Abstract
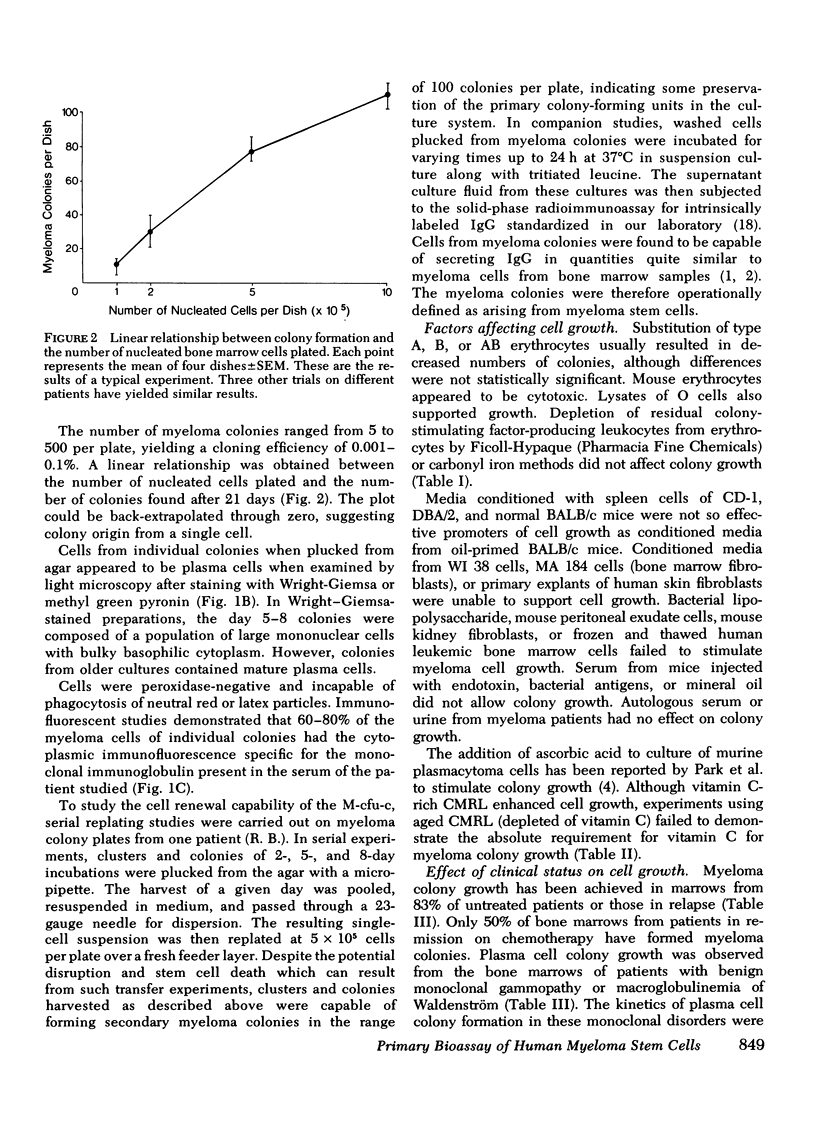
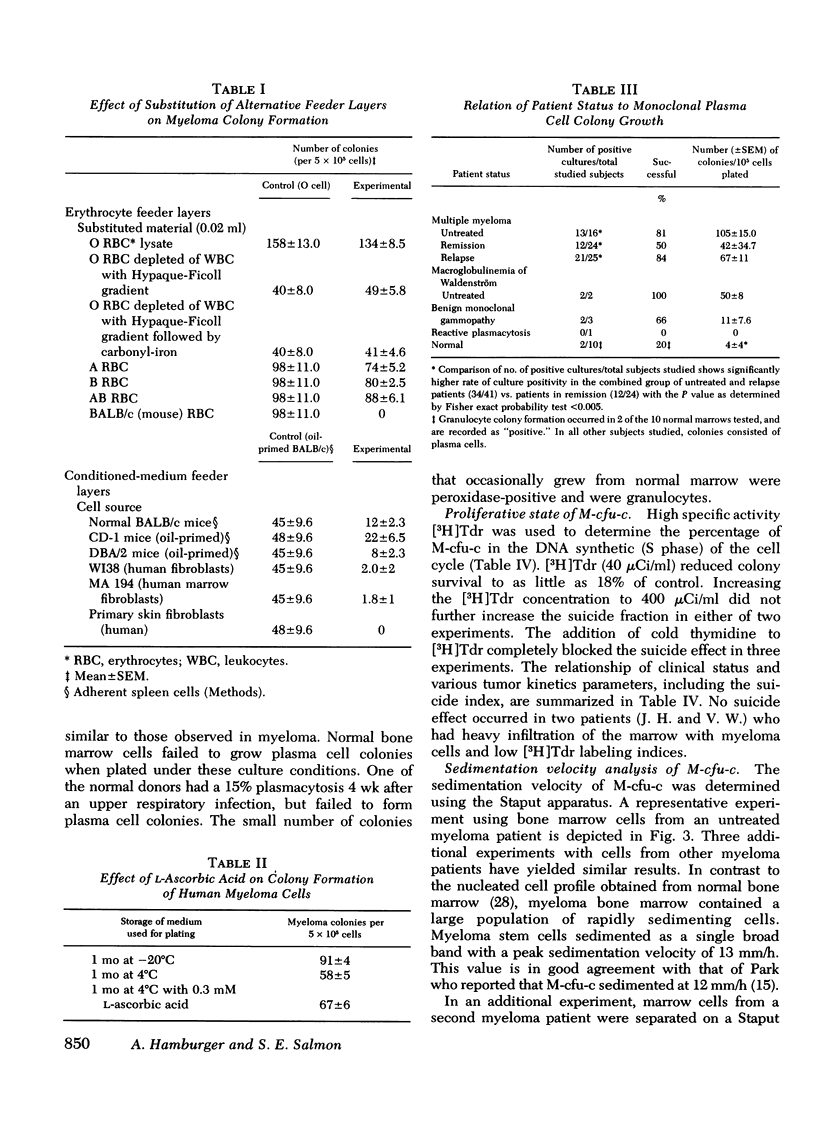
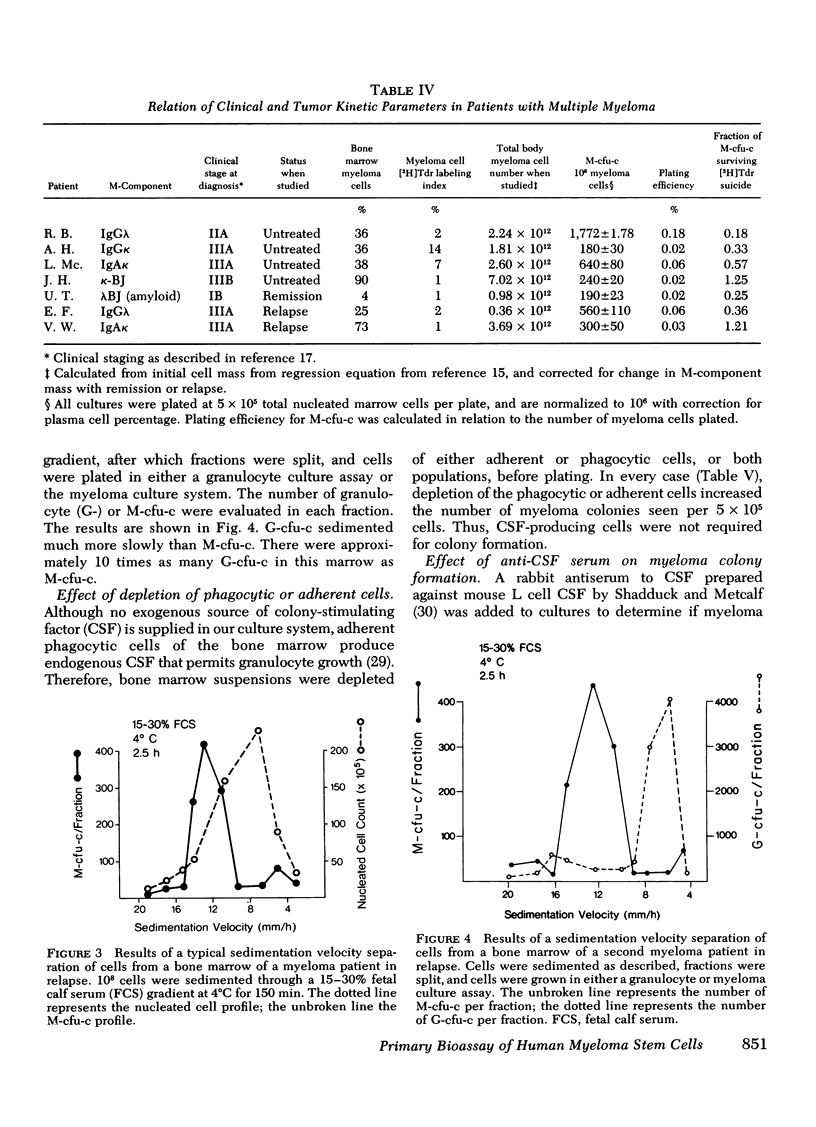
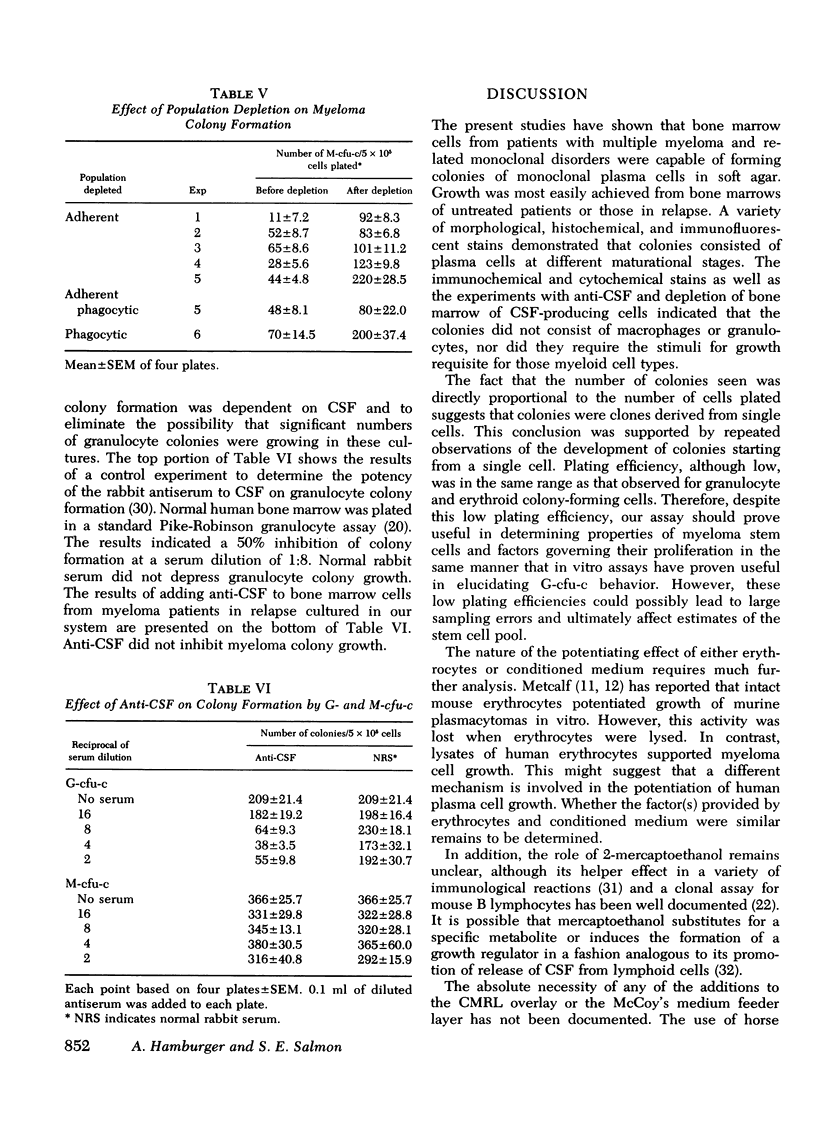
The ability to clone primary tumors in soft agar has proven useful in the study of the kinetics and biological properties of tumor stem cells. We report the development of an in vitro assay which permits formation of colonies of human monoclonal plasma cells in soft agar. Colony growth has been observed from bone marrow aspirates from 75% of the 70 patients with multiple myeloma or related monoclonal disorders studied. Growth was induced with either 0.02 ml of human type O erythrocytes or 0.25 ml of medium conditioned by the adherent spleen cells of mineral oil-primed BALB/c mice. 5-500 colonies appeared after 2-3 wk in culture yielding a plating efficiency of 0.001-0.1%. The number of myeloma colonies was proportional to the number of cells plated between concentrations of 105-106 and back-extrapolated through zero, suggesting that colonies were clones derived from single myeloma stem cells. Morphological, histochemical, and functional criteria showed the colonies to consist of immature plasmablasts and mature plasma cells. 60-80% of cells picked from colonies contained intracytoplasmic monoclonal immunoglobulin. Colony growth was most easily achieved from the bone marrow cells of untreated patients or those in relapse. Only 50% of bone marrow samples from patients in remission were successfully cultured. Tritiated thymidine suicide studies provided evidence that for most myeloma patients, a very high proportion of myeloma colony-forming cells was actively in transit through the cell cycle. Velocity sedimentation at 1 g showed myeloma stem cells sedimented in a broad band with a peak at 13 mm/h. Antibody to granulocyte colony-stimulating factor did not reduce the number or size of the colonies. Increased numbers of myeloma colonies were seen when the marrow was depleted of colony-stimulating factor elaborating adherent cells before plating. This bioassay should prove useful in studying the in vitro biological behavior of certain bone marrow-derived (B)-cell neoplasia. In addition, systematic and predictive studies of anticancer drug effects on myeloma stem cells should now be feasible.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberts D. S., Golde D. W. DNA synthesis in multiple myeloma cells following cell cycle-nonspecific chemotherapy. Cancer Res. 1974 Nov;34(11):2911–2914. [PubMed] [Google Scholar]
- Bradley T. R., Metcalf D. The growth of mouse bone marrow cells in vitro. Aust J Exp Biol Med Sci. 1966 Jun;44(3):287–299. doi: 10.1038/icb.1966.28. [DOI] [PubMed] [Google Scholar]
- Drewinko B., Brown B. W., Humphrey R., Alexanian R. Effect of chemotherapy in the labelling index of myeloma cells. Cancer. 1974 Sep;34(3):526–531. doi: 10.1002/1097-0142(197409)34:3<526::aid-cncr2820340308>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Durie B. G., Salmon S. E. A clinical staging system for multiple myeloma. Correlation of measured myeloma cell mass with presenting clinical features, response to treatment, and survival. Cancer. 1975 Sep;36(3):842–854. doi: 10.1002/1097-0142(197509)36:3<842::aid-cncr2820360303>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Durie B. G., Salmon S. E. High speed scintillation autoradiography. Science. 1975 Dec 12;190(4219):1093–1095. doi: 10.1126/science.1188385. [DOI] [PubMed] [Google Scholar]
- Heber-Katz E., Click R. E. Immune responses in vitro. V. Role of mercaptoethanol in the mixed-leukocyte reaction. Cell Immunol. 1972 Nov;5(3):410–418. doi: 10.1016/0008-8749(72)90067-6. [DOI] [PubMed] [Google Scholar]
- Iscove N. N., Till J. E., McCulloch E. A. The proliferative states of mouse granulopoietic progenitor cells. Proc Soc Exp Biol Med. 1970 May;134(1):33–36. doi: 10.3181/00379727-134-34721. [DOI] [PubMed] [Google Scholar]
- Jobin M. E., Fahey J. L., Price Z. Long-term establishment of a human plasmacyte cell line derived from a patient with IgD multiple myeloma. I. Requirement of a plasmacyte-stimulating factor for the proliferation of myeloma cells in tissue culture. J Exp Med. 1974 Aug 1;140(2):494–507. doi: 10.1084/jem.140.2.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudtzon S. Growth stimulation of normal human bone marrow cells in agar culture by human serum. Scand J Haematol. 1974;12(4):298–306. doi: 10.1111/j.1600-0609.1974.tb00213.x. [DOI] [PubMed] [Google Scholar]
- Messner H. A., McCulloch E. A. Interacting cell populations affecting granulopoietic colony formation by normal and leukemic human marrow cells. Blood. 1973 Nov;42(5):701–710. [PubMed] [Google Scholar]
- Messner H., Till J. E., McCulloch E. A. Density distributions of marrow cells from mouse and man. Ser Haematol. 1972;5(2):22–36. [PubMed] [Google Scholar]
- Metcalf D. Colony formation in agar by murine plasmacytoma cells: potentiation by hemopoietic cells and serum. J Cell Physiol. 1973 Jun;81(3):397–410. doi: 10.1002/jcp.1040810312. [DOI] [PubMed] [Google Scholar]
- Metcalf D. The serum factor stimulating colony formation in vitro by murine plasmacytoma cells: response to antigens and mineral oil. J Immunol. 1974 Jul;113(1):235–243. [PubMed] [Google Scholar]
- Miller R. G., Phillips R. A. Separation of cells by velocity sedimentation. J Cell Physiol. 1969 Jun;73(3):191–201. doi: 10.1002/jcp.1040730305. [DOI] [PubMed] [Google Scholar]
- Moore M. A., Williams N., Metcalf D. In vitro colony formation by normal and leukemic human hematopoietic cells: interaction between colony-forming and colony-stimulating cells. J Natl Cancer Inst. 1973 Mar;50(3):591–602. doi: 10.1093/jnci/50.3.591. [DOI] [PubMed] [Google Scholar]
- Namba Y., Hanaoka M. Immunocytology of cultured IgM-forming cells of mouse. I. Requirement of phagocytic cell factor for the growth of IgM-forming tumor cells in tissue culture. J Immunol. 1972 Dec;109(6):1193–1200. [PubMed] [Google Scholar]
- Nilsson K., Bennich H., Johansson S. G., Pontén J. Established immunoglobulin producing myeloma (IgE) and lymphoblastoid (IgG) cell lines from an IgE myeloma patient. Clin Exp Immunol. 1970 Oct;7(4):477–489. [PMC free article] [PubMed] [Google Scholar]
- Ogawa M., Bergsagel D. E., McCulloch E. A. Chemotherapy of mouse myeloma: quantitative cell cultures predictive of response in vivo. Blood. 1973 Jan;41(1):7–15. [PubMed] [Google Scholar]
- Ogawa M., Bergsagel D. E., McCulloch E. A. Differential effects of melphalan on mouse myeloma (adj. PC-5) and hemopoietic stem cells. Cancer Res. 1971 Dec;31(12):2116–2119. [PubMed] [Google Scholar]
- Ogawa M., Bergsagel D. E., McCulloch E. A. Sensitivity of human and murine hemopoietic precursor cells to chemotherapeutic agents assessed in cell culture. Blood. 1973 Dec;42(6):851–856. [PubMed] [Google Scholar]
- Park C. H., Bergsagel D. E., McCulloch E. A. Mouse myeloma tumor stem cells: a primary cell culture assay. J Natl Cancer Inst. 1971 Feb;46(2):411–422. [PubMed] [Google Scholar]
- Parker J. W., Metcalf D. Production of colony-stimulating factor in mitogen-stimulated lymphocyte cultures. J Immunol. 1974 Feb;112(2):502–510. [PubMed] [Google Scholar]
- Pike B. L., Robinson W. A. Human bone marrow colony growth in agar-gel. J Cell Physiol. 1970 Aug;76(1):77–84. doi: 10.1002/jcp.1040760111. [DOI] [PubMed] [Google Scholar]
- Pluznik D. H., Sachs L. The induction of clones of normal mast cells by a substance from conditioned medium. Exp Cell Res. 1966 Oct;43(3):553–563. doi: 10.1016/0014-4827(66)90026-7. [DOI] [PubMed] [Google Scholar]
- Salmon S. E. Expansion of the growth fraction in multiple myeloma with alkylating agents. Blood. 1975 Jan;45(1):119–129. [PubMed] [Google Scholar]
- Salmon S. E. Immunoglobulin synthesis and tumor kinetics of multiple myeloma. Semin Hematol. 1973 Apr;10(2):135–144. [PubMed] [Google Scholar]
- Salmon S. E., Smith B. A. Immunoglobulin synthesis and total body tumor cell number in IgG multiple myeloma. J Clin Invest. 1970 Jun;49(6):1114–1121. doi: 10.1172/JCI106327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadduck R. K., Metcalf D. Preparation and neutralization characteristics of an anti-CSF antibody. J Cell Physiol. 1975 Oct;86(2 Pt 1):247–252. doi: 10.1002/jcp.1040860208. [DOI] [PubMed] [Google Scholar]
- Stephenson J. R., Axelrad A. A., McLeod D. L., Shreeve M. M. Induction of colonies of hemoglobin-synthesizing cells by erythropoietin in vitro. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1542–1546. doi: 10.1073/pnas.68.7.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan P. W., Salmon S. E. Kinetics of tumor growth and regression in IgG multiple myeloma. J Clin Invest. 1972 Jul;51(7):1697–1708. doi: 10.1172/JCI106971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepperman A. D., Curtis J. E., McCulloch E. A. Erythropietic colonies in cultures of human marrow. Blood. 1974 Nov;44(5):659–669. [PubMed] [Google Scholar]
- Weinberg S. R., Stohlman F., Jr Growth of mouse yolk sac cells cultured in vivo. Br J Haematol. 1976 Apr;32(4):543–555. doi: 10.1111/j.1365-2141.1976.tb00958.x. [DOI] [PubMed] [Google Scholar]



