Abstract
Objective
Vascular smooth muscle cell (VSMC) proliferation is central to the development of vascular diseases, including hypertension, which is regulated by numerous hormones and humoral factors. Our previous study showed that the stimulatory effect of norepinephrine on VSMC proliferation is inhibited by D1-like receptors and the D3 dopamine receptor, a member of the D2-like receptor family. Insulin is a proliferative hormone but it is not known if there is any interaction between insulin and D1-like receptors. We hypothesized that Dl-like receptors may have an inhibitory effect on the insulin-induced VSMC proliferation; aberrant insulin and Dl-like receptor functions could be involved in the pathogenesis of essential hypertension.
Methods
VSMC proliferation was determined by [3H]-thymidine incorporation; insulin receptor mRNA and protein expressions were determined by RT-PCR, immunoblotting, and immunohistochemistry.
Results
Insulin increased VSMC proliferation in immortalized aortic A10 cells, determined by [3H]-thymidine incorporation. Although the D1-like receptor, by itself, had no effect on VSMC proliferation, stimulation with fenoldopam, a D1-like receptor agonist, inhibited the stimulatory effect of insulin. The inhibitory effect of fenoldopam on insulin-mediated VSMC proliferation was receptor specific, because its effect could be blocked by SCH23390, a D1-like receptor antagonist. Fenoldopam also inhibited insulin receptor mRNA and protein expression, which was time dependent and concentration dependent. A PKC or MAP kinase inhibitor blocked the inhibitory effect of fenoldopam on insulin receptor expression, indicating that PKC and MAP kinase were involved in the signaling pathway.
Conclusion
The inhibitory effect of D1-like receptors on insulin-mediated VSMC proliferation may play an important role in the regulation of blood pressure.
Keywords: dopamine receptor, insulin, proliferation, vascular smooth muscle cells
Introduction
Epidemiological evidence supports a link between insulin resistance and hypertension [1–3]. Insulin resistance refers to the decreased ability of insulin to exert its biological effects on cells, resulting in over secretion of insulin to compensate for insulin resistance, which leads to hyperinsulinemia [1–3]. The high levels of insulin may play an important role in the pathogenesis of hypertension by increasing renal sodium reabsorption [4–6] and by stimulating the proliferation of vascular smooth muscle cells (VSMCs) [7,8].
Dopamine is an endogenous catecholamine that regulates/modulates many cellular functions, including behavior, hormone synthesis and release, blood pressure, and transmembrane ion transport [9–11]. Dopamine receptors are classified into the D1-like and D2-like subtypes based on their structure and pharmacology. D1-like receptors, composed of D1 and D5 receptors, stimulate adenylyl cyclase activity, whereas D2-like receptors, composed of D2, D3, and D4 receptors, inhibit adenylyl cyclase activity and regulate/modulate the activity of several ion channels [9–11].
Previous studies have shown that stimulation of D1-like receptors causes vasodilation [12]. Vasodilator hormones, such as atrial natriuretic peptide, have been shown to act as antihypertrophic and antiproliferative factors [13,14]. We have reported that stimulation of the α1-adrenergic receptor increases VSMC proliferation, whereas in the presence of a D1-like receptor agonist, fenoldopam, the α1-adrenergic receptor-mediated proliferative effect is inhibited [15]. These findings led us to hypothesize that Dl-like receptors may have an inhibitory effect on the insulin-induced VSMC proliferation; aberrant insulin and Dl-like receptor functions could be involved in the pathogenesis of essential hypertension. Therefore, the present study was designed to investigate the role of Dl-like receptors on insulin-mediated VSMC proliferation, and to determine the mechanisms underlying this regulation in A10 cells, a rat thoracic aorta-derived smooth muscle cell line.
Methods
Cell culture and sample preparation
Embryonic thoracic aortic smooth muscle cells [12,16] (passages 10–20) from normotensive Berlin–Druckrey IX rats (A10; CRL 1476, ATCC) were cultured at 37°C in 95% air/5% CO2 atmosphere in Dulbecco’s Modified Eagle’s Medium. A10 cells (80% confluence) were homogenized in ice-cold lysis buffer (final pH 7.4) (5 ml/g tissue) (20 mmol/l Tris-HCl, pH 7.4; 2 mmol/l EDTA, pH 8.0; 2 mM EGTA; 100 mmol/l NaCl; 10µg/ml leupeptin; 10µg/ml aprotinin; 2 mmol/l phenylmethylsulfonyl fluoride; 1% NP-40), sonicated, kept on ice for 1 h, and centrifuged at 16 000 g for 30 min. The supernatants of the samples were stored at −70°C until use.
Immunoblotting
A10 cells were treated with vehicle (dH2O), a D1-like receptor agonist (fenoldopam) [11,12,15,17] (Sigma, St. Louis, Missouri, USA) or a D1-like receptor antagonist (SCH23390) [11,12,15,17,18] (Sigma, St. Louis, Missouri, USA), at the indicated concentrations and times. Immunoblotting was performed as previously reported [12,15,17,19]. Protein concentration was determined using a protein assay kit (Bio-Rad Laboratories, Hercules, California, USA) with bovine serum albumin as standard [20]. Cell lysates were boiled in sample buffer (35mmol/l Tris-HCl, pH 6.8, 4% SDS, 9.3% dithiothreitol, 0.01% bromophenol blue, 30% glycerol) at 95°C for 5 min. Samples containing 50 µg of cell protein were separated by SDS-PAGE with 10% polyacrylamide gel, and then electroblotted onto nitrocellulose membranes (Bio-Rad). Blots were blocked overnight with 5% nonfat dry milk in PBS-T [0.05% Tween 20 in 10 mmol/l phosphate buffered (isotonic) saline] at 4°C with constant shaking. The blots were then incubated with polyconal rabbit anti-human insulin receptor antibody (1:400, Santa Cruz Biotechnology, Inc; Santa Cruz, California, USA; catalog No. sc-711, lot No. C0107) [21,22] in 5% nonfat dry milk in TBST (tris buffered saline/Tween buffer) for 1 h at room temperature. Membranes were washed three times with TBST and then incubated with peroxidase-labeled goat antirabbit IgG (1 : 5000; Santa Cruz) for 1 h at room temperature and developed for the detection of the specific protein using enhanced chemiluminescence reagents (Amersham, Little Chalfont, UK). The amount of protein transferred onto the membranes was normalized by immunoblotting of α-actin (monoclonal α-actin antibody, Santa Cruz Biotechnology, Inc., Santa Cruz, California, USA, 1:400) [12,15,17,19].
All immunoblot bands in one group (receptor of interest or actin) were given a value of 100%. The density of each sample was calculated as a fraction of 100%. The ordinates indicate the ratio of the density of the protein of interest as a fraction of 100% and the density of actin as a fraction of 100%.
Reverse transcriptase-PCR of insulin receptors
A total of 2–3 µg of total RNA extracted from A10 cells was used to synthesize cDNA, which served as template for the amplification of insulin receptor and β-actin (as housekeeping gene) [23]. For β-actin, the forward primer was 5′-GTGGGTATGGGTCAGAAGGA-3′ and the reverse primer was 5′-AGCGCGTAACCCTCATA GAT-3′ (GenBank Accession No. NM031144). The amplification was performed with the following conditions: denaturation at 94°C for 30 s, annealing for 30 s at 608C, and extension for 45 s at 728C for 35 cycles. For insulin receptor, the forward primer was 5′-GGA CTG AAG GTA TGA ATG GAG-3′ and the reverse primer was 5′-TAA CAC AAG CCA AGG AAG GG-3′. (GenBank Accession No. d12rat56). The amplification was performed with the following conditions: denaturation at 948C for 30 s, annealing for 30 s at 60°C, and extension for 45 s at 728C for 35 cycles. The insulin receptor mRNA expression was normalized for β-actin mRNA.
Immunohistochemistry
Cells grown in 96-well plates were fixed for 30 min in PBS containing 4% paraformaldehyde and washed three times with PBS. Fixed cells were incubated with anti-insulin receptor antibody (1:200) at 4°C overnight. After incubation with the primary antibodies, cells were rinsed three times with PBS and incubated for 60 min at 37°C with 10 µg/ml of biotin-conjugated goat antirabbit IgG (Jackson ImmunoResearch Laboratories). The cells were again washed three times with PBS and incubated for 20 min at room temperature with an avidin-biotin-peroxidase complex (Elite ABC kit; Vector Laboratories Inc., Burlingame, California, USA). The peroxidase label was then developed for 10 min using 3-amino-9-ethylcar-bazole and peroxide with a kit from Calbiochem. Staining distribution and intensity were evaluated and scored by two independent reviewers unaware of the treatments. The mean optical density was measured at 400 × magnification in five random fields for every slide, each field contained about 30 cells, and five slides were assessed in each group.
Photographs were obtained using a Leica DMLS microscope (Leica Microsystems). Images were processed using Image Pro Plus 6.0 (Media Cybernetics, California, USA) advanced acquisition software. The intensity of expression was determined by the computer program and given as density units, the result of integrated optical density divided by the sum of the detected area [24].
3H-thymidine incorporation
Cell proliferation was determined by measuring the incorporation of [3H]-thymidine (Atomic Energy Research Establishment of China, Beijing City, China) into DNA of cells cultured in 96-well plates [16,25,26]. After induction of quiescence, the cells were stimulated with insulin (Huofen Co., Shanghai City, China) or vehicle (dH2O) in the presence or absence of fenoldopam (Sigma Co.), a D1-like receptor agonist, for 24 h. The antagonists and inhibitors were added to the medium 30 min prior to the corresponding agonists or activators. Thereafter, [3H]-thymidine (1 µCi/ml) was added to the growth medium of each well 6 h prior to the measurements. At the end of incubation, the medium was removed and the cells were treated with 0.25 ml of 0.05% trypsin−0.53 mmol/l EDTA (Gibco, Grand Island, New York, USA) for 5 min and diluted to 10 ml with a balanced electrolyte solution [21]. The cells were then treated with 10% trichloroacetic acid to precipitate acid-insoluble materials from which the DNA was extracted with 0.1 N NaOH. The DNA was collected on Whatman GF/B filter and washed twice with 5 ml ice-cold PBS. The filter was then cut and shaken in 3.5 ml scintillation fluid for 24 h before counting in a liquid scintillation counter (Beckman LS6500, Beckman, Missouri, USA). Protein concentrations were determined using a BCA protein assay kit (Pierce, Rockford, Illinois, USA), as previously described [20]. Data are presented as [3H]-thymidine uptake per microgram of protein.
To determine the specificity of the D1-like receptor agonist, the D1-like receptor antagonist SCH23390 (Sigma Co.) [11,12,15,17,18] was used to block the effect of fenoldopam.
Determination of the second messenger(s) involved in the antiproliferative effects of D1-like receptor
To determine the second messenger(s) involved in the effect of D1-like receptors, different agonists and antagonists were used in this study, including protein kinase C (PKC) inhibitor peptide 19–31 (catalog No: 05– 23–4904, Calbiochem Co., Darmstadt, Germany) [27], protein kinase A (PKA) inhibitor 14–22 amide (catalog No: 476485, Calbiochem Co.) [28], dihydropyridine calcium channel blocker, nicardipine (catalog No: N7510, Sigma Co.) [29], mitogen-activated protein (MAP) kinse inhibitor PD 98059 (catalog No: P215, Sigma Co.) [30], PKC activator phorbol 12-myristate 13-acetate (PMA)(catalog No: P8139, Sigma Co.) [31], PKA agonist Sp-cAMP-S (catalog No: C8990, Sigma Co.), and calcium channel activator BAY-K8644 (catalog No: K8644, Sigma Co.) [32].
Statistical analysis
The data are expressed as mean ± SEM. Comparison within groups was determined by repeated measures ANOVA (or paired t test when only two groups were compared), and comparison among groups (or t test when only two groups were compared) was determined by factorial ANOVA and Duncan’s test. A value of P<0.05 was considered significant.
Results
The insulin-mediated proliferation of A10 cells is attenuated by a D1-like receptor agonist
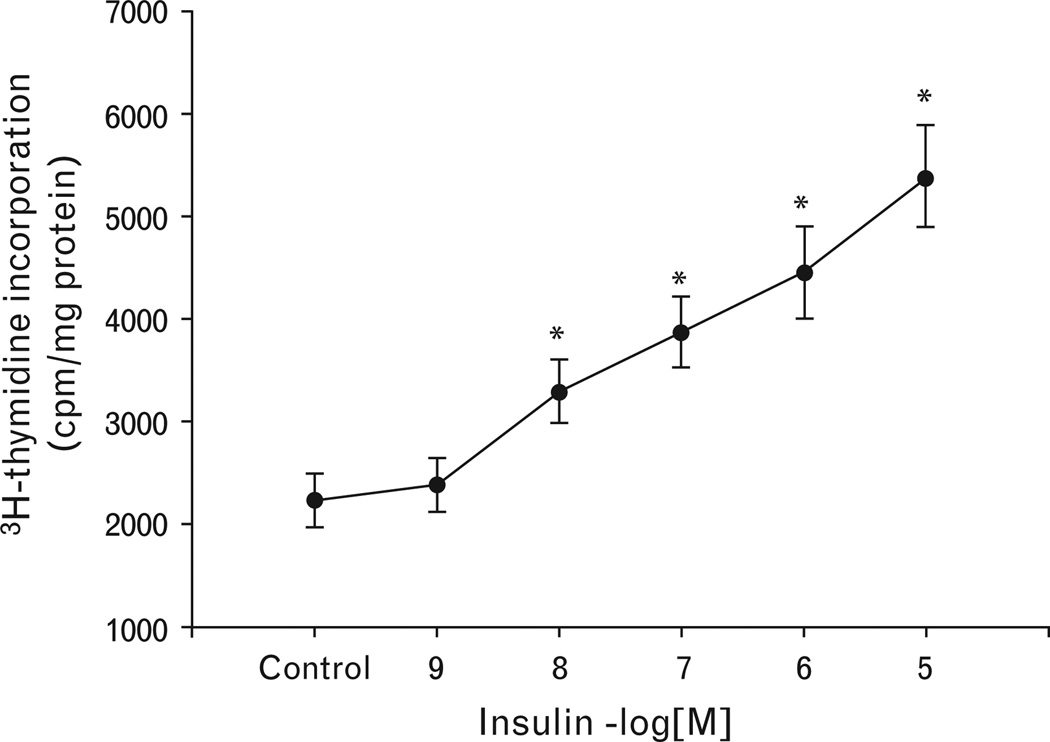
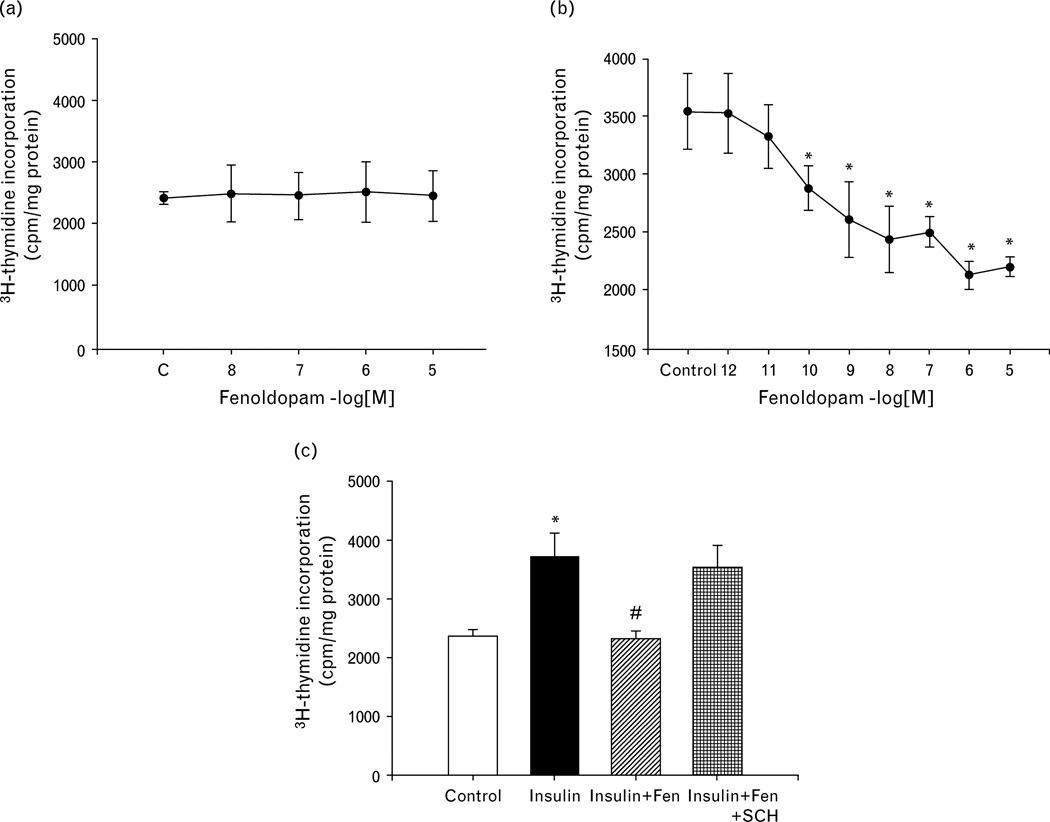
Treatment of VSMCs with varying concentrations of insulin (10−9 –10−5 mol/l) for 24 h resulted in a concentration-dependent increase in [3H]-thymidine incorporation in A10 cells (Fig. 1). Fenoldopam, by itself, had no effect on [ H]-thymidine incorporation (Fig. 2a), but dose-dependently (10−12 –10−5 mol/l) reduced the stimulatory effect of insulin (10−7 mol/l/24h; Fig. 2b). The effect of fenoldopam was via the D1-like receptor, because the inhibitory effect of fenoldopam on the insulin-mediated proliferation was blocked by the D1-like receptor antagonist, SCH23390 (Fig. 2c).
Fig. 1.
Concentration-dependent stimulatory effect of insulin on vascular smooth muscle cell proliferation in A10 cells. The proliferation of A10 cells was determined by [3H]-thymidine incorporation after incubation with the indicated concentrations of insulin (10−9 mol/l−10−5 mol/l) for 24 h. The control is vehicle (dH2O) in this figure. Results are expressed as counts per minute (cpm)/mg protein (n = 4, *P<0.05 vs. others, ANOVA, Duncan’s test).
Fig. 2.
Effect of D1-like receptor stimulation on insulin-induced proliferation of A10 cells. (a) Effect of a D1-like receptor agonist, fenoldopam, on the proliferation of A10 cells. The proliferation of A10 cells was determined by [3H]-thymidine incorporation after incubation with the indicated concentrations of fenoldopam (10−8mol/l−10−5 mol/l). The control (c) is vehicle (dH2O) in this figure. Results are expressed as cpm/mg protein (n = 4, ANOVA, Duncan’s test). (b) Effect of a D1-like receptor agonist, fenoldopam, on insulin-induced proliferation of A10 cells. The proliferation of A10 cells was determined by [3H]-thymidine incorporation after incubation with the indicated concentrations of insulin (10−7 mol/l) with or without fenoldopam (10−12 mol/l−10−5 mol/l). Control utilized vehicle (dH2O) instead of fenoldopam in this figure. Results are expressed as counts per minute (cpm)/mg protein (n= 5, *P<0.05 vs. control, ANOVA, Duncan’s test). (c) Effect of a D1-like receptor agonist (fenoldopam; Fen), and a D1-like receptor antagonist (SCH23390; SCH) on insulin-induced proliferation of A10 cells. The cells were incubated with the indicated reagents (fenoldopam, 10−6M; SCH23390, 10−6 mol/l) for 24 h. The control is vehicle (dH2O) in this figure. The proliferation of A10 cells was determined by [3H]-thymidine incorporation. Results are expressed as cpm/mg protein (n = 4, *P<0.05 vs. control; #P<0.05 vs. insulin, ANOVA, Duncan’s test).
Activation of D1-like receptor decreases insulin receptor expression in A10 cells
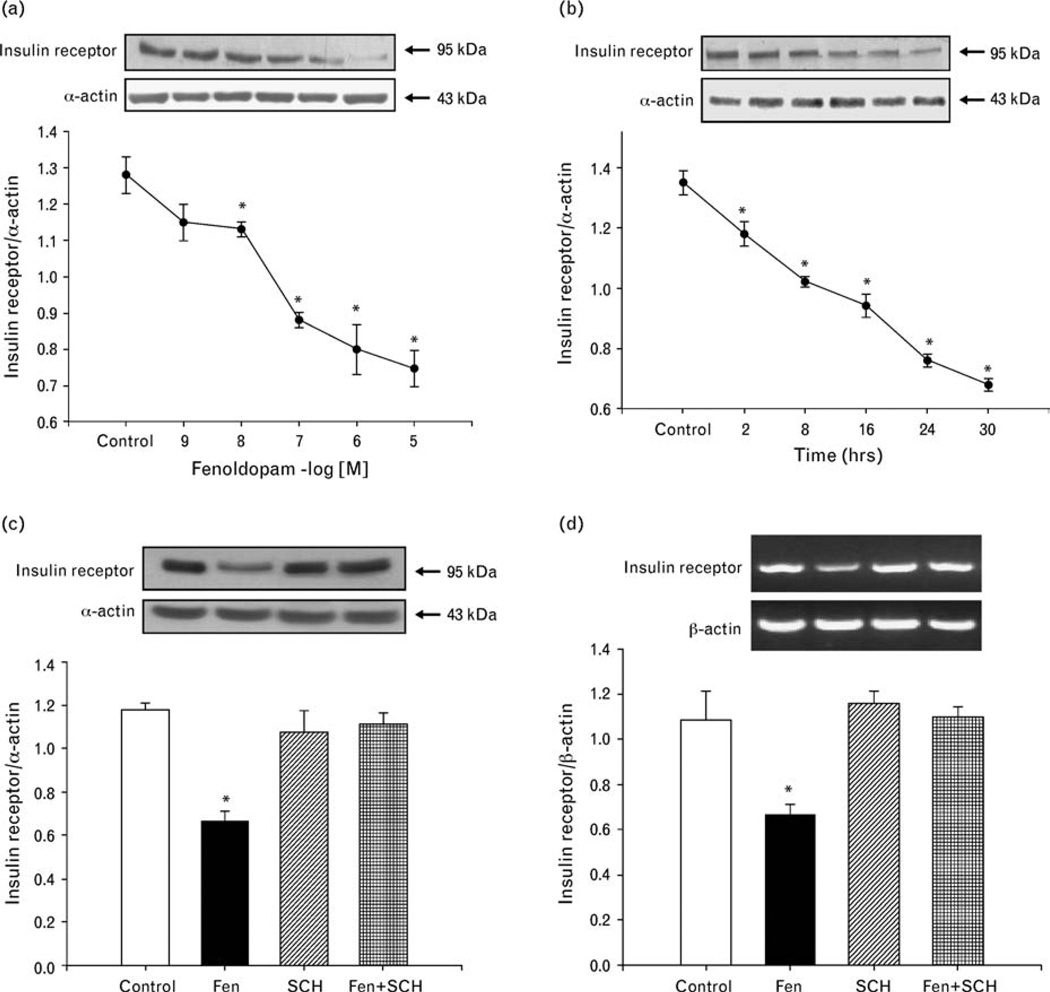
To determine the mechanisms underlying the inhibitory effect of fenoldopam on the insulin receptor-mediated proliferation, we studied the expression of insulin receptors in A10 cells. The D1-like receptor agonist, fenoldopam, decreased insulin receptor expression in a concentration-dependent and time-dependent manner (Fig. 3a and b).
Fig. 3.
Effect of D1-like receptor stimulation on insulin receptor expression in A10 cells. (a) Concentration-dependent effect of a D1-like receptor agonist, fenoldopam, on insulin receptor protein expression in A10 cells. The control is vehicle (dH2O) in this figure. Immunoreactive insulin receptor band was quantified after 24-h incubation with the indicated concentrations of fenoldopam (10−9 mol/l−10−5 mol/l). Results are expressed as the ratio of insulin receptor (as a fraction of 100%), to α-actin (as a fraction of 100%) densities (n = 4, *P<0.05 vs. control (dH2O), ANOVA, Duncan’s test). (b) Time-course of the effect of a D1-like receptor agonist, fenoldopam, on insulin receptor protein expression in A10 cells. The cells were incubated at the indicated times with 10−6 mol/l fenoldopam. The control is vehicle (dH2O) in this figure. Results are expressed as the ratio of insulin receptor (as a fraction of 100%), to α-actin (as a fraction of 100%) (n = 4, *P<0.05 vs. control [0 time], ANOVA, Duncan’s test). (c and d) Effect of a D1-like receptor agonist (fenoldopam) and a D1-like receptor antagonist (SCH23390) on insulin receptor protein (c) and mRNA (d) expression in A10 cells. The cells were incubated with the indicated reagents (fenoldopam, 10−6 mol/l; SCH23390, 10−6 mol/l) for 24 h. The control is vehicle (dH2O) in this figure. Results are expressed as the ratio of insulin receptor (as a fraction of 100%) to α-actin for immunoblotting or β-actin for RT-PCR (as a fraction of 100%) (n = 4, *P<0.05 vs. others, ANOVA, Duncan’s test).
The specificity of fenoldopam as a D1-like receptor agonist was also determined by studying the effect of the D1-like receptor antagonist, SCH23390. Consistent with the results shown in Fig. 3a and b, SCH23390. Consistent with the results shown in Fig. 3a and b, fenoldopam (10−6 mol/l/24h), decreased insulin receptor expression (control = 1.2 ±0.03 DU, fenoldopam = 0.66 ± 0.05 DU, n = 4; P<0.05). The D1-like receptor antagonist, SCH23390 (10−6 mol/l), by itself, had no effect on insulin receptor expression (SCH23390= 1.1 ±0.1 DU), but reversed the inhibitory effect of fenoldopam on insulin receptor expression (fenoldopam ± SCH23390 = 1.1 ± 0.06 DU, n = 4) (Fig. 3c). Consistent with the results determined by immunoblotting in Fig. 3c, fenoldopam (10−6 mol/l/24h), decreased insulin receptor mRNA expression (control = 1.08 ±0.12 DU, fenoldopam = 0.65 ±0.06 DU, n = 4; P<0.05), which was blocked in the presence of SCH23390 (SCH23390= 1.11 ±0.05 DU, fenoldopam + SCH23390= 1.09 ±0.04 DU, n = 4) (Fig. 3d).
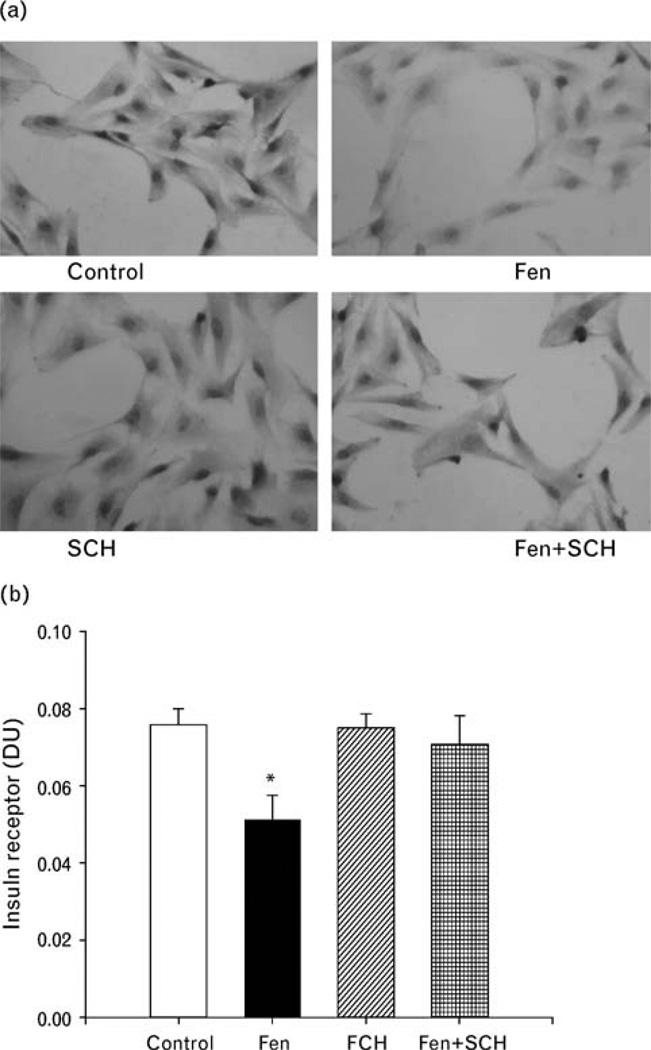
To confirm, further, the effect of fenoldopam on insulin receptor expression, we studied insulin receptor protein abundance by immunohistochemistry. Consistent with the results in Fig. 3c, stimulation of A10 cells with fenoldopam (10−6 mol/l) decreased insulin receptor protein expression in A10 cells, which was blocked by SCH23390 (10−6 mol/l) (control = 0.076 ±0.004 DU, fenoldopam = 0.050 ±0.007 DU, SCH23390 = 0.075 ± 0.003 DU, fenoldopam + SCH23390 = 0.070 ±0.007 DU, n = 3; P<0.05) (Fig. 4a and b).
Fig. 4.
Effect of D1-like receptor stimulation on insulin receptor expression in A10 cells determined by immunohistochemisty. The A10 cells were treated with fenoldopam (10−6 mol/l/24 h) with or without the presence of a D1-like receptor antagonist, SCH23390(10−6mol/l/24h). (a) Representative immunohistochemistry. (b) The optical density of the intensity of staining of the insulin receptor was determined and the integrated optical density was divided by the sum of the detected areas.
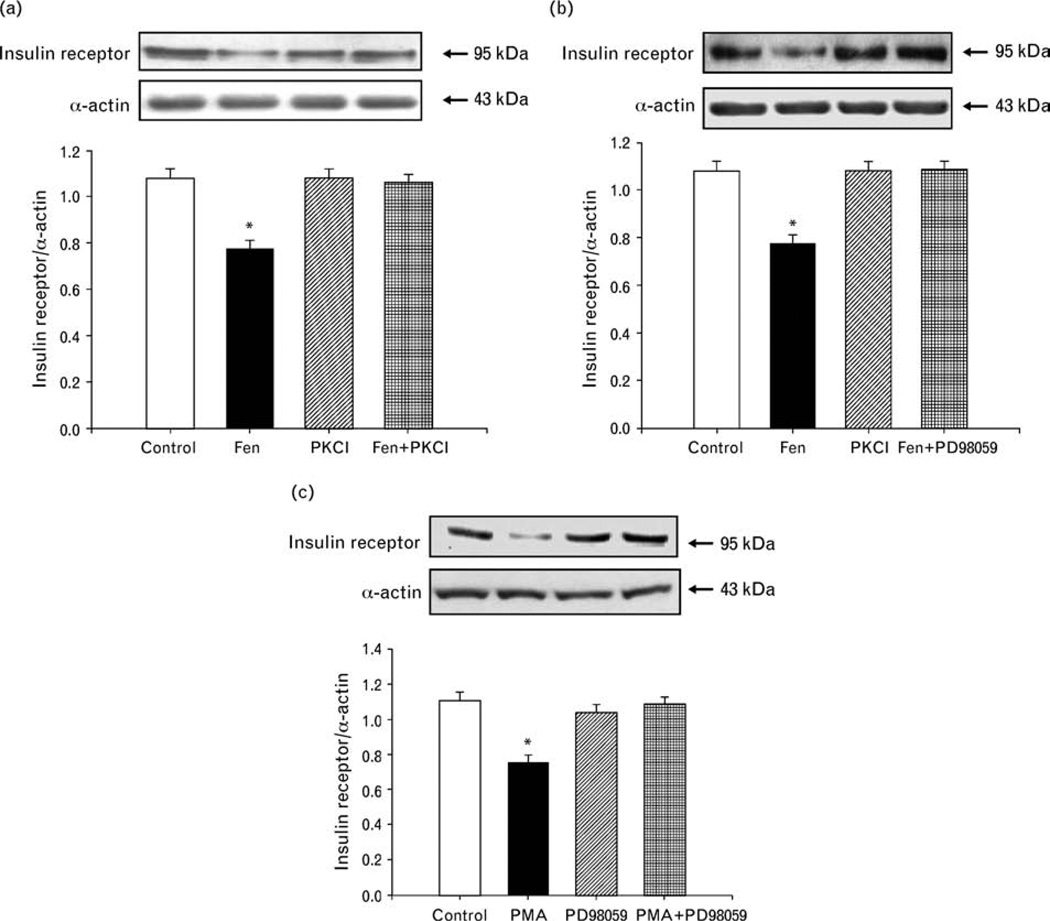
Role of PKC and MAP kinase in the inhibitory effect of fenoldopam on insulin receptor expression in A10 cells
The PKC inhibitor peptide 19–31 (10−6 mol/l) and MAP kinase inhibitor PD98059 (10−7 mol/l), by themselves, had no effect on insulin receptor expression, but blocked the inhibitory effect of fenoldopam on insulin receptor expression in A10 cells (Fig. 5a and b), indicating that PKC or MAP kinase was involved in the inhibitory action of fenoldopam. To determine the relationship between PKC and MAP kinase in the signaling pathway, we also studied the effect of a PKC agonist, PMA (10−7 mol/l), on insulin receptor expression in A10 cells. PMA inhibited insulin receptor expression but the inhibitory effect of PMA on insulin receptor expression was blocked by the MAP kinase inhibitor PD98059, indicating that PKC is upstream of MAP kinase (Fig. 5c).
Fig. 5.
Effect of PKC or MAP kinase on the inhibitory effect of fenoldopam on insulin receptor expression in A10 cells. (a) Effect of PKC on the inhibitory effect of fenoldopam on insulin receptor expression in A10 cells. A10 cells were treated with a D1-like receptor agonist (fenoldopam, 10−6 mol/l) or/and a PKC inhibitor, peptide 19–31 (PKCI, 10−6 mol/l) for 24 h. The control is vehicle (dH2O) in this figure. Results are expressed as the ratio of insulin receptor (as a fraction of 100%) to α-actin (as a fraction of 100%) (n = 4, *P< 0.05 vs. others, ANOVA, Duncan’s test). (b) Effect of MAP kinase on the inhibitory effect of fenoldopam on insulin receptor expression in A10 cells. A10 cells were treated with a D1-like receptor agonist (fenoldopam, 10−6 mol/l) and/or a MAP kinase inhibitor PD98059 (10−7 mol/l) for 24 h. The control is vehicle (dH2O) in this figure. Results are expressed as the ratio of insulin receptor (as a fraction of 100%) to α-actin (as a fraction of 100%) (n = 5, *P<0.05 vs. others, ANOVA, Duncan’s test). (c) Effect of PKC activator and MAP kinase inhibitor on insulin receptor expression in A10 cells. The cells were incubated with the indicated reagents: PKC activator PMA (10−7 mol/l), MAP kinase inhibitor PD98059 (10−7mol/l) for 24 h. The control is vehicle (dH2O) in this figure. Results are expressed as the ratio of insulin receptor (as a fraction of 100%), to α-actin (as a fraction of 100%) (n = 5, *P< 0.05 vs. others, ANOVA, Duncan’s test).
We also studied the effect of a PKA inhibitor (14–22), a PKA agonist (Sp-cAMP-S), a dihydropyridine calcium channel blocker (nicardipine), and a calcium channel activator (BAY-K8644) on fenoldopam-inhibited insulin expression in additional experiments. However, these reagents could not block the inhibitory effect of D1-like receptor on insulin receptor expression (data not shown).
Discussion
There are several novel observations in our study. We show that the proliferative effect of insulin in A10 cells is reduced by activation of D1-like receptors by fenoldopam, although the D1-like receptors, alone, have no effect on VSMC proliferation. The inhibitory effect of fenoldopam is via D1-like receptors, because its effect could be blocked in the presence of SCH23390, a D1-like receptor antagonist. The inhibitory effect of D1-like receptor stimulation of insulin on VSMC proliferation is caused by a D1-like receptor-mediated decrease in insulin receptor protein expression, probably at the level of transcription because fenoldopam also decreases insulin mRNA expression. The fenoldopam-mediated inhibition of insulin receptor protein expression is mediated by PKC and MAP kinase because either a PKC or MAP kinase inhibitor could block the inhibitory effect of fenoldopam on insulin receptor expression.
There is increasing evidence for interactions between insulin and dopamine receptors. Dopamine D2-like receptors in pancreatic beta cells inhibit insulin secretion [33]. Activation of D2-like receptors with bromocriptine decreases insulin levels and ameliorates several metabolic features in obese women [34]. Hyperinsulinemic animals and patients with noninsulin-dependent diabetes have defective renal dopaminergic system [35–37]. In obese Zucker rats, a model of type 2 diabetes, renal D1 receptors are downregulated and dopamine fails to induce diuresis and natriuresis. Treatment with an insulin sensitizer, rosiglitazone, decreases their plasma insulin levels and restores their renal D1 receptor function. Chronic exposure of cells to insulin causes a reduction in D1 receptor abundance and uncoupling from G proteins, and impairment of the inhibitory effect of dopamine on Na+-K+ ATPase activity [38]. The D1-like receptor agonist, fenoldopam, improves peripheral insulin sensitivity and renal function in streptozotocin-induced type 2 diabetes in rats [39]. The counter regulatory actions of the insulin and dopamine receptors extend to effects on vascular proliferation. Our previous study showed that the D1-like or D3 receptor has an inhibitory effect on norepinephrine-induced VSMC proliferation, and costimulation of D1-like and D3 receptors has an additive inhibitory effect, indicating the interaction between D1-like and D3 receptors in the artery [15]. Our current study shows an interaction between D1-like and insulin receptors because stimulation with a D1-like receptor agonist, fenoldopam, inhibits the proliferative effect of insulin in A10 cells.
The mechanisms of the D1-like receptor-mediated inhibition of the insulin-induced VSMC proliferation are not completely known. In the present study, fenoldopam does not affect the proliferation of nonstimulated A10 cells. In trypan blue exclusion tests, the 24-h treatment with fenoldopam does not increase the number of dead cells (data not shown). On the basis of these observations, it is unlikely that the inhibitory effect of fenoldopam on VSMC proliferation is due to any cytotoxic effect. We found that along with the inhibitory effect of fenoldopam on insulin-mediated proliferation, activation of D1-like receptor also decreases the insulin receptor expression, indicating that the downregulation of insulin receptor expression may be involved in the fenoldopam inhibitory effect.
The signaling pathway of D1-like receptor continues to evolve. The classic signaling pathway for D1-like receptors begins with activation of adenylyl cyclase, resulting in an increase in cAMP levels, and activation of PKA. PKA, in turn, causes phosphorylation and inhibition of NHE3 and Na+-K+-ATPase in renal tubular cells, effects that are nephron-segment specific [9,11,40–42]. Another signaling pathway of the D1 receptor involves the activation of phospholipase C [43], in the presence of the adaptor protein, calcyon [44], resulting in the production of inositol phosphates and diacylglycerol, activation of PKC and an increase in intracellular calcium concentration. This pathway, via phosphatidyl-inositol-3 kinase, is involved in D1-like receptor inhibition of Na+-K+-ATPase activity [45–47]. A previous study found that inhibition of PKC prevents hepatic insulin resistance [48]. To determine the mechanisms of D1-like receptor-mediated regulation of insulin receptor, we used different agonists or antagonists of PKA, PKC, and calcium channels. We found that the D1-like receptor-mediated inhibition of VSMC proliferation is blocked by the inhibition of PKC or MAP kinase activity, but not by the inhibition of PKA or blockade of L-type calcium channels, indicating that PKC and MAP kinase are involved in this action. This is in contrast to the study of Yasunari et al. [49], who found that the antiproliferative effect of D1-like receptors is mediated, in part, by inhibition of PKC activity. The reason for the difference between these two studies is not known; however, there are differences between our study and that reported by Yasunari et al. [49]. First, we used A10 cells, a rat thoracic aorta-derived smooth muscle cell line, whereas Yasunari et al. used VSMCs from the renal artery of Wistar rats. The differences may be vessel specific. Second, the proliferative agents used are different; we used insulin, whereas Yasunari et al. [49] used platelet-derived growth factor. It is possible that the signaling pathways are different between insulin and platelet-derived growth factor.
In summary, we have demonstrated that the proliferative effect of insulin in A10 cells is reduced by activation of the D1-like receptor. A D1-like receptor downregulation of the insulin receptor, via PKC and MAP kinase, may be involved in this process.
Perspectives.
VSMC proliferation plays an important role in the pathogenesis of VSM contractility and hypertension. Insulin increases VSMC proliferation, whereas the D1-like receptor reduces the insulin-mediated proliferative effects. The development of drugs that selectively activate dopamine receptor subtypes might be helpful in the prevention of the vessel wall hypertrophy and hyperplasia, reduce peripheral arterial resistance and normalize blood pressure, and improve conduit vessel function.
Acknowledgements
These studies were supported in part by grants from the National Institutes of Health (HL23081, DK39308, HL68686, HL62211, HL074940), the National Natural Science Foundation of China (30470728, 30672199) and the National Basic Research Program of China (973 Program, 2008CB517308).
Abbreviations
- MAP
kinase, mitogen-activated protein kinase
- PKA
protein kinase A
- PKC
protein kinase C
- PMA
phorbol 12-myristate 13-acetate
- VSMC
vascular smooth muscle cell
References
- 1.Kim JA, Wei Y, Sowers JR. Role of mitochondrial dysfunction in insulin resistance. Circ Res. 2008;102:401–414. doi: 10.1161/CIRCRESAHA.107.165472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sowers JR. Insulin resistance and hypertension. Am J Physiol Heart Circ Physiol. 2004;286:H1597–H1602. doi: 10.1152/ajpheart.00026.2004. [DOI] [PubMed] [Google Scholar]
- 3.Nigro J, Osman N, Dart AM, Little PJ. Insulin resistance and atherosclerosis. Endocr Rev. 2006;27:242–259. doi: 10.1210/er.2005-0007. [DOI] [PubMed] [Google Scholar]
- 4.Fuster DG, Bobulescu IA, Zhang J, Wade J, Moe OW. Characterization of the regulation of renal Na+/H+ exchanger NHE3 by insulin. Am J Physiol Renal Physiol. 2007;292:F577–F585. doi: 10.1152/ajprenal.00240.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song J, Hu X, Riazi S, Tiwari S, Wade JB, Ecelbarger CA. Regulation of blood pressure, the epithelial sodium channel (ENaC), and other key renal sodium transporters by chronic insulin infusion in rats. Am J Physiol Renal Physiol. 2006;290:F1055–F1064. doi: 10.1152/ajprenal.00108.2005. [DOI] [PubMed] [Google Scholar]
- 6.Nofziger C, Chen L, Shane MA, Smith CD, Brown KK, Blazer-Yost BL. PPARγ agonists do not directly enhance basal or insulin-stimulated Na+ transport via the epithelial Na+ channel. Pflugers Arch. 2005;451:445–453. doi: 10.1007/s00424-005-1477-4. [DOI] [PubMed] [Google Scholar]
- 7.White PW, Abularrage CJ, Weiswasser JM, Kellicut DC, Arora S, Sidawy AN. Hypoxia attenuates insulin-induced proliferation and migration of human diabetic infrapopliteal vascular smooth muscle cells. Ann Vasc Surg. 2006;20:381–386. doi: 10.1007/s10016-006-9057-4. [DOI] [PubMed] [Google Scholar]
- 8.Tuck ML, Bounoua F, Eslami P, Nyby MD, Eggena P, Corry DB. Insulin stimulates endogenous angiotensin II production via a mitogen-activated protein kinase pathway in vascular smooth muscle cells. J Hypertens. 2004;22:1779–1785. doi: 10.1097/00004872-200409000-00023. [DOI] [PubMed] [Google Scholar]
- 9.Zeng C, Armando I, Luo Y, Eisner GM, Felder RA, Jose PA. Dysregulation of dopamine-dependent mechanisms as a determinant of hypertension: studies in dopamine receptor knockout mice. Am J Physiol Heart Circ Physiol. 2008;294:H551–H569. doi: 10.1152/ajpheart.01036.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bertorello AM, Sznajder JI. The dopamine paradox in lung and kidney epithelia: sharing the same target but operating different signaling networks. Am J Respir Cell Mol Biol. 2005;33:432–437. doi: 10.1165/rcmb.2005-0297TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hussain T, Lokhandwala MF. Renal dopamine receptors and hypertension. Exp Biol Med (Maywood) 2003;228:134–142. doi: 10.1177/153537020322800202. [DOI] [PubMed] [Google Scholar]
- 12.Zeng C, Wang D, Yang Z, Wang Z, Asico LD, Wilcox CS, et al. D1 dopamine receptor augmentation of D3 receptor action in rat aortic or mesenteric vascular smooth muscles. Hypertension. 2004;43:673–679. doi: 10.1161/01.HYP.0000118958.27649.6f. [DOI] [PubMed] [Google Scholar]
- 13.Hutchinson HG, Trindade PT, Cunanan DB, Wu CF, Pratt RE. Mechanisms of natriuretic-peptide-induced growth inhibition of vascular smooth muscle cells. Cardiovasc Res. 1997;35:158–167. doi: 10.1016/s0008-6363(97)00086-2. [DOI] [PubMed] [Google Scholar]
- 14.Wolf G, Ziyadeh FN, Stahl RA. Atrial natriuretic peptide stimulates the expression of transforming growth factor-beta in cultured murine mesangial cells: relationship to suppression of proliferation. J Am Soc Nephrol. 1995;6:224–233. doi: 10.1681/ASN.V62224. [DOI] [PubMed] [Google Scholar]
- 15.Li Z, Zeng C, Han Y, Liu Y, He D, Huang H, et al. Inhibitory effect of D1 and D3 dopamine receptors on norepinephrine-induced proliferation in vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2008;294:H2761–H2768. doi: 10.1152/ajpheart.01344.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kimes BW, Brandt BL. Characterization of two putative smooth muscle cell lines from rat thoracic aorta. Exp Cell Res. 1976;98:349–366. doi: 10.1016/0014-4827(76)90446-8. [DOI] [PubMed] [Google Scholar]
- 17.Johansson GS, Arnqvist HJ. Insulin IGF-I action on insulin receptors, IGF-I receptors, and hybrid insulin/IGF-I receptors in vascular smooth muscle cells. Am J Physiol Endocrinol Metab. 2006;291:E1124–E1130. doi: 10.1152/ajpendo.00565.2005. [DOI] [PubMed] [Google Scholar]
- 18.Sachdev D, Singh R, Fujita-Yamaguchi Y, Yee D. Down-regulation of insulin receptor by antibodies against the type I insulin-like growth factor receptor: implications for antiinsulin-like growth factor therapy in breast cancer. Cancer Res. 2006;66:2391–2402. doi: 10.1158/0008-5472.CAN-05-3126. [DOI] [PubMed] [Google Scholar]
- 19.Yu P, Asico LD, Luo Y, Andrews P, Eisner GM, Hopfer U, et al. D1 dopamine receptor hyperphosphorylation in renal proximal tubules in hypertension. Kidney Int. 2006;70:1072–1079. doi: 10.1038/sj.ki.5001708. [DOI] [PubMed] [Google Scholar]
- 20.Sanada H, Asico LD, Shigetomi S, Tanaka K, Niimura S, Watanabe H, et al. The effect of docarpamine, a dopamine pro-drug, on blood pressure and catecholamine levels in spontaneously hypertensive rats. Clin Exp Hypertens. 2000;22:419–429. doi: 10.1081/ceh-100100081. [DOI] [PubMed] [Google Scholar]
- 21.Zeng C, Liu Y, Wang Z, He D, Huang L, Yu P, et al. Activation of D3 dopamine receptor decreases AT1 angiotensin receptor expression in rat renal proximal tubule cells. Circ Res. 2006;99:494–500. doi: 10.1161/01.RES.0000240500.96746.ec. [DOI] [PubMed] [Google Scholar]
- 22.Okamoto H, Fujioka Y, Takahashi A, Takahashi T, Taniguchi T, Ishikawa Y, Yokoyama M. Trichostatin A, an inhibitor of histone deacetylase, inhibits smooth muscle cell proliferation via induction of p21(WAF1) J Atheroscler Thromb. 2006;13:183–191. doi: 10.5551/jat.13.183. [DOI] [PubMed] [Google Scholar]
- 23.Parmentier JH, Smelcer P, Pavicevic Z, Basic E, Idrizovic A, Estes A, Malik KU. PKC-zeta mediates norepinephrine-induced phospholipase D activation and cell proliferation in VSMC. Hypertension. 2003;41:794–800. doi: 10.1161/01.HYP.0000047873.76255.0B. [DOI] [PubMed] [Google Scholar]
- 24.Li XC, Campbell DJ, Ohishi M, Yuan S, Zhuo JL. AT1 receptor-activated signaling mediates angiotensin IV-induced renal cortical vasoconstriction in rats. Am J Physiol Renal Physiol. 2006;290:F1024–F1033. doi: 10.1152/ajprenal.00221.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muthusamy T, Dhevika S, Murugesan P, Balasubramanian K. Testosterone deficiency impairs glucose oxidation through defective insulin and its receptor gene expression in target tissues of adult male rats. Life Sci. 2007;81:534–542. doi: 10.1016/j.lfs.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 26.Xapelli S, Bernardino L, Ferreira R, Grade S, Silva AP, Salgado JR, et al. Interaction between neuropeptide Y (NPY) and brain-derived neurotrophic factor in NPY-mediated neuroprotection against excitotoxicity: a role for microglia. Eur J Neurosci. 2008;27:2089–2102. doi: 10.1111/j.1460-9568.2008.06172.x. [DOI] [PubMed] [Google Scholar]
- 27.Johnson EA, Oldfield S, Braksator E, Gonzalez-Cuello A, Couch D, Hall KJ, et al. Agonist-selective mechanisms of mu-opioid receptor desensitization in human embryonic kidney 293 cells. Mol Pharmacol. 2006;70:676–685. doi: 10.1124/mol.106.022376. [DOI] [PubMed] [Google Scholar]
- 28.Bobalova J, Mutafova-Yambolieva VN. Activation of the adenylyl cyclase/protein kinase A pathway facilitates neural release of b-nicotinamide adenine dinucleotide in canine mesenteric artery. Eur J Pharmacol. 2006;536:128–132. doi: 10.1016/j.ejphar.2006.02.049. [DOI] [PubMed] [Google Scholar]
- 29.Curran MP, Robinson DM, Keating GM. Intravenous nicardipine: its use in the short-term treatment of hypertension and various other indications. Drugs. 2006;66:1755–1782. doi: 10.2165/00003495-200666130-00010. [DOI] [PubMed] [Google Scholar]
- 30.Michlig S, Mercier A, Doucet A, Schild L, Horisberger JD, Rossier BC, Firsov D. ERK1/2 controls Na,K-ATPase activity and transepithelial sodium transport in the principal cell of the cortical collecting duct of the mouse kidney. J Biol Chem. 2004;279:51002–51012. doi: 10.1074/jbc.M405674200. [DOI] [PubMed] [Google Scholar]
- 31.Lei J, Mariash CN, Bhargava M, Wattenberg EV, Ingbar DH. T3 increases Na-K-ATPase activity via a MAPK/ERK1/2-dependent pathway in rat adult alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2008;294:L749–L754. doi: 10.1152/ajplung.00335.2007. [DOI] [PubMed] [Google Scholar]
- 32.Inui T, Mori Y, Watanabe M, Takamaki A, Yamaji J, Sohma Y, et al. Physiological role of L-type Ca2+ channels in marginal cells in the stria vascularis of guinea pigs. J Physiol Sci. 2007;57:287–298. doi: 10.2170/physiolsci.RP006807. [DOI] [PubMed] [Google Scholar]
- 33.Rubí B, Ljubicic S, Pournourmohammadi S, Carobbio S, Armanet M, Bartley C, Maechler P. Dopamine D2-like receptors are expressed in pancreatic beta cells and mediate inhibition of insulin secretion. J Biol Chem. 2005;280:36824–36832. doi: 10.1074/jbc.M505560200. [DOI] [PubMed] [Google Scholar]
- 34.Kok P, Roelfsema F, Frölich M, van Pelt J, Stokkel MP, Meinders AE, Pijl H. Activation of dopamine D2 receptors simultaneously ameliorates various metabolic features of obese women. Am J Physiol Endocrinol Metab. 2006;291:E1038–F1043. doi: 10.1152/ajpendo.00567.2005. [DOI] [PubMed] [Google Scholar]
- 35.Segers O, Anckaert E, Gerlo E, Dupont AG, Somers G. Dopamine-sodium relationship in type 2 diabetic patients. Diabetes Res Clin Pract. 1996;34:89–98. doi: 10.1016/s0168-8227(96)01341-1. [DOI] [PubMed] [Google Scholar]
- 36.Shigetomi S, Yamada ZO, Ishii H, Sanada H, Watanabe H, Fukuchi S. Dopaminergic activity and endorenal dopamine synthesis in noninsulin dependent diabetes mellitus. Hypertens Res. 1995;18:S125–S130. doi: 10.1291/hypres.18.supplementi_s125. [DOI] [PubMed] [Google Scholar]
- 37.Tsuchida H, Imai G, Shima Y, Satoh T, Owada S. Mechanism of sodium load-induced hypertension in noninsulin dependent diabetes mellitus model rats: defective dopaminergic system to inhibit Na-K-ATPase activity in renal epithelial cells. Hypertens Res. 2001;24:127–135. doi: 10.1291/hypres.24.127. [DOI] [PubMed] [Google Scholar]
- 38.Banday AA, Asghar M, Hussain T, Lokhandwala MF. Dopamine-mediated inhibition of renal Na,K-ATPase is reduced by insulin. Hypertension. 2003;41:1353–1358. doi: 10.1161/01.HYP.0000069260.11830.CD. [DOI] [PubMed] [Google Scholar]
- 39.Umrani DN, Goyal RK. Fenoldopam treatment improves peripheral insulin sensitivity and renal function in STZ-induced type 2 diabetic rats. Clin Exp Hypertens. 2003;25:221–233. doi: 10.1081/ceh-120020392. [DOI] [PubMed] [Google Scholar]
- 40.Zeng C, Sanada H, Watanabe H, Eisner GM, Felder RA, Jose PA. Functional genomics of the dopaminergic system in hypertension. Physiol Genomics. 2004;19:233–246. doi: 10.1152/physiolgenomics.00127.2004. [DOI] [PubMed] [Google Scholar]
- 41.Hussain T, Lokhandwala MF. Renal dopamine receptor function in hypertension. Hypertension. 1998;32:187–197. doi: 10.1161/01.hyp.32.2.187. [DOI] [PubMed] [Google Scholar]
- 42.Aperia AC. Intrarenal dopamine: a key signal in the interactive regulation of sodium metabolism. Annu Rev Physiol. 2000;62:621–647. doi: 10.1146/annurev.physiol.62.1.621. [DOI] [PubMed] [Google Scholar]
- 43.Felder CC, Blecher M, Jose PA. Dopamine-1-mediated stimulation of phospholipase C activity in rat renal cortical membranes. J Biol Chem. 1989;264:8739–8745. [PubMed] [Google Scholar]
- 44.Tang TS, Bezprozvanny I. Dopamine receptor-mediated Ca2+ signaling in striatal medium spiny neurons. J Biol Chem. 2004;279:42082–42094. doi: 10.1074/jbc.M407389200. [DOI] [PubMed] [Google Scholar]
- 45.Yao LP, Li XX, Yu PY, Xu J, Asico LD, Jose PA. Dopamine D1 receptor and protein kinase C isoforms in spontaneously hypertensive rats. Hypertension. 1998;32:1049–1053. doi: 10.1161/01.hyp.32.6.1049. [DOI] [PubMed] [Google Scholar]
- 46.Pedemonte CH, Efendiev R, Bertorello AM. Inhibition of Na,K-ATPase by dopamine in proximal tubule epithelial cells. Semin Nephrol. 2005;25:322–327. doi: 10.1016/j.semnephrol.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 47.Asghar M, Kansra V, Hussain T, Lokhandwala MF. Hyperphosphorylation of Na-pump contributes to defective renal dopamine response in old rats. J Am Soc Nephrol. 2001;12:226–232. doi: 10.1681/ASN.V122226. [DOI] [PubMed] [Google Scholar]
- 48.Samuel VT, Liu ZX, Wang A, Beddow SA, Geisler JG, Kahn M, et al. Inhibition of protein kinase C epsilon prevents hepatic insulin resistance in nonalcoholic fatty liver disease. J Clin Invest. 2007;117:739–745. doi: 10.1172/JCI30400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yasunari K, Kohno M, Hasuma T, Horio T, Kano H, Yokokawa K, et al. Dopamine as a novel antimigration and antiproliferative factor of vascular smooth muscle cells through dopamine D1-like receptors. Arterioscler Thromb Vasc Biol. 1997;17:3164–3173. doi: 10.1161/01.atv.17.11.3164. [DOI] [PubMed] [Google Scholar]