Abstract
Introduction
Primary cilia are microtubule-based extensions of the plasma membrane in nearly all cell types. In vertebrate photoreceptors, the sensory cilium develops as outer segment (OS) that contains the photopigment Rhodopsin and other proteins necessary for phototransduction. The distinct composition of proteins and lipids in the OS membrane is maintained by the selective barrier located at the border between the basal body and the ciliary compartment, called the Transition Zone (TZ).
Areas covered
In this review, we will discuss the identification and function of two ciliary TZ proteins, RPGR (retinitis pigmentosa GTPase regulator) and CEP290. Mutations in these proteins account for a majority of retinopathies due to ciliary dysfunction. We will also discuss the potential of such information in designing therapeutic approaches to treat cilia-dependent photoreceptor degenerative diseases.
Expert opinion
RPGR and CEP290 perform overlapping yet distinct functions in regulating trafficking of cargo via the TZ of photoreceptors. While RPGR modulates the trafficking by acting as a GEF for the small GTPase RAB8A, CEP290 may be involved in maintaining the polarized distribution of proteins in the OS by modulating intracellular levels of selected proteins involved in inhibiting OS formation.
Keywords: Ciliopathies, cilia, retina, retinitis pigmentosa, retinal degeneration, Leber congenital amaurosis, Ciliary trafficking, connecting cilium, transition zone
1. Introduction
Cilia are microtubule based microscopic extensions of the plasma membrane present in almost all cell types and are involved in various developmental processes, including signal transduction, left right axis determination, sensory perception and adult homeostasis 1-3. Consequently, defects in cilia development or function result in diverse developmental and degenerative disorders, collectively termed “ciliopathies”. These include a wide range of disorders, such as Nephronophthisis (NPHP), Retinitis Pigmentosa (RP), Bardet Biedl Syndrome (BBS), Joubert Syndrome (JBTS) and Meckel Gruber Syndrome (MKS) 4.
Cilia consist of a microtubule-based ciliary axoneme, which is assembled from the basal body or mother centriole. During ciliogenesis, the mother centriole docks at the apical plasma membrane and extends a microtubule-based structure called axoneme 5. Vesicular trafficking is required for the transport of membrane proteins and lipids to the ciliary base. Once at the base, the proteins have to pass through a narrow transition zone (TZ), to enter the axoneme. The TZ therefore acts as a docking site and selection point for the proteins destined for the distal cilium 6. The cargo proteins are transported from the TZ to the distal tip of the cilium by the action of macromolecular protein complexes by an evolutionary conserved transport process, called Intraflagellar Transport (IFT) 7. The IFT was first described in Chlamydomonas and has now been identified in almost all ciliary cell types 8, 9.
2. Photoreception and Cilia
Vision, a process of detecting and perceiving light, is initiated in the neural retina. The retina is part of the central nervous system and acts as a hub to receive light and initiate the visual transduction cascade that is mediated by five major neuronal cell types and one type of cilia. The major neurons are photoreceptors, bipolar cells, horizontal cells and ganglion cells. These cell types are arranged in the form of a laminated structure, with photoreceptors in the outer retina 10. Light passes through inner retinal layers to reach the photoreceptors where it isomerizes the chromophore in the visual pigment and initiates a signal transduction cascade accompanied by changes in the membrane potential. Such information is transmitted to the next order of inner neurons, subsequently reaching the ganglion cells and then to the brain via the optic nerve.
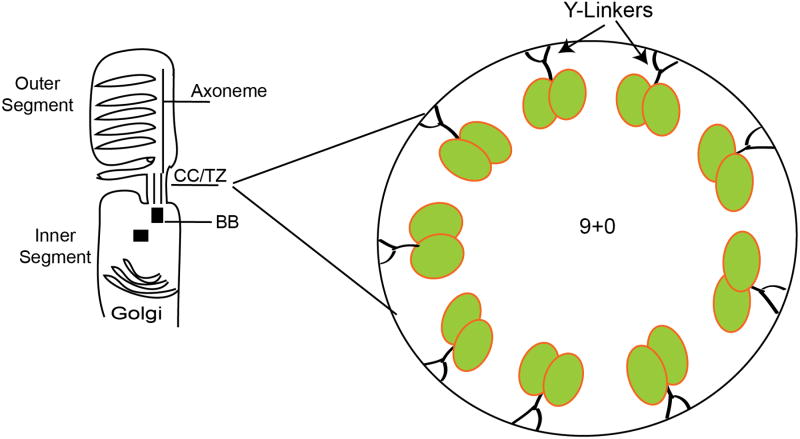
Photoreceptors (PR's) account for 65–70 % of all cell types in the retina. PR's are highly polarized neurons, with a distinct inner segment (IS) and an outer segment (OS). The IS contains the cellular machinery of protein synthesis and trafficking while the OS is the hub of the selected set of proteins that are involved in visual transduction. The OS is arranged in the form of membranous discs arranged in a coin-stack like fashion. In photoreceptors, the OS is the sensory cilium, which concentrates more than 109 molecules of the light-sensory G-protein coupled receptor rhodopsin in stacked membranous discs 11. In addition, there is enrichment of other visual cascade proteins, such as beta and delta subunits of phosphodiesterase (PDEα and PDEβ respectively) 12, 13, cyclic nucleotide gated (CNG) channel 14 and membrane Guanylate cyclases (GC) 15, in OS. The photoreceptors have to synthesize all the OS membrane proteins in the IS because no biosynthetic machinery is present in the cilia. These proteins must pass through a narrow bridge-like structure of the cilium, called the connecting cilium (CC; also called transition zone; TZ), between the BB and the OS 16, 17 (Figure 1). A property that makes the photoreceptor cilia unique among all ciliated cells is its ability to undergo shedding at the tips and renewal of at the base. The tips of the OS undergo periodic shedding due to phagocytosis by the juxtaposed retinal pigment epithelial (RPE) cells 11, 18-20. The photoreceptors constantly shed their distal discs (∼10% of discs are shed each day) 11, 20-23 and must transport approximately 2000 opsin molecules per second through the CC to renew those discs 11. Photoreceptors also exhibit massive light-dependent translocation of soluble moieties of the phototransduction cascade including transducin and arrestin, likely by diffusion 24-27. Nonetheless, all of the intersegmental transport must occur through the TZ.
Figure 1. Schematic representation of a rod photoreceptor.
The polarized nature of the photoreceptors is represented by an elaborate generation of a distinct membrane component called the outer segment. This compartment contains membranous discs and is generated from the basal body (BB) in the apical inner segment. The region between the axoneme and the BB is called the connecting cilium (CC) or transition zone (TZ). A detailed schematic representation of the CC/TZ of photoreceptors shows the presence of Y-linkers that connect the ciliary microtubules to the plasma membrane. This region acts as a gate to selectively regulate trafficking of proteins to the OS.
3. Membrane trafficking to PR cilia: role of small GTPases
PR's are specialized and polarized neurons. The polar structure and distribution of membrane proteins in PR's is essential for their development and function. The polarity is achieved by targeted trafficking of the vesicles from the Golgi apparatus to the base of cilia. IFT proteins, particularly IFT20 localizes to the Golgi and seems to be involved in the trafficking of membrane proteins to OS 28, 29. In addition, IFT20 interacts with IFT54, which in turn associates with the small GTPase RAB8A 30.
Small GTPases are critical regulators of membrane trafficking. There are numerous members of GTPase families. Among these, small GTPases of Arf/Arl and Rab subfamilies have been implicated in regulating cilia assembly and function 31, 32. These GTPases function by assisting in hydrolysis of GTP. The Rab family of GTPase is the largest family with at least 38 functionally distinct moieties. The function of GTPases relies on two states: active or GTP- bound state, and inactive or GDP-bound form. The active or inactive state also influences the localization of the GTPases in membrane versus cytosol. Such dynamic conformations are further regulated by accessory factors, including guanine nucleotide exchange factors (GEF's); GTPase activating protein (GAP's). During cargo trafficking, Rab GTPases are targeted to the cargo membrane, and activated by GEF's. The activated GTPase subsequently facilitates vesicle docking to the acceptor membrane. Multiple GTPases include RAB6, RAB11, and RAB8A are involved in the trafficking to the cilium, in association with the exocyst complex 33, 34. Recent studies have shown that the potential handover mechanisms may exist between RAB11 and RAB8 at the base of the cilium 35. RAB8A is involved in regulating ciliogenesis and photoreceptor OS formation and function 36, 37. It has been shown that expression of the dominant negative mutant of RAB8A results in the accumulation of the rhodopsin containing vesicles at the base of the OS in frog photoreceptors 36.
4. Ciliary OS of PR's
The region of the cilium between the BB and the axoneme in the OS is called connecting cilium (CC). It is essentially the only link between the IS and OS and acts as a conduit for trafficking of proteins 38, 39. As previously described, the CC is analogous to the TZ of other eukaryotic cilia. The TZ consists of doublet microtubules, which are anchored to the PM by Y–shaped cross-linkers 38. Although these structures were described more than 40 years ago, their molecular composition is still not completely understood. Several proteins mutated in human ciliary diseases are localized to the TZ. These include NPHP proteins, MKS modules, and RP-related proteins, RPGR, and CEP290/NPHP6 4, 40-44. The TZ, owing to the Y–links and transition fibers, appears to form a gate like structure, and is involved in selective trafficking of proteins into the cilia. In this review article, we will focus specifically on recent studies that have provided new knowledge on the function of ciliary TZ proteins RPGR and CEP290/NPHP6 mutated in retinal degeneration and their role in regulating protein trafficking via the TZ of photoreceptors.
5. RPGR
The RPGR gene was cloned in 1996 as a causative gene for X-linked RP 45, 46. Patients with RPGR mutations exhibit early-onset visual defects starting in the second decade of life 45-48. There are multiple isoforms of RPGR 49 but two major subtypes are: the constitutive isoform (CI) and a retina-enriched ORF15-isoform. The CI consists of 19 exons and encodes a protein of 815 amino acids. Mutations in this isoform account for 10-15% of XLRP cases and are located within exons 1-15. No mutation has to date been identified in exons 16-19. On the other hand, the retina-enriched ORF15-isoform terminates in intron 15. The terminal exon of this isoform is termed ORF15 (exon 15+part of intron 15) and consists of purine-rich repeats (GAAAGGAA…..). The corresponding unusual amino-acid sequence exhibits highly acidic domain with glutamic acid and glycine-rich repeats (Glu/Gly repeats: EEEGEGE repeat in mouse, EEEGEGEGE repeat in human). A majority (50-60%) of RPGR mutations are found in exon ORF15 and are mostly large deletions or duplications that result in premature truncation of the protein 45, 46, 50-52. Hence, this exon was termed a mutational hotspot.
The primary structure analysis of the RPGR protein revealed interesting clues to its potential function in photoreceptors. The amino-terminal domain of RPGR, encoded by exons 2-11 is highly homologous to RCC1 (regulator of chromosome condensation 1; RCC1-like domain; RLD), which is a GEF for Ran GTPases and is involved in regulating nucleo-cytoplasmic trafficking of proteins. It was based on this homology that the RPGR gene was given the name of a GTPase regulator 45, 46, 53. The discovery of the alternative ORF15-containing isoform shifted the focus of research on RPGR to this unusual transcript. The primary structure of this isoform consists of the RLD and the acidic ORF15-encoded domain. Owing to highly acidic nature of this domain, it has been an uphill task to delineate the function of the ORF15 domain. Nonetheless, remarkable studies of Tiansen Li and colleagues provided first clues to a possible role of this isoform in photoreceptors 54. They showed that ORF15 acts as an exon splicing enhancer and undergoes additional splicing in cells to produce multiple spliced transcripts of RPGR-ORF15. All these transcripts exhibited in-frame deletions of parts of ORF15 while retaining the carboxyl terminal sequence terminating in –LELK.
A major breakthrough in understanding the role of RPGR in photoreceptors came from the studies on its intracellular localization in photoreceptors. Studies from the labs of Tiansen Li and Paulo Ferreira showed that RPGR localizes to the TZ of photoreceptor and other cilia 54, 55. If such localization is species dependent is still a debatable issue. The ciliary and centrosomal localization of RPGR was further validated by multiple investigators and by using heterologous systems, including cultured hTERT-RPE cells, Madin Darby Canine Kidney cells and rodent kidneys 43, 56-59. Although RPGR is widely expressed, mutations in RPGR predominantly affect photoreceptor function, with few documented cases of hearing loss, sino-respiratory infections and sperm dysfunction 60-63. Such information prompted functional analysis of different isoforms of RPGR. Isoform-specific antibodies against RPGR were used to investigate potential differences in the function or localization of the different RPGR isoforms. An interesting observation was that RPGR protein isoforms seem to be considerably less in number as compared to the RNA isoforms, indicating that all RNA isoforms may not be translated into proteins and that some of them may act to stabilize the translation of RPGR and perform additional as yet unknown regulatory functions in photoreceptors 64. Another possible scenario is that the distinct isoforms of RPGR may be translated in a tissue-specific manner to meet with the demand of ciliary function. As photoreceptors rely immensely on regulated protein trafficking, a majority of RPGR isoforms expressed in photoreceptors participates in orchestrating polarized transport of proteins to the OS. However, in other cell types, RPGR isoforms may play a relatively less crucial role as the demand of ciliary transport is not as high as in photoreceptors and other compensatory moieties could act to stabilize ciliary function in the absence of RPGR.
All the extensive studies described above beg the question: What is the precise function of RPGR? In the next section, we will go over the studies that have provided insights into the function of RPGR.
5.1. Identification of RPGR-interacting proteins
Initial studies identified delta subunit of cGMP phosphodiesterase (PDEdelta) as an interacting partner of RPGR 65. Notably, PDEdelta is involved in the extraction of prenylated proteins (such as Rab proteins) from the membrane and subsequent transport of membrane proteins; hence RPGR was proposed to regulate protein transport and localization in photoreceptors. Supporting this hypothesis, the carboxyl-terminal sequence of the CI of RPGR, -CTIL, forms a conserved isoprenylation sequence (CAAX box). The CI of mouse RPGR was shown to localize to the Golgi and mutagenesis of the CTIL sequence resulted in mislocalization of RPGR in transiently transfected cultured cells 66.
Different groups while working independently, identified a coiled-coil domain containing novel interacting partner of RPGR, which was termed RPGR-interacting protein 1 (RPGRIP1) 67-69. The RPGRIP protein localizes to the TZ of photoreceptors, binds directly to the ciliary microtubules and is required for the recruitment to or retention of RPGR at the cilium 69. Further studies showed that RPGRIP1 also localizes to centrosomes and basal bodies along with RPGR 59. A breakthrough study revealed that the RPGRIP1 gene is mutated in patients with Leber congenital amaurosis (LCA) 70. LCA is a severe early-onset childhood blindness disorder that is caused by defective development or maturation of photoreceptors 71. As RPGR is not detected at the cilium in Rpgrip1-null retinas, these studies pointed towards a potentially significant role of RPGR in the cilium and suggested that localization of RPGR at the cilium is critical for photoreceptor function. Further insights into a ciliary role of RPGR came from subsequent co-immunoprecipitation and mass spectrometry studies 42, 43. These studies not only identified RPGR as part of multiprotein complexes of ciliary and microtubule-based assemblies, it also revealed its association with the IFT complex. Moreover, RPGR was found to directly interact with novel ciliary proteins Structural Maintenance of Chromosomes (SMC) proteins, SMC1 and SMC3. Although a direct correlation between SMC proteins and ciliary function still needs to be established, the fact that these proteins contain large coiled coil domains and other ciliary proteins also contain SMC-like coiled-coil domains, it is intriguing to speculate the SMC-like sequences may act as signature moieties to target to the cilium.
5.2. RPGR interacts with RAB8A
Although RPGR's homology to the GEF RCC1 was identified back in 1996; it was not until 2010 that we obtained first direct evidence for an interaction of RPGR with small GTPases. It was found that RPGR, particularly the RLD, interacts with RAB8A 57. This interaction is stabilized when RAB8A is present in a GDP-locked manner. Given that GEFs typically interact with GDP-bound GTPases and that RPGR has homology to a GEF, the next obvious step was to interrogate a potential activity of RPGR as a GEF for RAB8A. Concomitantly, the authors further demonstrated that RPGR could indeed act as a GEF and catalyze the conversion of RAB8A-GDP to RAB8A-GTP.
5.3. Role of RPGR in cilia
In the year 2000, Tiansen Li and colleagues reported the generation of Rpgr-knock out (Rpgr-ko) mouse 72. The authors targeted exons 4-6 encoding part of the RLD of RPGR and replaced them with a Neomycin cassette. Phenotypic analysis revealed retinal dysfunction and degeneration starting at around 6 months of age. This phenotype was associated by a mild mislocalization of cone opsins and decreased content of rhodopsin in the OS of photoreceptors. Another breakthrough in understanding RPGR-related disease came from the identification and characterization of two canine models of RPGR mutation, named X-linked Progressive Retinal Atrophy (XLPRA) 1 and XLPRA2 73. These mutant dogs carried distinct microdeletions in exon ORF15 of RPGR and displayed disparate phenotypes: XLPRA1 exhibits a deletion of carboxyl-terminal 230 residues and is associated with a delayed onset and slowly progressing mild but post-developmental photoreceptor dysfunction and degeneration. On the other hand, the mutation in XLPRA2 causes a frameshift and results in the addition of 34 residues and premature truncation of the RPGR protein. This mutant exhibits a relatively early onset, severe and fast progressing disease. Both canine and mouse models of RPGR represent the disease spectrum of XLRP observed in patients with mutations in the RPGR gene. These models offer an excellent platform to study RPGR-associated disease progression and pathogenesis and design treatment strategies. In fact, an exciting study has demonstrated that expressing the RPGR-ORF15 protein in the retina can ameliorate retinal degeneration in the canine models of RPGR 74. Nonetheless, the mechanism of associated photoreceptor degeneration was still unclear; such information is a prerequisite to development of rational therapeutic modalities for such a clinically heterogeneous disease.
A clue to the mechanism of action of RPGR was obtained when it was found that knockdown of RPGR results in shorter cilia but spares their development and generation 56. In zebrafish embryos, silencing of rpgr culminates in shorter cilia of Kupffer's vesicles (KV), which are transient structures that form during zebrafish development and regulate body axis asymmetry. Notably, no effect on the development of retina and photoreceptors was detected in a majority of embryos. In another study, Alan Wright and colleagues showed that depletion of rpgr in zebrafish could result in abnormal retinal development 75. Although such a phenotype is expected based on its role in ciliary functions, it does not correlate with the phenotype observed in patients or in larger animal models of RPGR mutation. Studies using RPGR patient samples have revealed no effect on the development of photoreceptors 76. In early life, a majority of RPGR patients seems to have a close to normal vision and exhibit deterioration with progression in age. If the defect in RPGR results in a post-developmental phenotype and is associated with a slow but progressive effect on cilia function, it is conceivable that the insult is exacerbated with age and hence, a severe phenotype is exhibited at a later age in these patients and in animal models.
6. CEP290/NPHP6
CEP290, also termed NPHP6 is one of the twelve ciliary genes mutated in Nephronophthisis-associated ciliopathies 77. During investigations on identifying new ciliary disease genes, groups of Friedhelm Hildebrandt and colleagues and of Joseph Gleeson and colleagues independently identified a cilia-centrosomal protein of 290 kDa (CEP290) as a causative gene for developmental ciliopathies JBTS and MKS 78, 79. These patients exhibited cerebellar vermis aplasia, retinal coloboma and several other developmental and life-threatening anomalies. Another exciting study identified a naturally occurring mouse mutant called rd16 (retinal degeneration 16), which carries an in-frame deletion in the Cep290 gene 40. The domain deleted in the rd16 mouse is termed deleted in rd16 domain (DRD) 80. Contrary to the human phenotype, the rd16 mouse revealed no major extra-retinal anomalies, other than mild olfactory dysfunction 81. However, the retinal phenotype of the rd16 mouse revealed interesting observations. The rd16 retina exhibits early onset photoreceptor degeneration with initial signs of rod and cone photoreceptor dysfunction starting as early as postnatal day (P) 18. This group went on to show that the CEP90 mutation in the rd16 mouse is in fact a hypomorphic allele; residual levels of the deleted CEP290 protein could be detected in the mutant retina. Using molecular cell biological approaches, it was found that CEP290 localizes predominantly to the TZ of photoreceptors and interacts with several ciliary transport proteins in photoreceptors. The rd16 retina exhibited mislocalization of the OS proteins, rhodopsin, arrestin and transducin, implicating a transport defect due to CEP290 mutation. Moreover, CEP290 was found to associate with RPGR; the deleted variant of CEP290 showed increased association with RPGR and concomitant mislocalization of RPGR in the rd16 retina.
Further analysis of the retinal phenotype of patients with CEP290 mutations and that of the rd16 mouse prompted Koenekoop, den Hollander and colleagues to investigate the involvement of CEP290 mutations in early onset retinal degenerative diseases 82. A pioneering study from this group reported that CEP290 mutations are found in 22-25% of patients with Leber congenital amaurosis (LCA), a childhood blindness disorder due to defective photoreceptor development or maturation. The most frequent CEP290 mutation was a splice site defect that resulted in aberrant splicing of the CEP290 gene in patient lymphocytes and production of a truncated transcript 82. Notably, these authors also detected the expression of the full-length CEP290 transcript in those lymphocytes, albeit at very low levels. Some CEP290 patients also exhibited olfactory deficits. These and additional such observations led to a hypothesis that hypomorphic CEP290 mutations may be associated with a tissue-restricted phenotype, with majority of the defect in sensory neurons. However, a loss of function mutation in CEP290 results in severe developmental disorders, including JBTS, and MKS. Detailed analysis of the genotype-phenotype correlation studies is required to test this hypothesis. With the recent development of a knockout mouse of Cep290, it will be interesting to investigate the overlapping and distinct features between this and the rd16 mouse 83. Such studies promise to reveal insights into progression and pathogenesis of a tissue-restricted versus systemic effects of CEP290 mutations.
6.1. Functional analysis of CEP290
Studies on silencing of CEP290 in cultured cells and in zebrafish have revealed an important role of CEP290 in regulating cilia assembly and vertebrate ciliary development. In cultured cells, knockdown of CEP290 results in defective ciliogenesis, which is accompanied by mislocalization of RAB8A 84, 85. It was also found that for CEP290 to mediate cilia assembly, another centrosomal protein CP110 should be removed from the basal bodies. As CEP290 interacts with CP110, it is hypothesized that CEP290 functions to remove CP110 during cilia formation and entry of RAB8A. Commensurate with this, CP110 acts as a negative regulator of ciliogenesis 86.
A recent study proposed a model of CEP290 function in photoreceptors and suggests that CEP290 is involved in targeting inhibitors of ciliogenesis to degradation. Using co-immunoprecipitation and tandem mass spectrometry analysis of proteins precipitated using anti-CEP290 antibody the authors identified proteins that are differentially associated with CEP290 in the WT but no rd16 retinas 80. This analysis revealed an association of CEP290 with a novel ciliary protein RKIP (Raf1 Kinase Inhibitory Protein). The RKIP protein was identified predominantly at the TZ and the apical inner segment of photoreceptors. Interestingly, the rd16 retina as well as cep290-knockdown zebrafish embryos and cultured hTERT-RPE1 cells exhibited a more than 2-fold increase in the intracellular levels of RKIP. This accumulation was not due to upregulated transcription or translation of RKIP, and pointed towards increased stabilization of the RKIP protein. The authors went on to show that RKIP interacts directly with RAB8A and that accumulation of RKIP sequesters RAB8A from cilia and results in defective cilia formation in cultured cells and zebrafish embryos.
7. Ciliary Protein Interactomes and Retinopathies
Ciliopathies are almost always associated with multi-systemic phenotypes although the phenotype varies with tissue type. If the basic components of ciliary assembly and function are conserved, it is perplexing to observe variations in the degree of severity in distinct cell types due to ciliary dysfunction. In order to understand this dilemma, studies are being conducted to interrogate how multiple ciliary proteins are assembled in distinct protein complexes in a tissue-specific manner.
Recent years have seen an explosion of information about the protein components of the cilia and their involvement in regulating protein trafficking inside the cilia 32, 39, 87-90. Pioneering work by Peter Jackson and colleagues described a multiprotein complex called BBSome, which consists of a subset of BBS proteins that regulate cilia formation and maturation 37. Later studies identified distinct complexes of NPHP proteins that are proposed to function in concert to regulate the docking and entry of proteins inside the cilium 91, 92. Such studies have provided novel clues to the mechanism of cilia formation and function and to understanding the mode of ciliary dysfunction when assembly of the complexes is perturbed. One such clue is that multiple protein complexes act in concert at the TZ to regulate protein sorting and entry into the cilia.
7.1. Photoreceptor ciliary Interactomes
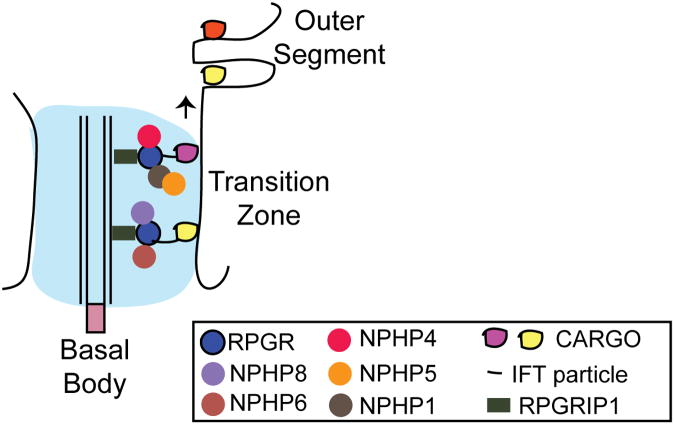
After the groundbreaking work of Eric Pierce and colleagues on reporting components of the photoreceptor sensory cilium proteome 39, it has become clear that a majority of retinal disease proteins localize to the cilia in photoreceptors. Moreover, this database is widely used to cross-reference any new retinal disease genes or novel interacting partners of known ciliary proteins for their potential localization and function at the cilia. However, a study on the existence of distinct multiprotein complexes of ciliary proteins in photoreceptors had not been performed. Initial clues to the existence of such complexes came from co-immunoprecipitation experiments in which RPGR and CEP290/NPHP6 were found to associate with overlapping as well as distinct set of ciliary and transport proteins in mammalian retina 40, 43 (Figure 2). More recently, it was found that RPGR and CEP290 indeed exist in multiple distinct protein complexes; however, the individual components of such complexes can be overlapping as well as distinct for each complex 93, 94. Using serial immunodepletion experiments, it was reported that in bovine retina, RPGR exists in at least two distinct complexes with selected NPHP proteins. Some of the interactions of the NPHP proteins with RPGR were direct while others were found to be indirect associations.
Figure 2. Model of involvement of discrete multiprotein complexes of RPGR and CEP290/NPHP6 in photoreceptor cilia.
The transition zone (TZ) of photoreceptors is largely composed of several protein complexes involved in degenerative disease. They form distinct complexes that may mediate sorting and trafficking of specific cargo to the OS. RPGRIP1 anchors RPGR at the TZ. The cargo is transported via the IFT particle.
8. Expert Opinion
RPGR and CEP290 are two of the commonly mutated ciliary proteins in retinal ciliopathies. Both these proteins associate with each other; however, mutations are found in a wide spectrum of disorders, ranging from delayed onset post-developmental photoreceptor degeneration (RPGR) to childhood onset blindness disorder, LCA (CEP290). A major hurdle in developing treatment strategies for these diseases is the considerable phenotypic heterogeneity observed in patients. Such heterogeneity is not only restricted to the severity and onset of retinal degeneration but is also observed in multiple tissue types. The phenotypes observed in patients and in experimental models point to the existence of a wide range of effects of mutations on the function of RPGR and CEP290. Some severe mutations seem to have a loss of function effect on the protein while others behave as a hypomorphic variation resulting in reduced function. Developing assay systems to test such effects is a prerequisite to understanding the mechanisms of heterogenic disease presentation.
With the identification of RPGR as a GEF for RAB8A and its effect on cilia extension 56, 57, it is now possible to test the pathogenic potential of disease-causing mutations in RPGR. Additional studies on the role of the GEF activity of RPGR specifically in protein trafficking in photoreceptors will assist in developing novel assay systems to test the pathogenicity of RPGR mutations and classification into loss, gain or hypomorphic effect on protein function. As ORF15 is a hotspot of mutations in RPGR, future studies should be designed to delineate the role of this domain in RPGR. Initial studies have shown that cilia-related functions of RPGR can be carried out in the absence of ORF15; however, such studies were performed in cultured cell systems and should be carefully extrapolated to in vivo scenarios. A promising study to design therapies for RPGR-XLRP was recently reported wherein a construct encoding the full-length RPGR-ORF15 protein was delivered to the photoreceptors of canine models of RPGR ORF15 mutations using adeno-associated viral (AAV)-vector based system 74. If such studies can be designed for mutations in the amino-terminus of RPGR awaits further investigations.
Examination of the function of CEP290 in photoreceptors revealed interesting observations that overlap with proposed functions of other ciliary proteins. Previously, BBS proteins, specifically BBS4, were shown to associate with the protein degradation machinery to regulate ciliary signaling cascades during vertebrate development 95. However, a direct correlation with the degenerative diseases associated with BBS remains to be delineated. Notably, the recent report that CEP290 is involved in regulating intracellular levels of another ciliary protein RKIP in photoreceptors and that abrogation of such function results in photoreceptor degeneration offers clues to the mechanisms of disease progression in retinopathies due to CEP290 mutations 80. Additional studies, including the mechanism by which CEP290 alters the intracellular levels of RKIP and identification of additional proteins that act as negative regulators of cilia formation (such as CP110) will provide new knowledge of the mode of regulation of ciliary development and function in photoreceptors. These studies will also reveal novel pathway intermediates, such as the protein degradation machinery or inhibitors of ciliogenesis that can be targeted to develop therapeutic strategies for CEP290 and associated disorders. In order to develop a gene therapeutic approach for treating CEP290-associated disease will require a detailed characterization of the effect of mutations on its function. This is particularly important in case of CEP290 because mutations in CEP290 are associated with wide-range of retinal and extra-retinal disorders. If a gene augmentation strategy were to be utilized to treat CEP290-LCA, a crucial hurdle is the limitation of the size of the cDNA that can be packaged into a viral vector. Given the long (∼8 kb) cDNA of CEP290, it is going to be an uphill task to generate modified viral vectors to accommodate such long cDNAs. Another strategy would be to identify critical but smaller regions of CEP290 that can ameliorate tissue-specific phenotypes. Two such approaches have been reported that utilized an amino-terminal domain of CEP290 and another using the DRD of CEP290 80, 96. Both these studies have reported potentially significant effect on rescuing cep290-associated retinal degeneration in zebrafish. However, additional studies, including their likely toxic effects and their usage in a larger animal model need to be performed before developing a therapeutic paradigm using such approach. Another negative effect of such an approach could arise from the fact that majority of CEP290-LCA patients carry a splice site defect and exhibit expression of the wild-type CEP290 transcript, albeit at low levels. A potential interaction between the wild-type protein and the smaller overexpressed domain may result in deleterious effect on photoreceptor function. Another approach is to utilize antisense oligonucleotides to correct the splice site defect. Such studies were successfully utilized to correct a splice site defect in the RPGR gene 97.
Article Highlights Box.
Targeted trafficking of protein from photoreceptor inner segment to sensory outer segment is critical for its development and function.
The transition zone of photoreceptors, which is a narrow bridge-like structure between the IS and the OS, regulates the docking and selection of cargo destined for the OS.
Recent studies have identified macromolecular complexes of ciliary proteins that function at the TZ to regulate its structure and function. This review focuses on understanding the function of two such proteins, RPGR and CEP290.
While RPGR mutations result in X-linked RP, mutations in CEP290 are associated with relatively early onset retinal degeneration called Leber congenital amaurosis.
Studies on understanding the function of RPGR and CEP290 have revealed novel clues to their mode of action in photoreceptors and mechanism of associated disease.
Acknowledgments
The authors apologize to those scientists whose outstanding work could not be cited due to space limitations.
This work was supported by grants from the National Institutes of Health and Foundation Fighting Blindness.
Abbreviations
- RPGR
retinitis pigmentosa GTPase regulator
- LCA
Leber congenital amaurosis
- RKIP
Raf1 Kinase Inhibitory Protein
- OS
outer segment
- IS
inner segment
- CC
connecting cilium
- TZ
transition zone
- BB
basal body
Footnotes
Declaration of interest: The authors declare no other conflicts of interest.
References
- 1.Singla V, Reiter JF. The primary cilium as the cell's antenna: signaling at a sensory organelle. Science. 2006 Aug;313:629–33. doi: 10.1126/science.1124534. [DOI] [PubMed] [Google Scholar]
- 2.Berbari NF, O'Connor AK, Haycraft CJ, Yoder BK. The primary cilium as a complex signaling center. Curr Biol. 2009 Jul;19:R526–35. doi: 10.1016/j.cub.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gerdes JM, Davis EE, Katsanis N. The vertebrate primary cilium in development, homeostasis, and disease. Cell. 2009 Apr;137:32–45. doi: 10.1016/j.cell.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hildebrandt F, Benzing T, Katsanis N. Ciliopathies. N Engl J Med. 2011 Apr;364:1533–43. doi: 10.1056/NEJMra1010172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sorokin S. Centrioles and the formation of rudimentary cilia by fibroblasts and smooth muscle cells. J Cell Biol. 1962 Nov;15:363–77. doi: 10.1083/jcb.15.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nachury MV, Seeley ES, Jin H. Trafficking to the ciliary membrane: how to get across the periciliary diffusion barrier? Annu Rev Cell Dev Biol. 2010 Nov;26:59–87. doi: 10.1146/annurev.cellbio.042308.113337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenbaum J. Intraflagellar transport. Curr Biol. 2002 Feb;12:R125. doi: 10.1016/s0960-9822(02)00703-0. [DOI] [PubMed] [Google Scholar]
- 8.Rosenbaum JL, Cole DG, Diener DR. Intraflagellar transport: the eyes have it. J Cell Biol. 1999 Feb;144:385–8. doi: 10.1083/jcb.144.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pazour GJ, Witman GB. The vertebrate primary cilium is a sensory organelle. Curr Opin Cell Biol. 2003 Feb;15:105–10. doi: 10.1016/s0955-0674(02)00012-1. [DOI] [PubMed] [Google Scholar]
- 10.Jeon CJ, Strettoi E, Masland RH. The major cell populations of the mouse retina. J Neurosci. 1998 Nov;18:8936–46. doi: 10.1523/JNEUROSCI.18-21-08936.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Besharse JC, Hollyfield JG, Rayborn ME. Turnover of rod photoreceptor outer segments. II. Membrane addition and loss in relationship to light. J Cell Biol. 1977 Nov;75:507–27. doi: 10.1083/jcb.75.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang H, Fan J, Li S, Karan S, et al. Trafficking of membrane-associated proteins to cone photoreceptor outer segments requires the chromophore 11-cis-retinal. J Neurosci. 2008 Apr;28:4008–14. doi: 10.1523/JNEUROSCI.0317-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang H, Li S, Doan T, Rieke F, et al. Deletion of PrBP/delta impedes transport of GRK1 and PDE6 catalytic subunits to photoreceptor outer segments. Proc Natl Acad Sci U S A. 2007 May;104:8857–62. doi: 10.1073/pnas.0701681104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaupp UB, Seifert R. Cyclic nucleotide-gated ion channels. Physiol Rev. 2002 Jul;82:769–824. doi: 10.1152/physrev.00008.2002. [DOI] [PubMed] [Google Scholar]
- 15.Baehr W, Karan S, Maeda T, Luo DG, et al. The function of guanylate cyclase 1 and guanylate cyclase 2 in rod and cone photoreceptors. J Biol Chem. 2007 Mar;282:8837–47. doi: 10.1074/jbc.M610369200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16**.Young RW. Passage of newly formed protein through the connecting cilium of retina rods in the frog. J Ultrastruct Res. 1968 Jun;23:462–73. doi: 10.1016/s0022-5320(68)80111-x. The data presented in this paper highlight the importance of the connecting cilium in polarized protein trafficking to the outer segment of photoreceptors. [DOI] [PubMed] [Google Scholar]
- 17.Horst CJ, Johnson LV, Besharse JC. Transmembrane assemblage of the photoreceptor connecting cilium and motile cilium transition zone contain a common immunologic epitope. Cell Motil Cytoskeleton. 1990;17:329–44. doi: 10.1002/cm.970170408. [DOI] [PubMed] [Google Scholar]
- 18.Besharse JC. The Retina: A Model for Cell Biological Studies Part I: Academic. New York: 1986. [Google Scholar]
- 19.Besharse JC, Hollyfield JG. Ultrastructural changes during degeneration of photoreceptors and pigment epithelium in the Ozark cave salamander. J Ultrastruct Res. 1977 Apr;59:31–43. doi: 10.1016/s0022-5320(77)80026-9. [DOI] [PubMed] [Google Scholar]
- 20.LaVail MM. Kinetics of rod outer segment renewal in the developing mouse retina. J Cell Biol. 1973 Sep;58:650–61. doi: 10.1083/jcb.58.3.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson DH, Fisher SK, Steinberg RH. Mammalian cones: disc shedding, phagocytosis, and renewal. Invest Ophthalmol Vis Sci. 1978 Feb;17:117–33. [PubMed] [Google Scholar]
- 22.Besharse JC, Hollyfield JG. Turnover of mouse photoreceptor outer segments in constant light and darkness. Invest Ophthalmol Vis Sci. 1979 Oct;18:1019–24. [PubMed] [Google Scholar]
- 23.Bok D, Young RW. The renewal of diffusely distributed protein in the outer segments of rods and cones. Vision Res. 1972 Feb;12:161–8. doi: 10.1016/0042-6989(72)90108-3. [DOI] [PubMed] [Google Scholar]
- 24.Sokolov M, Lyubarsky AL, Strissel KJ, Savchenko AB, et al. Massive light-driven translocation of transducin between the two major compartments of rod cells: a novel mechanism of light adaptation. Neuron. 2002 Mar;34:95–106. doi: 10.1016/s0896-6273(02)00636-0. [DOI] [PubMed] [Google Scholar]
- 25.Strissel KJ, Lishko PV, Trieu LH, Kennedy MJ, et al. Recoverin undergoes light-dependent intracellular translocation in rod photoreceptors. J Biol Chem. 2005 Aug;280:29250–5. doi: 10.1074/jbc.M501789200. [DOI] [PubMed] [Google Scholar]
- 26.Strissel KJ, Sokolov M, Trieu LH, Arshavsky VY. Arrestin translocation is induced at a critical threshold of visual signaling and is superstoichiometric to bleached rhodopsin. J Neurosci. 2006 Jan;26:1146–53. doi: 10.1523/JNEUROSCI.4289-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang H, Constantine R, Vorobiev S, Chen Y, et al. UNC119 is required for G protein trafficking in sensory neurons. Nat Neurosci. 2011 Jul;14:874–80. doi: 10.1038/nn.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Follit JA, Tuft RA, Fogarty KE, Pazour GJ. The intraflagellar transport protein IFT20 is associated with the Golgi complex and is required for cilia assembly. Mol Biol Cell. 2006 Sep;17:3781–92. doi: 10.1091/mbc.E06-02-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Follit JA, Xu F, Keady BT, Pazour GJ. Characterization of mouse IFT complex B. Cell Motil Cytoskeleton. 2009 Aug;66:457–68. doi: 10.1002/cm.20346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Omori Y, Zhao C, Saras A, Mukhopadhyay S, et al. Elipsa is an early determinant of ciliogenesis that links the IFT particle to membrane-associated small GTPase Rab8. Nat Cell Biol. 2008 Apr;10:437–44. doi: 10.1038/ncb1706. [DOI] [PubMed] [Google Scholar]
- 31.Barr F, Lambright DG. Rab GEFs and GAPs. Curr Opin Cell Biol. 2010 Aug;22:461–70. doi: 10.1016/j.ceb.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Inglis PN, Boroevich KA, Leroux MR. Piecing together a ciliome. Trends Genet. 2006 Sep;22:491–500. doi: 10.1016/j.tig.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 33.Deretic D. Rab proteins and post-Golgi trafficking of rhodopsin in photoreceptor cells. Electrophoresis. 1997 Dec;18:2537–41. doi: 10.1002/elps.1150181408. [DOI] [PubMed] [Google Scholar]
- 34.Mazelova J, Ransom N, Astuto-Gribble L, Wilson MC, et al. Syntaxin 3 and SNAP-25 pairing, regulated by omega-3 docosahexaenoic acid, controls the delivery of rhodopsin for the biogenesis of cilia-derived sensory organelles, the rod outer segments. J Cell Sci. 2009 Jun;122:2003–13. doi: 10.1242/jcs.039982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Westlake CJ, Baye LM, Nachury MV, Wright KJ, et al. Primary cilia membrane assembly is initiated by Rab11 and transport protein particle II (TRAPPII) complex-dependent trafficking of Rabin8 to the centrosome. Proc Natl Acad Sci U S A. 2011 Feb;108:2759–64. doi: 10.1073/pnas.1018823108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36**.Moritz OL, Tam BM, Hurd LL, Peranen J, et al. Mutant rab8 Impairs docking and fusion of rhodopsin-bearing post-Golgi membranes and causes cell death of transgenic Xenopus rods. Mol Biol Cell. 2001 Aug;12:2341–51. doi: 10.1091/mbc.12.8.2341. This paper illustrates the role of Rab GTPases in the traffikcing of rhodopsin to the outer segment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37**.Nachury MV, Loktev AV, Zhang Q, Westlake CJ, et al. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell. 2007 Jun;129:1201–13. doi: 10.1016/j.cell.2007.03.053. The authors of this stuy demonstrated the existence of a multiprotein complex of BBS proteins and its role in regulating cilia formation and function. [DOI] [PubMed] [Google Scholar]
- 38.Insinna C, Besharse JC. Intraflagellar transport and the sensory outer segment of vertebrate photoreceptors. Dev Dyn. 2008 Aug;237:1982–92. doi: 10.1002/dvdy.21554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu Q, Tan G, Levenkova N, Li T, et al. The proteome of the mouse photoreceptor sensory cilium complex. Mol Cell Proteomics. 2007 Aug;6:1299–317. doi: 10.1074/mcp.M700054-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40**.Chang B, Khanna H, Hawes N, Jimeno D, et al. In-frame deletion in a novel centrosomal/ciliary protein CEP290/NPHP6 perturbs its interaction with RPGR and results in early-onset retinal degeneration in the rd16 mouse. Hum Mol Genet. 2006 Jun;15:1847–57. doi: 10.1093/hmg/ddl107. The authors demonsgtrate the identification and charcaterization of a naturally occurring mouse model of Cep290 mutation. They show that a hypomorphic mutation in Cep290 results in a tissue restricted phenotype in the retina. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41**.Hong DH, Pawlyk B, Sokolov M, Strissel KJ, et al. RPGR isoforms in photoreceptor connecting cilia and the transitional zone of motile cilia. Invest Ophthalmol Vis Sci. 2003 Jun;44:2413–21. doi: 10.1167/iovs.02-1206. In this paper, the authors show that RPGR localizes to the photoreceptor transition zone or connecting cilium. [DOI] [PubMed] [Google Scholar]
- 42.Khanna H, Davis E, Murga-Zamalloa C. A common allele in RPGRIP1L is a modifier of retinal degeneration in ciliopathies. Nature Genetics. 2009 Jun;41:739–45. doi: 10.1038/ng.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43**.Khanna H, Hurd TW, Lillo C, Shu X, et al. RPGR-ORF15, which is mutated in retinitis pigmentosa, associates with SMC1, SMC3, and microtubule transport proteins. J Biol Chem. 2005 Sep;280:33580–7. doi: 10.1074/jbc.M505827200. This study demonstrates that RPGR exists in multiprotein complexes in photoreceptors and localizes to sperm flagella. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Williams CL, Li C, Kida K, Inglis PN, et al. MKS and NPHP modules cooperate to establish basal body/transition zone membrane associations and ciliary gate function during ciliogenesis. J Cell Biol. 2011 Mar;192:1023–41. doi: 10.1083/jcb.201012116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45**.Meindl A, Dry K, Herrmann K, Manson F, et al. A gene (RPGR) with homology to the RCC1 guanine nucleotide exchange factor is mutated in X-linked retinitis pigmentosa (RP3) Nat Genet. 1996 May;13:35–42. doi: 10.1038/ng0596-35. Independently from Roepman et al. 1996, this study identified the RPGR gene as the causative gene for the RP3 form of XLRP. [DOI] [PubMed] [Google Scholar]
- 46**.Roepman R, van Duijnhoven G, Rosenberg T, Pinckers AJ, et al. Positional cloning of the gene for X-linked retinitis pigmentosa 3: homology with the guanine-nucleotide-exchange factor RCC1. Hum Mol Genet. 1996 Jul;5:1035–41. doi: 10.1093/hmg/5.7.1035. Independently from Meindl et al. 1996, this study identified the RPGR gene as the causative gene for the RP3 form of XLRP. [DOI] [PubMed] [Google Scholar]
- 47.Fishman GA, Farber MD, Derlacki DJ. X-linked retinitis pigmentosa. Profile of clinical findings. Arch Ophthalmol. 1988 Mar;106:369–75. doi: 10.1001/archopht.1988.01060130395029. [DOI] [PubMed] [Google Scholar]
- 48.Heckenlively JR, Yoser SL, Friedman LH, Oversier JJ. Clinical findings and common symptoms in retinitis pigmentosa. Am J Ophthalmol. 1988 May;105:504–11. doi: 10.1016/0002-9394(88)90242-5. [DOI] [PubMed] [Google Scholar]
- 49.Kirschner R, Rosenberg T, Schultz-Heienbrok R, Lenzner S, et al. RPGR transcription studies in mouse and human tissues reveal a retina-specific isoform that is disrupted in a patient with X-linked retinitis pigmentosa. Hum Mol Genet. 1999 Aug;8:1571–8. doi: 10.1093/hmg/8.8.1571. [DOI] [PubMed] [Google Scholar]
- 50.Breuer DK, Yashar BM, Filippova E, Hiriyanna S, et al. A comprehensive mutation analysis of RP2 and RPGR in a North American cohort of families with X-linked retinitis pigmentosa. Am J Hum Genet. 2002 Jun;70:1545–54. doi: 10.1086/340848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shu X, Black GC, Rice JM, Hart-Holden N, et al. RPGR mutation analysis and disease: an update. Hum Mutat. 2007 Apr;28:322–8. doi: 10.1002/humu.20461. [DOI] [PubMed] [Google Scholar]
- 52*.Vervoort R, Lennon A, Bird AC, Tulloch B, et al. Mutational hot spot within a new RPGR exon in X-linked retinitis pigmentosa. Nat Genet. 2000 Aug;25:462–6. doi: 10.1038/78182. This study identified the alternative exon ORF15 of RPGR, which accounted for a majority of mutations identified in XLRP patients. [DOI] [PubMed] [Google Scholar]
- 53.Renault L, Nassar N, Vetter I, Becker J, et al. The 1.7 A crystal structure of the regulator of chromosome condensation (RCC1) reveals a seven-bladed propeller. Nature. 1998 Mar;392:97–101. doi: 10.1038/32204. [DOI] [PubMed] [Google Scholar]
- 54.Hong DH, Li T. Complex expression pattern of RPGR reveals a role for purine-rich exonic splicing enhancers. Invest Ophthalmol Vis Sci. 2002 Nov;43:3373–82. [PubMed] [Google Scholar]
- 55.Mavlyutov TA, Zhao H, Ferreira PA. Species-specific subcellular localization of RPGR and RPGRIP isoforms: implications for the phenotypic variability of congenital retinopathies among species. Hum Mol Genet. 2002 Aug;11:1899–907. doi: 10.1093/hmg/11.16.1899. [DOI] [PubMed] [Google Scholar]
- 56**.Ghosh AK, Murga-Zamalloa CA, Chan L, Hitchcock PF, et al. Human retinopathy-associated ciliary protein retinitis pigmentosa GTPase regulator mediates cilia-dependent vertebrate development. Hum Mol Genet. 2010 Jan;19:90–8. doi: 10.1093/hmg/ddp469. This paper demonstrates that RPGR regulates cilia extension and functions in zebrafish. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57**.Murga-Zamalloa CA, Atkins SJ, Peranen J, Swaroop A, et al. Interaction of retinitis pigmentosa GTPase regulator (RPGR) with RAB8A GTPase: implications for cilia dysfunction and photoreceptor degeneration. Hum Mol Genet. 2010 Sep;19:3591–8. doi: 10.1093/hmg/ddq275. The authors show that RPGR is indeed a GEF for RAB8A and this function is critical for cilia extension. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Patil SB, Verma R, Venkatareddy M, Khanna H. Expression and localization of the ciliary disease protein retinitis pigmentosa GTPase regulator in mammalian kidney. Kidney Int. 2010 Sep;78:622–3. doi: 10.1038/ki.2010.252. [DOI] [PubMed] [Google Scholar]
- 59.Shu X, Fry AM, Tulloch B, Manson FD, et al. RPGR ORF15 isoform co-localizes with RPGRIP1 at centrioles and basal bodies and interacts with nucleophosmin. Hum Mol Genet. 2005 May;14:1183–97. doi: 10.1093/hmg/ddi129. [DOI] [PubMed] [Google Scholar]
- 60.Iannaccone A, Wang X, Jablonski MM, Kuo SF, et al. Increasing evidence for syndromic phenotypes associated with RPGR mutations. Am J Ophthalmol. 2004 Apr;137:785–6. doi: 10.1016/j.ajo.2003.11.050. author reply 6. [DOI] [PubMed] [Google Scholar]
- 61.Koenekoop RK, Loyer M, Hand CK, Al Mahdi H, et al. Novel RPGR mutations with distinct retinitis pigmentosa phenotypes in French-Canadian families. Am J Ophthalmol. 2003 Oct;136:678–87. doi: 10.1016/s0002-9394(03)00331-3. [DOI] [PubMed] [Google Scholar]
- 62.Zito I, Downes SM, Patel RJ, Cheetham ME, et al. RPGR mutation associated with retinitis pigmentosa, impaired hearing, and sinorespiratory infections. J Med Genet. 2003 Aug;40:609–15. doi: 10.1136/jmg.40.8.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van Dorp DB, Wright AF, Carothers AD, Bleeker-Wagemakers EM. A family with RP3 type of X-linked retinitis pigmentosa: an association with ciliary abnormalities. Hum Genet. 1992 Jan;88:331–4. doi: 10.1007/BF00197269. [DOI] [PubMed] [Google Scholar]
- 64.He S, Parapuram SK, Hurd TW, Behnam B, et al. Retinitis Pigmentosa GTPase Regulator (RPGR) protein isoforms in mammalian retina: Insights into X-linked Retinitis Pigmentosa and associated ciliopathies. Vision Res. 2008 Feb;48:366–76. doi: 10.1016/j.visres.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Linari M, Ueffing M, Manson F, Wright A, et al. The retinitis pigmentosa GTPase regulator, RPGR, interacts with the delta subunit of rod cyclic GMP phosphodiesterase. Proc Natl Acad Sci U S A. 1999 Feb;96:1315–20. doi: 10.1073/pnas.96.4.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yan D, Swain PK, Breuer D, Tucker RM, et al. Biochemical characterization and subcellular localization of the mouse retinitis pigmentosa GTPase regulator (mRpgr) J Biol Chem. 1998 Jul;273:19656–63. doi: 10.1074/jbc.273.31.19656. [DOI] [PubMed] [Google Scholar]
- 67.Boylan JP, Wright AF. Identification of a novel protein interacting with RPGR. Hum Mol Genet. 2000 Sep;9:2085–93. doi: 10.1093/hmg/9.14.2085. [DOI] [PubMed] [Google Scholar]
- 68.Roepman R, Bernoud-Hubac N, Schick DE, Maugeri A, et al. The retinitis pigmentosa GTPase regulator (RPGR) interacts with novel transport-like proteins in the outer segments of rod photoreceptors. Hum Mol Genet. 2000 Sep;9:2095–105. doi: 10.1093/hmg/9.14.2095. [DOI] [PubMed] [Google Scholar]
- 69.Zhao Y, Hong DH, Pawlyk B, Yue G, et al. The retinitis pigmentosa GTPase regulator (RPGR)- interacting protein: subserving RPGR function and participating in disk morphogenesis. Proc Natl Acad Sci U S A. 2003 Apr;100:3965–70. doi: 10.1073/pnas.0637349100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dryja TP, Adams SM, Grimsby JL, McGee TL, et al. Null RPGRIP1 alleles in patients with Leber congenital amaurosis. Am J Hum Genet. 2001 May;68:1295–8. doi: 10.1086/320113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Koenekoop RK. An overview of Leber congenital amaurosis: a model to understand human retinal development. Surv Ophthalmol. 2004 Jul-Aug;49:379–98. doi: 10.1016/j.survophthal.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 72**.Hong DH, Pawlyk BS, Shang J, Sandberg MA, et al. A retinitis pigmentosa GTPase regulator (RPGR)-deficient mouse model for X-linked retinitis pigmentosa (RP3) Proc Natl Acad Sci U S A. 2000 Mar;97:3649–54. doi: 10.1073/pnas.060037497. This study demonstrates the generation and characterization of a knockout mouse model of Rpgr and showsits involvement in regulating. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang Q, Acland GM, Wu WX, Johnson JL, et al. Different RPGR exon ORF15 mutations in Canids provide insights into photoreceptor cell degeneration. Hum Mol Genet. 2002 May;11:993–1003. doi: 10.1093/hmg/11.9.993. [DOI] [PubMed] [Google Scholar]
- 74.Beltran A, Cideciyan AV, Lewin, et al. Gene therapy rescues photoreceptor blindness in dogs and paves way for treating human X-linked retinitis pigmentosa. Proc Natl Acad Sci U S A. 2012 Feb;109:2132–7. doi: 10.1073/pnas.1118847109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shu X, Zeng Z, Gautier P, Lennon A, et al. Zebrafish Rpgr is required for normal retinal development and plays a role in dynein-based retrograde transport processes. Hum Mol Genet. 2010 Feb;19:657–70. doi: 10.1093/hmg/ddp533. [DOI] [PubMed] [Google Scholar]
- 76.Szczesny PJ. Retinitis pigmentosa and the question of photoreceptor connecting cilium defects. Graefes Arch Clin Exp Ophthalmol. 1995 May;233:275–83. doi: 10.1007/BF00177649. [DOI] [PubMed] [Google Scholar]
- 77.Hildebrandt F, Attanasio M, Otto E. Nephronophthisis: disease mechanisms of a ciliopathy. J Am Soc Nephrol. 2009 Jan;20:23–35. doi: 10.1681/ASN.2008050456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78**.Sayer JA, Otto EA, O'Toole JF, Nurnberg G, et al. The centrosomal protein nephrocystin-6 is mutated in Joubert syndrome and activates transcription factor ATF4. Nat Genet. 2006 Jun;38:674–81. doi: 10.1038/ng1786. The authors report the identification of CEP290 as a causative gene for a form of Joubert Syndrome in humans. [DOI] [PubMed] [Google Scholar]
- 79**.Valente EM, Silhavy JL, Brancati F, Barrano G, et al. Mutations in CEP290, which encodes a centrosomal protein, cause pleiotropic forms of Joubert syndrome. Nat Genet. 2006 Jun;38:623–5. doi: 10.1038/ng1805. Independently from Sayer et al. 2006, this study also identified Joubert Syndrome associated mutations in CEP290. [DOI] [PubMed] [Google Scholar]
- 80**.Murga-Zamalloa CA, Ghosh AK, Patil SB, Reed NA, et al. Accumulation of the Raf-1 kinase inhibitory protein (Rkip) is associated with Cep290-mediated photoreceptor degeneration in ciliopathies. J Biol Chem. 2011 Aug;286:28276–86. doi: 10.1074/jbc.M111.237560. The authors show by protein protein interaction and biochemical analyses that CEP290 modulates intracellular protein levels of its interacting protein RKIP. They also show that a small domain of CEP290, which interacts with RKIP, can ameliorate CEP290-mediated photoreceptor degeneration in zebrafish. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McEwen DP, Koenekoop RK, Khanna H, Jenkins PM, et al. Hypomorphic CEP290/NPHP6 mutations result in anosmia caused by the selective loss of G proteins in cilia of olfactory sensory neurons. Proc Natl Acad Sci U S A. 2007 Oct;104:15917–22. doi: 10.1073/pnas.0704140104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82**.den Hollander AI, Koenekoop RK, Yzer S, Lopez I, et al. Mutations in the CEP290 (NPHP6) gene are a frequent cause of Leber congenital amaurosis. Am J Hum Genet. 2006 Sep;79:556–61. doi: 10.1086/507318. The authors of this study deminstrate that hypomorphic mutations in CEP290 are associated with Leber congenital amaurosis, a childhood blindness disorder. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lancaster MA, Gopal DJ, Kim J, Saleem SN, et al. Defective Wnt-dependent cerebellar midline fusion in a mouse model of Joubert syndrome. Nat Med. 2011 Jun;17:726–31. doi: 10.1038/nm.2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kim J, Krishnaswami SR, Gleeson JG. CEP290 interacts with the centriolar satellite component PCM-1 and is required for Rab8 localization to the primary cilium. Hum Mol Genet. 2008 Dec;17:3796–805. doi: 10.1093/hmg/ddn277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tsang WY, Bossard C, Khanna H, Peranen J, et al. CP110 suppresses primary cilia formation through its interaction with CEP290, a protein deficient in human ciliary disease. Dev Cell. 2008 Aug;15:187–97. doi: 10.1016/j.devcel.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Spektor A, Tsang WY, Khoo D, Dynlacht BD. Cep97 and CP110 suppress a cilia assembly program. Cell. 2007 Aug;130:678–90. doi: 10.1016/j.cell.2007.06.027. [DOI] [PubMed] [Google Scholar]
- 87.Gherman A, Davis EE, Katsanis N. The ciliary proteome database: an integrated community resource for the genetic and functional dissection of cilia. Nat Genet. 2006 Sep;38:961–2. doi: 10.1038/ng0906-961. [DOI] [PubMed] [Google Scholar]
- 88.Marshall WF. Human cilia proteome contains homolog of zebrafish polycystic kidney disease gene qilin. Curr Biol. 2004 Nov;14:R913–4. doi: 10.1016/j.cub.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 89.Ostrowski LE, Blackburn K, Radde KM, Moyer MB, et al. A proteomic analysis of human cilia: identification of novel components. Mol Cell Proteomics. 2002 Jun;1:451–65. doi: 10.1074/mcp.m200037-mcp200. [DOI] [PubMed] [Google Scholar]
- 90.Pazour GJ, Agrin N, Leszyk J, Witman GB. Proteomic analysis of a eukaryotic cilium. J Cell Biol. 2005 Jul;170:103–13. doi: 10.1083/jcb.200504008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Garcia-Gonzalo FR, Corbit KC, Sirerol-Piquer MS, Ramaswami G, et al. A transition zone complex regulates mammalian ciliogenesis and ciliary membrane composition. Nat Genet. 2011 Aug;43:776–84. doi: 10.1038/ng.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sang L, Miller JJ, Corbit KC, Giles RH, et al. Mapping the NPHP-JBTS-MKS protein network reveals ciliopathy disease genes and pathways. Cell. 2011 May;145:513–28. doi: 10.1016/j.cell.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Murga-Zamalloa C, Swaroop A, Khanna H. Multiprotein Complexes of Retinitis Pigmentosa GTPase Regulator (RPGR), a Ciliary Protein Mutated in X-Linked Retinitis Pigmentosa (XLRP) Adv Exp Med Biol. 2010;664:105–14. doi: 10.1007/978-1-4419-1399-9_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Murga-Zamalloa CA, Desai NJ, Hildebrandt F, Khanna H. Interaction of ciliary disease protein retinitis pigmentosa GTPase regulator with nephronophthisis-associated proteins in mammalian retinas. Mol Vis. 2010 Jul;16:1373–81. [PMC free article] [PubMed] [Google Scholar]
- 95*.Gerdes JM, Liu Y, Zaghloul NA, Leitch CC, et al. Disruption of the basal body compromises proteasomal function and perturbs intracellular Wnt response. Nat Genet. 2007 Nov;39:1350–60. doi: 10.1038/ng.2007.12. This study demonstrates the role of ciliary proteins in regulating proteasomal degradation of selected signaling proteins. [DOI] [PubMed] [Google Scholar]
- 96*.Baye LM, Patrinostro X, Swaminathan S, Beck JS, et al. The N-terminal region of centrosomal protein 290 (CEP290) restores vision in a zebrafish model of human blindness. Hum Mol Genet. 2011 Apr;20:1467–77. doi: 10.1093/hmg/ddr025. The authors in this study demonstrate a role of an amino-terminal domain of CEP290 in rescuing Cep290-associated retinopathy in zebrafish. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Glaus E, Schmid F, Da Costa R, Berger W, et al. Gene therapeutic approach using mutation-adapted U1 snRNA to correct a RPGR splice defect in patient-derived cells. Mol Ther. 2011 May;19:936–41. doi: 10.1038/mt.2011.7. [DOI] [PMC free article] [PubMed] [Google Scholar]