Abstract
Clonal proliferation is an obligatory component of adipogenesis. Although several cell cycle regulators are known to participate in the transition between pre-adipocyte proliferation and terminal adipocyte differentiation, how the core DNA synthesis machinery is coordinately regulated in adipogenesis remains elusive. PCNA (proliferating cell nuclear antigen) is an indispensable component for DNA synthesis during proliferation. Here we show that PCNA is subject to phosphorylation at the highly conserved tyrosine residue 114 (Y114). Replacing the Y114 residue with phenylalanine (Y114F), which is structurally similar to tyrosine but cannot be phosphorylated, does not affect normal animal development. However, when challenged with high fat diet, mice carrying homozygous Y114F alleles (PCNAF/F) are resistant to adipose tissue enlargement in comparison to wild-type (WT) mice. Mouse embryonic fibroblasts (MEFs) harboring WT or Y114F mutant PCNA proliferate at similar rates. However, when subjected to adipogenesis induction in culture, PCNAF/F MEFs are not able to re-enter the cell cycle and fail to form mature adipocytes, while WT MEFs undergo mitotic clonal expansion in response to the adipogenic stimulation, accompanied by enhanced Y114 phosphorylation of PCNA, and differentiate to mature adipocytes. Consistent with the function of Y114 phosphorylation in clonal proliferation in adipogenesis, fat tissues isolated from WT mice contain significantly more adipocytes than those isolated from PCNAF/F mice. This study identifies a critical role for PCNA in adipose tissue development, and for the first time identifies a role of the core DNA replication machinery at the interface between proliferation and differentiation.
Keywords: PCNA, tyrosine phosphorylation, adipogenesis, proliferation
INTRODUCTION
Obesity is an epidemic worldwide. When energy intake exceeds expenditure, excess energy is stored in the form of triglycerides in adipocytes and results in adipose tissue expansion. The number and size of adipocytes in fat depots determine whether an individual remains lean or becomes obese [1–3]. Unlike adipocyte size, which changes with the status of food intake and energy expenditure, the number of adipocytes generally remains constant throughout adult life. However, deregulated increase of adipose cell number, termed hyperplasia, is a known pathological contributor to obesity in many children and adults [4, 5]. Recent studies have also demonstrated that adipocytes in adults undergo dynamic turnover with a relatively rapid proliferation [6, 7]. These findings suggest that a tight regulation of adipocyte number homeostasis is a critical contributor to obesity.
Much of our knowledge on the progression of adipocyte differentiation, termed adipogenesis, is derived from studies using primary or established rodent pre-adipocyte or adipocytes [8]. When preadipocytes are grown to confluence, they withdraw from the cell cycle and enter a growth-arrested state due to contact inhibition. Treatment of confluent cells with a cocktail of hormonal and mitogenic agents induces the cells to re-enter the cell cycle and engage in synchronous rounds of DNA replication and cell division, termed mitotic clonal expansion (MCE), to clonally expand committed cells [1, 9–12]. Post-mitotic cells then irreversibly exit the mitotic cycle and undergo terminal differentiation to form mature adipocytes. There is abundant evidence for the obligatory role of MCE for adipogenesis, and MCE may also contribute to the mechanism that controls adipocyte numbers [1, 2]. However, the mechanisms regulating DNA synthesis in growth-arrested cells are not well understood.
The Proliferating Cell Nuclear Antigen (PCNA) is a DNA synthesis processivity factor during cell proliferation and is responsible for replication of the majority of our genome [13]. Due to its indispensable role in cell proliferation, homozygous deletion of PCNA in mice causes embryonic lethality [14]. During DNA replication, three PCNA monomers form a homotrimeric ring encircling the DNA double helix as a sliding platform to assemble and coordinate the core DNA synthesis machinery at the replication fork [13, 15–18]. At the interaction junction of the homotrimeric ring, two monomers of PCNA interact though hydrogen bonds conferred by a different β sheet and a different helix along the interface of each monomer [19]. The hydrophobic tyrosine 114 residue (Y114), located in the strand βI1 of one monomer interface, is conserved in mammals, fly, and Xenopus and appears to be a critical interaction point (Fig. 1A). Substituting this residue with alanine (Y114A) abolishes trimerization of the mutant PCNA in vitro, thereby supporting the important role of this tyrosine residue [20]. However, given the drastic structural difference between tyrosine and alanine, the mutant protein appears to have very different thermodynamic properties compared with the wild type. Therefore, the physiological role of the Y114 residue in PCNA function remains unclear. We identified that PCNA is subject to phosphorylation at Y114, and generated a knock-in mouse model in which the Y114 residue is changed to a phenylalanine (Y114F). Molecular characterization of the mutant mice and the derived MEFs led us to the unexpected discovery that this phosphorylation is an important signaling event for MCE during adipogenesis and the development of adiposity in response to an energy-rich diet.
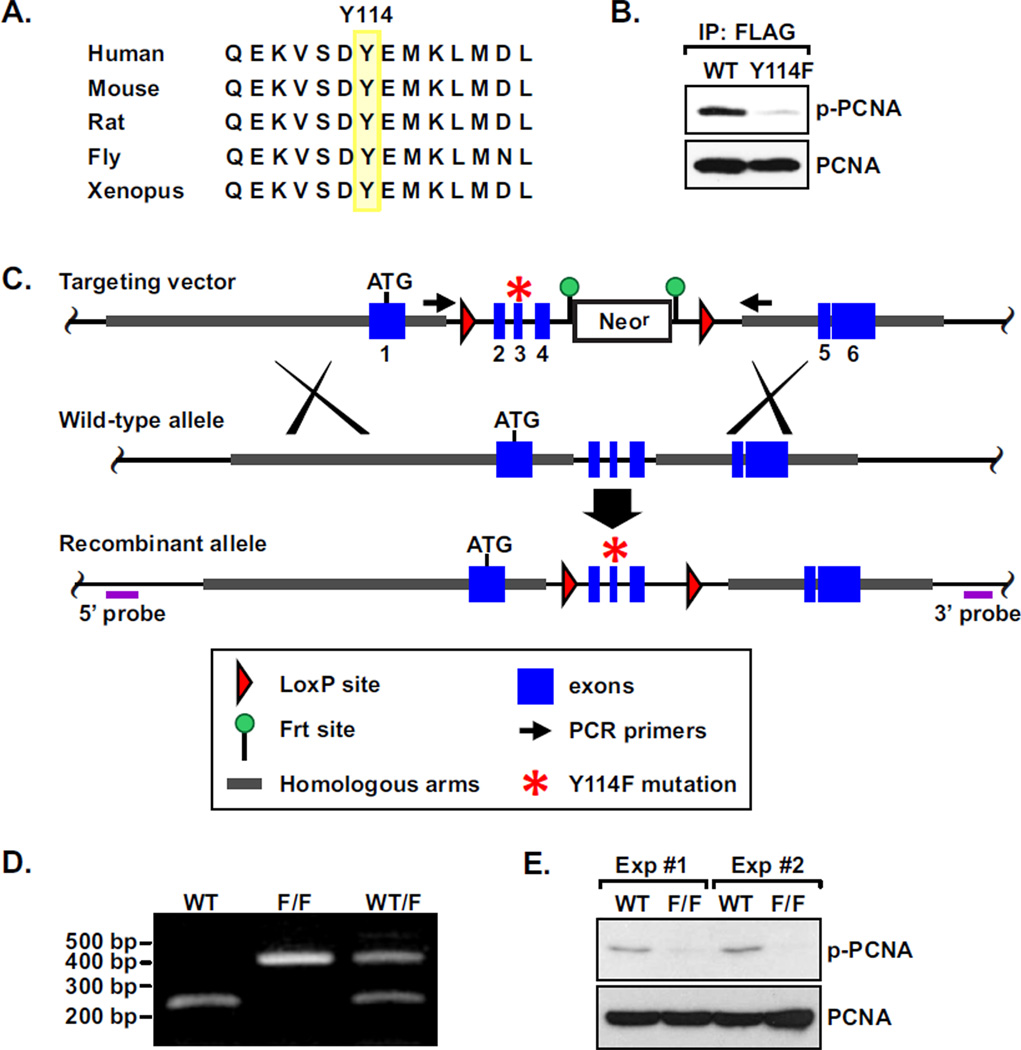
Figure 1. Y114 phosphorylation of PCNA and the knock-in mouse model.
A, The Y114 residue of PCNA is evolutionarily conserved. B, HKE293T cells were transfected with FLAG-tagged PCNA of WT or Y114F mutant. The ectopic PCNA was immunoprecipitated with an anti-FLAG antibody and the blot was probed with the anti-phospho-Y114 PCNA antibody. C, Targeting strategy to generate Y114F knock-in mutation. D, PCR analysis to confirm the WT and the Y114F allele in WT, heterozygous (W/F) and homozygous (F/F) mutant mice. E, Protein extracts of WT and F/F MEF cells were examined for total PCNA and phospho-Y114 PCNA (p-PCNA) by Western analysis.
MATERIALS AND METHODS
Generation of Y114F PCNA mice
Heterozygote Y114F knock-in mice were generated through a collaboration contract with Xenogen/Taconic. Briefly, the mouse BAC clone (RP23-161C23) was used to generate the homology arms containing the 5’ homology arm (4.9 kb), 3’ homology arm (3.0 kb) and the conditional knock-out (KO) region (1.4 kb). The Tyr to Phe (A to T) mutation of Y114 in exon 3 was then introduced into the conditional KO region (Y114F) by PCR-based site-directed mutagenesis (QuickChange II, Stratagene/Agilent Technology). The final vector also contains LoxP sequences flanking the conditional KO region, Frt sequences flanking the neomycin-resistant marker expression cassette (for positive selection of the ES cells), and a DTA expression cassette (for negative selection of the ES cells). The final vector was verified by both restriction digestion and end sequencing analysis. The targeting plasmid was linearized with NotI prior to electroporation into FVB ES cells. Cell clones were selected with G418, and screened with PCR. Homologous recombination was confirmed with Southern analysis and sequencing. Flp electroporation was then performed to delete the neomycin cassette. The ES cells were then injected into FVB blastocysts.
Chemicals and antibodies
3-isobutyl-1-methylxanthine (IBMX), dexamethasone, insulin, and Oil Red O were purchased from Sigma. Antibodies were purchased: anti-PCNA from Santa Cruz, anti-BrdU from Millipore. The anti-phospho-Y114 PCNA antibody was generated by immunizing rabbits with the KLH-conjugated synthetic peptide CNQEKVSD-pY-EMKLMD (Yenzym). The serum was purified in two rounds by a phosphopeptide affinity matrix, followed by clean-up with an unphosphorylated peptide-conjugated affinity matrix.
Cell proliferation analysis
Colorimetric BrdU proliferation was performed using a kit (Roche). Immunofluorescent detection of BrdU was performed by incubating cells with BrdU for 4 hours. Cells were then fixed with chilled methanol, and treated with two consecutive incubations with 4M HCl for 15 minutes at room temperature. BrdU was detected with an anti-BrdU antibody, followed by a FITC-conjugated secondary antibody.
In vitro adipogenesis
Induction of MEF cells to adipogenesis was conducted following the protocol established by Dr. Daniel Lane’s laboratory [21]. Briefly, MEF cells were plated in 6-well plates in growth media (DMEM/F12 with 10% fetal bovine serum (FBS)) until confluence. To induce adipocyte differentiation, growth media was changed to media containing 10% FBS, 10 µg/ml insulin, 1 M dexamethasone, and 0.5 mM IBMX for 2 days, followed by media with 10% FBS and 10 g/ml insulin for 6 days. At the end of differentiation, cells were fixed with 4% paraformaldehyde. Adipocytes with accumulated lipid were visualized using 0.1% Oil Red O staining.
Adipocyte number in fat pads
Peri-ovarian adipose tissues were dissected, sliced into small pieces, washed twice with PBS and then fixed with OsO4 for 72 hours. The adipocytes were released by digestion with 8M urea. Cell numbers were determined using an automatic cell counter (Invitrogen).
RESULTS
To examine the status of phosphorylation at the highly conserved Y114 residue, an antibody was raised against a synthetic peptide containing the epitope of phosphorylated Y114 (see Materials and Methods). Enzyme-linked immunosorbent assay (ELISA) analysis showed that this antibody specifically recognizes the phosphorylated but not the unphosphorylated wild-type Y114 epitope (data not shown). To further verify this antibody in vivo, HEK-293T cells were transiently transfected with cDNA of FLAG-tagged wild-type or Y114F mutant PCNA, and ectopic FLAG-PCNA was immunoprecipitated with an anti-FLAG antibody. The precipitate was separated by electrophoresis followed by Western analysis using the purified anti-phospho-Y114 antibody. The results demonstrated that the antibody specifically recognized a sub-population of the wild-type but not the phosphorylation-incompetent Y114F mutant PCNA (Fig. 1B).
To investigate the physiological role of Y114 phosphorylation, we generated mice bearing a knock-in PCNA allele with the tyrosine 114 residue replaced with a phenylalanine (Fig. 1, C and D). Intercross of the F1 heterozygotes PCNAWT/114F yielded all three genotypes in F2 litters roughly followed a Mendelian inheritance pattern. PCNAF/F pups are fertile and developed normally, indicating that the Y114F mutant PCNA maintained its normal functions in cell growth and development. To study the functions of Y114 at the cellular level, mouse embryonic fibroblasts (MEFs) were isolated from WT and PCNAF/F embryos at day 13.5. The cells were immortalized through a standard 3T3 protocol of spontaneous immortalization [22]. Consistent with the observation in HEK293T cells, Y114 phosphorylation of PCNA was detected in WT but not in PCNAF/F MEFs using the anti-phospho-Y114 antibody (Figure 1E; two independent experiments are shown).
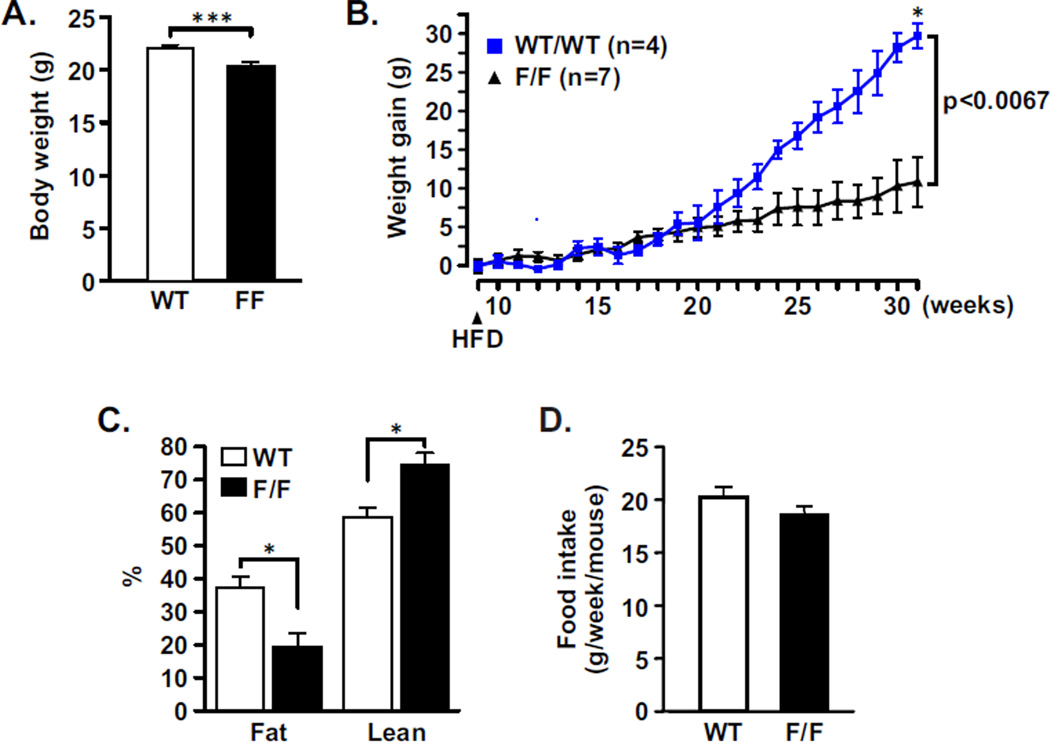
We noticed that body weights of adult (9 weeks old) strain-matched WT mice were slightly but significantly heavier than the PCNA114F/114F animals when fed with a normal chow diet (Fig. 2A). To test whether wild-type and mutant animals responded differently to fat-enriched food, mice were fed with a high fat diet (HFD; 45% calories from fat) up to 31 weeks of age. Feeding of WT mice with HFD fostered a more significant weight gain than in PCNA114F/114F animals (Fig. 2B). Fat and lean components of body composition were analyzed with a non-invasive quantitative magnetic resonance technique [23]. Only the fat component was significantly increased in the WT animals (Fig. 2C). Food intake by both types of animals was not different (Fig. 2D). These results demonstrated that development of adiposity on an energy-rich diet is diminished in the PCNA114F/114F animals.
Figure 2. PCNA114F/114F mice are resistant to diet-induced obesity.
A, Body weights of 9-week old female PCNA114F/114F mice (n=21) are slightly but significantly lower than those of WT mice (n=18) when fed with a normal chow diet. ***, P = 0.001. B, 9-week old FVB WT (n=5) and PCNA114F/114F (n=7) female mice were fed with a HFD (arrow). Gain of body weight was measured weekly and plotted. Average body weights of the cohort of mice were 23.1 g (WT) and 18.2 g (PCNA114F/114F). **, P=0.0067. C, Body composition of WT (n=2) and PCNA114F/114F (n=4) female mice analyzed by quantitative magnetic resonance. *, P=0.04. D, Food intake normalized by body weight was measured for the WT (n=5) and PCNA114F/114F (n=7) 9-week old mice on a high-fat diet (HFD) with 45% of total calories derived from fat. P=0.127.
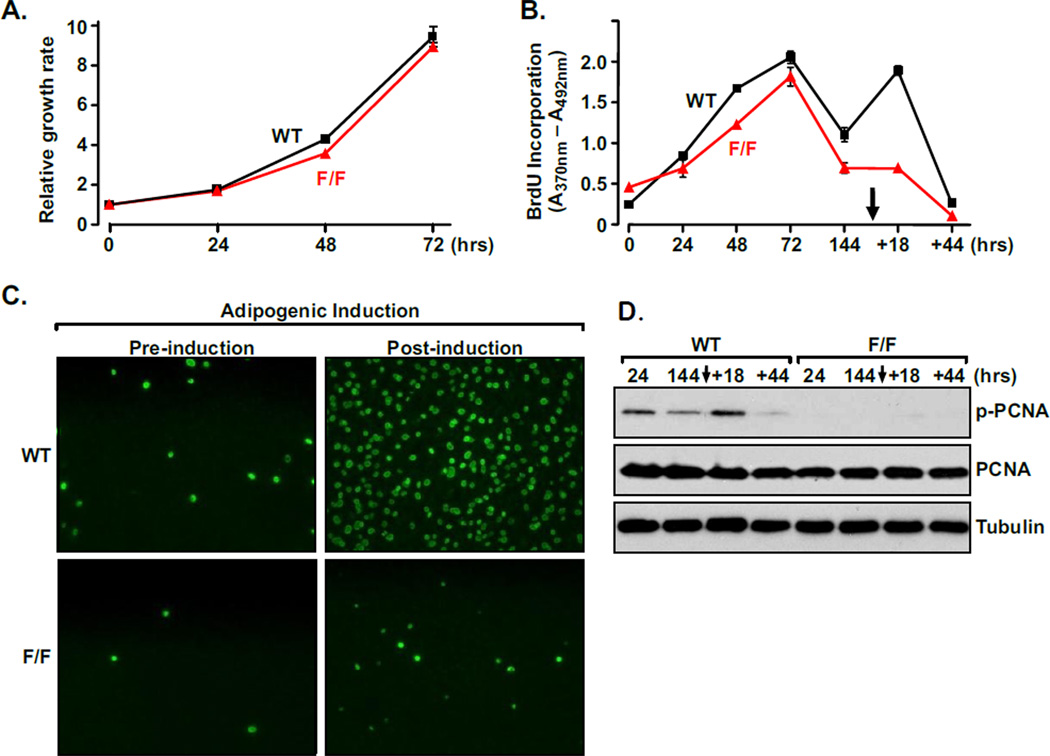
To dissect out the underlying mechanism of the obese phenotype under HFD, isolated MEFs were analyzed. WT and PCNA114F/114F MEFs showed no significant difference in growth rate and the capacity to proliferate under normal culture conditions (Fig. 3A). As previously reported [21], DNA synthesis measured by BrdU incorporation showed that MEFs plated in tissue culture plates continue to proliferate (72-hour time point in Fig. 3B) until reaching confluence, when they become growth-arrested due to contact inhibition (144-hour time point in Fig. 3B). At this point, MEFs can be induced to undergo adipocyte differentiation following simulation with an adipogenic cocktail, which contains insulin, 3-isobutyl-1-methylxanthine (IBMX), and dexamethasone [1, 10, 12]. Application of the adipogenic cocktail induced growth-arrested WT MEFs to synchronously re-enter the cell cycle and undergo active DNA synthesis (as evident by the peak of BrdU incorporation at the +18-hour time point in Fig. 3B). In drastic contrast, confluent PCNA114F/114F MEFs remain quiescent and fail to re-enter the cell cycle upon adipogenic induction. This phenotype was confirmed in a separate experiment using an anti-BrdU antibody and immunofluorescence microscopy to detect incorporated BrdU before and after the stimulation of the adipogenic cocktail (Fig. 3C).
Figure 3. PCNAF/F MEFs do not undergo mitotic clonal expansion in response to adipogenic stimuli.
A, WT and PCNA114F/114F MEFs have similar growth rates under normal culture conditions. Standard deviation (SD) of triplicate cell counts was <1%, unless shown via error bars. Viable cells were measured by CellTiter-Glo assay (Promega). B, WT and PCNA114F/114F MEFs actively incorporate BrdU until they reached confluence. Adding the adipogenic cocktail (arrow) stimulates WT but not PCNA114F/114F MEFs to re-enter the cell cycle for clonal expansion. Incorporated BrdU was quantitated using a colorimetric analysis. SD of triplicate counts was <1%, unless shown as error bars. The time point scale after induction is not drawn to scale. C, Proliferating WT and PCNA114F/114F MEFs at confluency were visualized using a GFP-conjugated anti-BrdU antibody (green) prior to (−) and following (+) adipogenic stimulation. D, Phosphorylation of Y114 of PCNA was then detected with Western analysis using a phospho-Y114-specific antibody.
To further assess the role of PCNA Y114 phosphorylation in adipocyte generation, we examined Y114 phosphorylation levels in cells sampled at pre- and post-induction time points (Fig. 3D). In WT MEFs, the Y114 phosphorylation level is low in confluent cells (144-hour time point), and was significantly induced by the adipogenic cocktail when the cells re-enter the proliferation phase (+18-hour time point). On the other hand, no Y114 phosphorylation was detected in PCNA114F/114F lysates. Collectively, these results show that Y114 phosphorylation of PCNA is an important signaling event required for MCE in response to adipogenic induction.
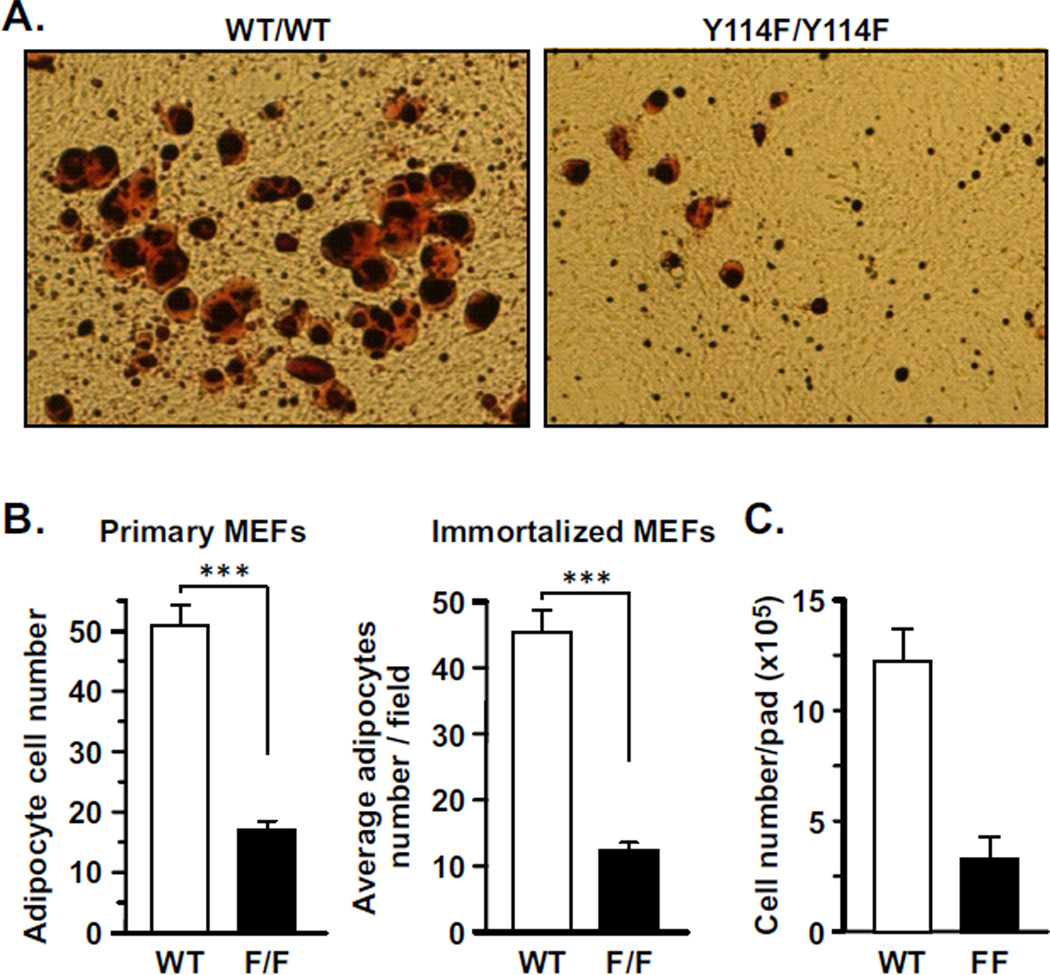
The defect of PCNA114F/114F MEFs in MCE predicts that these cells are incapable of adipogenesis, which would be consistent with the lean phenotype under HFD. Mature adipocytes are capable of accumulating fat droplets, which can be visualized by staining with the lipid dye Oil Red O. Using this assay, we found that while WT MEFs readily differentiated into mature adipocytes after adipogenic stimulation, PCNA114F/114F MEFs failed to do so (Fig. 4A). Similar results were observed in primary as well as immortalized MEFs (Fig. 4A). This experiment demonstrates that PCNA114F/114F MEFs are deficient in adipogenesis, contributing to the obesity-resistant phenotype of PCNA114F/114F mice fed with a HFD. These results also predict a lower number of adipocytes in the fat pads of PCNA114F/114F mice than in WT mice. Indeed, PCNA114F/114F mice had significantly less adipocytes in the peri-ovarian fat pad in comparison with the WT mice (Fig. 4B).
Figure 4. The ability of MEFs to differentiate into mature adipocytes is diminished in PCNA114F/114F MEFs.
A, Representative fields comparing non-immortalized WT and PCNA114F/114F MEFs in adipocyte differentiation. B, The numbers of adipocytes derived from primary and immortalized MEFs were counted in six independent fields and the average cell numbers per field are plotted. ***, P < 0.005. C, Adipocyte numbers in peri-ovarian fat depots of WT and PCNA114F/114F mice fed with HFD for 30 weeks (n=3). Bar, standard deviation.
DISCUSSION
The functions of PCNA are known to be regulated by post-translational modifications, such as ubiquitylation, sumoylation, and phosphorylation [13]. Our previous studies have identified that phosphorylation of PCNA at tyrosine Y211 enhances its stability on the chromatin and plays an important role in cancer cell proliferation [24–26]. In this report, we show that phosphorylation of PCNA at Y114 is involved in the development of adiposity on energy-rich diet. We show that this signaling event is not required for cell growth or normal development, but is important for facilitating adipogenesis upon adipogenic stimulation in vitro and in vivo. Additional studies of this molecular event can clarify how proliferative activities in cell growth and differentiation are differentially regulated.
While many studies have been performed to address the roles of cell cycle regulators with adipogenesis, a direct link between cell cycle control and adipogenesis remains to be further elucidated. Expression of cyclin-dependent kinase inhibitors p18Ink4c, p21Cip1 and p27Kip1 has been shown to couple with the progression of adipogenesis [27, 28]. The transcription factor peroxisome proliferator-activated receptor (PPAR), a master regulator of adipogenesis, plays a key role in regulating the expression of p21 and p18 at different stages through MCE, indicating that these cell cycle inhibitors are involved in adipogenesis [28]. However, contradictory results regarding the roles of p21 and p27 have been reported [29–31]. The reason for the discrepancy remains to be determined. These cell cycle inhibitors control entry into S phase by suppressing the activity of cyclin-dependent kinases (CDKs) [32]. Although cyclins and CDKs have been shown to promote adipogenesis, the mechanism can be attributed to their transcriptional functions in a mode independent from cell cycle [33–36]. These findings prompt continued investigation of the mechanisms coordinating mitotic proliferation and adipogenesis. Furthermore, it should be noted that all these cell cycle regulators also function in proliferating cells grown in normal culture conditions that do not convert to adipocytes even with adipogenic stimulation. Thus, while these studies demonstrated the complex and dynamic relationship between cell cycle regulation and adipogenesis, the fundamental mechanisms integrating cell proliferation with adipogenesis remained to be determined. In this regard, virtually nothing is unknown about how DNA replication activity, as mediated by the core DNA synthesis machinery, is differentially regulated during adipocyte differentiation.
Both hyperplasia (increase in cell number) and hypertrophy (increase in cell size) of adipocytes contribute to the expansion of fat mass and have a life-long influence on the susceptibility to obesity [1, 2]. The number of adipocytes was previously thought to be determined during childhood and stays constant throughout adulthood [37]. Until recently, the prevalent model stipulated that adipocyte hypertrophy is the most critical factor which determines fat mass in adult humans [4, 38]. This notion was challenged by a recent study demonstrating a highly dynamic turnover of adipocytes through cell proliferation (DNA replication) [7]. It remains to be determined how cell proliferation is facilitated in adipogenesis and how adipocyte cellularity is maintained. Our results show that phosphorylation at Y114 of PCNA plays a critical role in regulating adipocyte number.
An important question is whether the function of Y114 phosphorylation is specific to adipogenesis. To answer this question, detailed understanding of how this signaling event is regulated and how it functions during the formation of mature adipocytes will be necessary so that the impact of modulating the mechanism in other biological systems can be assessed. It is noteworthy that lack of Y114 phosphorylation does not affect normal cell growth in cell culture nor the development of the entire organism, indicating that the signaling event is dispensable for these processes. The phosphorylation event may be particularly important under metabolic stress such as feeding with high-fat diet. Identification of the tyrosine kinase responsible for Y114 phosphorylation should facilitate addressing this question. We envision that the manipulation of adipose depots by altering adipocyte turnover through regulation of PCNA Y114 phosphorylation may lead to effective approaches for the prevention and treatment of obesity and associated metabolic diseases. The fact that lack of Y114 phosphorylation of PCNA does not affect cell growth or normal development suggests that targeting Y114 phosphorylation could be a safe approach.
HIGHLIGHTS.
Proliferating Cell Nuclear Antigen (PCNA) is phosphorylated at Y114.
Phospho-Y114 of PCNA is not required for cell proliferation for normal growth.
MCE during adipogenesis is abolished in the lack of the phosphorylation.
Homozygous Y114F mice are resistant to high fat diet induced obesity.
Our results shed light on the interface between proliferation and differentiation.
ACKNOWLEDGEMENT
The authors thank Dr. Belinda Peace and Glenn Doerman for assistance in editing the manuscript and preparing the graphics. This work was supported in part by the Center for Clinical and Translational Science and Training (CCTST) of University of Cincinnati and Cincinnati Children’s Hospital, and the start-up funding of the University of Cincinnati (to S.-C. W.), and by PHS Grant P30 DK078392.
ABBREVIATIONS
- GFP
green fluorescence protein
- HFD
high fat diet
- MCE
mitotic clonal expansion
- MEF
mouse embryonic fibroblast
- PCNA
proliferating cell nuclear antigen
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Otto TC, Lane MD. Adipose development: from stem cell to adipocyte. Critical reviews in biochemistry and molecular biology. 2005;40:229–242. doi: 10.1080/10409230591008189. [DOI] [PubMed] [Google Scholar]
- 2.Hausman DB, DiGirolamo M, Bartness TJ, Hausman GJ, Martin RJ. The biology of white adipocyte proliferation. Obes Rev. 2001;2:239–254. doi: 10.1046/j.1467-789x.2001.00042.x. [DOI] [PubMed] [Google Scholar]
- 3.Avram MM, Avram AS, James WD. Subcutaneous fat in normal and diseased states 3. Adipogenesis: from stem cell to fat cell. J Am Acad Dermatol. 2007;56:472–492. doi: 10.1016/j.jaad.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 4.Hirsch J, Batchelor B. Adipose tissue cellularity in human obesity. Clin Endocrinol Metab. 1976;5:299–311. doi: 10.1016/s0300-595x(76)80023-0. [DOI] [PubMed] [Google Scholar]
- 5.Hager A, Sjostrm L, Arvidsson B, Bjorntorp P, Smith U. Body fat and adipose tissue cellularity in infants: a longitudinal study. Metabolism. 1977;26:607–614. doi: 10.1016/0026-0495(77)90082-8. [DOI] [PubMed] [Google Scholar]
- 6.Arner E, Westermark PO, Spalding KL, Britton T, Ryden M, Frisen J, Bernard S, Arner P. Adipocyte turnover: relevance to human adipose tissue morphology. Diabetes. 2010;59:105–109. doi: 10.2337/db09-0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O, Blomqvist L, Hoffstedt J, Naslund E, Britton T, Concha H, Hassan M, Ryden M, Frisen J, Arner P. Dynamics of fat cell turnover in humans. Nature. 2008;453:783–787. doi: 10.1038/nature06902. [DOI] [PubMed] [Google Scholar]
- 8.Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol. 2006;7:885–896. doi: 10.1038/nrm2066. [DOI] [PubMed] [Google Scholar]
- 9.Cornelius P, MacDougald OA, Lane MD. Regulation of adipocyte development. Annu Rev Nutr. 1994;14:99–129. doi: 10.1146/annurev.nu.14.070194.000531. [DOI] [PubMed] [Google Scholar]
- 10.MacDougald OA, Lane MD. Transcriptional regulation of gene expression during adipocyte differentiation. Annu Rev Biochem. 1995;64:345–373. doi: 10.1146/annurev.bi.64.070195.002021. [DOI] [PubMed] [Google Scholar]
- 11.MacDougald OA, Mandrup S. Adipogenesis: forces that tip the scales. Trends in Endocrinology and Metabolism. 2002;13:5–11. doi: 10.1016/s1043-2760(01)00517-3. [DOI] [PubMed] [Google Scholar]
- 12.Tang QQ, Otto TC, Lane MD. Mitotic clonal expansion: a synchronous process required for adipogenesis. Proc Natl Acad Sci U S A. 2003;100:44–49. doi: 10.1073/pnas.0137044100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moldovan GL, Pfander B, Jentsch S. PCNA, the Maestro of the Replication Fork. Cell. 2007;129:665–679. doi: 10.1016/j.cell.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 14.Roa S, Avdievich E, Peled JU, MacCarthy T, Werling U, Kuang FL, Kan R, Zhao C, Bergman A, Cohen PE, Edelmann W, Scharff MD. Ubiquitylated PCNA plays a role in somatic hypermutation and class-switch recombination and is required for meiotic progression. Proceedings of the National Academy of Sciences. 2008;105:16248–16253. doi: 10.1073/pnas.0808182105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maga G, Hubscher U. Proliferating cell nuclear antigen (PCNA): a dancer with many partners. J Cell Sci. 2003;116:3051–3060. doi: 10.1242/jcs.00653. [DOI] [PubMed] [Google Scholar]
- 16.Paunesku T, Mittal S, Protic M, Oryhon J, Korolev SV, Joachimiak A, Woloschak GE. Proliferating cell nuclear antigen (PCNA): ringmaster of the genome. Int J Radiat Biol. 2001;77:1007–1021. doi: 10.1080/09553000110069335. [DOI] [PubMed] [Google Scholar]
- 17.Kelman Z. PCNA: structure, functions and interactions. Oncogene. 1997;14:629–640. doi: 10.1038/sj.onc.1200886. [DOI] [PubMed] [Google Scholar]
- 18.Komatsu K, Wharton W, Hang H, Wu C, Singh S, Lieberman HB, Pledger WJ, Wang HG. PCNA interacts with hHus1/hRad9 in response to DNA damage and replication inhibition. Oncogene. 2000;19:5291–5297. doi: 10.1038/sj.onc.1203901. [DOI] [PubMed] [Google Scholar]
- 19.Krishna TS, Kong XP, Gary S, Burgers PM, Kuriyan J. Crystal structure of the eukaryotic DNA polymerase processivity factor PCNA. Cell. 1994;79:1233–1243. doi: 10.1016/0092-8674(94)90014-0. [DOI] [PubMed] [Google Scholar]
- 20.Jonsson ZO, Podust VN, Podust LM, Hubscher U. Tyrosine 114 is essential for the trimeric structure and the functional activities of human proliferating cell nuclear antigen. Embo J. 1995;14:5745–5751. doi: 10.1002/j.1460-2075.1995.tb00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang Q-Q, Otto TC, Lane MD. CCAAT/enhancer-binding protein b is required for mitotic clonal expansion during adipogenesis. Proc Natl Acad Sci USA. 2003;100:850–855. doi: 10.1073/pnas.0337434100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu J. Current Protocols in Molecular Biology. John Wiley & Sons, Inc.; 2001. Preparation, Culture, and Immortalization of Mouse Embryonic Fibroblasts; pp. 28.21.21–28.21.27. [DOI] [PubMed] [Google Scholar]
- 23.Tinsley FC, Taicher GZ, Heiman ML. Evaluation of a Quantitative Magnetic Resonance Method for Mouse Whole Body Composition Analysis. Obes. Res. 2004;12:150–160. doi: 10.1038/oby.2004.20. [DOI] [PubMed] [Google Scholar]
- 24.Wang S-C, Nakajima Y, Yu Y-L, Xia W, Chen C-T, Yang C-C, McIntush EW, Li L-Y, Hawke DH, Kobayashi R, Hung M-C. Tyrosine phosphorylation controls PCNA function through protein stability. Nat Cell Biol. 2006;8:1359–1368. doi: 10.1038/ncb1501. [DOI] [PubMed] [Google Scholar]
- 25.Zhao H, Lo Y-H, Ma L, Waltz SE, Gray JK, Hung M-C, Wang S-C. Targeting tyrosine phosphorylation of PCNA inhibits prostate cancer growth. Molecular Cancer Therapeutics. 2011;10:29–36. doi: 10.1158/1535-7163.MCT-10-0778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao H, Ho P-C, Lo Y-H, Espejo A, Bedford MT, Hung M-C, Wang S-C. Interaction of proliferation cell nuclear antigen (PCNA) with c-Abl in cell proliferation and response to DNA damages in breast cancer. PLoS ONE. 2012;7:e29416. doi: 10.1371/journal.pone.0029416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Auld CA, Morrison RF. Evidence for cytosolic p27(Kip1) ubiquitylation and degradation during adipocyte hyperplasia. Obesity (Silver Spring) 2006;14:2136–2144. doi: 10.1038/oby.2006.250. [DOI] [PubMed] [Google Scholar]
- 28.Morrison RF, Farmer SR. Role of PPARgamma in regulating a cascade expression of cyclin-dependent kinase inhibitors, p18(INK4c) and p21(Waf1/Cip1), during adipogenesis. J Biol Chem. 1999;274:17088–17097. doi: 10.1074/jbc.274.24.17088. [DOI] [PubMed] [Google Scholar]
- 29.Naaz A, Holsberger DR, Iwamoto GA, Nelson A, Kiyokawa H, Cooke PS. Loss of cyclin-dependent kinase inhibitors produces adipocyte hyperplasia and obesity. FASEB J. 2004;18:1925–1927. doi: 10.1096/fj.04-2631fje. [DOI] [PubMed] [Google Scholar]
- 30.Lin J, Della-Fera MA, Li C, Page K, Choi YH, Hartzell DL, Baile CA. P27 knockout mice: Reduced myostatin in muscle and altered adipogenesis. Biochemical and Biophysical Research Communications. 2003;300:938–942. doi: 10.1016/s0006-291x(02)02949-2. [DOI] [PubMed] [Google Scholar]
- 31.Inoue N, Yahagi N, Yamamoto T, Ishikawa M, Watanabe K, Matsuzaka T, Nakagawa Y, Takeuchi Y, Kobayashi K, Takahashi A, Suzuki H, Hasty AH, Toyoshima H, Yamada N, Shimano H. Cyclin-dependent kinase inhibitor, p21WAF1/CIP1, is involved in adipocyte differentiation and hypertrophy, linking to obesity, and insulin resistance. J Biol Chem. 2008;283:21220–21229. doi: 10.1074/jbc.M801824200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Besson A, Dowdy SF, Roberts JM. CDK Inhibitors: Cell Cycle Regulators and Beyond. Developmental Cell. 2008;14:159–169. doi: 10.1016/j.devcel.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 33.Abella A, Dubus P, Malumbres M, Rane SG, Kiyokawa H, Sicard A, Vignon F, Langin D, Barbacid M, Fajas L. Cdk4 promotes adipogenesis through PPARgamma activation. Cell Metab. 2005;2:239–249. doi: 10.1016/j.cmet.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 34.Aguilar V, Annicotte J-S, Escote X, Vendrell J, Langin D, Fajas L. Cyclin G2 Regulates Adipogenesis through PPARγ Coactivation. Endocrinology. 2010;151:5247–5254. doi: 10.1210/en.2010-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sarruf DA, Iankova I, Abella A, Assou S, Miard S, Fajas L. Cyclin D3 Promotes Adipogenesis through Activation of Peroxisome Proliferator-Activated Receptor γ. Molecular and Cellular Biology. 2005;25:9985–9995. doi: 10.1128/MCB.25.22.9985-9995.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li X, Kim JW, Gronborg M, Urlaub H, Lane MD, Tang QQ. Role of cdk2 in the sequential phosphorylation/activation of C/EBPbeta during adipocyte differentiation. Proc Natl Acad Sci U S A. 2007;104:11597–11602. doi: 10.1073/pnas.0703771104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prins JB, O'Rahilly S. Regulation of adipose cell number in man. Clin Sci (Lond) 1997;92:3–11. doi: 10.1042/cs0920003. [DOI] [PubMed] [Google Scholar]
- 38.Bjorntorp P. Effects of age, sex, and clinical conditions on adipose tissue cellularity in man. Metabolism. 1974;23:1091–1102. doi: 10.1016/0026-0495(74)90076-6. [DOI] [PubMed] [Google Scholar]