Abstract
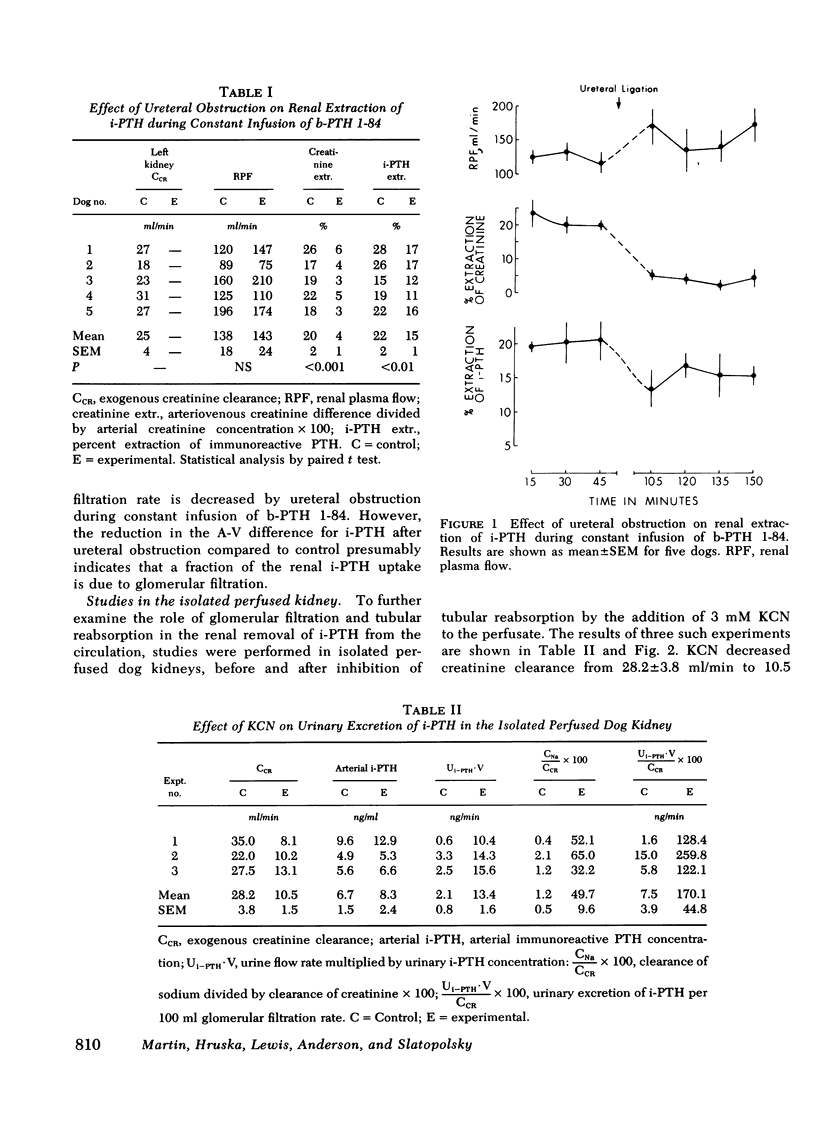
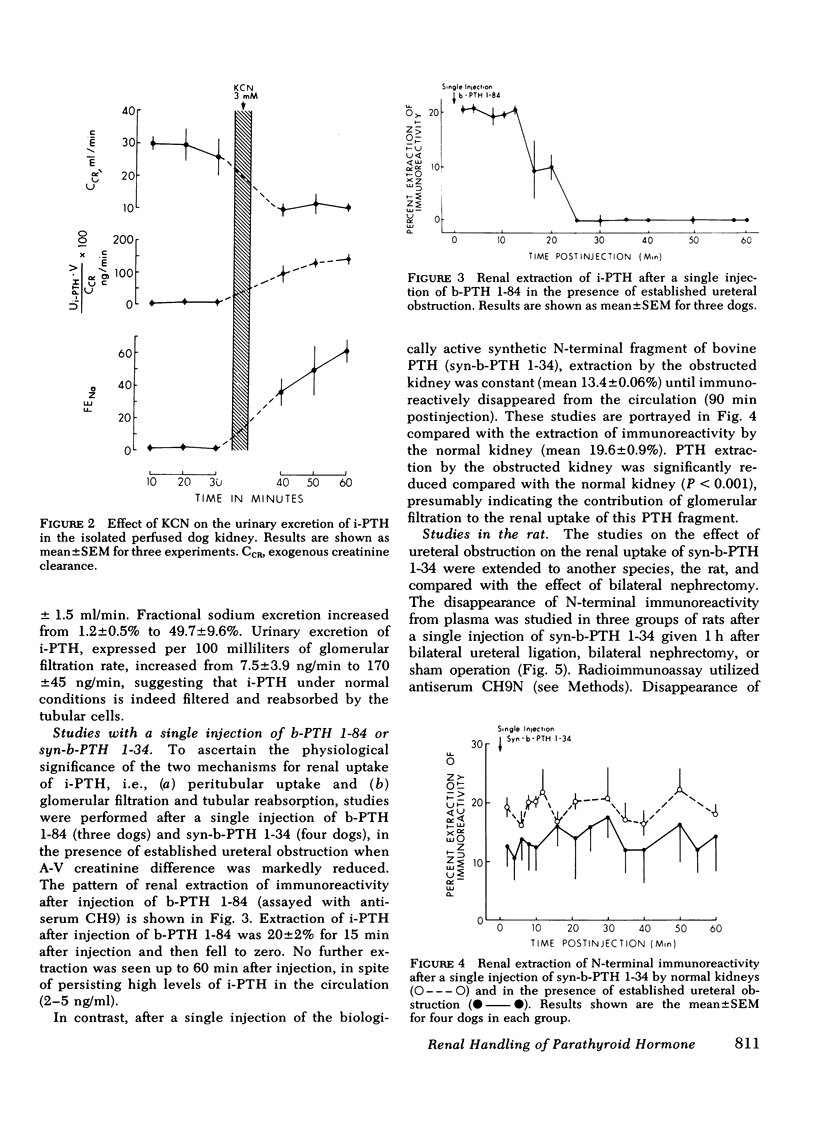
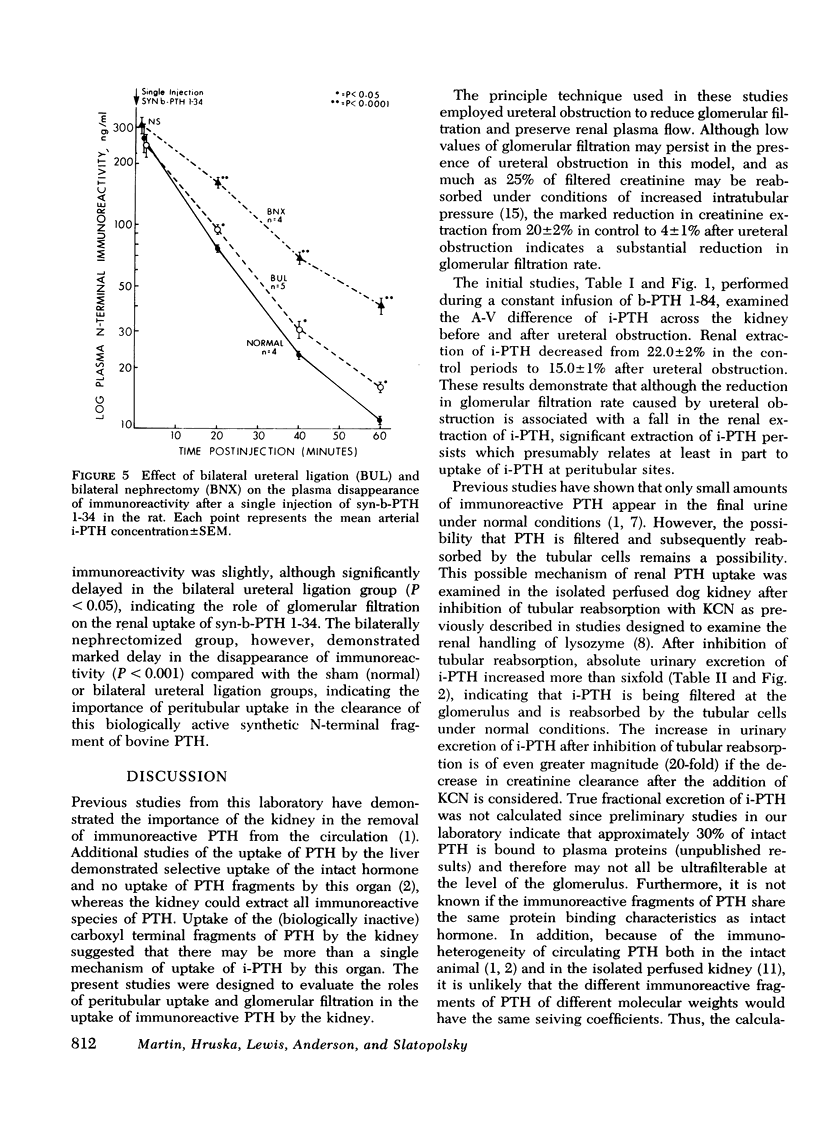
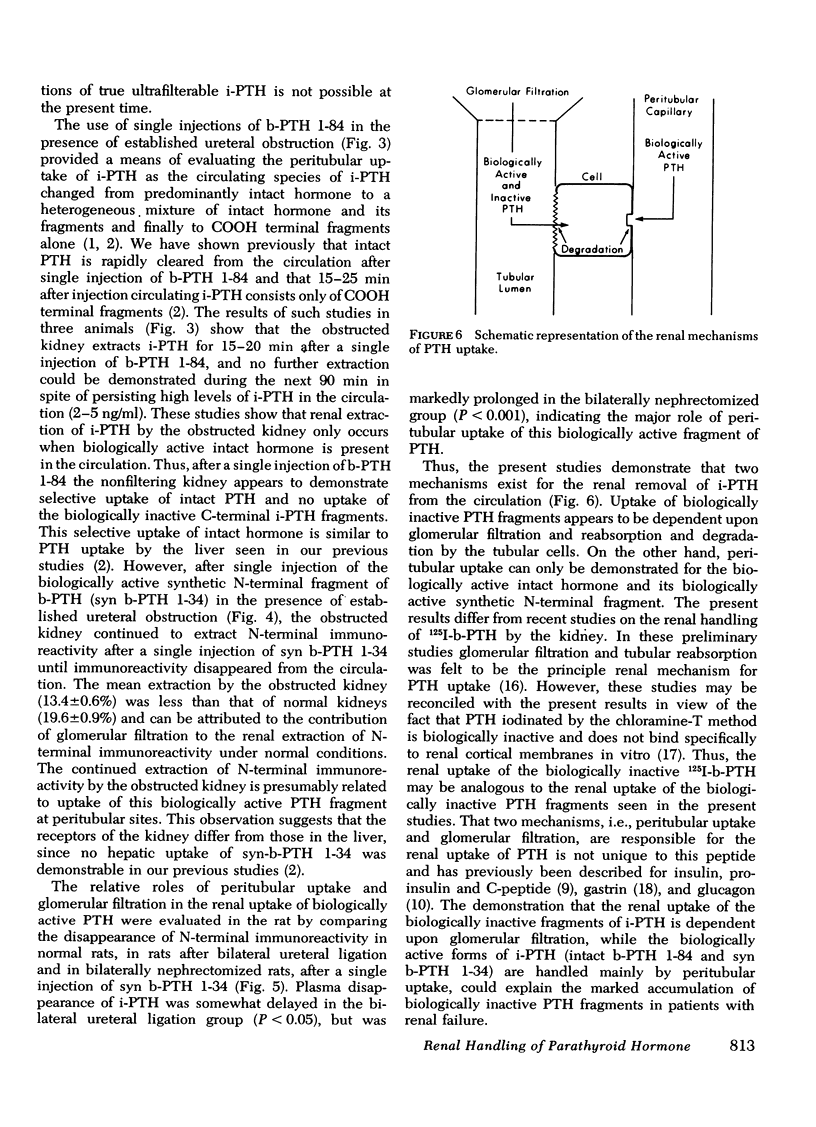
The mechanisms of uptake of parathyroid hormone (PTH) by the kidney was studied in anesthetized dogs before and after ureteral ligation. During constant infusion of bovine PTH (b-PTH 1-84), the renal arteriovenous (A-V) difference for immunoreactive PTH (i-PTH) was 22±2%. After ureteral ligation and no change in renal plasma flow, A-V i-PTH fell to 15±1% (P < 0.01), indicating continued and significant uptake of i-PTH at peritubular sites and a lesser role of glomerular filtration (GF) in the renal uptake of i-PTH. Since, under normal conditions, minimal i-PTH appears in the final urine, the contribution of GF and subsequent tubular reabsorption was further examined in isolated perfused dog kidneys before and after inhibition of tubular reabsorption by potassium cyanide. Urinary i-PTH per 100 ml GF rose from 8±4 ng/min (control) to 170±45 ng/min after potassium cyanide. Thus, i-PTH is normally filtered and reabsorbed by the tubular cells. The physiological role of these two mechanisms of renal PTH uptake was examined by giving single injections of b-PTH 1-84 or synthetic b-PTH 1-34 in the presence of established ureteral ligation. After injection of b-PTH 1-84, renal A-V i-PTH was 20% only while biologically active intact PTH was present (15-20 min). No peritubular uptake of carboxyl terminal PTH fragments was demonstrable. In contrast, after injection of synthetic b-PTH 1-34, renal extraction of N-terminal i-PTH after ureteral ligation (which was 13.4±0.6% vs. 19.6±0.9% in controls) continued for as long as i-PTH persisted in the circulation. These studies indicate that both GF and peritubular uptake are important mechanisms for renal PTH uptake. Renal uptake of carboxyl terminal fragments of PTH is dependent exclusively upon GF and tubular reabsorption, whereas peritubular uptake can only be demonstrated for biologically active b-PTH 1-84 and synthetic b-PTH 1-34.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chu L. L., Forte L. R., Anast C. S., Cohn D. V. Interaction of parathyroid hormone with membranes of kidney cortex: degradation of the hormone and activation of adenylate cyclase. Endocrinology. 1975 Oct;97(4):1014–1023. doi: 10.1210/endo-97-4-1014. [DOI] [PubMed] [Google Scholar]
- Di Bella F. P., Dousa T. P., Miller S. S., Arnaud C. D. Parathyroid hormone receptors of renal cortex: specific binding of biologically active, 125I-labeled hormone and relationship to adenylate cyclase activation. Proc Natl Acad Sci U S A. 1974 Mar;71(3):723–726. doi: 10.1073/pnas.71.3.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmanouel D. S., Jaspan J. B., Kuku S. F., Rubenstein A. H., Katz A. I., Huen A. H. Pathogenesis and characterization of hyperglucagonemia in the uremic rat. J Clin Invest. 1976 Nov;58(5):1266–1272. doi: 10.1172/JCI108581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace S. G., Davidson W. D., State D. Renal mechanisms for removal of gastrin from the circulation. Surg Forum. 1974;25(0):323–325. [PubMed] [Google Scholar]
- HARVEY R. B., BROTHERS A. J. Renal extraction of para-aminohippurate and creatinine measured by continuous in vivo sampling of arterial and renal-vein blood. Ann N Y Acad Sci. 1962 Oct 31;102:46–54. doi: 10.1111/j.1749-6632.1962.tb13624.x. [DOI] [PubMed] [Google Scholar]
- Hruska K. A., Kopelman R., Rutherford W. E., Klahr S., Slatopolsky E., Greenwalt A., Bascom T., Markham J. Metabolism in immunoreactive parathyroid hormone in the dog. The role of the kidney and the effects of chronic renal disease. J Clin Invest. 1975 Jul;56(1):39–48. doi: 10.1172/JCI108077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruska K. A., Martin K., Mennes P., Greenwalt A., Anderson C., Klahr S., Slatopolsky E. Degradation of parathyroid hormone and fragment production by the isolated perfused dog kidney. The effect of glomerular filtration rate and perfusate CA++ concentrations. J Clin Invest. 1977 Sep;60(3):501–510. doi: 10.1172/JCI108802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz A. I., Rubenstein A. H. Metabolism of proinsulin, insulin, and C-peptide in the rat. J Clin Invest. 1973 May;52(5):1113–1121. doi: 10.1172/JCI107277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorentz W. B., Jr, Lassiter W. E., Gottschalk C. W. Renal tubular permeability during increased intrarenal pressure. J Clin Invest. 1972 Mar;51(3):484–492. doi: 10.1172/JCI106836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maack T. Renal handling of low molecular weight proteins. Am J Med. 1975 Jan;58(1):57–64. doi: 10.1016/0002-9343(75)90533-1. [DOI] [PubMed] [Google Scholar]
- Malbon C. G., Zull J. E. Interactions of parathyroid hormone and plasma membranes from rat kidney. Biochem Biophys Res Commun. 1974 Feb 27;56(4):952–958. doi: 10.1016/s0006-291x(74)80281-0. [DOI] [PubMed] [Google Scholar]
- Martin K., Hruska K., Greenwalt A., Klahr S., Slatopolsky E. Selective uptake of intact parathyroid hormone by the liver: differences between hepatic and renal uptake. J Clin Invest. 1976 Oct;58(4):781–788. doi: 10.1172/JCI108529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordquist R. E., Palmieri M. A. Intracellular localization of parathyroid hormone in the kidney. Endocrinology. 1974 Jul;95(1):229–237. doi: 10.1210/endo-95-1-229. [DOI] [PubMed] [Google Scholar]
- Singer F. R., Segre G. V., Habener J. F., Potts J. T., Jr Peripheral metabolism of bovine parathyroid hormone in the dog. Metabolism. 1975 Feb;24(2):139–144. doi: 10.1016/0026-0495(75)90014-1. [DOI] [PubMed] [Google Scholar]
- Sutcliffe H. S., Martin T. J., Eisman J. A., Pilczyk R. Binding of parathyroid hormone to bovine kidney-cortex plasma membranes. Biochem J. 1973 Aug;134(4):913–921. doi: 10.1042/bj1340913. [DOI] [PMC free article] [PubMed] [Google Scholar]



