimulation improves functional status and relieves symptoms in patients with refractory angina pectoris: The first placebo-controlled randomised study. Heart 2007; 93: 585–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nachit-Ouinekh F, Dartigues JF, Henry P, et al. Use of the headache impact test (HIT-6) in general practice: Relationship with quality of life and severity. Eur J Neurol 2005; 12: 189–193 [DOI] [PubMed] [Google Scholar]
- 30.Yang M, Rendas-Baum R, Varon SF, et al. Validation of the Headache Impact Test (HIT-6) across episodic and chronic migraine. Cephalalgia 2011; 31: 357–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bjorner JB, Turner-Bowker DM.Kattan MW. SF-36 and SF-12 Health Surveys. Encyclopedia of medical decision making Thousand Oaks, CA, USA: SAGE Publications, 2009 [Google Scholar]
- 32.Bahra A, May A, Goadsby PJ. Cluster headache: A prospective clinical study with diagnostic implications. Neurology 2002; 58: 354–361 [DOI] [PubMed] [Google Scholar]
- 33.Coeytaux RR, Kaufman JS, Chao R, et al. Four methods of estimating the minimal important difference score were compared to establish a clinically significant change in Headache Impact Test. J Clin Epidemiol 2006; 59: 374–380 [DOI] [PubMed] [Google Scholar]
- 34.Hays RD, Morales LS. The RAND-36 measure of health-related quality of life. Ann Med 2001; 33: 350–357 [DOI] [PubMed] [Google Scholar]
- 35.Ekbom K, Hardebo JE. Cluster headache: Aetiology, diagnosis and management. Drugs 2002; 62: 61–69 [DOI] [PubMed] [Google Scholar]
- 36.Rozen TD, Fishman RS. Inhaled oxygen and cluster headache sufferers in the United States: Use, efficacy and economics: Results from the United States Cluster Headache Survey. Headache 2011; 51: 191–200 [DOI] [PubMed] [Google Scholar]
- 37.Kunz R, Oxman AD. The unpredictability paradox: Review of empirical comparisons of randomised and non-randomised clinical trials. BMJ 1998; 317: 1185–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mueller OM, Gaul C, Katsarava Z, et al. Occipital nerve stimulation for the treatment of chronic cluster headache —lessons learned from 18 months experience. Cent Eur Neurosurg 2011; 72: 84–89 [DOI] [PubMed] [Google Scholar]
- 39.Al-Din OF, Coghlan KM, Magennis P. Sensory nerve disturbance following Le Fort I osteotomy. Int J Oral Maxillofac Surg 1996; 25: 13–19 [DOI] [PubMed] [Google Scholar]
- 40.Thygesen TH, Bardow A, Norholt SE, et al. Surgical risk factors and maxillary nerve function after Le Fort I osteotomy. J Oral Maxillofac Surg 2009; 67: 528–536 [DOI] [PubMed] [Google Scholar]
- 41.Ueki K, Nakagawa K, Marukawa K, et al. Evaluation of upper lip hypoesthesia with a trigeminal somatosensory-evoked potential following Le Fort I osteotomy in combination with mandibular osteotomy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2007; 103: 169–174 [DOI] [PubMed] [Google Scholar]
- 42.Saper JR, Dodick DW, Silberstein SD, et al. Occipital nerve stimulation for the treatment of intractable chronic migraine headache: ONSTIM feasibility study. Cephalalgia 2011; 31: 271–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Silberstein S, Dodick D, Saper J, et al. The safety and efficacy of occipital nerve stimulation for the management of chronic migraine. Cephalalgia 2011; 31: 117–118 [Google Scholar]
- 44.Ekström J, Brodin E, Ekman R, et al. Depletion of neuropeptides in rat parotid glands and declining atropine‐resistant salivary secretion upon continuous parasympathetic nerve stimulation. Regul Pept 1985; 11: 353–359 [DOI] [PubMed] [Google Scholar]
- 45.Delépine L, Aubineau P. Plasma protein extravasation induced in the rat dura mater by stimulation of the parasympathetic sphenopalatine ganglion. Exp Neurol 1997; 147: 389–400 [DOI] [PubMed] [Google Scholar]
- 46.Suzuki N, Hardebo JE, Kåhrström J, et al. Effect on cortical blood flow of electrical stimulation of trigeminal cerebrovascular nerve fibres in the rat. Acta Physiol Scand 1990; 138: 307–316 [DOI] [PubMed] [Google Scholar]
- 47.Yu JH, Schneyer CA. Effects of varying frequency of parasympathetic nerve stimulation on flow rate and ion concentrations of rat submandibular saliva. Arch Oral Biol 1984; 29: 551–553 [DOI] [PubMed] [Google Scholar]
- 48.Norton NS.Netter's head and neck anatomy for dentistry Philadelphia, PA: Saunders, 2006 [Google Scholar]
- 49.May A, Goadsby PJ. Cluster headache: Imaging and other developments. Curr Opin Neurol 1998; 11: 199–203 [DOI] [PubMed] [Google Scholar]
- 50.Magis D, Gerardy PY, Remacle JM, et al. Sustained effectiveness of occipital nerve stimulation in drug-resistant chronic cluster headache. Headache 2011; 51: 1191–1201 [DOI] [PubMed] [Google Scholar]