Abstract
OBJECTIVE
We sought to evaluate the risk of intrauterine fetal death (IUFD) in small-for-gestational-age (SGA) fetuses.
STUDY DESIGN
We analyzed a retrospective cohort of all births in the United States in 2005, as recorded in a national database. We calculated the risk of IUFD within 3 sets of SGA threshold categories as well as within non-SGA pregnancies using the number of at-risk fetuses as the denominator.
RESULTS
The risk of IUFD increased with gestational age and was inversely proportional to percentile of birthweight for gestational age. The risk for IUFD in those <3rd percentile was as high as 58.0 IUFDs per 10,000 at-risk fetuses, 43.9 for <5th percentile, and 26.3 for <10th percentile compared to 5.1 for non-SGA gestations.
CONCLUSION
There is an increase in the risk of IUFD in SGA fetuses compared to non-SGA fetuses at all gestational ages with the greatest risk demonstrated in the lowest percentile cohort evaluated.
Keywords: birthweight, fetal death, small for gestational age, stillbirth
The concept of appropriate weight for gestational age was first described in the 1960s with the development of birthweight nomograms according to gestational week.1 Subsequently, small-for-gestational- age (SGA) infants were defined as those with birthweights ≤10th percentile for their gestational age.2 Using this classification system, it was observed that infants born SGA had increased rates of perinatal morbidity and mortality at each gestational age relative to those infants not SGA.3
While these studies and others have made the case that altered fetal growth is associated with adverse perinatal outcomes,1–4 recent studies have attempted to determine whether or not the 10th percentile is a clinically useful cutoff.5–8 Additionally, research has focused on identifying indicators of pathologic growth restriction compared to normal growth in a constitutionally small fetus. The addition of Doppler velocimetry to perinatal assessment has greatly enhanced clinicians’ ability to identify fetuses with pathologic growth restriction related to altered umbilical artery blood flow.9–12 Moreover, the routine use of Doppler in high-risk pregnancies decreases induction of labor and antepartum admission, suggesting that in the absence of abnormal Doppler findings SGA pregnancies may benefit from expectant management rather than early delivery.13
Despite these advances, clinical decision making related to timing of delivery for SGA pregnancies remains a challenge for many clinicians. Consider, for instance, a 34-week pregnancy with reassuring umbilical artery Doppler, but an estimated fetal weight (EFW) indicating growth <3rd percentile. The morbidity associated with late preterm delivery is significant and yet many clinicians would be hesitant to commit to expectant management in a fetus at such a low centile. Given this clinical uncertainty, we have attempted to focus on the risk of intrauterine fetal death (IUFD) in SGA pregnancies with a goal of providing estimates of risk for fetuses <3rd, <5th, and <10th percentiles compared to non-SGA pregnancies by week of gestation. To further evaluate and refine the significance of fetal growth, we have also compared the risk of fetal death by cohorts of fetuses<3rd percentile, 3rd–5th percentile, and 5th–10th percentile compared to non-SGA pregnancies. It is our hope that having greater resolution of the risks faced by SGA pregnancies will aid in patient counseling and clinical decision making.
MATERIALS AND METHODS
To examine the risk of IUFD at a given week of gestation based on fetal growth, we conducted a retrospective cohort study of all singleton neonates born to women in the United States in 2005. The period-linked live birth and fetal death files from the National Center for Health Statistics (NCHS) (Centers for Disease Control and Prevention) for the year 2005 were exported and aggregated to form a single database comprising all fetal deaths and births from Jan. 1 through Dec. 31, 2005. Data were divided based on calculations used to categorize fetuses ≤3rd, 3rd–5th, or 5th– 10th percentile for birthweights and those fetuses at or above all remaining percentiles for birthweights. We excluded all multiple gestations and major congenital anomalies. Approval for this study was obtained from the Oregon Health and Science University Institutional Review Board.
The NCHS data set included month and year of birth, gestational age at delivery, birthweight, delivery method, and plurality. Gestational age was calculated according to delivery date and last menstrual period (LMP). If that information was unavailable the clinical estimate of gestational age on the birth certificate was used (the standard technique for data presentation in NCHS publications).
Fetal death was defined as IUFD prior to delivery, excluding cases of voluntary termination. Multiple gestations, anomalous fetuses, and all deliveries <24 weeks’ gestation and >41 6/7 weeks’ gestation were excluded.
Using the entire population of 2005 singleton births without congenital anomalies as the reference, we generated 3 sets of SGA thresholds based on percentiles of birthweight for gestational age: the<10th percentile (as SGA is commonly defined), the<5th percentile, and the<3rd percentile. The risk of IUFD was calculated out of the population of ongoing pregnancies representing at-risk fetuses at a particular gestational age. Thus, risk of fetal death was calculated as the number of IUFDs at a particular week of gestation divided by all ongoing pregnancies at a given gestational age and expressed as rates per 10,000. The number of ongoing pregnancies at the beginning of each week of gestation was calculated by consecutive subtractions of deliveries from the previous week of gestation, live born or otherwise. When examining the risk of IUFD at the 3rd percentile, this cohort included all neonates and stillbirths born with a birthweight <3rd percentile. This was similar for the 5th percentile, 10th percentile, and the non-SGA groups.
RESULTS
The NCHS database included 3,399,816 nonanomalous singletons delivered between 24 0/7–41 6/7 weeks’ gestation. Of these, 96,825 were <3rd percentile, 157,922 were <5th percentile, 322,161 were <10th percentile, and3,077,655were ≥10th percentile. Maternal age, parity, race, smoking history, and educational status differed in the birthweight groups (Table 1).
TABLE 1.
Maternal characteristics
| Characteristic | <3rd percentile n (%) |
<5th percentile n (%) |
<10th percentile n (%) |
≥10th percentile n (%) |
|
|---|---|---|---|---|---|
| Parity | |||||
| Nulliparous | 47,988 (50.5) | 77,968 (50.1) | 155,889 (48.9) | 1,136,112 (39.1) | |
| Primiparous, multiparous | 47,040 (49.5) | 77,583 (49.9) | 162,699 (51.1) | 1,763,472 (60.9) | |
| Maternal age, y | |||||
| <35 | 85,559 (88.4) | 139,871 (88.6) | 285,855 (87.6) | 2,504,635 (86.0) | |
| ≥35 | 11,266 (11.6) | 18,051 (11.4) | 36,306 (12.4) | 408,781 (14.0) | |
| Maternal race | |||||
| White (non-Hispanic) | 43,457 (44.9) | 71,303 (45.2) | 148,169 (46.0) | 1,659,279 (57) | |
| Black | 23,780 (24.6) | 37,506 (23.7) | 72,010 (22.4) | 373,685 (12.8) | |
| Hispanic | 20,399 (21.1) | 33,644 (21.3) | 69,770 (21.7) | 652,888 (22.4) | |
| Asian/Pacific Islander | 6592 (6.8) | 11,347 (7.2) | 24,048 (7.5) | 157,947 (5.4) | |
| Native American | 883 (0.9) | 1373 (0.9) | 2732 (0.8) | 28,014 (1) | |
| Other | 1714 (1.8) | 2749 (1.7) | 5432 (1.7) | 41,603 (1.4) | |
| Marital status | |||||
| Unwed | 50,255 (52.4) | 80,309 (51.2) | 156,714 (48.9) | 1,030,732 (35.4) | |
| Married | 45,685 (47.6) | 76,522 (48.8) | 164,056 (51.1) | 1,881,112 (64.6) | |
| Educational status | |||||
| No college | 60,299 (63.3) | 96,632 (62.2) | 191,150 (60.2) | 1,420,154 (49.3) | |
| Some college | 34,892 (36.7) | 58,787 (37.8) | 126,267 (39.8) | 1,458,554 (50.7) | |
| Tobacco use | |||||
| None | 61,348 (79.3) | 100,950 (80.2) | 211,183 (82.1) | 2,113,975 (90.8) | |
| Any | 15,974 (20.7) | 24,922 (19.8) | 46,063 (17.9) | 215,031 (9.2) | |
Pilliod. The risk of intrauterine fetal death in the SGA fetus. Am J Obstet Gynecol 2012.
The risk of IUFD is greater for lower percentile thresholds of SGA pregnancies at all weeks of gestation (Table 2). The 3rd-percentile risk reaches a nadir at 31 weeks’ gestational age with a risk of 12.2 IUFDs per 10,000 at-risk fetuses. The 5th, 10th, and ≥10th percentiles all reached nadirs at 32 weeks with risks of 9.3, 6.4, and 0.8 IUFDs per 10,000 at-risk fetuses, respectively. As the risk begins to climb after 32 weeks, the rate of change is fairly stable until 39 weeks, after which point the steepness of the slope increases. Maximum risk of IUFD was among postterm pregnancies for all percentile groups with the 3rd percentile as high as 58.0 IUFDs per 10,000 at-risk fetuses, 43.9 for the 5th percentile, and 26.3 for the 10th percentile compared to 5.1 for non-SGA gestations.
TABLE 2.
Intrauterine fetal death risk by <3rd, <5th, <10th, and ≥10th percentiles
|
GA, wk |
Deliveries | OP | Fetal deaths |
Risk of fetal death (per 10,000 at-risk pregnancies) |
95% CI | GA, wk |
Deliveries | OP | Fetal deaths |
Risk of fetal death (per 10,000 OP) |
95% CI | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <3rd percentile | <5th percentile | |||||||||||
| 24 | 282 | 96,825 | 207 | 21.38 | 18.47–24.29 | 24 | 382 | 157,922 | 253 | 16.02 | 14.05–17.99 | |
| 25 | 260 | 96,543 | 174 | 18.02 | 15.35–20.70 | 25 | 343 | 157,540 | 210 | 13.33 | 11.53–15.13 | |
| 26 | 261 | 96,283 | 168 | 17.45 | 14.81–20.08 | 26 | 375 | 157,197 | 211 | 13.42 | 11.61–15.23 | |
| 27 | 239 | 96,022 | 135 | 14.06 | 11.69–16.43 | 27 | 336 | 156,822 | 164 | 10.46 | 8.86–12.06 | |
| 28 | 273 | 95,783 | 127 | 13.26 | 10.95–15.56 | 28 | 400 | 156,486 | 155 | 9.91 | 8.35–11.46 | |
| 29 | 322 | 95,510 | 148 | 15.50 | 13.00–17.99 | 29 | 469 | 156,086 | 176 | 11.28 | 9.61–12.94 | |
| 30 | 399 | 95,188 | 156 | 16.39 | 13.82–19.96 | 30 | 588 | 155,617 | 179 | 11.50 | 9.82–13.19 | |
| 31 | 406 | 94,789 | 116 | 12.24 | 10.01–14.46 | 31 | 667 | 155,029 | 153 | 9.87 | 8.31–11.43 | |
| 32 | 554 | 94,383 | 119 | 12.61 | 10.43–14.87 | 32 | 861 | 154,362 | 143 | 9.26 | 7.75–10.78 | |
| 33 | 852 | 93,829 | 152 | 16.20 | 13.63–18.77 | 33 | 1331 | 153,501 | 185 | 12.05 | 10.32–13.79 | |
| 34 | 1427 | 92,977 | 161 | 17.32 | 14.64–19.99 | 34 | 2300 | 152,170 | 193 | 12.68 | 10.89–14.47 | |
| 35 | 2319 | 91,550 | 168 | 18.35 | 15.58–21.12 | 35 | 3708 | 149,870 | 207 | 13.81 | 11.93–16.69 | |
| 36 | 4187 | 89,231 | 191 | 21.41 | 18.37–24.44 | 36 | 6665 | 146,162 | 214 | 14.64 | 12.68–16.60 | |
| 37 | 9018 | 85,044 | 159 | 18.70 | 15.79–21.60 | 37 | 14,045 | 139,497 | 193 | 13.84 | 11.88–15.79 | |
| 38 | 18,376 | 76,026 | 176 | 23.15 | 19.73–26.57 | 38 | 32,016 | 125,452 | 225 | 17.94 | 15.59–20.28 | |
| 39 | 27,426 | 57,650 | 186 | 32.26 | 27.63–36.89 | 39 | 42,776 | 93,436 | 212 | 22.69 | 19.64–25.74 | |
| 40 | 20,567 | 30,224 | 98 | 32.42 | 26.02–38.83 | 40 | 35,490 | 50,660 | 127 | 25.07 | 20.71–29.42 | |
| 41 | 9657 | 9657 | 56 | 57.99 | 42.84–73.13 | 41 | 15,170 | 15,170 | 65 | 42.85 | 32.45–53.24 | |
| <10th percentile | ≥10th percentile | |||||||||||
| 24 | 578 | 322,161 | 315 | 9.78 | 8.70–10.86 | 24 | 2929 | 3,077,655 | 423 | 1.37 | 1.24–1.51 | |
| 25 | 567 | 321,583 | 277 | 8.61 | 7.60–9.63 | 25 | 3142 | 3,074,726 | 368 | 1.20 | 1.07–1.32 | |
| 26 | 590 | 321,016 | 257 | 8.01 | 7.03–8.98 | 26 | 3514 | 3,071,584 | 342 | 1.11 | 1.00–1.23 | |
| 27 | 552 | 320,426 | 214 | 6.68 | 5.78–7.57 | 27 | 3651 | 3,068,070 | 333 | 1.09 | 0.97–1.20 | |
| 28 | 705 | 319,874 | 214 | 6.69 | 5.79–7.59 | 28 | 4974 | 3,064,419 | 300 | 0.98 | 0.87–1.09 | |
| 29 | 805 | 319,169 | 228 | 7.14 | 6.22–8.07 | 29 | 5879 | 3,059,445 | 278 | 0.91 | 0.80–1.02 | |
| 30 | 1069 | 318,364 | 243 | 7.63 | 6.67–8.59 | 30 | 8165 | 3,053,566 | 297 | 0.97 | 0.86–1.08 | |
| 31 | 1216 | 317,295 | 209 | 6.59 | 5.69–7.48 | 31 | 10,233 | 3,045,401 | 284 | 0.93 | 0.82–1.04 | |
| 32 | 1679 | 316,079 | 203 | 6.42 | 5.54–7.31 | 32 | 14,523 | 3,035,168 | 237 | 0.78 | 0.68–0.88 | |
| 33 | 2527 | 314,400 | 242 | 7.70 | 6.73–8.67 | 33 | 22,535 | 3,020,645 | 283 | 0.94 | 0.83–1.05 | |
| 34 | 4348 | 311,873 | 251 | 8.05 | 7.05–9.04 | 34 | 40,488 | 2,998,110 | 294 | 0.98 | 0.87–1.09 | |
| 35 | 7462 | 307,525 | 255 | 8.29 | 7.27–9.31 | 35 | 69,196 | 2,957,622 | 310 | 1.05 | 0.93–1.16 | |
| 36 | 13,214 | 300,063 | 278 | 9.26 | 8.18–10.35 | 36 | 131,742 | 2,888,426 | 364 | 1.26 | 1.13–1.39 | |
| 37 | 28,767 | 286,849 | 253 | 8.82 | 7.73–9.91 | 37 | 281,877 | 2,756,684 | 403 | 1.46 | 1.32–1.60 | |
| 38 | 66,016 | 258,082 | 287 | 11.12 | 9.83–12.41 | 38 | 626,335 | 2,474,807 | 473 | 1.91 | 1.74–2.08 | |
| 39 | 91,295 | 192,066 | 256 | 13.33 | 11.70–14.96 | 39 | 869,260 | 1,848,472 | 420 | 2.27 | 2.05–2.49 | |
| 40 | 68,862 | 100,771 | 165 | 16.37 | 13.88–18.87 | 40 | 673,874 | 979,212 | 328 | 3.35 | 2.99–3.71 | |
| 41 | 31,909 | 31,909 | 84 | 26.32 | 20.70–31.95 | 41 | 305,338 | 305,338 | 155 | 5.08 | 4.28–5.88 | |
CI, confidence interval; GA, gestational age; OP, ongoing pregnancies.
Pilliod. The risk of intrauterine fetal death in the SGA fetus. Am J Obstet Gynecol 2012.
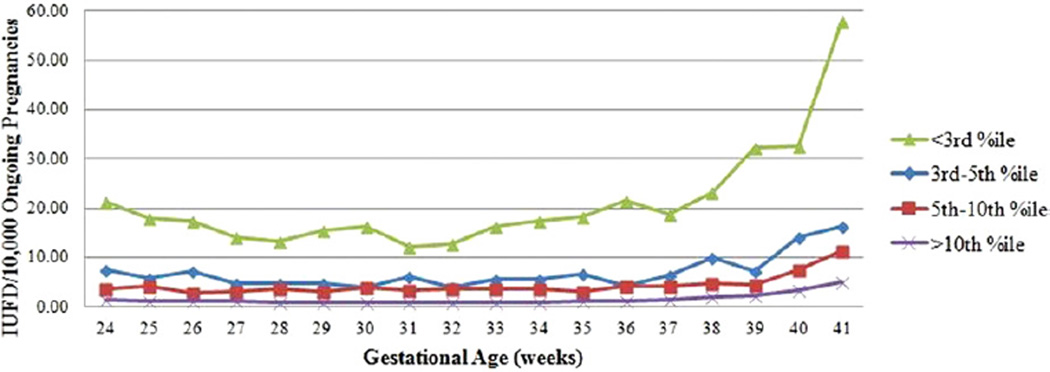
Tofurther characterize those fetuses facing the greatest risk of IUFD, additional comparisons were made using birthweights between the 3rd–5th percentiles and between the 5th–10th percentiles (Table 3). Unsurprisingly, the 3rd-percentile group faced the highest risk with an approximately 3-fold increased risk over the 3rd–5th–percentile group in nearly all gestational ages and a 4- to 7-fold increased risk over the 5th–10th–percentile group. When presented graphically, the risk of IUFD is J-shaped for each category with the greatest risk of IUFD in the late-term and postterm periods (Figure).
TABLE 3.
Intrauterine fetal death risk by 3rd–5th and 5th–10th percentile groups
| 3rd–5th percentiles | 5th–10th percentiles | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| GA, wk |
Deliveries | Ongoing pregnancies |
Fetal deaths |
Risk of fetal death (per 10,000 at-risk pregnancies) |
95% CI | GA, wk |
Deliveries | Ongoing pregnancies |
Fetal deaths |
Risk of fetal death (per 10,000 at-risk pregnancies) |
95% CI |
| 24 | 54 | 60,529 | 46 | 7.60 | 5.40–9.80 | 24 | 132 | 163,269 | 61 | 3.74 | 2.80–4.67 |
| 25 | 47 | 60,475 | 36 | 5.95 | 4.01–7.90 | 25 | 155 | 163,137 | 67 | 4.11 | 3.12–5.09 |
| 26 | 67 | 60,428 | 43 | 7.12 | 4.99–9.24 | 26 | 169 | 162,982 | 46 | 2.82 | 2.01–3.64 |
| 27 | 68 | 60,361 | 29 | 4.80 | 3.06–6.55 | 27 | 166 | 162,813 | 50 | 3.07 | 2.22–3.92 |
| 28 | 99 | 60,293 | 28 | 4.64 | 2.92–6.36 | 28 | 246 | 162,647 | 59 | 3.63 | 2.70–4.55 |
| 29 | 119 | 60,194 | 28 | 4.65 | 2.93–6.37 | 29 | 284 | 162,401 | 52 | 3.20 | 2.33–4.07 |
| 30 | 166 | 60,075 | 23 | 3.83 | 2.26–5.39 | 30 | 417 | 162,117 | 64 | 3.95 | 2.98–4.91 |
| 31 | 224 | 59,909 | 37 | 6.18 | 4.19–8.17 | 31 | 493 | 161,700 | 56 | 3.46 | 2.56–4.37 |
| 32 | 283 | 59,685 | 24 | 4.02 | 2.41–5.63 | 32 | 758 | 161,207 | 60 | 3.72 | 2.78–4.66 |
| 33 | 446 | 59,402 | 33 | 5.56 | 3.66–7.45 | 33 | 1139 | 160,449 | 57 | 3.55 | 2.63–4.47 |
| 34 | 841 | 58,956 | 32 | 5.43 | 3.55–7.31 | 34 | 1990 | 159,310 | 58 | 3.64 | 2.70–4.58 |
| 35 | 1350 | 58,115 | 39 | 6.71 | 4.61–8.82 | 35 | 3706 | 157,320 | 48 | 3.05 | 2.19–3.91 |
| 36 | 2455 | 56,765 | 23 | 4.05 | 2.40–5.71 | 36 | 6485 | 153,614 | 64 | 4.17 | 3.15–5.19 |
| 37 | 4997 | 54,310 | 34 | 6.26 | 4.16–8.36 | 37 | 14,662 | 147,129 | 60 | 4.08 | 3.05–5.11 |
| 38 | 13,591 | 49,313 | 49 | 9.94 | 7.16–12.72 | 38 | 33,938 | 132,467 | 62 | 4.68 | 3.52–5.85 |
| 39 | 15,324 | 35,722 | 26 | 7.28 | 4.48–10.08 | 39 | 48,475 | 98,529 | 44 | 4.47 | 3.15–5.78 |
| 40 | 14,894 | 20,398 | 29 | 14.22 | 9.05–19.39 | 40 | 33,334 | 50,054 | 38 | 7.59 | 5.18–10.00 |
| 41 | 5504 | 5504 | 9 | 16.35 | 5.68–27.03 | 41 | 16,720 | 16,720 | 19 | 11.36 | 6.26–16.47 |
GA, gestational age.
Pilliod. The risk of intrauterine fetal death in the SGA fetus. Am J Obstet Gynecol 2012.
Figure.
Risk of IUFD by gestational age
IUFD, intrauterine fetal death.
Pilliod. The risk of intrauterine fetal death in the SGA fetus. Am J Obstet Gynecol 2012.
Number needed to treat (NNT) analysis was conducted to estimate the number of immediate deliveries required to prevent 1 additional IUFD. Comparing the 3rd-percentile group and non-SGA pregnancies, 612 immediate deliveries at 34 weeks, 496 at 36 weeks, and 333 at 39 weeks were required to prevent an additional IUFD among the smallest fetuses. This is compared to 2248 immediate deliveries at 34 weeks, 3582 at 36 weeks, and 1997 at 39 weeks in the 3rd–5th–percentile group and 3759 immediate deliveries at 34 weeks, 3441 at 36 weeks, and 4558 at 39 weeks in the 5th–10th–percentile group.
COMMENT
These findings substantiate previous studies regarding the risk of IUFD in SGA and non-SGA pregnancies, and then further delineate this risk by week of gestation and in 3 percentile ranges within the <10th-percentile group.2–8 In all cohorts of SGA pregnancies the risk for fetal death was greater at all gestational ages than for non- SGA pregnancies. In the late preterm period, the risk rose for all pregnancies with the greatest risk faced by the smallest fetuses in late term and postterm pregnancies. When looking at different cohorts within the<10th percentile we found that those with birthweights in the <3rd percentile had a much greater probability of IUFD with each advancing week of gestation and that the magnitude of this risk was consistently 3 times higher than the closest cohort of the 3rd–5th percentile and 4–7 times higher than the 5th–10th percentile.
Such a dramatic difference in IUFD rates raises the question of whether or not the 10th-percentile cutoff continues to be a clinically relevant threshold for classifying SGA pregnancies. The intent of classifying SGA fetuses is to identify those most at risk for poor outcomes, with IUFD being the gravest in utero concern. Overestimating the risk of IUFD based on a narrow percentile classification system comes with the possibility of increased interventions, costs, and iatrogenic morbidity and mortality. Conversely, underestimating the IUFD risk secondary to a broad spercentile classification system comes with the chance of fetal death that may have been avoided with earlier delivery. If there is to be utility to SGA classification, it needs to be set at a point where more benefit than harm comes from intervening in those pregnancies at greatest risk. In our study, the risk of IUFD was clearly greatest in the <3rd-percentile cohort. Comparing this in terms of NNT, the number of immediate deliveries required to prevent 1 IUFD at 34 weeks’ gestation was 3.7 times higher in the 3rd–5th–percentile cohort and 6.1 higher in the 5th–10th percentile than the <3rd. By 39 weeks’ gestation, the difference was 6.0 times higher in the 3rd–5th–percentile cohort and 13.7 times higher in the 5th–10th– percentile cohort compared to the <3rd percentile. Meanwhile the difference between the 3rd–5th–percentile and 5th– 10th–percentile cohorts were nearly equivalent until 39 weeks’ gestation when the 3rd–5th–percentile cohort was 2.2 times higher than the 5th–10th percentile. Thus, instead of a single 10th-percentile cutoff, there may be more clinical utility in having different thresholds that would trigger different levels of intervention because of the varying risk, dependent on gestational age.
We acknowledge that our NNT calculations are limited in scope because they only account for 2 interventions, delivery or expectant management, and 2 outcomes, fetal death or live birth. This is a simplification of clinical practice and we recognize that many other risk factors, assessments, including Doppler ultrasound and antenatal testing, the impact of interventions and other perinatal outcomes are necessary for medical decision making. Nonetheless, we believe that our NNT calculations were justified in their ability to further demonstrate the magnitude of the increased risk faced by the lower percentile cohorts.
Once pregnancies have been appropriately identified as SGA, clinicians must still determine the optimal timing of delivery for fetuses facing increased risk of IUFD. After reaching a nadir around 32 weeks’ gestation, the IUFD risk begins to rise among all pregnancies. This rate of change is most dramatic in the late preterm period. As the rates of IUFD begin to rise it becomes necessary to consider neonatal morbidity and mortality associated with immediate delivery vs expectant management. While the risks of neonatal morbidity and mortality in the preterm and late preterm period have been well established, it remains unclear to what degree size for gestational age impacts these risks.14–18 Further complicating decision making, clinicians managing SGA pregnancies must differentiate pathologic growth restriction from constitutionally small fetuses. Uterine, umbilical, and fetal Doppler velocimetry are being combined with customized or established growth curves to help providers better estimate fetal survival and optimal timing of delivery.12,13 Although there is no set algorithm for determining the optimal timing of delivery of the SGA infant, this study adds granularity to the existing data on the risks faced by expectant management.
Even as this study is novel in its examination of fetal death in pregnancies categorized by the 3rd, 5th, and 10th percentiles, it is not without limitations. One of our study limitations was the use of birthweight rather than EFW. Accurate fetal weight estimation is the cornerstone of making management decisions based on morbidity and mortality associated with lower birthweight groups. A 2005 metaanalysis examined 11 studies comparing ultrasound EFW with birthweight and found the EFWs to be compromised by large intraobserver and interobserver variability.19 Given the inconsistency of the errors in EFW, it is difficult to approximate the direction or degree of impact this has on our findings.
Additionally, our study is limited by the dating available in the US natality database, which is based primarily on LMP. Studies show that using LMP alone to estimate gestational age is subject to both random error and a tendency to overestimate the duration of gestation compared to dating using ultrasound findings or clinical judgment.20–23 This suggests that the nadir of IUFD risk may be earlier and that the rate of increase in IUFD risk may be slightly steeper at term than this study reports. Moreover, our birthweight categories may erroneously capture appropriately sized infants who measure small for their misassigned gestational age. Furthermore, the data captured in the national database is recorded at time of delivery, which may overestimate gestational age if fetal death occurred before delivery or alter the birthweight if the fetus lost or gained weight in utero postmortem.
Finally, these findings use observational data and are based on only one aspect of neonatal outcome in SGA pregnancies. We acknowledge the absence of neonatal and maternal morbidity and mortality in our examination. Ideally, future studies will include decision and cost analyses to allow for simultaneous comparisons for short- and long-term neonatal outcomes, maternal preferences, and costs to the health care system as well as studies aimed at evaluating and improving the sensitivity and specificity of tools and tests used to identify and qualify risk groups such as Doppler velocimetry, methods for fetal estimation, and customized growth chart.
Despite these limitations, we believe this study contributes to the literature related to identification and evaluation of the SGA fetus. To the extent that algorithms exist for prompting additional fetal assessment in the setting of possible growth restriction, we hope that this more refined estimate of the risk of IUFD facilitates higher suspicion of risk of fetal death among the smallest percentile fetus. Additionally, we hope these findings may spur discussion regarding revision of EFW cutoffs for determining SGA.
Acknowledgments
Y.W.C. is supported by the National Institute of Child Health and Human Development, grant number HD01262, as a Women′s Reproductive Health Research Scholar.
Footnotes
The authors report no conflict of interest.
Presented as a poster at the 32nd annual meeting of the Society for Maternal-Fetal Medicine, Dallas, TX, Feb. 6-11, 2012.
REFERENCES
- 1.Lubchenco LO, Hansman C, Dressler M, Boyd E. Intrauterine growth as estimated from live born birth-weight data at 24 to 42 weeks of gestation. Pediatrics. 1963;32:793–800. [PubMed] [Google Scholar]
- 2.Battaglia FC, Lubchenco LO. A practical classification of newborn infants by weight and gestational age. J Pediatr. 1967;71:159–163. doi: 10.1016/s0022-3476(67)80066-0. [DOI] [PubMed] [Google Scholar]
- 3.Lubchenco LO, Searls DT, Brazie JV. Neonatal mortality rate: relationship to birth weight and gestational age. J Pediatr. 1972;81:814–822. doi: 10.1016/s0022-3476(72)80114-8. [DOI] [PubMed] [Google Scholar]
- 4.Koops BL, Morgan LJ, Battaglia FC. Neonatal mortality risk in relation to birth weight and gestational age: an update. J Pediatr. 1982;101:969–977. doi: 10.1016/s0022-3476(82)80024-3. [DOI] [PubMed] [Google Scholar]
- 5.Seeds JW, Peng T. Impaired growth and risk of fetal death: is the tenth percentile the appropriate standard. Am J Obstet Gynecol. 1998;178:658–669. doi: 10.1016/s0002-9378(98)70475-2. [DOI] [PubMed] [Google Scholar]
- 6.McIntire DD, Bloom SL, Casey BM, et al. Birthweight in relation to morbidity and mortality among newborn infants. N Engl J Med. 1999;340:1234–1238. doi: 10.1056/NEJM199904223401603. [DOI] [PubMed] [Google Scholar]
- 7.Myers SA, Ferguson R. A population study of the relationship between fetal death and altered fetal growth. Obstet Gynecol. 1989;74:325–331. [PubMed] [Google Scholar]
- 8.Ferguson R, Myers SA. Population study of the risk of fetal death and its relationship to birthweight, gestational age, and race. Am J Perinatol. 1994;11:267–272. doi: 10.1055/s-2007-994589. [DOI] [PubMed] [Google Scholar]
- 9.Erskine RL, Ritchie JW. Umbilical artery blood flow characteristics in normal and growth-retarded fetuses. Br J Obstet Gynaecol. 1985;92:605–610. doi: 10.1111/j.1471-0528.1985.tb01399.x. [DOI] [PubMed] [Google Scholar]
- 10.Gudmundsson S, Marsal K. Umbilical and uteroplacental blood flow velocity waveforms in pregnancies with fetal growth retardation. Eur J Obstet Gynecol Reprod Biol. 1988;27:187–196. doi: 10.1016/0028-2243(88)90122-0. [DOI] [PubMed] [Google Scholar]
- 11.Reuwer PJ, Bruinse HW, Stoutenbeek P, Haspels AA. Doppler assessment of the feto-placental circulation in normal and growth-retarded fetuses. Eur J Obstet Gynecol Reprod Biol. 1984;18:199–205. doi: 10.1016/0028-2243(84)90117-5. [DOI] [PubMed] [Google Scholar]
- 12.Shaffer BL, Parer JT. Chapter 11. Antepartum fetal monitoring. In: Queenan JT, Spong CY, Lockwood CJ, editors. Management of high risk pregnancy. 5th ed. Malden, MA: Blackwell; 2007. [Google Scholar]
- 13.Neilson JP, Alfirevic Z. Doppler ultrasound for fetal assessment in high risk pregnancies. Cochrane Database Syst Rev. 2000;2:CD000073. doi: 10.1002/14651858.CD000073. [DOI] [PubMed] [Google Scholar]
- 14.Gilbert WM, Danielsen B. Pregnancy outcomes associated with intrauterine growth restriction. Am J Obstet Gynecol. 2003;188:1596–1599. doi: 10.1067/mob.2003.384. [DOI] [PubMed] [Google Scholar]
- 15.Morse SB, Zheng H, Tang Y, Roth J. Early school-age outcomes of late preterm infants. Pediatrics. 2009;123:e622–e629. doi: 10.1542/peds.2008-1405. [DOI] [PubMed] [Google Scholar]
- 16.Surman G, Hemming K, Platt MJ, et al. Children with cerebral palsy: severity and trends over time. Paediatr Perinat Epidemiol. 2009;23:513–21. doi: 10.1111/j.1365-3016.2009.01060.x. [DOI] [PubMed] [Google Scholar]
- 17.Jacobsson B, Ahlin K, Francis A, Hagberg G, Hagberg H, Gardosi J. Cerebral palsy and restricted growth status at birth: population-based case-control study. BJOG. 2008;115:1250–1255. doi: 10.1111/j.1471-0528.2008.01827.x. [DOI] [PubMed] [Google Scholar]
- 18.Pulver LS, Guest-Warnick G, Stoddard GJ, Byington CL, Young PC. Weight for gestational age affects the mortality of late preterm infants. Pediatrics. 2009;123:e1072–e1077. doi: 10.1542/peds.2008-3288. [DOI] [PubMed] [Google Scholar]
- 19.Dudley NJ. A systematic review of the ultrasound estimation of fetal weight. Ultrasound Obstet Gynecol. 2005;25:80–89. doi: 10.1002/uog.1751. [DOI] [PubMed] [Google Scholar]
- 20.Ananth CV. Menstrual versus clinical estimate of gestational age dating in the United States: temporal trends and variability in indices of perinatal outcomes. Paediatr Perinat Epidemiol. 2007;21(Suppl):22–30. doi: 10.1111/j.1365-3016.2007.00858.x. [DOI] [PubMed] [Google Scholar]
- 21.Dietz PM, England LJ, Callaghan WM, Pearl M, Wier ML, Kharrazi M. A comparison of LMP-based and ultrasound-based estimates of gestational age using linked California livebirth and prenatal screening records. Paediatr Perinat Epidemiol. 2007;21(Suppl):62–71. doi: 10.1111/j.1365-3016.2007.00862.x. [DOI] [PubMed] [Google Scholar]
- 22.Pearl M, Wier ML, Kharrazi M. Assessing the quality of last menstrual period date on California birth records. Paediatr Perinat Epidemiol. 2007;21(Suppl):50–61. doi: 10.1111/j.1365-3016.2007.00861.x. [DOI] [PubMed] [Google Scholar]
- 23.Savitz DA, Terry JW, Jr, Dole N, Thorp JM, Jr, Siega-Riz AM, Herring AH. Comparison of pregnancy dating by last menstrual period, ultrasound scanning, and their combination. Am J Obstet Gynecol. 2002;187:1660–1666. doi: 10.1067/mob.2002.127601. [DOI] [PubMed] [Google Scholar]