Abstract
Current approaches to develop skin equivalents often only include the epidermal and dermal components. Yet, full thickness skin includes the hypodermis, a layer below the dermis of adipose tissue containing vasculature, nerves and fibroblasts, necessary to support the epidermis and dermis. In the present study, we developed a full thickness skin equivalent including an epidermis, dermis and hypodermis that could serve as an in vitro model for studying skin development, disease or as a platform for consumer product testing as a means to avoid animal testing. The full thickness skin equivalent was easy to handle and was maintained in culture for greater than 14 days while expressing physiologically relevant morphologies of both the epidermis and dermis, as seen by keratin 10, collagen I and collagen IV expression. The skin equivalent produced glycerol and leptin, markers of adipose tissue metabolism. This work serves as a foundation for our understanding of some of the necessary factors needed to develop a stable, functional model of full-thickness skin.
Introduction
Skin is the largest organ of the human body and is responsible for the protecting the body from the environment. However, when this barrier is disrupted, as in the case of large wounds, burns and chronic, diabetic ulcers, the clinical use of grafted skin has shown therapeutic efficacy. However, such skin autografts are limited by healthy donor site availability. As a result, many approaches have been explored for skin replacement therapy, including cadaver skin. While these options offer temporary benefit, they only persist at the grafting site for a limited amount of time. In response to these limitations, new approaches for skin engineering have been tested and developed in recent years. These advances have led to the development of in vitro models to study wound healing, skin cancer, and skin biology. Since skin barrier function is recapitulated in these in vitro skin equivalents, they are now an important part of consumer product testing. For example, since the European Union will no longer allow the sale of new consumer products tested on animals (European Union Council Directive 76/768/EEC), it is essential that U.S. companies developing consumer products who wish to sell them abroad must find alternative modes of testing that avoids the use of animals. In order to replace animal testing, engineered skin needs to closely mimic the complexity of the in vivo tissue and must respond to toxic agents in a fashion that mimics native skin.
Current commercially available skin equivalents contain only keratinocytes and dermal fibroblasts (see 1 for review). Yet, skin is a complex tissue harboring multiple cell types and much of the research in tissue engineering of skin has focused on developing the epidermal and dermal layers2–5 and on the dynamic interactions between these 2 tissue types. However, multiple cell types are present below the dermis that have not been incorporated into engineered skin. In particular, the adipose tissue that comprises the hypodermis, would allow the development of a full-thickness, tri-layered engineered skin harboring epidermis, dermis, and hypodermis would serve as a more physiologically relevant system, that would likely sustain physiological function for more extended time periods in ways that would permit both acute, short-term and chronic, long-term studies of skin development and pathogenesis. The ability of skin to respond to toxic agents is mediated by cross talk between the multiple cell types present 6. As a result, it has long been a goal to develop a full-thickness, tri-layer skin model5,7, whose morphology and organization that would be able to secrete appropriate levels of cytokines such as leptin5,7 and mimic the full spectrum of biological functions of skin that harbors subcutaneous adipose tissue.
In the present study, we sought to develop a full thickness human skin model with appropriate morphology and metabolic activity, which was also responsive to different drugs or consumer products. Previously, we have constructed human skin equivalents harboring dermal and epidermal compartments that closely resemble the morphology of human skin by demonstrating a fully-differentiated, stratified squamous epithelium overlaid on a collagen gel containing human dermal fibroblasts2,3. However, these constructs do not incorporate a viable layer of subcutaneous adipose tissue that are needed to further normalize the proliferation and differentiation of keratinocytes in the epidermal compartment, when compared to live human skin. Further, skin equivalents harboring only dermal and epidermal compartments are only functional for up to 7-10 days and have a limited ability to mimic chronic conditions affecting skin. We expect that the increased complexity of incorporating a viable adipose layer will provide cell-cell interactions, such as those mediated by paracrine-acting factors that will improve the physiological relevance of skin equivalents to enable them to more closely mimic features of human skin. The construction of such tri-layered tissues is based on our previous success in the construction of 3D tissue models of vascular adipose tissue that combine human adipocytes and endothelial cells, co-cultured on porous silk fibroin protein sponge scaffolds8–10. These constructs demonstrated physiological lipogenic and lipolytic responses, an important milestone in adipose tissue engineering11,12.
The goal of this study was to identify optimal conditions to combine 3D adipose constructs with the epidermal-dermal engineered tissues to establish a more physiologically-relevant and sustainable 3D skin equivalent tissue. Challenges associated with developing such a 3D tissue model include meeting the distinct growth requirements of 4 different cell types in a complex tissue microenvironment, the integration of these multiple cell types into a viable, functioning tissue, and the need to fabricate a tissue microenvironment that will provide endogenous growth support to allow optimal tissue maturation and differentiation leading to tissue longevity.
Materials and Methods
Materials
Bombyx mori silkworm cocoons were supplied by Tajimia Shoji Co. (Yokohama, Japan). All cell culture supplies and collagenase type I were purchased from Invitrogen (Carlsbad, CA) unless otherwise noted. Human recombinant insulin, dexamethasone, pantothenate, biotin, Rosiglitazone, 3-isobutyl-1-methylxanthine (IBMX), bovine serum albumin (BSA), vascular endothelial growth factor (VEGF) and laminin from human placenta were purchased from Sigma-Aldrich (St. Louis, MO). Primary human adult microvascular endothelial cells and complete endothelial cell media (EGM-2MV) were purchased from Lonza (Walkersville, MD). Histological solvents were purchased from Fisher Scientific (Pittsburgh, PA) and histological reagents were purchased from Sigma-Aldrich. Enzyme- linked immunosorbant assay (ELISA) kits for human leptin were purchased from R&D Systems (Minneapolis, MN). Glycerol quantification kit was purchased from Sigma-Aldrich.
Silk Scaffold Preparation
Silk solution was prepared as published13. Briefly, cocoons were chopped and placed in boiling 0.02M NaCO2 for 30 minutes to remove sericin, and then washed 3 times in ultrapure water. The resulting silk fibroin fibers were left to dry overnight. The dry silk was solubilized in 9.3 M LiBr in 20% w/v at 60°C for 4 hours. The silk solution was then dialyzed in ultrapure water in 3,500 MWCO membrane for 2 days with a total of 6 water changes, to remove the LiBr. Salt crystals were sieved to the desired range of 500-600 microns, poured into Teflon coated petri dishes and aqueous silk solution was added. The ratio of salt to aqueous silk solution was 2g NaCl for every mL aqueous silk solution. At this ratio, the NaCl is insoluble, and such that the silk gels around the porogen. Scaffolds were allowed to form at room temperature and then after 2 days were placed in water to leach out the salt particles. The water was changed 3 times a day for 2 days. The scaffolds were removed from the petri dish, cut to the desired dimension, 14 mm diameter × 2 mm height, using a biopsy punch. The scaffolds were left to dry, autoclaved, then kept at 4°C until used. The dry scaffolds were placed into EGM-2MV media for 1 hour before seeding with cells.
Adipose Derived Stem Cell Isolation
Subcutaneous adipose tissue was obtained from abdominoplasties approved under Tufts University IRB (Tufts University IRB Protocol #0906007) from Tufts Medical Center, Department of Plastic Surgery. No identifying information about the patient was obtained. The specimens were kept at room temperature in saline and used the same day. The adipose tissue was separated from the skin by blunt dissection and chopped. Chopped adipose tissue was placed into 50 mL conical tubes and minced well with scissors. The tissues were washed in equal volumes of warmed PBS, until essentially free of blood. An equal volume of 1 mg/mL collagenase I in 1% bovine serum albumin in PBS was added to the tissue and placed under gentle agitation at 37°C for 1 hour. The tissue samples were centrifuged at 300 × G for 10 minutes at room temperature. The supernatant containing the tissue was removed and the pellet resuspended in PBS and centrifuged at the same settings to remove the collagenase solution. The pellet was resuspended in growth media and plated so that 70 g of initial tissue volume was plated per T225 cm2 tissue culture flask.
Cell Culture and 3D (Static) Culture
Normal human keratinocytes (NHK) were first grown in 2D, monolayer culture on irradiated 3T3 fibroblasts and foreskin fibroblasts were grown in media containing Dulbecco's Modified Eagle's Medium (DMEM) and 10% fetal calf serum. The dermal component of the tri-layer construct was grown by adding fibroblasts to neutralized Type I collagen (Organogenesis, Canton, MA) to a final concentration of 2.5 × 104 cells/mL. Three ml of this mixture was added to each 35mm well of a 6 well plate (Organogenesis, Canton, MA) and incubated for 6 d in media containing DMEM and 10% fetal calf serum, until the collagen matrix showed no further shrinkage. Keratinocytes grown on feeder layers in monolayer culture were trypsinized and 500,000 cells were seeded onto these contracted collagen matrices and allowed to attach for 2 hours. Tissues were maintained submerged in low calcium epidermal growth media (EGM) for 2d, submerged for 2d in normal calcium EGM and raised to the air-liquid interface for an additional 5d by feeding from below.
Endothelial cells were cultured and expanded according to the manufacturers’ protocols. Adipose derived stem cells were expanded in growth media comprised of DMEM/F12, 10% FBS, 1% PSF until confluence. At 2 days post-confluence, the cells were switched to adipogenic induction media comprised of DMEM/F12, 3% FBS, 1% PSF, 500 μM IBMX, 5 μM Rosiglitazone, 1 μM dexamethasone, 17 μM pantothenate, 33 mM biotin and 1 μM insulin until seeded on scaffolds. The endothelial cells were added in 20 μL seedings with a total of 2,000,000 cells/scaffold. The seeded scaffolds were placed in an incubator for 2 hours before media was added to the well. The induced ASCs were added after 1 week, but with 1,000,000 cells/scaffold. TGF-beta was included after 1 day in culture in the adipogenic media at a final concentration of 10 ng/mL. Cells and cultures were fed twice per week, and maintained in 37°C, humidified incubator.
Constructs were combined after 4 days of dermal/epidermal differentiation and 14 days of adipose construct culture.
Histology and immunohistochemistry
Constructs were processed according standard histology protocols. Formalin fixed samples were put through a series of dehydration solvents and finally paraffin using an automated tissue processor. Samples were embedded in paraffin, cut in 10 micron sections, and let to adhere on glass slides. The sections were rehydrated and stained with hematoxylin and eosin. For immunohistochemical staining, tissues were serial-sectioned at 6 μm and mounted onto gelatin-chrome alum-coated slides. Sections were incubated with monoclonal antibodies to Keratin 1 (AE2, ICN, Cosa Mesa, CA) and this differentiation-associated protein was detected with Alexa 488™-conjugated rabbit anti-mouse IgG (Molecular Probes, Eugene, OR). For proliferation assays, bromodeoxyuridine (BrdU) (Sigma, St. Louis, MO) was added to 3D cultures 8h prior to harvesting at a final concentration of 10 μM. BrdU was detected in tissue sections using an anti-bromodeoxyuridine antibody (Boehringer Mannheim, Indianapolis, IN) and proliferation of was measured as the percentage of BrdU positive nuclei in the basal layer (LI = Labeling Index) after counting stained nuclei in five serial sections.
Detection of Soluble Factors by ELISA assay
Stored frozen media samples were thawed and immediately assayed according to manufacturer's protocols for both leptin quantification using an ELISA kit, and glycerol concentration using an enzymatic detection kit.
Gene Expression
RNA was isolated using Qiagen RNeasy purification kit (Qiagen, Valencia, CA), and then converted to cDNA with the iScript cDNA synthesis kit (Biorad, Hercules, CA) using 0.5μg RNA. Real-time RT-PCR reactions were carried out using 20ng of cDNA, 200nM of each primer and 2X SYBRgreen Supermix (Biorad, Hercules, CA) at a total sample volume of 12.5 μL and samples were run in triplicate on a iQ5 Real-Time PCR detection system (Bioad, Hercules, CA). PCR products were amplified to 30 cycles at 94 °C for 30 s, 60 °C for 30 s and 72 °C for 60 s. The relative level of gene expression was assessed using the 2−ΔΔCt method. The oligonucleotide primer sequences used are found in Table 3.
Table 3.
Gene expression of various dermal and adipose markers. Dermal markers-collagen I and collagen IV increased from day 5 to 9. Fat markers remained consistent from day 5 to 9.
| Gene | Region | Primers (forward/reverse) | Time | Expression |
|---|---|---|---|---|
| Collagen I, a1 | Dermal | 5′ TCC CCA GCT GTC TTA TGG CT | Day5 | + |
| 5′ CAG GCA CGG AAA TTC CTC C | Day9 | ++ | ||
| Collagen IV, a1 | Dermal | 5′ CGC TTA CAG CTT TTG GCT CG | Day5 | ++ |
| 5′ GAC GGC GTA GGC TTC TTG AA | Day9 | +++ | ||
| Elastin | Dermal | 5′ GGT ATC CCA TCA AGG CCC C | Day5 | +/− |
| 5′ TTT CCC TGT GGT GTA GGG CA | Day9 | +/− | ||
| Fatty Acid Synthase (FAS) | Adipose | 5′ TGT GGA CAT GGT CAC GGA C | Day5 | + |
| 5′ GGC ATC AAA CCT AGA CAG GTC | Day9 | + | ||
| Fatty Acid Binding Protein (FABP4) | Adipose | 5′ AGC ACC ATA ACC TTA GAT GGG | Day5 | +/− |
| 5′ CGT GGA AGT GAC GCC TTT CA | Day9 | +/− | ||
| Glucose transporter 4 (Glut4) | Adipose | 5′ CTC AGC AGC GAG TGA CTG G | Day5 | ++ |
| 5′ CCC CAA TGT TGT ACC CAA ACT | Day9 | ++ |
(–) means no cDNA detected, (+/−) means borderline detection, (+) means low cDNA level, (++) means moderate cDNA level, and (+++) means high cDNA level. Levels are representative of n=2 per group.
Results
Optimization of growth conditions
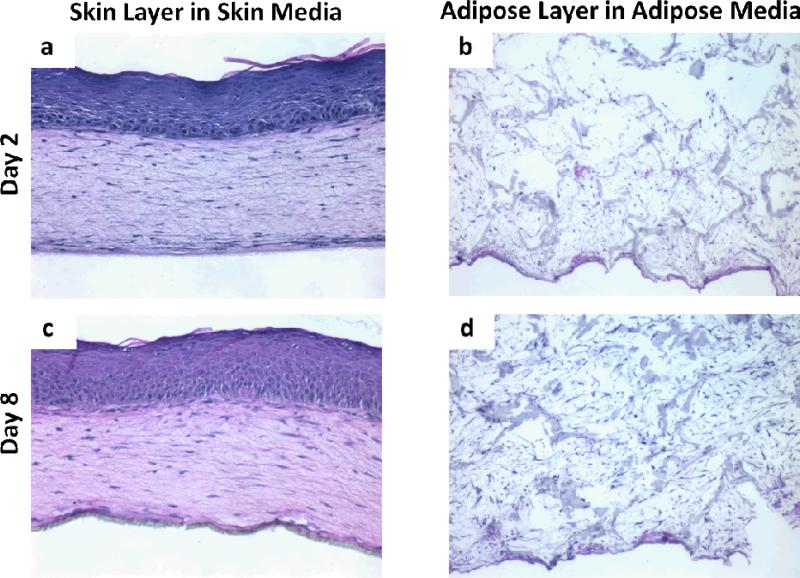
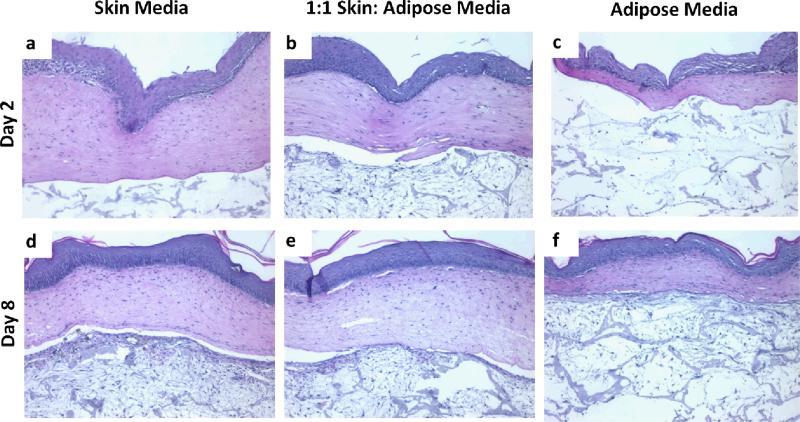
To develop and optimize a tri-layer, full thickness human skin equivalent, after dermalepidermal constructs were cultured at an air-liquid interface for 4 days and adipose tissues were grown for 14 days separately and then combined (Fig. 1). These tri-layered tissues were then grown for an additional 21 days. Figure 2 demonstrates the morphology of individual constructs for adipose tissue (Fig. 2b, 2d) and skin (Fig. 2a, 2c) had a normal morphologic appearance for each tissue type in their respective media. By day 8 of adipose culture, cells and matrix filled in pores of the silk sponges (Fig. 2d). Two days after being raised to the air-liquid interface, bi-layer skin constructs demonstrated keratinocytes that generated a multi-layered epithelial tissue with distinct basal cells, a well-formed spinous layer and the presence of a thin stratum granulosum and stratum corneum. At day 8 of skin culture, tissues were further developed as seen by a thickened spinous layer and S. granulosum. At this time point, basal cells demonstrated a polarized morphology (Fig. 2c), suggestive of the elevated proliferation of these epidermal basal cells (Fig. 2c). In order to determine optimal growth conditions for tissues that harbor these multiple cell types, after they were combined on day 14, three media formulations were evaluated: epidermal media (Fig. 3a, 3d), adipose media (Fig. 3c, 3f) and a 1:1 mix of epidermal to adipose media (Fig. 3b, 3e). We found that 2 and 8 days of culture of tri-layer constructs in adipose media were not sufficient to support the normal development of skin equivalents (Fig. 3c, 3f). In contrast, normalized epithelial tissue morphology was seen for both skin media (Fig. 3a, 3d) and the 1:1 epidermal:adipose media (Fig. 3b, 3e) as seen by the strat-specific differentiation at day 8 (Fig. 3e). The separation seen between the dermal and adipose layers is an artifact of histological processing (Fig. 3). Since adipose tissue organization was improved when tissues were grown in 1:1 skin: adipose media, it was decided that this media was optimal to support tri-layer tissue development. This was seen by a loss of epidermal tissue organization that was characterized by disorganization of the basal cell layer at day 2 (Fig. 3c) and incomplete morphologic differentiation at day 8 (Fig. 3f).
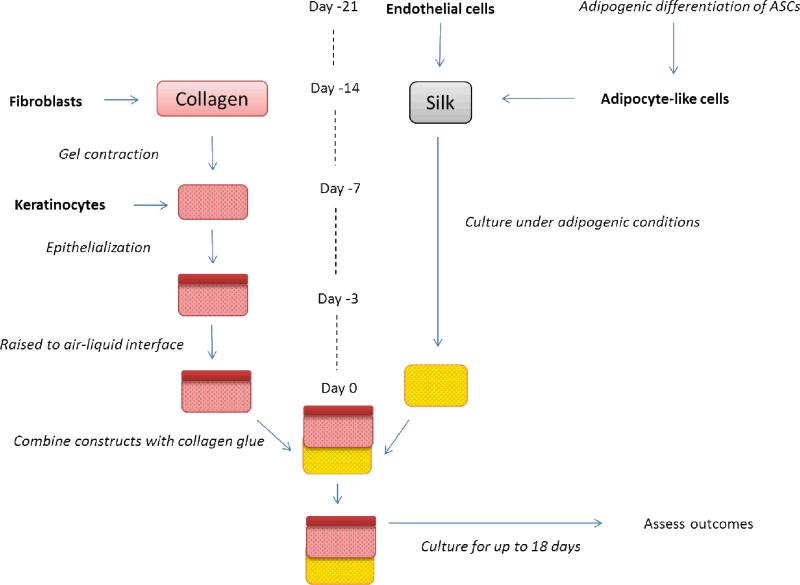
Figure 1.
Experimental outline for tri-layer culture. Skin and adipose tissues were cultured independently prior to being combined at ‘day 0’. At day -21, endothelial cells are seeded onto silk sponges, while adipose derived stem cells undergo adipogenic differentiation in 2D. At day 14, the adipocyte-like cells are added to the endothelial seeded silk sponges and the co-cultures were maintained in a 1:1 adipogenic:endothelial cell media. Also at day 14, fibroblasts are cast within a collagen gel. After the cell contracts, keratinocytes are seeded on top of the collagen gel. After epithelialization has occurred the construct was raised to the air-liquid interface for 3 days. On day 0, the 2 constructs were combined with a thin layer of collagen gel as a binder. At this point the tri-layer construct was cultured in a 1:1 mix of the co-culture media to skin media.
Figure 2.
H&E images of positive controls for skin and adipose constructs after 2 and 8 days in culture. Skin constructs demonstrated a well-organized, multilayer epithelium by day 2 (a) that demonstrated all 4 morphological layers and a collagen lattice in which resident fibroblasts showed their characteristic spindled morphology. This epithelium was further developed by day 8 (c), where it showed a columnar basal cell layer and more complete development of the spinous cell layer. By day 8, cells populated the entire silk sponge in the adipose construct. Images are representative of n=2 per group.
Figure 3.
Constructs cultured in 1:1 skin: adipose media had more physiologically relevant features. H&E images of tri-layer constructs in different media after 2 and 8 days in culture. Images are representative of n=2 per group.
Functional adipose tissue outcomes
In order to characterize adipose tissue function under these growth conditions to further establish optimal tissue function, leptin (Table 1) and glycerol (Table 2) levels secreted into the media were determined at 0, 2, 6 and 8 days after combining skin and adipose constructs. When tri-layer constructs were cultured in epidermal media, leptin levels were greater than in tissues consisting of adipose constructs alone during the first 6 days of culture (Table 1). When tri-layer constructs were cultured in adipose media, leptin levels were lower than the adipose constructs alone at day 0 and 8, with no changes from days 2-6 (Table 1). When tri-layer constructs were cultured in 1:1 epidermal: adipose media, leptin levels did not vary from adipose constructs alone on days 2-8 (Table 1). Glycerol levels were consistently greater in tri-layer constructs than in individual constructs (Table 2).
Table 1.
Leptin release varied with media and construct type. Leptin is an adipocytokine released by mature adipocytes. When tri-layer constructs were cultured in skin media, leptin levels remained the same as or increased over adipose constructs alone. Leptin levels in tri-layer constructs decreased or remained the same over adipose constructs when cultured in adipose media. When tri-layer constructs were cultured in 1:1 skin:adipose media, leptin levels remained constant over time when compared to adipose construct alone. No protein was detected in epidermal-dermal constructs alone.
| Leptin in media | Tissue | |||
|---|---|---|---|---|
| Day | Media | Epidermal-dermal | Adipose | Tri-layer constructs |
| 0 | Skin | − | ++ | +++ |
| 1:1 | − | +++ | ++ | |
| Adipose | − | +++ | ++ | |
| 2 | Skin | − | ++ | +++ |
| 1:1 | − | +++ | +++ | |
| Adipose | − | ++ | ++ | |
| 6 | Skin | − | ++ | +++ |
| 1:1 | − | ++ | ++ | |
| Adipose | − | ++ | ++ | |
| 8 | Skin | − | ++ | ++ |
| 1:1 | − | ++ | ++ | |
| Adipose | − | ++ | + | |
| Controls | Media only | Skin | − | − |
| 1:1 | − | − | ||
| Adipose | − | − | ||
(–) means no protein detected, (+/−) means borderline detection, (+) means low protein level, (++) means moderate protein level, and (+++) means high protein level. Levels are representative of n=2 per group.
Table 2.
Glycerol levels were greater in tri-layer groups but did not vary with media type. Glycerol levels were consistently greater in the tri-layer equivalent groups suggesting metabolic demands were not negatively impacted when all three layers were in contact.
| Glycerol in media | Tissue | |||
|---|---|---|---|---|
| Day | Media | Epidermal-dermal | Adipose | Tri-layer equivalent |
| 0 | Skin | ++ | + | ++ |
| 1:1 | + | + | ++ | |
| Adipose | + | ++ | ++ | |
| 2 | Skin | ++ | + | + |
| 1:1 | + | + | ++ | |
| Adipose | + | ++ | ++ | |
| 6 | Skin | + | + | ++ |
| 1:1 | + | + | ++ | |
| Adipose | + | + | ++ | |
| 8 | Skin | + | + | ++ |
| 1:1 | + | + | ++ | |
| Adipose | ++ | ++ | ++ | |
| Controls | Media only | Skin | − | − |
| 1:1 | − | − | ||
| Adipose | − | − | ||
(–) means no glycerol detected, (+/−) means borderline detection, (+) means low glycerol level, (++) means moderate glycerol level, and (+++) means high glycerol level. Levels are representative of n=2 per group.
Characterization of skin and adipose constructs within tri-layer construct
After determination of optimal media type (1:1 epidermal:adipose media), tri-layer constructs were cultured in this media for 5 and 9 days and gene expression for dermal and adipose markers were determined by RT-PCR. Structural, extracellular matrix proteins, Collagen Type I and Collagen Type IV levels increased from day 5 to 9 (Table 3), while expression levels of elastin did not change with time (Table 3). Markers of mature adipose tissue, including fatty acid synthase (FAS), fatty acid binding protein 4 (FABP4) and glucose transporter 4 (Glut4) were maintained at similar levels from day 5 to 9 (Table 3). This temporal analysis of gene expression is shown in Table 4. Only FABP4 remained constant through 18 days of culture (Table 4) Other dermal and adipose related genes studied increased over the 18 day culture period (Table 4).
Table 4.
Temporal gene expression levels increased with culture time except for fatty acid binding protein 4 (FABP4) which remained stable. The expression of dermal and adipose layer transcripts are shown as fold increase relative to expression levels of day 5. Levels are representative of n=2 per group.
| Dermal Markers | Adipose Markers | ||||||
|---|---|---|---|---|---|---|---|
| Gene | Collagen I | Collagen IV | Elastin | FAS | FABP4 | Glut4 | PPAR-γ |
| Day5 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Day9 | 1.11 | 1.48 | 1.32 | 1.17 | 1.18 | 1.22 | 3.04 |
| Day11 | 1.10 | 1.86 | 1.43 | 1.90 | 0.62 | 4.45 | 5.39 |
| Day13 | 1.47 | 3.36 | 2.23 | 1.45 | 1.25 | 4.46 | 1.07 |
| Day15 | 1.19 | 2.38 | 1.56 | 1.83 | 0.44 | 4.18 | 5.23 |
| Day18 | 1.66 | 2.86 | 2.68 | 3.44 | 1.01 | 11.02 | 5.70 |
Keratin 10 (K10), a marker of epidermal differentiation, was assessed by immunohistochemistry. We found that K10 levels were highest through day 9, then decreased and remained constant for days 11-18 (Table 6). Incorporation of bromodeoxyuridine (BrdU) was used to determine rate of proliferation. BrdU levels mirrored those of Keratin 10, highest expression during days 5-9, with a subsequent decrease and constant expression for days 11-18 (Table 6).
Tri-layer constructs respond to various treatments
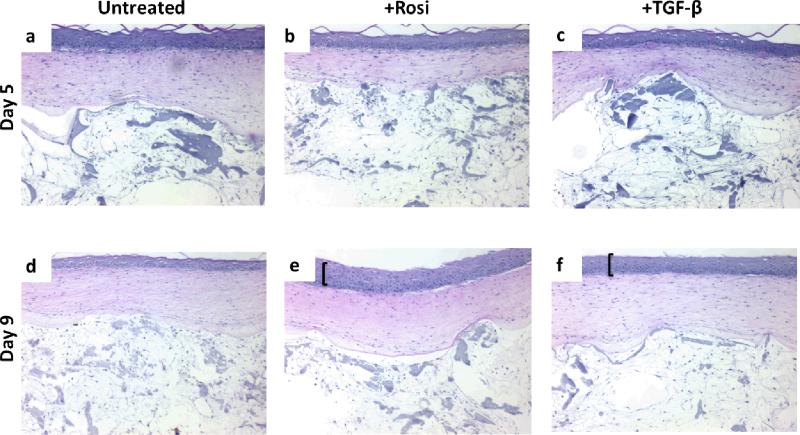
Tri-layer constructs were exposed to rosiglitazone and transforming growth factor beta (TGF-β) for 5 or 9 days and subsequently assessed for morphological changes. Rosiglitazone, an activator of adipogenesis, led to hyper-proliferation of the basal layer of the epidermis after 9 days exposure (Fig. 4e). Similarly, TGF-β caused hyper-proliferation after 9 days exposure (Fig. 4f). Hyper-proliferation was not seen in the untreated control groups at either time point (Fig. 4a, 4d). Exposure to these drugs did not appear to affect the dermal or adipose regions over time (Fig. 4).
Figure 4.
Tri-layer constructs were responsive to various drugs. Rosiglitizone (Rosi), an activator of the adipogenic program, caused hyper-proliferation (bracket) at the basal layer of the epidermis at 9 days. TGF-β, also caused hyper-proliferation, to a lesser extent than the Rosi group, at the basal layer of the epidermis at 9 days. The adipose regions did not appear affected, morphologically, by the drug treatments. Images are representative of n=2 per group.
Discussion
The goal of this work was to develop a full-thickness human skin equivalent which is physiologically relevant and easily handled and manipulated for various assays. The constructs were easily handled as the silk sponge served as a robust scaffold. It is crucial that all cell types are organized and sustainable in the system. For this reason, the first part of our study focused on defining the appropriate media formulation. We examined three media that support individual adipose or skin constructs or a 1:1 combination of skin to adipose media. Not surprisingly, adipose media alone did not support the skin construct in the tri-layer equivalent, as adipose media lacks critical factors for skin development, such as KGF and HGF. Soluble factors, leptin and glycerol were also examined. Leptin, an adipocytokine produced by mature adipocytes, release was not negatively impacted in the presence of skin layers or the 1:1 skin: adipose media. Yet, in tri-layer constructs cultured in adipose media, leptin levels were lower than their adipose construct only controls, while levels were greater in skin media. This is evidence of cross-talk between the various cell types of the skin and adipose constructs, but the mechanism is unknown. Glycerol release, a byproduct of lipolysis in adipocytes, was not affected by media type and the levels were highest in the tri-layer constructs. It is unclear from these data if the increase in glycerol was due to increased numbers of cells producing glycerol in the tri-layer or if the skin with adipose tissue contact led to increased lipolysis rates.
In the second set of studies, we examined transcript levels for various dermal and adipose markers. Collagen I is produced by dermal fibroblasts to provide structural support to the overlaying epidermis and collagen IV is a major component of the basement membrane aiding in anchoring each layer together4. In this study, collagen transcript levels increased likely due to the dynamic remodeling and reorganization of the tissue. Adipose tissue markers increased over an 18 day culture period in the tri-layer constructs. Similar trends were seen in our previous studies (unpublished results) with adipose only constructs. Keratin 10, a marker of epidermal differentiation was higher early in culture and then decreased and was stable at later time points. These results, along with a similar trend in BrdU levels, are significant, as in other tissue engineering models of skin, hyper-proliferation is problematic. The tri-layer combination apparently helps to normalize these hyper-proliferative effects.
Once the tissue model was established, it was exposed to either Rosiglitazone or TGF-β, which both led to alterations in epidermal tissue architecture and hyperproliferation in the basal cell layer, while no morphological changes were seen in the rest of the tissue. This finding was interesting after establishing that this construct had no hyper-proliferation in its normal state. Rosiglitazone slows the inflammatory process, and the result that rosiglitazone addition caused hyperplasia is counterintuitive14. Future studies should expand the range of drugs tested as well to further elucidate the reason for the findings.
The decision to proceed with cost-effective clinical trials following the discovery of new biological therapeutics needs to be based on establishing their efficacy and safety profile in ways that will be highly predictive of their clinical response. However, most experimental assay systems designed to do so have been based primarily on 2D, monolayer culture systems. Unfortunately, the power of these biological systems to simulate biological processes in human tissues is very limited, as it is known that biologically-meaningful function is optimal when cells are spatially organized in 3D tissues, but is lost in lost in these rudimentary 2D culture systems. To overcome these limitations, 3D tissues now serve as human, pre-clinical, surrogate biological systems that act as an important translational platform that provide more meaningful correlations between in vitro screening assays for toxicity and efficacy and in vivo tissue outcomes in human clinical trials. However, no 3D tissue models currently exist that can efficiently screen drug responses in complex adipose tissues to dramatically impact the identification of optimal, new biological therapies to most effectively treat obesity and diabetes. The model is robust enough to be handled and manipulated for toxicity testing, while future studies should evaluate if applying shear forces to the construct causes delamination. While we have taken an important step towards this goal, tissues we have generated still lack complexity seen in their in vivo counterparts. To better screen the safety and efficacy of candidate drugs in the future, such tissues should include immune cells, such as dendritic cells found in the epidermis or incorporation of nerve tissue. In addition, another factor that should be explored in our system is a viable vascular component. Classically in tissue engineering, vascularity is needed for supporting the tissues mass transport needs, especially if the tissue will be implanted. In the present tissue, the vascular component is important for conferring immunity in addition to supporting the tissue. Even though we included endothelial cells at the start of the culture, they were not detectable at the end of the culture. Previous studies from our group showed lumen formation when adipocytes and endothelial cells were co-cultured in silk sponges8. Future studies should improve the vascular portion of the system by better supporting endothelial cell survival and vasculogenesis.
Table 5.
Proliferation and differentiation capacity of the epidermal layer was modulated in the tri-layer constructs. The metabolic activity and differentiation capacity of the epidermal region of the tri-layer constructs were studied using immunohistochemical staining for keratin 10, an epidermal differentiation marker. BrdU incorporation was used to document epidermal proliferation. The expression of keratin 10 confirmed the differentiation capacity of the epidermal layers. Unexpectedly, there was higher expression of keratin 10 at earlier time points, and reduced keratin 10 expression at later time points, with similar results for BrdU incorporation. These unexpected results point to the formation of a more biologically relevant epidermal layer.
| Epidermal Marker | ||
|---|---|---|
| Keratin 10 | BrdU | |
| Day5 | +++ | ++ |
| Day9 | +++ | ++ |
| Day11 | + | + |
| Day13 | + | + |
| Day15 | + | + |
| Day18 | + | + |
(–) means no protein detected, (+/−) means borderline detection, (+) means low protein level, (++) means moderate protein level, and (+++) means high protein level. Levels are representative of n=2 per group.
Acknowledgements
The authors would like to thank Shumin Dong and Christophe Egles for their helpful insight and assistance. Funding from J&J and the NIH - P41 EB002520 – is greatly appreciated.
References
- 1.Welss T, Basketter D. a, Schröder KR. In vitro skin irritation: facts and future. State of the art review of mechanisms and models. Toxicology in vitro: an international journal published in association with BIBRA. 2004;18:231–43. doi: 10.1016/j.tiv.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 2.Egles C, Garlick JA, Shamis Y. Chapter 24 Three-Dimensional Human Tissue Models of Wounded Skin. Methods in Molecular Biology. :345–359. doi: 10.1007/978-1-60761-380-0_24. doi:10.1007/978-1-60761-380-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carlson MW, Alt-holland A, Egles C, Garlick JA. Three-Dimensional Tissue Models of Normal and Diseased Skin. 2008:1–17. doi: 10.1002/0471143030.cb1909s41. doi:10.1002/0471143030.cb1909s41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Metcalfe AD, Ferguson MWJ. Tissue engineering of replacement skin: the crossroads of biomaterials, wound healing, embryonic development, stem cells and regeneration. Journal of the Royal Society, Interface / the Royal Society. 2007:413–37. doi: 10.1098/rsif.2006.0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trottier V, Marceau-Fortier G, Germain L, Vincent C, Fradette J. IFATS collection: Using human adipose-derived stem/stromal cells for the production of new skin substitutes. Stem cells. 2008;26:2713–23. doi: 10.1634/stemcells.2008-0031. [DOI] [PubMed] [Google Scholar]
- 6.Sun T, Jackson S, Haycock JW, MacNeil S. Culture of skin cells in 3D rather than 2D improves their ability to survive exposure to cytotoxic agents. Journal of biotechnology. 2006;122:372–81. doi: 10.1016/j.jbiotec.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 7.Monfort A, Soriano-Navarro M, García-Verdugo JM, Izeta A. Production of human tissue-engineered skin trilayer on a plasma-based hypodermis. Journal of Tissue Engineering and Regenerative Medicine n/a-n/a. 2012 doi: 10.1002/term.548. doi:10.1002/term.548. [DOI] [PubMed] [Google Scholar]
- 8.Kang JH, Gimble JM, Kaplan DL. In vitro 3D model for human vascularized adipose tissue. Tissue engineering. Part A. 2009;15:2227–36. doi: 10.1089/ten.tea.2008.0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mauney JR, et al. Engineering adipose-like tissue in vitro and in vivo utilizing human bone marrow and adipose-derived mesenchymal stem cells with silk fibroin 3D scaffolds. Biomaterials. 2007;28:5280–5290. doi: 10.1016/j.biomaterials.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi J, Bellas E, Vunjak-Novakovic G, Kaplan DL. Adipose-Derived Stem Cells. Methods in molecular. 2011;702:319–330. doi: 10.1007/978-1-61737-960-4_23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi JH, Gimble JM, Vunjak-Novakovic G, Kaplan DL. Effects of Hyperinsulinemia on Lipolytic Function of Three-Dimensional Adipocyte/Endothelial Co-Cultures. Tissue Engineering Part C: Methods. 2010;16:1157–1165. doi: 10.1089/ten.tec.2009.0760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi JH, Bellas E, Gimble JM, Vunjak-Novakovic G, Kaplan DL. Lipolytic Function of Adipocyte/Endothelial Cocultures. Tissue Engineering Part A. 2011;17:1437–1444. doi: 10.1089/ten.tea.2010.0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rockwood DN, et al. Materials fabrication from Bombyx mori silk fibroin. Nature Protocols. 2011;6:1612–1631. doi: 10.1038/nprot.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taira BR, et al. Rosiglitazone, a PPAR-γ Ligand, Reduces Burn Progression in Rats. Journal of Burn Care & Research. 2009;30:499–504. doi: 10.1097/BCR.0b013e3181a28e37. [DOI] [PubMed] [Google Scholar]