Abstract
Dopamine is important in the pathogenesis of hypertension because of abnormalities in receptor-mediated regulation of renal sodium transport. Dopamine receptors are classified into D1-like (D1, D5) and D2-like (D2, D3, D4) subtypes, all of which are expressed in the kidney. Mice deficient in specific dopamine receptors have been generated to provide holistic assessment on the varying physiological roles of each receptor subtype. This review examines recent studies on these mutant mouse models and evaluates the impact of individual dopamine receptor subtypes on blood pressure regulation.
Keywords: Dopamine receptor, Hypertension, Knockout mice, Renal function
Introduction
Hypertension, which affects a quarter of all middle-aged adults, is one of the most common risk factors of cardiovascular disease. High blood pressure is a phenotype expressed by several disorders, and results from an aberrant interaction between genetic and environmental factors. Hypertension occurs because of the increased activity of pro-hypertensive systems and/or decreased activity of anti-hyrtensive systems [1–4], one of which is the dopaminergic system [1, 2]. Therefore, a dysfunction in the dopaminergic pathway may be a mechanism in the development of essential hypertension.
Mammalian dopamine receptors are G protein-coupled receptors (GPCRs) belonging to the α group of the rhodopsin family (Class A, Family A, or Family 1) and include five subtypes identified by molecular cloning [5, 6]. The D1 and D5 receptor subtypes are known as D1-like receptors and couple to G proteins Gs and Golf to stimulate adenylyl cyclase activity. The D2, D3, and D4 receptor subtypes are known as D2-like receptors and couple to GI and Go to inhibit adenylyl cyclase activity [1, 2]. D1-like receptors are typically expressed post-synaptically/post-junctionally, whereas D2-like receptors are also expressed pre-synaptically/pre-junctionally. Distinct cells within the same organ may co-express different receptor subtypes, rendering cell-specific assignment of receptor subtypes difficult [2, 7–14]. Dopamine receptor agonists and antagonists have poor selectivity for individual dopamine receptors and, consequently, hinder investigation of the specific roles of each dopamine receptor subtype. To circumvent this problem, gene-targeted mutant mouse models lacking one or more dopamine receptor subtypes [8–13, 15–26] have been generated. Studies using these mouse models have shed light on the specific physiological role of each receptor at the cell, organ, and whole animal level.
The long-term regulation of blood pressure is accomplished by both renal and non-renal mechanisms [1, 2, 27– 31]. Abnormalities in renal epithelial ion transport play important roles in the pathogenesis of essential hypertension, especially in salt-sensitive hypertension. In this review, we summarize the role of the dopamine receptor subtypes in the regulation of blood pressure in humans and rodents.
Renal distribution of the dopamine receptors
D1 receptor
Human
D1 receptor mRNA is expressed in cultured human renal proximal tubule cells [32]. In the human kidney [33], D1 receptor immunostaining is found in the apical and basolateral membrane of the proximal and distal convoluted tubules, medullary thick ascending limb of Henle, macula densa, and cortical collecting duct, but not in the glomerulus and juxtaglomerular cell. D1 receptor protein is also present in the large intrarenal arteries in humans [33], but not in renal veins. Immunoblotting studies have revealed the presence of several forms of D1 receptors, varying in size from 50 to 210 kDa, in rat and human renal proximal tubules [34], which is in agreement with data obtained in brain tissue [35].
Rat
Radioligand binding studies of the rat have shown the presence of D1-like receptors in the renal cortex, but not in glomeruli or medulla [36–41]. However, ligands to both D1-like receptors have similar affinities and, therefore, cannot pharmacologically distinguish the D1 from the D5 receptor. D1 receptor mRNA is expressed in the rat proximal and distal tubule, arteriole, juxtaglomerular apparatus, but not in the glomerulus or medulla [42–44]. It is also expressed in cultured renal proximal tubule cells from the rat [45], mouse (unpublished studies), opossum [46], human [32], and pig [47]. D1 receptor immunostaining is present in the rat apical and basolateral membrane of the proximal and distal convoluted tubules, medullary thick ascending limb of Henle, macula densa, and cortical collecting duct [7, 48]. Most of the D1 receptors in the rat proximal tubules are located in the S3 segment (unpublished observations, 2007). The protein expression of D1 receptors in the inner medullary collecting duct has not been documented, but dopamine does not stimulate cyclic adenosine monophosphate (cAMP) production in this nephron segment [49]. The expression of D1 receptors in the juxtaglomerular cell [7, 44, 48] has been reported in the rat, but not in the human or mouse kidney. Consistent with the results from mRNA studies and adenylyl cyclase activation in response to D1-like receptor agonist [2], D1 receptor proteins are not expressed in the rat glomerulus.
Mouse
The distribution of D1 receptor protein in the renal tubular segments in the mouse ([2], unpublished studies) is similar to that in the human and rat kidney.
D2 receptor
There are two D2 receptor isoforms, D2short and D2long, which have been suggested to function pre- and post-synaptically, respectively [50]. The D2short receptor is expressed more in pre- than in post-synaptic junctions [51, 52].
Rat
D2long mRNA is expressed in renal tubules and glomeruli [53]. Immunoblotting [54] revealed that D2 receptor protein is expressed in rat kidney cortex at a major band of 63 kDa and a minor protein band of 95 kDa (probably a D2 receptor dimer). By indirect immunofluorescence microscopy, D2 receptor immunostaining is found in the rat renal cortical proximal tubule, collecting duct, and the mesangial cell of the glomerulus [54]. The D2 receptor is expressed in the intercalated—but not in the principal cell— of the medullary collecting duct. D2 receptor protein is also expressed in an opossum proximal tubule cell line, which also exhibits distal tubular cell characteristics [55]. There are no reports of renal tubular D2 receptor expression in the human and mouse.
D3 receptor
Human
There are no published studies on the location of the D3 receptor in non-embryonic human kidney.
Rat
Radioligand binding of 7-OH-DPAT, a selective D2 and D3 receptor ligand, has been reported in rat cortical tubules and mesangial cells [56]. D3 receptor mRNA is expressed in the glomeruli, renal cortical and medullary tubular, and intrarenal vascular tissues [53]. In renal proximal tubules, D3 receptor protein is mainly located in the apical and subapical areas, but not in the basolateral membrane [57]. However, results on the expression of the rat D3 receptor along the nephron and blood vessels have not been consistent. Nurnberger et al. [57] found D3 receptor protein to be limited to the proximal tubule, whereas O’Connell et al. [58] found the receptor protein in the glomerulus, renal blood vessel, renal proximal tubule, the apical membrane of the distal convoluted tubule, in the intercalated cell of the cortical collecting duct, and in the macula densa of the rat kidney, but not in juxtaglomerular apparatus. However, D3 receptor mRNA and functional D3 receptors have been described in primary cultures of rat juxtaglomerular cells [59]. The discrepancy between the findings in the kidney in situ and juxtaglomerular cells in vitro suggests that the expression of D3 receptors is conditional (e.g. culturedependent). Two species of D3 receptor (45 and 90 kDa) are expressed in rat renal proximal tubule brush border membranes [60] and cells [61], as detected by immunoblotting studies.
Mouse
D3 receptor immunostaining is observed at the apical membrane of the S1 segment of the proximal convoluted tubule. The staining is also found in the macula densa, cytoplasm of the thick ascending limb of Henle, distal convoluted tubule, and the glomerulus, but not in the medullary collecting duct [62].
D4 receptor
Human
D4 receptor mRNA is expressed in the human kidney [63].
Rat
D4 receptor mRNA is not expressed in the glomerulus or the loop of Henle [63], but the D4 receptor protein is found in cultured rat juxtaglomerular cells [59]. Immunoblotting analysis of the renal cortex has revealed a minor protein band of 49 kDa and a major protein band of 38 kDa [54]. D4 receptor immunostaining is present in the S1 segment of the proximal tubule, the distal convoluted tubule [64], and especially in the cortical and medullary collecting ducts, where it is more prominent at the basolateral area than at the luminal areas. D4 receptor staining is weak in the glomerulus [54].
Mouse
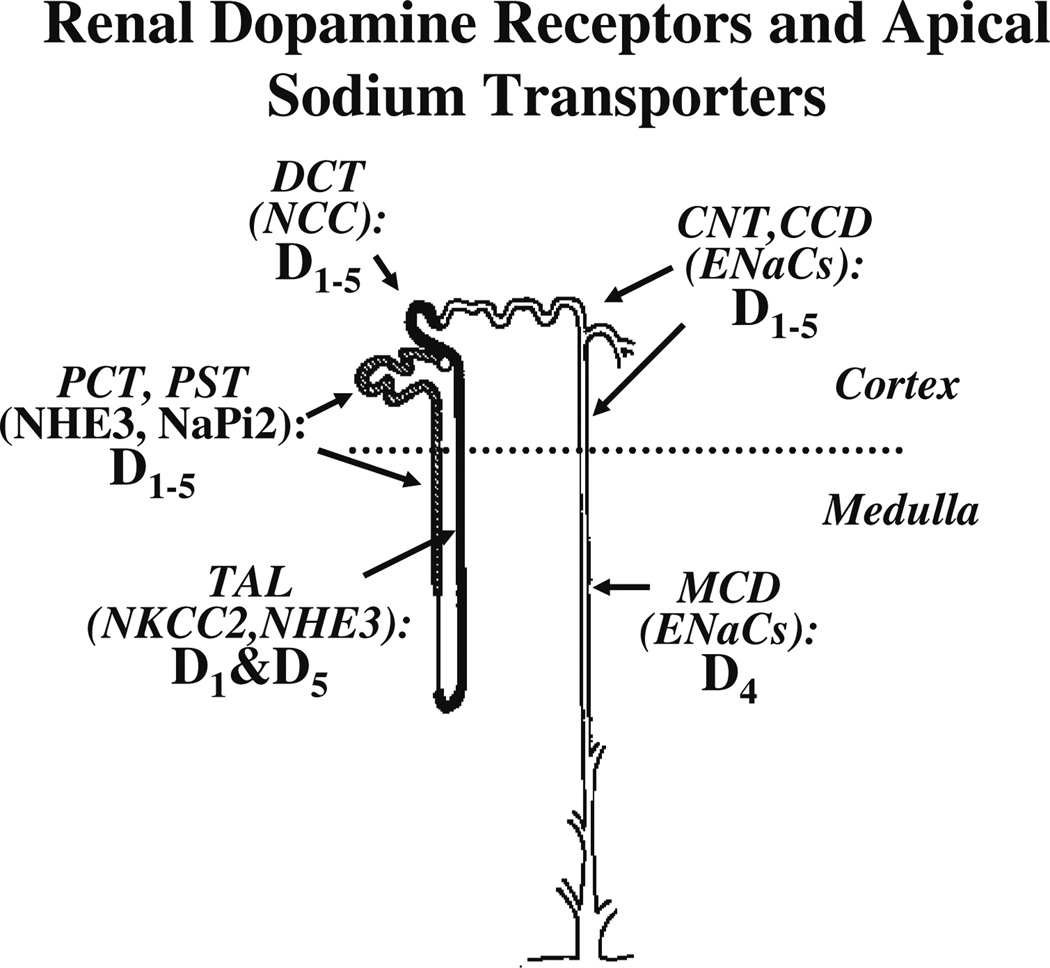
There are no reports on the expression of the D4 receptor in the mouse kidney. Our unpublished studies indicate that the D4 receptor is expressed throughout the nephron, except at the thick ascending limb of Henle (Fig. 1).
Fig. 1.
Distribution of dopamine receptor subtypes (D1–5) and apical sodium transporters in rat tubules. PCT Proximal convoluted tubule, PST proximal straight tubule, TAL thick ascending limb of Henle’s loop, DCT distal convoluted tubule, CNT cortical connecting tubule, CCD cortical collecting duct, MCD medullar collecting duct, NHE3 sodium hydrogen exchanger type 3, NaPi2 sodium phosphate cotransporter type 2, NKCC2 sodium potassium two chloride cotransporter, NCC sodium chloride cotransporter, ENaC epithelial sodium channel
D5 receptor
Human
D5 receptor protein is expressed in cultured proximal tubule cells from the human kidney [65].
Rat
The D5 receptor protein is expressed in the proximal and distal convoluted tubules, and arteriole, but not in the juxtaglomerular cell, glomerulus, or macula densa [7, 66– 68]. The D5 receptor may be expressed preferentially over the D1 receptor in the thick ascending limb of Henle and the cortical collecting duct, while the D1 receptor is preferentially expressed in the proximal tubule [69, 70]. Immortalized rat renal proximal tubule cells express D5 receptors of molecular sizes similar to those of the D1 receptor [71, 72]. The D5 receptor is also expressed in the opossum kidney, but not in the opossum kidney cell line [46].
Mouse
The D5 receptor immunostaining is located in the apical and brush border membrane of mouse renal cortical tubule [72].
Role of the dopamine receptors in renal physiology
The D1-like receptors
Dopamine produced by the renal proximal tubule plays an important role in the regulation of sodium excretion, especially when there is a surfeit of sodium in the system [1, 2]. The D1-like dopamine receptors, D1R and D5R, account for 50–70% of renal sodium excretion [1, 2] during moderate sodium intake. The inhibition of sodium transport exerted by endogenous renal dopamine is due mainly to renal tubular effects [2, 38, 73], in contrast to exogenously administered dopamine and D1-like receptor agonists which regulate sodium excretion by both hemodynamic and tubular mechanisms [1, 2, 14, 38, 74–76].
As aforementioned, there are no available ligands that can distinguish the D1 from the D5 receptor. However, it is likely that the natriuretic effect of dopamine is exerted mainly through the D1 receptor in renal proximal tubule cells since the cAMP production caused by dopamine in these cells is mostly due to D1 receptor activity [77]; the inhibition of renal ion transport by D1-like receptors is, in part, mediated by cAMP. Chronic intrarenal cortical infusion of D1 receptor antisense oligodeoxynucleotides, which selectively decrease D1 receptor protein expression without affecting the D5 receptor expression [78], provides direct evidence of the specific D1 receptor role in the inhibition of renal sodium transport. In this study, the selective renal cortical inhibition of D1 receptor expression decreased sodium excretion during a normal or high salt intake.
The stimulation of D1-like receptors decreases sodium transport in the proximal tubule by inhibiting the activity of several sodium transporters, including the sodium hydrogen exchanger 3 (NHE3, SLC9A3), sodium phosphate cotransporter (NaPi-IIa/SLC34A1 and NaPi-IIc/SLC34A3), and Cl−/ HCO3− exchanger (SLC26A6) at the apical membrane, and electrogenic Na+/HCO3− co-transporter (NBCe1A, SLC4A4), and Na+-K+ ATPase at the basolateral membrane [16, 75, 79–86]. In the medullary thick ascending limb of Henle, D1-like receptors decrease sodium transport [87] by inhibiting Na+–K+ ATPase activity, although dopamine increases NKCC2 (SLC12A1) activity in this nephron segment [88]. We have suggested that the D1-like receptor stimulation of NKCC2 may be important in K+ recycling. Eicosanoids (20-hydroxyeicosatetraenoic acid, 20-HETE) may act synergistically with D1-like receptors to inhibit NKCC2 activity. G protein-dependent, cAMP/protein kinase A (PKA)-dependent, and protein kinase C (PKC)-independent mechanisms are involved in the D1-like receptor inhibition of Na+/Pi2, NHE3, Na/HCO3− co-transporter and Cl−/HCO3− exchanger activity, including their translocation out of the brush border membranes [82, 89–94]. On the other hand, D1-like receptors inhibit Na+–K+ ATPase activity via cAMP/PKA, certain PKC isoforms, and 20-HETE that internalizes Na+–K+ ATPase subunits [2, 83, 95–104]. The mechanism of the inhibitory effect of D1-like receptors on Na+–K+ ATPase is nephron-segment specific. Protein kinase A is involved in D1-like receptor-mediated inhibition of sodium transporters in the cortical collecting duct, while PKC is involved in the proximal convoluted tubule, and the eicosanoids are involved in all nephron segments [1, 95–98, 100–103, 105–107]. D1-like receptors inhibit NHE3 activity via PKA in rat renal proximal tubule cells [91], while PKC may also be involved in opossum kidney cells [107]. Interestingly, the ability of D1-like receptors to stimulate calcium uptake in renal proximal tubule cells, via cAMP and PKC, may play a role in the inhibition of NHE3 activity [108].
In addition to the direct inhibition of sodium excretion by the D1-like receptors per se, these receptors interact with the D2-like receptors to enhance their inhibitory effect. We have reported that the increase in sodium excretion induced by Z-1046, a dopamine receptor agonist with a rank order potency D4 ≥ D3 > D2 > D5 > D1, is blocked by either a D1-like or D2-like receptor antagonist [60]. D2-like receptors may potentiate the inhibitory effect of D1-like receptors on Na+-Pi co-transport, NHE3, and Na+-K+ ATPase activities in renal proximal tubules (see below).
D1 receptor and renal function
The D1 receptor interacts with other systems, such as the renin–angiotensin–aldosterone and the sympathetic nervous systems, to tightly regulate renal and cardiovascular functions. Angiotensin II exerts its effects on angiotensin II type I receptors (AT1R) and opposes the effects of dopamine exerted mainly through the D1-like receptors. D1-like and AT1 receptors have contrasting effects on the generation of second messengers, i.e. D1-like receptors stimulate adenylyl cyclases, while the AT1 receptor inhibits them. Both D1-like and AT1 receptors stimulate phospholipase C activity; however, they stimulate different PKC isoforms [1, 2]. Activation of AT1 receptors stimulates all renal proximal tubular ion-transporting proteins that are inhibited by the D1-like dopamine receptors [106, 109–111]. Thus, the effect of the D1-like receptors on natriuresis is enhanced when angiotensin II production is decreased or when AT1 receptors are blocked [112, 113]. The D1-like receptors can also counteract the renal vasoconstrictor effect of angiotensin II [114]. Activation of the D1-like receptors decreases AT1 receptor expression and the number of angiotensin II binding sites in renal proximal tubule cells from normotensive Wistar–Kyoto (WKY) rats [72, 115]. The use of antisense oligonucleotides against D1 or D5 receptors points to D5R as the major negative regulator of AT1R expression [65]. However, the D1 receptor can also inhibit AT1 receptor function by direct physical interaction [116]. In contrast to AT1 receptors, AT2 receptors participate in the natriuresis mediated by the D1-like receptors [117].
D5 receptor and renal function
Activation of D1-like receptors induces diuresis and natriuresis in WKY rats [1, 2, 38, 39, 117]. Due to the lack of selective D1 and D5 receptor ligands, the relative contribution of D1 and D5 receptors on natriuresis in response to D1-like receptor agonist stimulation is not known. It is presumed that both D1 and D5 receptors are involved because both receptors increase cAMP production and cAMP mediates the D1-like receptor-mediated inhibition of ion transport [77]. However, as aforementioned, cAMP production is increased to a greater extent by the D1 receptor than by the D5 receptor in renal proximal tubule cells [77] and, thus, the D1 receptor, conceivably, mediates most of the natriuretic effect of dopamine [78].
D2-like receptors and renal function
The D2-like receptor family consists of the D2, D3, and D4 dopamine receptors. These receptors variably regulate sodium transport [1, 2]. The D2-like receptors can modulate renal function by regulating dopamine transporter activity [118] and renal dopamine production [119]. Bromocriptine, a D2-like receptor agonist with a tenfold greater affinity for D2 over D3 receptors, stimulates Na+–K+ ATPase activity by increasing its alpha subunit in the plasma membrane [120, 121]. Bromocriptine also increases chloride transport in the medullary thick ascending limb of Henle in rat renal proximal tubules [122]. LY171555, a D2-like receptor agonist with a higher affinity to the D2 receptors, stimulates Na+–K+ ATPase activity in murine fibroblasts heterologously expressing D2Long [123]. Interestingly, dopamine engenders antinatriuresis in sodium-depleted women [124]. Sulpiride, a D2-like receptor antagonist with equal affinity to all D2-like receptors, impairs the dopamine-mediated natriuresis in volume-expanded women [125]. Thus, activation of D2-like receptors results in an antinatriuresis in conditions of volume depletion or in a natriuresis in volume-expanded states. Whether this is a direct effect of the D2-like receptors or due to their interaction with the D1-like receptors remains to be determined. D2-like receptors have also been shown to possess vasodilatory effects, presumably by a pre-junctional inhibition of catecholamine release [2].
D2 receptor and renal function
In the LTK2 cells expressing the D2Long receptor, activation of D2-like receptors decreases cAMP levels, resulting in increased Na+–K+ ATPase activity [123]. The selective agonists for the D2 receptor, bromocriptine, and LY171555 inhibit adenylyl cyclase activities. The resulting decrease in cAMP production/PKA activation probably mediates the stimulation of Na+–K+ ATPase activity [120, 123]. The D2 receptor can inhibit adenylyl cyclase activity even in the absence of adenylyl cyclase V [126], the isoform not expressed in the kidney [127]. D1 receptor-mediated activation of PKC could lead to a D2long-mediated sensitization of adenylyl cyclase VI [128]. In Chinese hamster ovary (CHO) cells heterologously expressing tenfold more D2 receptors than D1 receptors, stimulation of either receptor results in the potentiation of arachidonic acid release compared to those cells expressing either receptor only [129, 130]; arachidonic acid cytochrome P-450 products have been shown to inhibit renal sodium transport [83, 98, 131, 132]. This synergism between D1 and D2 receptors in the production of cAMP, phospholipase C, and arachidonic acid products might explain the observed D2 receptor-mediated natriuretic effect. The D1 and D2 receptors can heterodimerize [133] and interact with each other [130, 134] to increase c-fos [135] and inhibit Na+-K+ ATPase activity [136]. It is conceivable that the D2-like receptors stimulate sodium transport under conditions of “low” sodium intake and inhibit Na+-K+ ATPase activity in renal proximal tubules cells under conditions of “moderate” sodium excess (in concert with D1-like receptors) to synergistically increase sodium excretion [124, 125, 137–139].
D3 receptor and renal function
The ability of D3 receptors to mediate natriuresis is supported by the decreased ability of D3 receptor-deficient mice to excrete an acute saline load [17] and corroborated by several pharmacological studies. The D3 receptor agonists 7-OH-DPAT and PD128907 [140] have been shown to decrease renal sodium transport [141]. Acute intravenous administration of 7-OH-DPAT in salt-resistant Dahl rats increases glomerular filtration rate and sodium and water excretion without affecting blood pressure [141]. PD128907, a selective D3 receptor agonist with a 120-fold selectivity over the D2 receptor, infused directly into the renal artery, dose-dependently increases fractional sodium excretion in WKY rats [but not in spontaneously hypertensive rats (SHR)] fed a normal or high salt diet [142]. Quinerolane, a D2-like receptor agonist with 21-fold selectivity for the D3 over the D2 receptor, opens K+ channels, resulting in hyperpolarization and inhibition of Na+–K+ ATPase activity [143]. The renal effects of D3 receptor activation are mediated by actions on post-junctional glomerular and tubular receptors, and pre-synaptic modulation of norepinephrine release because renal denervation attenuates the effects of 7-OH-DPAT [144].
D4 receptor and renal function
The D4 receptor exerts an antagonistic effect on vasopressin- and aldosterone-dependent water and sodium reabsorption in the cortical collecting duct [145, 146]. In the rabbit cortical collecting duct, the D4 receptor-mediated decrease in sodium transport is exerted mainly at the basolateral membrane despite of greater expression of D4 receptors at the luminal membrane [146].
The distribution of dopamine receptors and apical sodium transporters in rat renal tubules is summarized in Fig. 1.
Aberrant dopamine receptor function and hypertension
D1-like receptors and hypertension
The diuretic and natriuretic responses to administration of D1-like receptor agonists are impaired in rodents with polygenic hypertension (Dahl salt-sensitive rats and SHRs) [1, 2, 38, 75, 147]. The natriuretic effect of parathyroid hormone and cholecystokinin is not impaired in the SHRs, indicating that the impaired D1-like receptor effect is receptor-specific [60]. More importantly, the natriuretic effect of dopamine synthesized in the kidney is also impaired in rodent polygenic hypertension [38, 60, 147]. In human essential hypertensive subjects, the actions of D1-like receptors on renal hemodynamics and distal renal tubule function (see below) are preserved [148, 149], and for this reason, the overall natriuretic effect of administered D1 receptor agonists may be similar or even greater than that in normotensive subjects, even when the ability of D1-like receptor agonists to inhibit proximal tubular reabsorption is impaired [148].
The ability of D1-like receptors to inhibit NHE3, Cl−/ HCO3- exchanger, Na+/HCO3- cotransporter, and Na+–K+ ATPase activities is decreased in rodent genetic hypertension which is due, in part, to decreased production of second messengers (e.g. cAMP, diacylglycerol, eicosanoids) in the renal proximal tubule [16, 37, 71, 75, 86, 92, 93, 98, 150–153] and the thick ascending limb of Henle [75]. The D1-like receptor-mediated stimulation of cAMP production, however, is not impaired in the cortical collecting duct of SHRs [154].
The impaired ability of D1-like receptors to stimulate cAMP production in renal proximal tubules is receptor specific, since the ability of parathyroid hormone to stimulate cAMP production or stimulate G proteins is intact [153, 155, 156] and, at least in young SHRs, β-adrenergic function is also intact [157]. Increased serine phosphorylation and decreased expression of D1 receptors at the plasma membrane [71, 158], are responsible for the impaired ability of D1-like receptors to stimulate cAMP production in renal proximal tubules in genetic hypertension. However, the impairment of D1-like receptor function is organ specific because D1-like receptor function in the brain striatum is intact [37]. The D1-like receptor defect is genetic because it co-segregates with high blood pressure and precedes the onset of hypertension [16, 37, 159, 160].
Deficiency of the expression of D1-like receptors could be involved in human essential hypertension. The human D1 gene locus on chromosome 5 at q35.1 is linked to human essential hypertension [161]. Although there are as yet no reports of the association of polymorphisms of the coding region of the D1 receptor and essential hypertension, a polymorphism, A-48G, in the 5′-untranslated region is associated with essential hypertension in Japanese [162]— but not in Caucasians [163]. However, the D1 receptor A-48G polymorphism has been reported to be associated with albuminuria [164].
The D5 receptor gene locus (chromosome 4p15.1–16.1) is also linked to essential hypertension [165]; the human D5 receptor gene has polymorphisms that code for receptors abnormally coupled to adenylyl cyclase [166]. The role of the D5 receptor in hypertension has been difficult to determine, mostly due to the fact that the D1 and D5 receptors are pharmacologically indistinguishable. As mentioned above, the lack of selective ligands makes it impossible to activate or block only the D1 or D5 receptor in vivo. For this reason, genetic approaches to this problem have been employed by investigators who used gene silencing techniques to inhibit D1 or D5 receptor expression and generate D1 or D5 receptor-deficient mice [9, 12, 16, 22, 23].
D1 receptor mutant mice
D1 receptor knockout (D1−/−) mice were generated by targeted mutagenesis with 7.0 kb of the 129/Sv-derived D1 receptor genomic construct in pPNT [16, 22]. The homozygous D1−/− mice have no binding sites for D1-like receptor antagonists, and in response to D1-like receptor agonist stimulation, they do not increase cAMP accumulation although their response to parathyroid hormone is intact. Systolic, diastolic, and mean arterial pressures are higher in both homozygous and heterozygous mice than in wild-type mice. These data indicate that defective D1 receptor/signal transduction results in increased blood pressure in mice [16]. However, there is no report available on the renal function or the activity of the renin-angiotensin II–aldosterone system in D1−/− mice. It is also unknown whether the hypertension of the null mice is salt-dependent.
D5 receptor-deficient mice
D5 receptor deficient (D5−/−) mice were generated by injecting into C57BL/6 mouse blastocysts 129/SV embryonic stem cells containing the targeting construct generated by ligating the neomycin resistance gene in reverse orientation at the unique SfiI site in the second intracellular loop of the D5 receptor. D5−/− mice are viable, develop normally, and have normal expression of the other dopamine receptors, including the D1 receptor [23, 167].
Congenic D5−/− mice are hypertensive and salt-sensitive. Blood pressure is also increased in D5+/− mice (Table 1, unpublished data). D5−/− mice have a greater epinephrine/ norepinephrine ratio and a greater hypotensive response to the acute administration of an α-adrenergic blocker [23] than their D5+/+ littermates, indicating that increased sympathetic activity plays a role in the elevated blood pressure. However, the percentage decrease in systolic blood pressure, after adrenalectomy, is similar in D5−/− and D5+/+ mice, indicating that central nervous system mechanisms are involved in the hypertension of D5−/− mice [23, 168]. In addition, these central nervous system mechanisms, AT1 receptors, and the increased production of reactive oxygen species (ROS) are also involved in the hypertension of D5−/− mice [14, 66, 72, 169]. However, renal renin protein expression and serum aldosterone level are not altered in D5−/− mice (unpublished data). D5−/− mice also exhibit proteinuria [170].
Table 1.
Phenotypes of dopamine receptor (DR)-deficient mice and renal mechanism(s) involved in the pathogenesis of hypertension
| Mouse genotypes |
D1R |
D5R |
D2R |
D3R |
D4R |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| −/− | +/− | −/− | +/− | −/− | +/− | −/− | +/− | −/− | +/− | |
| Blood pressure on 0.8% NaCl diet | Increased [16] | Increased [16] | Increased [23,168, 169] | Increased # | Increased [24] | Increased [24] | Increased [17] | Increased [17] | Increased [20] | Normal # |
| Blood pressure on 4–6% NaCl diet | Increased [68, 169] | Increased # | Increased # | Increased # | ||||||
| Kidney deformities | None # | None | None # | None # | None # | |||||
| Creatinine clearance | &# | Normal # | Normal [184] | Normal # | Normal [20] | |||||
| Ability to excrete an acute NaCl load | Not impaired # | Not impaired [24] | Not impaired # | Impaired [17] | Not impaired [17] | Not Impaired # | Not impaired # | |||
| Ability to excrete a chronic NaCl load | Not impaired # | Not impaired # | Impaired [186] | Impaired # | ||||||
| Proteinuria Plasma/Kidneyrenin | Yes [170] Normal # | No # | No # Increased [17] | Increased [17] | No # Normal [20] | |||||
| Serum /Urine aldosterone | Normal # | Increased [184] | Normal [185] | Normal# | Normal # | |||||
| Renal AT1R | Increased [72] | Normal # | Increased [17] | Increased [20] | ||||||
Blank cell indicates no report available;
indicates normal blood urea nitrogen (BUN);
indicates unpublished
D2-like receptors and hypertension
Abnormalities of D2-like receptor function have been reported in hypertension. One of the polymorphisms of the D2 receptor, which results in decreased D2 receptor expression, is associated with hypertension [171, 172]. Moreover, transfer of the segment of chromosome 8 containing the D2 receptor gene from the normotensive Brown-Norway rat onto an SHR background decreases blood pressure [173].
The D3 Ser9Gly receptor variant heterologously expressed in CHO cells impairs D3 receptor-mediated inhibition of cAMP production, although these cell do possess the ability to decrease prostaglandin E2 (PGE2) production [174]. However, there is no association between the D3 Ser9Gly, or other D3 receptor gene variants with hypertension [175], despite the chromosome locus of the D3 receptor gene (3q13.3) having been linked to human essential hypertension [176, 177]. Several studies have demonstrated that D3 receptor deficiency may be important in the development of salt-sensitive hypertension. Salt-resistant Dahl rats on a high sodium diet and chronically treated with a highly selective D3 receptor antagonist have increased blood pressure [178]. The natriuretic effect of a D3 agonist is not evident in hypertensive salt-sensitive Dahl rats [178]. The same research group did not find differences in the natriuretic effect of a D3 agonist between WKY rats and SHRs [179]. In this study, however, the D3 agonist is given systemically, and activation of D3 receptors outside the kidney may have had compensating role, thereby obscuring a differential effect on sodium excretion between WKY and SHRs. When the selective D3 receptor agonist, PD128907, is infused directly into the renal artery in WKY rats and SHRs fed a high salt diet, fractional sodium excretion is dose-dependently increased in WKY rats but not in SHRs [142]. The mechanisms causing the impaired D3 receptor function in the kidney in hypertension are not completely known. The stimulatory effect of the D3 receptor agonist, PD128907, on D3 receptor expression is no longer evident in renal proximal tubule cells from the SHRs [180]. It is possible that mechanisms which impair D1 receptor function in SHR also impair D3 receptor—for example, by increased G protein-coupled receptor-related kinase 4 (GRK4) activity [181]—or that impaired D1 receptor function results in impaired synergistic interaction between the D1 and D3 receptor. In addition, the impaired natriuretic function of the D3 receptor in SHRs may, in part, be related to the aberrant D3 and AT1 receptor interaction in renal proximal tubule cells [180]. D3 receptor stimulation decreases AT1 receptor expression in renal proximal tubule cells from the WKY rat, while D3 receptor stimulation increases AT1 receptor expression in the SHR [180].
A locus near the D4 receptor gene (11p15.5) has been linked to hypertension. The most intensively studied D4 receptor polymorphism is a 48-bp repeat located in exon 3 of the D4 receptor gene. This variant codes for a 16-amino acid sequence located in the third intracellular loop of the D4 receptor protein, a region that appears to interact with G proteins and influence intracellular levels of cAMP [182]. The number of repeats at the D4 site varies from two to ten, but the four and seven repeat lengths are the most common in Caucasians in whom the long variant of the D4 gene is associated with increased systolic and diastolic blood pressures [183].
The effects of D4 agonists and antagonists on cardiovascular and renal function in genetically hypertensive rats have not been reported. The D4 receptor protein is increased in the renal cortex—but not in the inner medulla—of SHRs relative to WKY rats [54].
D2 receptor mutant mice
The D2 receptor gene deficient (D2−/−) mice were generated by homologous recombination in embryonic stem cells with deletion of exon 7 and the 5′ half of exon 8. These regions encode the majority of the putative third intracellular loop, the last two transmembrane domains, and the carboxy terminus [24]. D2−/− mice have decreased initiation of movement but otherwise normal basic motor skills without tremor, ataxia, or abnormal stance or posture. D2−/− and D2−/+ mice have higher systolic and diastolic blood pressures than D2 wild-type (D2+/+) mice. The ability of D2−/− mice to excrete an acute saline load is similar to that of D2+/+ mice regardless of the elevated blood pressure, indicating that D2−/− mice have a blunted pressure-natriuresis response. α-Adrenergic blockade decreases blood pressure to a greater extent in D2−/− mice than in D2+/+ mice. Epinephrine excretion is greater in D2−/− mice than in D2+/+ mice, and acute adrenalectomy decreases blood pressure to a similar level in D2−/− and D2+/+ mice. Endothelin B (ETB) receptor expression is greater in D2−/−mice than in D2+/+ mice, and an ETB receptor blocker decreases blood pressure in D2−/− mice but not D2+/+ mice. D2−/− mice may be hypertensive because of enhanced vascular reactivity caused by increased sympathetic and ETB receptor activities [24] as well as by increased ROS production [184]. The D2−/− mice also have increased production of aldosterone, and treatment with a mineralocorticoid receptor blocker normalizes blood pressure in these mice [184]. In contrast, D2+/− mice have normal urinary aldosterone levels [185].
In another strain of D2−/− mice, blood pressure is only increased when the animals are fed a high-salt diet, and this condition is associated with a decrease in renal aromatic L-amino acid decarboxylase (AADC) activity and renal dopamine production [26]; AADC converts L-DOPA (levodopa), which is taken up by renal brush border membranes, to dopamine. Sympathetic activity is not increased in these D2−/− mice. Basal urine flow and sodium excretion are lower in D2−/− mice than in D2+/+ mice. However, administration of dopamine increases urine volume and sodium excretion in D2−/− mice to levels similar to those found in D2+/+ mice [118]. The differences between the two strains of D2−/− mice could be related to differences in their genetic backgrounds.
D3 receptor mutant mice
D3 receptor gene deficient (D3−/−) mice have been generated by targeted mutagenesis. The targeting construct has a 7-kb 129/sv-derived D3 receptor genomic sequence in the GKNeo cassette in antisense orientation at the SalI site in exon 2 [15]. The locomotor phenotype of D3 mutants does not resemble that of D2 mutants. D3−/− mice develop normally, are fertile, and may show a transient locomotor hyperactivity in a novel environment. D3−/− and D3-/+ mice have higher systolic and diastolic blood pressures than their wild-type littermates, either on a mixed C57BL/6 and B129 background [17] or in congenic C57BL/6 background [62]. In contrast, Staudacher et al. reported that D3−/− mice in a congenic C57BL/6 background and on a low, normal, or high salt intake have normal blood pressure [186]. This report has to be interpreted with caution because C57BL/6 mice from Jackson Laboratories develop hypertension when fed a high NaCl diet [187], while those from Taconic [169] do not. The two strains of D3−/− mice, however, have a decreased ability to excrete an acute or a chronic sodium chloride load [17, 186], which may lead to an expansion of the extracellular fluid volume. Thus, disruption of the D3 receptor results in sodium retention, but the hypertension is also related to increased renal renin production. Renal renin activity and AT1 receptor expression are greater in D3−/−than in wild-type mice, and blockade of AT1 receptors decreases systolic blood pressure more effectively in D3−/−mice than in wild-type ones [17]. Urinary aldosterone levels are not altered in D3−/− mice (unpublished data).
D4 receptor mutant mice
D4 receptor deficient (D4−/−) mice backcrossed to C57BL/6J mice for more than six generations have increased systolic and diastolic blood pressures that are increased yet further with increased sodium intake [20]. D4−/− mice do not have altered circulating or renal renin levels [20] although juxtaglomerular cells in culture also express D4 receptors [59]. Serum/urinary aldosterone levels are not different between D4−/− and D4+/+ mice on a normal or increased sodium chloride diet (unpublished data). The expression of AT1 receptors is increased in homogenates of kidney and brain of D4−/− mice relative to that shown by similar homogenates of D4+/+ mice, but AT1 receptor expression in the heart is similar in the two strains. However, the hypotensive effect of a bolus intravenous injection of losartan, an AT1 receptor antagonist, dissipates after 10 min in D4+/+ mice, whereas the effect has been found to persist for more than 45 min in D4−/− mice. Thus, hypertension brought about by the absence of the D4 receptor is mediated, in part, by increased AT1 receptor expression [20]. While on a normal salt diet (unpublished data), the heterozygote mutant D1, D2, D3, and D5 receptor mice have increased blood pressure, while D4+/− mice have normal blood pressure.
Some phenotypes related to renal function in dopamine receptor deficient mice are summarized in Table 1.
Development of renal dopamine
Development of renal dopamine synthesis
Dopamine-mediated natriuresis and renal vascular effects are less in younger animals than in older ones. An inverse correlation between urinary dopamine and age has been reported in adults [188, 189]. The excretion of dopamine is higher in the pediatric population than in elderly subjects [190]. However, urinary dopamine increases postnatally in very low birth weight infants [191, 192]. In rats, intrarenal levels of dopamine have a tendency to increase between 3 and 20 days after birth, but significantly decrease between 20 to 80 days after birth [193]. Urinary excretions of dopamine and its deaminated metabolite 3,4-dihydroxyphe-nylacetic acid (DOPAC) [194], as well as dopamine formation in kidney slices, are markedly decreased in old rats, which have been attributed to decreased uptake of L-DOPA by tubular brush-border membranes [194, 195]. The uptake of L-DOPA is the rate-limiting step in the renal synthesis of dopamine [194]. The activity of aromatic AADC, the enzyme involved in the renal synthesis of dopamine, however, increases with age [195]. The activity of AADC is higher in the adult dog kidney than in 10-day or 2-month-old dogs [196]. In the rat, AADC immunore-activity can be observed in renal tubule cells as early as gestational day 18 and is present in proximal tubules in the outer and inner cortex 5 days after birth. Aromatic L-amino acid decarboxylase mRNA is present in the entire cortex by the sixth post-natal day [193].
Development of renal dopamine receptors
In the rat, renal dopamine D1-like and D2-like receptor densities approach adult values by 3 weeks of age [197, 198]. Rat renal D1 receptors are present by the 15th day of gestational age and increase with maturation [199]. Renal D1 receptors are also expressed in fetal sheep [200]. However, in the sheep, the renal cortical affinity or maximum receptor of D1-like receptors, as determined by radioligand binding, does not change with maturation [201].
In rats, renal D2-like receptor binding does not change within the first 2 weeks of life but increases after 3 weeks [202]. In contrast, renal D2-like receptor binding decreases with age in sheep [201].
Renal effects of dopaminergic agonists
The decreased natriuretic action of dopamine and D1-like receptor agonists in the young and the aged has been attributed to a decreased renal generation of cAMP and inhibition of NHE3 and Na+/K+ ATPase activity in response to D1-like receptor stimulation [198, 201–208]. Even though renal D1-like receptor densities are not different between 3- and 10-week-old rats, there is an impaired ability of the D1-like agonist fenoldopam to stimulate adenylyly cyclase activity in the young [198, 203]. The decreased inhibitory effect of D1-like receptors on NHE activity in young rats is caused, in part, by the increased expression and activity of the G protein subunit G β/γ [208].
In neonatal dogs, pigs, and sheep, the intravenous or intrarenal arterial administration of dopamine alone may not always increase renal blood flow or decrease renal vascular resistance [209–213]. The systemic or intrarenal arterial infusion of dopamine alone, even in low doses, may result in a renal vasoconstriction that is more pronounced in the fetal and neonatal animals [209, 211, 213]. This is due to the occupation of renal α adrenergic receptors that increase in number in the perinatal dog and sheep. A low dose of dopamine injected into the renal artery increases renal blood flow and decreases renal vascular resistance in adult dogs [214], but not in young puppies [215]. Even in the presence of α- and β-adrenergic blockade, dopamine does not increase renal blood flow in 1- to 2-week-old puppies, but it does increase renal blood flow and glomerular filtration rate in older puppies [215]. Similar results are observed following intravenous dopamine administration in young piglets [211]. In spite of the absence of any change in renal blood flow following intrarenal arterial infusion of dopamine, sodium excretion increases, but this effect is less evident in the younger puppy than in the older one [215]. Dopamine also increases sodium excretion in the fetal sheep without increasing renal blood flow [213, 216]. Thus, in the very young, the natriuretic effect of dopamine is probably due mainly to tubular factors.
The effects of the D2-like receptor agonist are not well studied. However, in the developing animal, selective D2-like receptor agonists increase glomerular filtration rate to a similar extent as that found in younger and older rats [217]. In premature infants, in contrast to the findings in adults, a predominantly D2-like receptor antagonist increases urine flow, sodium excretion, and osmolar clearance, suggesting that D2-like receptor effects may predominate in the human infant [218].
Ability to excrete a salt load in human neonates
Pre-term infants have a decreased ability to retain sodium, but both full-term and pre-term infants have a decreased ability to excrete a salt load [219]. In the pediatric age group, a decrease in sodium intake results in an increase in urinary dopamine, while in adults, a high salt intake results in an increase in urinary dopamine [212, 218]. Low doses of dopamine, however, can induce a natriuresis in preterm infants, but it is not known if the magnitude of the response is different from that reported in the adult [220]. Deficits in renal dopamine function may be responsible for the diminished ability of the aged kidney to excrete a salt load [221]. Whether this is also true in the neonate has not been determined.
Overall summary
In summary, dopamine receptors are important players in the regulation of renal function and blood pressure. Each dopamine receptor subtype exerts a unique regulatory function and interacts with other GPCRs, especially those related to angiotensin II. All dopamine receptor subtypes are present in the kidney, showing a distinct distribution along the nephron. Renal sodium transport is regulated by all subtypes, either directly or indirectly by interaction with other GPCRs, to maintain sodium balance. Deletion of any of the dopamine receptor subtypes results in elevated blood pressure and impaired pressure natriuresis in the mutant mice. Abnormalities of dopamine receptors in their genes/ proteins or in their regulation of renal function play an important role in the pathogenesis of hypertension.
Acknowledgment
This work is supported in part by grants from the National Institutes of Health, HL23081, DK39308, HL074940, and HL68686.
Contributor Information
Xiaoyan Wang, Department of Pediatrics, Georgetown University Medical Center, Washington, DC, USA.
Van Anthony M. Villar, Department of Pediatrics, Georgetown University Medical Center, Washington, DC, USA
Ines Armando, Department of Pediatrics, Georgetown University Medical Center, Washington, DC, USA.
Gilbert M. Eisner, Department of Medicine, Georgetown University Medical Center, Washington, DC, USA
Robin A. Felder, Department of Pathology, University of Virginia Health Sciences Center, Charlottesville, VA, USA
Pedro A. Jose, Email: pjose01@georgetown.edu, Department of Pediatrics, Georgetown University Medical Center, Washington, DC, USA; Department of Physiology and Biophysics, Georgetown University Medical Center, Washington, DC, USA.
References
- 1.Hussain T, Lokhandwala MF. Renal dopamine receptors and hypertension. Exp Biol Med (Maywood) 2003;228:134–142. doi: 10.1177/153537020322800202. [DOI] [PubMed] [Google Scholar]
- 2.Zeng C, Sanada H, Watanabe H, Eisner GM, Felder RA, Jose PA. Functional genomics of the dopaminergic system in hypertension. Physiol Genomics. 2004;19:233–246. doi: 10.1152/physiolgenomics.00127.2004. [DOI] [PubMed] [Google Scholar]
- 3.Dickson ME, Sigmund CD. Genetic basis of hypertension: revisiting angiotensinogen. Hypertension. 2006;48:14–20. doi: 10.1161/01.HYP.0000227932.13687.60. [DOI] [PubMed] [Google Scholar]
- 4.Osborn JW, Jacob F, Guzman P. A neural set point for the long-term control of arterial pressure: beyond the arterial baroreceptor reflex. Am J Physiol Regul Integr Comp Physiol. 2005;288:R846–R855. doi: 10.1152/ajpregu.00474.2004. [DOI] [PubMed] [Google Scholar]
- 5.Premont RT, Gainetdinov RR. Physiological roles of G protein-coupled receptor kinases and arrestins. Annu Rev Physiol. 2007;69:511–534. doi: 10.1146/annurev.physiol.69.022405.154731. [DOI] [PubMed] [Google Scholar]
- 6.Schioth HB, Fredriksson R. The GRAFS classification system of G-protein coupled receptors in comparative perspective. Gen Comp Endocrinol. 2005;142:94–101. doi: 10.1016/j.ygcen.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 7.Amenta F, Barili P, Bronzetti E, Ricci A. Dopamine D1-like receptor subtypes in the rat kidney: a microanatomical study. Clin Exp Hypertens. 1999;21:17–23. doi: 10.3109/10641969909068645. [DOI] [PubMed] [Google Scholar]
- 8.Glickstein SB, Schmauss C. Dopamine receptor functions: lessons from knockout mice [corrected] Pharmacol Ther. 2001;91:63–83. doi: 10.1016/s0163-7258(01)00145-0. [DOI] [PubMed] [Google Scholar]
- 9.Holmes A, Lachowicz JE, Sibley DR. Phenotypic analysis of dopamine receptor knockout mice; recent insights into the functional specificity of dopamine receptor subtypes. Neuropharmacology. 2004;47:1117–1134. doi: 10.1016/j.neuropharm.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 10.Joseph JD, Wang YM, Miles PR, Budygin EA, Picetti R, Gainetdinov RR, Caron MG, Wightman RM. Dopamine autoreceptor regulation of release and uptake in mouse brain slices in the absence of D3 receptors. Neuroscience. 2002;112:39–49. doi: 10.1016/s0306-4522(02)00067-2. [DOI] [PubMed] [Google Scholar]
- 11.Schmitz Y, Schmauss C, Sulzer D. Altered dopamine release and uptake kinetics in mice lacking D2 receptors. J Neurosci. 2002;22:8002–8009. doi: 10.1523/JNEUROSCI.22-18-08002.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sibley DR. New insights into dopaminergic receptor function using antisense and genetically altered animals. Annu Rev Pharmacol Toxicol. 1999;39:313–341. doi: 10.1146/annurev.pharmtox.39.1.313. [DOI] [PubMed] [Google Scholar]
- 13.Zapata A, Shippenberg TS. Lack of functional D2 receptors prevents the effects of the D3-preferring agonist (+)−PD 128907 on dialysate dopamine levels. Neuropharmacology. 2005;48:43–50. doi: 10.1016/j.neuropharm.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 14.Zeng C, Felder RA, Jose PA. A new approach for treatment of hypertension: modifying D1 dopamine receptor function. Cardiovasc Hematol Agents Med Chem. 2006;4:369–377. doi: 10.2174/187152506778520727. [DOI] [PubMed] [Google Scholar]
- 15.Accili D, Fishburn CS, Drago J, Steiner H, Lachowicz JE, Park BH, Gauda EB, Lee EJ, Cool MH, Sibley DR, Gerfen CR, Westphal H, Fuchs S. A targeted mutation of the D3 dopamine receptor gene is associated with hyperactivity in mice. Proc Natl Acad Sci USA. 1996;93:1945–1949. doi: 10.1073/pnas.93.5.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Albrecht FE, Drago J, Felder RA, Printz MP, Eisner GM, Robillard JE, Sibley DR, Westphal HJ, Jose PA. Role of the D1A dopamine receptor in the pathogenesis of genetic hypertension. J Clin Invest. 1996;97:2283–2288. doi: 10.1172/JCI118670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asico LD, Ladines C, Fuchs S, Accili D, Carey RM, Semeraro C, Pocchiari F, Felder RA, Eisner GM, Jose PA. Disruption of the dopamine D3 receptor gene produces renin-dependent hypertension. J Clin Invest. 1998;102:493–498. doi: 10.1172/JCI3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Azdad K, Piet R, Poulain DA, Oliet SH. Dopamine D4 receptor-mediated presynaptic inhibition of GABAergic transmission in the rat supraoptic nucleus. J Neurophysiol. 2003;90:559–565. doi: 10.1152/jn.00226.2003. [DOI] [PubMed] [Google Scholar]
- 19.Baik JH, Picetti R, Saiardi A, Thiriet G, Dierich A, Depaulis A, Le Meur M, Borrelli E. Parkinsonian-like locomotor impairment in mice lacking dopamine D2 receptors. Nature. 1995;377:424–428. doi: 10.1038/377424a0. [DOI] [PubMed] [Google Scholar]
- 20.Bek MJ, Wang X, Asico LD, Jones JE, Zheng S, Li X, Eisner GM, Grandy DK, Carey RM, Soares-da-Silva P, Jose PA. Angiotensin-II type 1 receptor-mediated hypertension in D4 dopamine receptor-deficient mice. Hypertension. 2006;47:288–295. doi: 10.1161/01.HYP.0000198427.96225.36. [DOI] [PubMed] [Google Scholar]
- 21.Dickinson SD, Sabeti J, Larson GA, Giardina K, Rubinstein M, Kelly MA, Grandy DK, Low MJ, Gerhardt GA, Zahniser NR. Dopamine D2 receptor-deficient mice exhibit decreased dopamine transporter function but no changes in dopamine release in dorsal striatum. J Neurochem. 1999;72:148–156. doi: 10.1046/j.1471-4159.1999.0720148.x. [DOI] [PubMed] [Google Scholar]
- 22.Drago J, Gerfen CR, Lachowicz JE, Steiner H, Hollon TR, Love PE, Ooi GT, Grinberg A, Lee EJ, Huang SP, Bartlett PF, Jose PA, Sibley DR, Westphal H. Altered striatal function in a mutant mouse lacking D1A dopamine receptors. Proc Natl Acad Sci USA. 1994;91:12564–12568. doi: 10.1073/pnas.91.26.12564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hollon TR, Bek MJ, Lachowicz JE, Ariano MA, Mezey E, Ramachandran R, Wersinger SR, Soares-da-Silva P, Liu ZF, Grinberg A, Drago J, Young WS, 3rd, Westphal H, Jose PA, Sibley DR. Mice lacking D5 dopamine receptors have increased sympathetic tone and are hypertensive. J Neurosci. 2002;22:10801–10810. doi: 10.1523/JNEUROSCI.22-24-10801.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li XX, Bek M, Asico LD, Yang Z, Grandy DK, Goldstein DS, Rubinstein M, Eisner GM, Jose PA. Adrenergic and endothelin B receptor-dependent hypertension in dopamine receptor type-2 knockout mice. Hypertension. 2001;38:303–308. doi: 10.1161/01.hyp.38.3.303. [DOI] [PubMed] [Google Scholar]
- 25.Rubinstein M, Phillips TJ, Bunzow JR, Falzone TL, Dziewczapolski G, Zhang G, Fang Y, Larson JL, McDougall JA, Chester JA, Saez C, Pugsley TA, Gershanik O, Low MJ, Grandy DK. Mice lacking dopamine D4 receptors are supersensitive to ethanol, cocaine, and methamphetamine. Cell. 1997;90:991–1001. doi: 10.1016/s0092-8674(00)80365-7. [DOI] [PubMed] [Google Scholar]
- 26.Ueda A, Ozono R, Oshima T, Yano A, Kambe M, Teranishi Y, Katsuki M, Chayama K. Disruption of the type 2 dopamine receptor gene causes a sodium-dependent increase in blood pressure in mice. Am J Hypertens. 2003;16:853–858. doi: 10.1016/s0895-7061(03)01013-6. [DOI] [PubMed] [Google Scholar]
- 27.Crowley SD, Gurley SB, Herrera MJ, Ruiz P, Griffiths R, Kumar AP, Kim HS, Smithies O, Le TH, Coffman TM. Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc Natl Acad Sci USA. 2006;103:17985–17990. doi: 10.1073/pnas.0605545103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Granger JP, Schnackenberg CG. Renal mechanisms of angiotensin II-induced hypertension. Semin Nephrol. 2000;20:417–425. [PubMed] [Google Scholar]
- 29.Nasjletti A. The role of eicosanoids in angiotensin-dependent hypertension. Hypertension. 1998;31:194–200. doi: 10.1161/01.hyp.31.1.194. [DOI] [PubMed] [Google Scholar]
- 30.Raizada MK, Der Sarkissian S. Potential of gene therapy strategy for the treatment of hypertension. Hypertension. 2006;47:6–9. doi: 10.1161/01.HYP.0000196685.91424.01. [DOI] [PubMed] [Google Scholar]
- 31.Touyz RM. Intracellular mechanisms involved in vascular remodelling of resistance arteries in hypertension: role of angiotensin II. Exp Physiol. 2005;90:449–455. doi: 10.1113/expphysiol.2005.030080. [DOI] [PubMed] [Google Scholar]
- 32.Watanabe H, Xu J, Bengra C, Jose PA, Felder RA. Desensitization of human renal D1 dopamine receptors by G protein-coupled receptor kinase 4. Kidney Int. 2002;62:790–798. doi: 10.1046/j.1523-1755.2002.00525.x. [DOI] [PubMed] [Google Scholar]
- 33.Ozono R, O’Connell DP, Wang ZQ, Moore AF, Sanada H, Felder RA, Carey RM. Localization of the dopamine D1 receptor protein in the human heart and kidney. Hypertension. 1997;30:725–729. doi: 10.1161/01.hyp.30.3.725. [DOI] [PubMed] [Google Scholar]
- 34.Yu P, Yang Z, Jones JE, Wang Z, Owens SA, Mueller SC, Felder RA, Jose PA. D1 dopamine receptor signaling involves caveolin-2 in HEK-293 cells. Kidney Int. 2004;66:2167–2180. doi: 10.1111/j.1523-1755.2004.66007.x. [DOI] [PubMed] [Google Scholar]
- 35.Jarvie KR, Booth G, Brown EM, Niznik HB. Glycoprotein nature of dopamine D1 receptors in the brain and parathyroid gland. Mol Pharmacol. 1989;36:566–574. [PubMed] [Google Scholar]
- 36.Felder RA, Jose PA. Dopamine1 receptors in rat kidneys identified with 125I-Sch 23982. Am J Physiol. 1988;255:F970–F976. doi: 10.1152/ajprenal.1988.255.5.F970. [DOI] [PubMed] [Google Scholar]
- 37.Felder RA, Kinoshita S, Ohbu K, Mouradian MM, Sibley DR, Monsma FJ, Jr, Minowa T, Minowa MT, Canessa LM, Jose PA. Organ specificity of the dopamine1 receptor/adenylyl cyclase coupling defect in spontaneously hypertensive rats. Am J Physiol. 1993;264:R726–R732. doi: 10.1152/ajpregu.1993.264.4.R726. [DOI] [PubMed] [Google Scholar]
- 38.Felder RA, Seikaly MG, Cody P, Eisner GM, Jose PA. Attenuated renal response to dopaminergic drugs in spontaneously hypertensive rats. Hypertension. 1990;15:560–569. doi: 10.1161/01.hyp.15.6.560. [DOI] [PubMed] [Google Scholar]
- 39.Hedge SS, Ricci A, Amenta F, Lokhandwala MF. Evidence from functional and autoradiographic studies for the presence of tubular dopamine-1 receptors and their involvement in the renal effects of fenoldopam. J Pharmacol Exp Ther. 1989;251:1237–1245. [PubMed] [Google Scholar]
- 40.Huo T, Healy DP. Autoradiographic localization of dopamine DA1 receptors in rat kidney with [3H]Sch 23390. Am J Physiol. 1989;257:F414–F423. doi: 10.1152/ajprenal.1989.257.3.F414. [DOI] [PubMed] [Google Scholar]
- 41.Takemoto F, Satoh T, Cohen HT, Katz AI. Localization of dopamine-1 receptors along the microdissected rat nephron. Pflugers Arch. 1991;419:243–248. doi: 10.1007/BF00371102. [DOI] [PubMed] [Google Scholar]
- 42.O’Connell DP, Aherne AM, Lane E, Felder RA, Carey RM. Detection of dopamine receptor D1A subtype-specific mRNA in rat kidney by in situ amplification. Am J Physiol. 1998;274:F232–F241. doi: 10.1152/ajprenal.1998.274.1.F232. [DOI] [PubMed] [Google Scholar]
- 43.Yamaguchi I, Jose PA, Mouradian MM, Canessa LM, Monsma FJ, Jr, Sibley DR, Takeyasu K, Felder RA. Expression of dopamine D1A receptor gene in proximal tubule of rat kidneys. Am J Physiol. 1993;264:F280–F285. doi: 10.1152/ajprenal.1993.264.2.F280. [DOI] [PubMed] [Google Scholar]
- 44.Yamaguchi I, Yao L, Sanada H, Ozono R, Mouradian MM, Jose PA, Carey RM, Felder RA. Dopamine D1A receptors and renin release in rat juxtaglomerular cells. Hypertension. 1997;29:962–968. doi: 10.1161/01.hyp.29.4.962. [DOI] [PubMed] [Google Scholar]
- 45.Zeng C, Luo Y, Asico LD, Hopfer U, Eisner GM, +Felder RA, Jose PA. Perturbation of D1 dopamine and AT1 receptor interaction in spontaneously hypertensive rats. Hypertension. 2003;42:787–792. doi: 10.1161/01.HYP.0000085334.34963.4E. [DOI] [PubMed] [Google Scholar]
- 46.Nash SR, Godinot N, Caron MG. Cloning and characterization of the opossum kidney cell D1 dopamine receptor: expression of identical D1A and D1B dopamine receptor mRNAs in opossum kidney and brain. Mol Pharmacol. 1993;44:918–925. [PubMed] [Google Scholar]
- 47.Kruse MS, Adachi S, Scott L, Holtback U, Greengard P, Aperia A, Brismar H. Recruitment of renal dopamine 1 receptors requires an intact microtubulin network. Pflugers Arch. 2003;445:534–539. doi: 10.1007/s00424-002-0899-5. [DOI] [PubMed] [Google Scholar]
- 48.O’Connell DP, Botkin SJ, Ramos SI, Sibley DR, Ariano MA, Felder RA, Carey RM. Localization of dopamine D1A receptor protein in rat kidneys. Am J Physiol. 1995;268:F1185–F1197. doi: 10.1152/ajprenal.1995.268.6.F1185. [DOI] [PubMed] [Google Scholar]
- 49.Maeda Y, Terada Y, Nonoguchi H, Knepper MA. Hormone and autacoid regulation of cAMP production in rat IMCD subsegments. Am J Physiol. 1992;263:F319–F327. doi: 10.1152/ajprenal.1992.263.2.F319. [DOI] [PubMed] [Google Scholar]
- 50.Monsma FJ, Jr, McVittie LD, Gerfen CR, Mahan LC, Sibley DR. Multiple D2 dopamine receptors produced by alternative RNA splicing. Nature. 1989;342:926–929. doi: 10.1038/342926a0. [DOI] [PubMed] [Google Scholar]
- 51.Jomphe C, Tiberi M, Trudeau LE. Expression of D2 receptor isoforms in cultured neurons reveals equipotent autoreceptor function. Neuropharmacology. 2006;50:595–605. doi: 10.1016/j.neuropharm.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 52.Lindgren N, Usiello A, Goiny M, Haycock J, Erbs E, Greengard P, Hokfelt T, Borrelli E, Fisone G. Distinct roles of dopamine D2L and D2S receptor isoforms in the regulation of protein phosphorylation at presynaptic and postsynaptic sites. Proc Natl Acad Sci USA. 2003;100:4305–4309. doi: 10.1073/pnas.0730708100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gao DQ, Canessa LM, Mouradian MM, Jose PA. Expression of the D2 subfamily of dopamine receptor genes in kidney. Am J Physiol. 1994;266:F646–F650. doi: 10.1152/ajprenal.1994.266.4.F646. [DOI] [PubMed] [Google Scholar]
- 54.Shin Y, Kumar U, Patel Y, Patel SC, Sidhu A. Differential expression of D2-like dopamine receptors in the kidney of the spontaneously hypertensive rat. J Hypertens. 2003;21:199–207. doi: 10.1097/00004872-200301000-00030. [DOI] [PubMed] [Google Scholar]
- 55.Narkar VA, Hussain T, Pedemonte C, Lokhandwala MF. Dopamine D2 receptor activation causes mitogenesis via p44/42 mitogen-activated protein kinase in opossum kidney cells. J Am Soc Nephrol. 2001;12:1844–1852. doi: 10.1681/ASN.V1291844. [DOI] [PubMed] [Google Scholar]
- 56.Barili P, Ricci A, Baldoni E, Mignini F, Amenta F. Pharmacological characterizsation and autoradiographic localisation of a putative dopamine D3 receptor in the rat kidney. Eur J Pharmacol. 1997;338:89–95. doi: 10.1016/s0014-2999(97)01303-4. [DOI] [PubMed] [Google Scholar]
- 57.Nurnberger A, Rabiger M, Mack A, Diaz J, Sokoloff P, Muhlbauer B, Luippold G. Subapical localization of the dopamine D3 receptor in proximal tubules of the rat kidney. J Histochem Cytochem. 2004;52:1647–1655. doi: 10.1369/jhc.4A6359.2004. [DOI] [PubMed] [Google Scholar]
- 58.O’Connell DP, Vaughan CJ, Aherne AM, Botkin SJ, Wang ZQ, Felder RA, Carey RM. Expression of the dopamine D3 receptor protein in the rat kidney. Hypertension. 1998;32:886–895. doi: 10.1161/01.hyp.32.5.886. [DOI] [PubMed] [Google Scholar]
- 59.Sanada H, Yao L, Jose PA, Carey RM, Felder RA. Dopamine D3 receptors in rat juxtaglomerular cells. Clin Exp Hypertens. 1997;19:93–105. doi: 10.3109/10641969709080807. [DOI] [PubMed] [Google Scholar]
- 60.Ladines CA, Zeng C, Asico LD, Sun X, Pocchiari F, Semeraro C, Pisegna J, Wank S, Yamaguchi I, Eisner GM, Jose PA. Impaired renal D1-like and D2-like dopamine receptor interaction in the spontaneously hypertensive rat. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1071–R1078. doi: 10.1152/ajpregu.2001.281.4.R1071. [DOI] [PubMed] [Google Scholar]
- 61.Zeng C, Asico LD, Wang X, Hopfer U, Eisner GM, Felder RA, Jose PA. Angiotensin II regulation of AT1 and D3 dopamine receptors in renal proximal tubule cells of spontaneously hypertensive rats. Hypertension. 2003;41:724–729. doi: 10.1161/01.HYP.0000047880.78462.0E. [DOI] [PubMed] [Google Scholar]
- 62.Wang X, Armando I, Asico LD, Jones JE, Escano CS, Jose PA. Hypertension in dopamine receptor D3 deficient mice is associated with increased Na transporters in kidney (abstract) J Am Soc Nephrol. 2005;16:350A. [Google Scholar]
- 63.Matsumoto T, Ozono R, Sasaki N, Oshima T, Matsuura H, Kajiyama G, Carey Matsumoto M, Hidaka K, Tada S, Tasaki Y, Yamaguchi T. Full-length cDNA cloning and distribution of human dopamine D4 receptor. Mol Brain Res. 1995;29:157–162. doi: 10.1016/0169-328x(94)00245-a. [DOI] [PubMed] [Google Scholar]
- 64.Ricci A, Marchal-Victorion S, Bronzetti E, Parini A, Amenta F, Tayebati SK. Dopamine D4 receptor expression in rat kidney: evidence for pre- and postjunctional localization. Histochem Cytochem. 2002;50:1091–1096. doi: 10.1177/002215540205000811. [DOI] [PubMed] [Google Scholar]
- 65.Gildea JJ, Wang X, Jose PA, Felder RA. D5 dopamine receptor inhibition of the angiotensin type I receptor. Hypertension. 2008;51:1–7. doi: 10.1161/HYPERTENSIONAHA.107.100099. [DOI] [PubMed] [Google Scholar]
- 66.Yang Z, Asico LD, Yu P, Wang Z, Jones JE, Bai RK, Sibley DR, Felder RA, Jose PA. D5 dopamine receptor regulation of phospholipase D. Am J Physiol Heart Circ Physiol. 2005;288:H55–H61. doi: 10.1152/ajpheart.00627.2004. [DOI] [PubMed] [Google Scholar]
- 67.Yao L, Ruan X, Arendshorst WJ, Jose PA. Dopamine receptor subtype (D1A and D1B) expression in rat renal microvessels (abstract) Pediatr Res. 1995;37:374A. [Google Scholar]
- 68.Zheng S, Yu P, Zeng C, Wang Z, Yang Z, Andrews PM, Felder RA, Jose PA. Gα12- and Gα13-protein subunit linkage of D5 dopamine receptors in the nephron. Hypertension. 2003;41:604–610. doi: 10.1161/01.HYP.0000057422.75590.D7. [DOI] [PubMed] [Google Scholar]
- 69.Amenta F. Light microscope autoradiography of peripheral dopamine receptor subtypes. Clin Exp Hypertens. 1997;19:27–41. doi: 10.3109/10641969709080802. [DOI] [PubMed] [Google Scholar]
- 70.Yao LP, Huque E, Baraniuk J, Carey RM, Felder RA, Jose PA. Dopamine receptor subtype expression (D1A and D1B) in rat nephron segments (abstract) J Invest Med. 1996;44:305A. [Google Scholar]
- 71.Yu P, Asico LD, Luo Y, Andrews P, Eisner GM, Hopfer U, Felder RA, Jose PA. D1 dopamine receptor hyper-phosphorylation in renal proximal tubules in hypertension. Kidney Int. 2006;70:1072–1079. doi: 10.1038/sj.ki.5001708. [DOI] [PubMed] [Google Scholar]
- 72.Zeng C, Yang Z, Wang Z, Jones J, Wang X, Altea J, Mangrum AJ, Hopfer U, Sibley DR, Eisner GM, Felder RA, Jose PA. Interaction of AT1 and D5 dopamine receptors in renal proximal tubule cells. Hypertension. 2005;45:804–810. doi: 10.1161/01.HYP.0000155212.33212.99. [DOI] [PubMed] [Google Scholar]
- 73.Carey RM, Siragy HM, Ragsdale NV, Howell NL, Felder RA, Peach MJ, Chevalier RL. Dopamine-1 and dopamine-2 mechanisms in the control of renal function. Am J Hypertens. 1990;3:59S–63S. doi: 10.1093/ajh/3.6.59s. [DOI] [PubMed] [Google Scholar]
- 74.Felder RA, Wang X, Gildea J, Bengra C, Sasaki M, Zeng C, Jones JE, Zheng W, Asico LD, Jose PA. Human renal angiotensin type 1 receptor regulation by the D1 dopamine receptor (abstract) Hypertension. 2003;42:438A. [Google Scholar]
- 75.Nishi A, Eklöf AC, Bertorello AM, Aperia A. Dopamine regulation of renal Na+,K+-ATPase activity is lacking in Dahl salt-sensitive rats. Hypertension. 1993;21:767–771. doi: 10.1161/01.hyp.21.6.767. [DOI] [PubMed] [Google Scholar]
- 76.Teirstein PS, Price MJ, Mathur VS, Madyoon H, Sawhney N, Baim DS. Differential effects between intravenous and targeted renal delivery of fenoldopam on renal function and blood pressure in patients undergoing cardiac catheterization. Am J Cardiol. 2006;97:1076–1081. doi: 10.1016/j.amjcard.2005.10.053. [DOI] [PubMed] [Google Scholar]
- 77.Sanada H, Xu J, Watanabe H, Jose PA, Felder RA. Differential expression and regulation of dopamine-1 (D-1) and dopamine-5 (D-5) receptor function in human kidney (abstract) Am J Hypertens. 2000;13:156A. [Google Scholar]
- 78.Wang ZQ, Felder RA, Carey RM. Selective inhibition of the renal dopamine subtype D 1A receptor induces antinatriuresis in conscious rats. Hypertension. 1999;33:504–510. doi: 10.1161/01.hyp.33.1.504. [DOI] [PubMed] [Google Scholar]
- 79.Aperia A, Bertorello A, Seri I. Dopamine causes inhibition of Na+-K+-ATPase activity in rat proximal convoluted tubule segments. Am J Physiol. 1987;252:F39–F45. doi: 10.1152/ajprenal.1987.252.1.F39. [DOI] [PubMed] [Google Scholar]
- 80.Asghar M, Hussain T, Lokhandwala MF. Overexpression of PKC-βI and -δ contributes to higher PKC activity in the proximal tubules of old Fischer 344 rats. Am J Physiol Renal Physiol. 2003;285:F1100–F1107. doi: 10.1152/ajprenal.00198.2003. [DOI] [PubMed] [Google Scholar]
- 81.Bacic D, Kaissling B, McLeroy P, Zou L, Baum M, Moe OW. Dopamine acutely decreases apical membrane Na/H exchanger NHE3 protein in mouse renal proximal tubule. Kidney Int. 2003;64:2133–2141. doi: 10.1046/j.1523-1755.2003.00308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Banday AA, Hussain T, Lokhandwala MF. Renal dopamine D1 receptor dysfunction is acquired and not inherited in obese Zucker rats. Am J Physiol Renal Physiol. 2004;287:F109–F116. doi: 10.1152/ajprenal.00396.2003. [DOI] [PubMed] [Google Scholar]
- 83.Grider JS, Ott CE, Jackson BA. Dopamine D1 receptor-dependent inhibition of NaCl transport in the rat thick ascending limb: mechanism of action. Eur J Pharmacol. 2003;473:185–190. doi: 10.1016/s0014-2999(03)01965-4. [DOI] [PubMed] [Google Scholar]
- 84.Hu MC, Fan L, Crowder LA, Karim-Jimenez Z, Murer H, Moe OW. Dopamine acutely stimulates Na+/H+ exchanger (NHE3) endocytosis via clathrin-coated vesicles: dependence on protein kinase A-mediated NHE3 phosphorylation. J Biol Chem. 2001;276:26906–26915. doi: 10.1074/jbc.M011338200. [DOI] [PubMed] [Google Scholar]
- 85.Gomes P, Soares-da-Silva P. Dopamine acutely decreases type 3 Na(+)/H(+) exchanger activity in renal OK cells through the activation of protein kinases A and C signalling cascades. Eur J Pharmacol. 2004;488:51–59. doi: 10.1016/j.ejphar.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 86.Xu J, Li XX, Albrecht FE, Hopfer U, Carey RM, Jose PA. D1 receptor, Gsα, and Na+/H+ exchanger interactions in the kidney in hypertension. Hypertension. 2000;36:395–399. doi: 10.1161/01.hyp.36.3.395. [DOI] [PubMed] [Google Scholar]
- 87.Fryckstedt J, Meister B, Aperia A. Control of electrolyte transport in the kidney through a dopamine- and cAMP-regulated phosphoprotein, DARPP-32. J Auton Pharmacol. 1992;12:183–189. doi: 10.1111/j.1474-8673.1992.tb00376.x. [DOI] [PubMed] [Google Scholar]
- 88.Aoki Y, Albrecht FE, Bergman KR, Jose PA. Stimulation of Na+-K+-2Cl- cotransport in rat medullary thick ascending limb by dopamine. Am J Physiol. 1996;271:R1561–R1567. doi: 10.1152/ajpregu.1996.271.6.R1561. [DOI] [PubMed] [Google Scholar]
- 89.Bacic D, Capuano P, Baum M, Zhang J, Stange G, Biber J, Kaissling B, Moe OW, Wagner CA, Murer H. Activation of dopamine D1-like receptors induces acute internalization of the renal Na+/phosphate cotransporter NaPi-IIa in mouse kidney and OK cells. Am J Physiol Renal Physiol. 2005;288:F740–F747. doi: 10.1152/ajprenal.00380.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Baines AD, Drangova R. Does dopamine use several signal pathways to inhibit Na-Pi transport in OK cells? J Am Soc Nephrol. 1998;9:1604–1612. doi: 10.1681/ASN.V991604. [DOI] [PubMed] [Google Scholar]
- 91.Felder CC, Campbell T, Albrecht F, Jose PA. Dopamine inhibits Na+-H+ exchanger activity in renal BBMV by stimulation of adenylate cyclase. Am J Physiol. 1990;259:F297–F303. doi: 10.1152/ajprenal.1990.259.2.F297. [DOI] [PubMed] [Google Scholar]
- 92.Kunimi M, Seki G, Hara C, Taniguchi S, Uwatoko S, Goto A, Kimura S, Fujita T. Dopamine inhibits renal Na+:HCO3 − cotransporter in rabbits and normotensive rats but not in spontaneously hypertensive rats. Kidney Int. 2000;57:534–543. doi: 10.1046/j.1523-1755.2000.00873.x. [DOI] [PubMed] [Google Scholar]
- 93.Pedrosa R, Jose PA, Soares-Da-Silva P. Defective D1-like receptor-mediated inhibition of Cl/HCO3 exchanger in immortalized SHR proximal tubular epithelial cells. Am J Physiol Renal Physiol. 2004;286:F1120–F1126. doi: 10.1152/ajprenal.00433.2003. [DOI] [PubMed] [Google Scholar]
- 94.Kocinsky HS, Girardi AC, Biemesderfer D, Nguyen T, Mentone S, Orlowski J, Aronson PS. Use of phospho-specific antibodies to determine the phosphorylation of endogenous Na+/ H+ exchanger NHE3 at PKA consensus sites. Am J Physiol Renal Physiol. 2005;289:F249–F258. doi: 10.1152/ajprenal.00082.2004. [DOI] [PubMed] [Google Scholar]
- 95.Asghar M, Hussain T, Lokhandwala MF. Activation of dopamine D1-like receptor causes phosphorylation of alpha1-subunit of Na+,K+-ATPase in rat renal proximal tubules. Eur J Pharmacol. 2001;411:61–66. doi: 10.1016/s0014-2999(00)00896-7. [DOI] [PubMed] [Google Scholar]
- 96.Horiuchi A, Takeyasu K, Mouradian MM, Jose PA, Felder RA. D1A dopamine receptor stimulation inhibits Na+-K+ ATPase activity through protein kinase A. Mol Pharmacol. 1993;43:281–285. [PubMed] [Google Scholar]
- 97.Efendiev R, Cinelli AR, Leibiger IB, Bertorello AM, Pedemonte CH. FRET analysis reveals a critical conformational change within the Na,K-ATPase alpha1 subunit N-terminus during GPCR-dependent endocytosis. FEBS Lett. 2006;580:5067–5070. doi: 10.1016/j.febslet.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 98.Hussain T, Lokhandwala MF. Altered arachidonic acid metabolism contributes to the failure of dopamine to inhibit Na+, K+-ATPase in kidney of spontaneously hypertensive rats. Clin Exp Hypertens. 1996;18:963–974. doi: 10.3109/10641969609097911. [DOI] [PubMed] [Google Scholar]
- 99.Kirchheimer C, Mendez CF, Acquier A, Nowicki S. Role of 20-HETE in D1/D2 dopamine receptor synergism resulting in the inhibition of Na+-K+-ATPase activity in the proximal tubule. Am J Physiol Renal Physiol. 2007;292:F1435–F1442. doi: 10.1152/ajprenal.00176.2006. [DOI] [PubMed] [Google Scholar]
- 100.Li D, Aperia A, Celsi G, da Cruze Silva EF, Greengard P, Meister B. Protein phosphatase-1 in the kidney: evidence for a role in the regulation of medullary Na+-K+-ATPase. Am J Physiol. 1995;269:F673–F680. doi: 10.1152/ajprenal.1995.269.5.F673. [DOI] [PubMed] [Google Scholar]
- 101.Nowicki S, Kruse MS, Brismar H, Aperia A. Dopamine-induced translocation of protein kinase C isoforms visualized in renal epithelial cells. Am J Physiol Cell Physiol. 2000;279:C1812–C1818. doi: 10.1152/ajpcell.2000.279.6.C1812. [DOI] [PubMed] [Google Scholar]
- 102.Ominato Satoh MT, Katz AI. Regulation of Na-KATPase activity in the proximal tubule: role of the protein kinase C pathway and of eicosanoids. J Membr Biol. 1996;152:235–243. doi: 10.1007/s002329900101. [DOI] [PubMed] [Google Scholar]
- 103.Satoh T, Cohen HT, Katz AI. Intracellular signaling in the regulation of renal Na-K-ATPase. I. Role of cyclic AMP and phospholipase A2. J Clin Invest. 1992;89:1496–1500. doi: 10.1172/JCI115740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hussain T, Abdul-Wahab R, Kotak DK, Lokhandwala MF. Bromocriptine regulates angiotensin II response on sodium pump in proximal tubules. Hypertension. 1998;32:1054–1059. doi: 10.1161/01.hyp.32.6.1054. [DOI] [PubMed] [Google Scholar]
- 105.Satoh T, Cohen HT, Katz AI. Different mechanisms of renal Na-K-ATPase regulation by protein kinases in proximal and distal nephron. Am J Physiol. 1993;265:F399–F405. doi: 10.1152/ajprenal.1993.265.3.F399. [DOI] [PubMed] [Google Scholar]
- 106.Yao LP, Li XX, Yu PY, Xu J, Asico LD, Jose PA. Dopamine D1 receptor and protein kinase C isoforms in spontaneously hypertensive rats. Hypertension. 1998;32:1049–1053. doi: 10.1161/01.hyp.32.6.1049. [DOI] [PubMed] [Google Scholar]
- 107.Pedrosa R, Gomes P, Soares-da-Silva P. Distinct signalling cascades downstream to Gsalpha coupled dopamine D1-like NHE3 inhibition in rat and opossum renal epithelial cells. Cell Physiol Biochem. 2004;14:91–100. doi: 10.1159/000076930. [DOI] [PubMed] [Google Scholar]
- 108.Han JY, Heo JS, Lee YJ, Lee JH, Taub M, Han HJ. Dopamine stimulates 45Ca2+ uptake through cAMP, PLC/PKC, and MAPKs in renal proximal tubule cells. J Cell Physiol. 2007;211:486–494. doi: 10.1002/jcp.20956. [DOI] [PubMed] [Google Scholar]
- 109.Sheikh-Hamad D, Wang YP, Jo OD, Yanagawa N. Dopamine antagonizes the actions of angiotensin II in renal brush-border membrane. Am J Physiol. 1993;264:F737–F743. doi: 10.1152/ajprenal.1993.264.4.F737. [DOI] [PubMed] [Google Scholar]
- 110.Gesek FA, Schoolwerth AC. Hormonal interactions with the proximal Na+-H+ exchanger. Am J Physiol. 1990;258:F514–F521. doi: 10.1152/ajprenal.1990.258.3.F514. [DOI] [PubMed] [Google Scholar]
- 111.Efendiev R, Budu CE, Cinelli AR, Bertorello AM, Pedemonte CH. Intracellular Na+ regulates dopamine and angiotensin II receptors availability at the plasma membrane and their cellular responses in renal epithelia. J Biol Chem. 2003;278:28719–28726. doi: 10.1074/jbc.M303741200. [DOI] [PubMed] [Google Scholar]
- 112.Chen C, Lokhandwala MF. Potentiation by enalaprilat of fenoldopam-evoked natriuresis is due to blockade of intrarenal production of angiotensin-II in rats. Naunyn Schmiedebergs Arch Pharmacol. 1995;352:194–200. doi: 10.1007/BF00176774. [DOI] [PubMed] [Google Scholar]
- 113.Clark KL, Hilditch A, Robertson MJ, Drew GM. Effects of dopamine DA1-receptor blockade and angiotensin converting enzyme inhibition on the renal actions of fenoldopam in the anaesthetized dog. J Hypertens. 1991;9:1143–50. [PubMed] [Google Scholar]
- 114.Chatziantoniou C, Ruan X, Arendshorst WJ. Interactions of cAMP-mediated vasodilators with angiotensin II in rat kidney during hypertension. Am J Physiol. 1993;265:F845–F852. doi: 10.1152/ajprenal.1993.265.6.F845. [DOI] [PubMed] [Google Scholar]
- 115.Cheng HF, Becker BN, Harris RC. Dopamine decreases expression of type-1 angiotensin II receptors in renal proximal tubule. J Clin Invest. 1996;97:2745–2752. doi: 10.1172/JCI118729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zeng C, Luo Y, Asico LD, Hopfer U, Eisner GM, Felder RA, Jose PA. Perturbation of D1 dopamine and AT1 receptor interaction in spontaneously hypertensive rats. Hypertension. 2003;42:787–792. doi: 10.1161/01.HYP.0000085334.34963.4E. [DOI] [PubMed] [Google Scholar]
- 117.Salomone LJ, Howell NL, McGrath HE, Kemp BA, Keller SR, Gildea JJ, Felder RA, Carey RM. Intrarenal dopamine D1-like receptor stimulation induces natriuresis via an angiotensin type-2 receptor mechanism. Hypertension. 2007;49:155–161. doi: 10.1161/01.HYP.0000251881.89610.ee. [DOI] [PubMed] [Google Scholar]
- 118.Lee FJ, Pei L, Moszczynska A, Vukusic B, Fletcher PJ, Liu F. Dopamine transporter cell surface localization facilitated by a direct interaction with the dopamine D2 receptor. EMBO J. 2007;26:2127–2136. doi: 10.1038/sj.emboj.7601656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ozono R, Ueda A, Oishi Y, Yano A, Kambe M, Katsuki M, Oshima T. Dopamine D2 receptor modulates sodium handling via local production of dopamine in the kidney. J Cardiovasc Pharmacol. 2003;42:S75–S79. doi: 10.1097/00005344-200312001-00017. [DOI] [PubMed] [Google Scholar]
- 120.Hussain T, Abdul-Wahab R, Lokhandwala MF. Bromocriptine stimulates Na+, K+-ATPase in renal proximal tubules via the cAMP pathway. Eur J Pharmacol. 1997;321:259–263. doi: 10.1016/s0014-2999(97)00039-3. [DOI] [PubMed] [Google Scholar]
- 121.Narkar VA, Hussain T, Lokhandwala MF. Activation of D2-like receptors causes recruitment of tyrosine-phosphorylated NKA alpha 1-subunits in kidney. Am J Physiol Renal Physiol. 2002;283:F1290–F1295. doi: 10.1152/ajprenal.00039.2002. [DOI] [PubMed] [Google Scholar]
- 122.Grider J, Kilpatrick E, Ott C, Jackson B. Effect of dopamine on NaCl transport in the medullary thick ascending limb of the rat. Eur J Pharmacol. 1998;342:281–284. doi: 10.1016/s0014-2999(97)01564-1. [DOI] [PubMed] [Google Scholar]
- 123.Yamaguchi I, Walk SF, Jose PA, Felder RA. Dopamine D2L receptors stimulate Na+/K+ ATPase activity in murine LTK2 cells. Mol Pharmacol. 1996;49:373–378. [PubMed] [Google Scholar]
- 124.Agnoli GC, Borgatti R, Cacciari M, Garutti C, Ikonomu E, Lenzi P. Antagonistic effects of sulpiride-racemic and enantiomerson renal response to low-dose dopamine infusion in normal women. Nephron. 1989;51:491–498. doi: 10.1159/000185382. [DOI] [PubMed] [Google Scholar]
- 125.Agnoli GC, Cacciari M, Garutti C, Ikonomu E, Lenzi P, Marchetti G. Effects of extracellular fluid volume changes on renal response to low-dose dopamine infusion in normal women. Clin Physiol. 1987;7:465–479. doi: 10.1111/j.1475-097x.1987.tb00189.x. [DOI] [PubMed] [Google Scholar]
- 126.Robinson SW, Caron MG. Selective inhibition of adenylyl cyclase type V by the dopamine D3 receptor. Mol Pharmacol. 1997;52:508–14. doi: 10.1124/mol.52.3.508. [DOI] [PubMed] [Google Scholar]
- 127.Bek MJ, Zheng S, Xu J, Yamaguchi I, Asico LD, Sun XG, Jose PA. Differential expression of adenylyl cyclases in the rat nephron. Kidney Int. 2001;60:890–899. doi: 10.1046/j.1523-1755.2001.060003890.x. [DOI] [PubMed] [Google Scholar]
- 128.Beazely MA, Watts VJ. Activation of a novel PKC isoform synergistically enhances D2L dopamine receptor-mediated sensitization of adenylate cyclase type 6. Cell Signal. 2005;17:647–653. doi: 10.1016/j.cellsig.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 129.Kanterman RY, Mahan LC, Briley EM, Monsma FJ, Jr, Sibley DR, Axelrod J, Felder CC. Transfected D2 dopamine receptors mediate the potentiation of arachidonic acid release in Chinese hamster ovary cells. Mol Pharmacol. 1991;39:364–369. [PubMed] [Google Scholar]
- 130.Piomelli D, Pilon C, Giros B, Sokoloff P, Martres MP, Schwartz JC. Dopamine activation of the arachidonic acid cascade as a basis for D1/D2 receptor synergism. Nature. 1991;353:164–167. doi: 10.1038/353164a0. [DOI] [PubMed] [Google Scholar]
- 131.Aperia A, Eklof AC, Holtback U, Nowicki S, Sundelof M, Greengard P. The renal dopamine system. Adv Pharmacol. 1998;42:870–873. doi: 10.1016/s1054-3589(08)60885-6. [DOI] [PubMed] [Google Scholar]
- 132.Sarkis A, Lopez B, Roman RJ. Role of 20-hydroxyeico-satetraenoic acid and epoxy- eicosatrienoic acids in hypertension. Curr Opin Nephrol Hypertens. 2004;13:205–214. doi: 10.1097/00041552-200403000-00009. [DOI] [PubMed] [Google Scholar]
- 133.Dziedzicka-Wasylewska M, Faron-Gorecka A, Andrecka J, Polit A, Kusmider M, Wasylewski Z. Fluorescence studies reveal heterodimerization of dopamine D1 and D2 receptors in the plasma membrane. Biochemistry. 2006;45:8751–8759. doi: 10.1021/bi060702m. [DOI] [PubMed] [Google Scholar]
- 134.Pollack A. Coactivation of D1 and D2 dopamine receptors: in marriage, a case of his, hers, and theirs. Sci STKE. 2004;2004:e50. doi: 10.1126/stke.2552004pe50. [DOI] [PubMed] [Google Scholar]
- 135.Cho DI, Quan W, Oak MH, Choi HJ, Lee KY, Kim KM. Functional interaction between dopamine receptor subtypes for the regulation of c-fos expression. Biochem Biophys Res Commun. 2007;357:1113–1138. doi: 10.1016/j.bbrc.2007.04.066. [DOI] [PubMed] [Google Scholar]
- 136.Bertorello AM, Hopfield JF, Aperia A, Greengard P. Inhibition by dopamine of (Na+-K+)ATPase activity in neostriatal neurons through D1 and D2 dopamine receptor synergism. Nature. 1990;347:386–388. doi: 10.1038/347386a0. [DOI] [PubMed] [Google Scholar]
- 137.Bertorello A, Aperia A. Inhibition of proximal tubule Na+-K+-ATPase activity requires simultaneous activation of DA1 and DA2 receptors. Am J Physiol. 1990;259:F924–F928. doi: 10.1152/ajprenal.1990.259.6.F924. [DOI] [PubMed] [Google Scholar]
- 138.Eklof AC. The natriuretic response to a dopamine DA1 agonist requires endogenous activation of dopamine DA2 receptors. Acta Physiol Scand. 1997;160:311–314. doi: 10.1046/j.1365-201X.1997.00166.x. [DOI] [PubMed] [Google Scholar]
- 139.Jose PA, Asico LD, Eisner GM, Pocchiari F, Semeraro C, Felder RA. Effects of costimulation of dopamine D1- and D2-like receptors on renal function. Am J Physiol. 1998;275:R986–R994. doi: 10.1152/ajpregu.1998.275.4.R986. [DOI] [PubMed] [Google Scholar]
- 140.Levesque D. Aminotetralin drugs and D3 receptor functions. What may partially selective D3 receptor ligands tell us about dopamine D3 receptor functions? Biochem Pharmacol. 1996;52:511–518. doi: 10.1016/0006-2952(96)00239-0. [DOI] [PubMed] [Google Scholar]
- 141.Luippold G, Kuster E, Joos TO, Muhlbauer B. Dopamine D3 receptor activation modulates renal function in anesthetized rats. Naunyn Schmiedebergs Arch Pharmacol. 1998;358:690–693. doi: 10.1007/pl00005314. [DOI] [PubMed] [Google Scholar]
- 142.Zeng C, Asico LD, Zheng S, Hopfer U, Eisner GM, Felder RA, Jose PA. Role of Gα12- and Gα13-protein subunit linkage of D3 dopamine receptors in the impaired natriuretic effect of D3 dopamine receptors in SHRs (abstract) Am J Hypertens. 2004;17:96A. [Google Scholar]
- 143.Gomes P, Soares-Da-Silva P. D2-like receptor-mediated inhibition of Na+-K+-ATPase activity is dependent on the opening of K+ channels. Am J Physiol Renal Physiol. 2002;283:F114–F123. doi: 10.1152/ajprenal.00244.2001. [DOI] [PubMed] [Google Scholar]
- 144.Muhlbauer B, Kuster E, Luippold G. Dopamine D3 receptors in the rat kidney: role in physiology and pathophysiology. Acta Physiol Scand. 2000;168:219–223. doi: 10.1046/j.1365-201x.2000.00665.x. [DOI] [PubMed] [Google Scholar]
- 145.Li L, Schafer JA. Dopamine inhibits vasopressindependent cAMP production in the rat cortical collecting duct. Am J Physiol. 1998;275:F62–F67. doi: 10.1152/ajprenal.1998.275.1.F62. [DOI] [PubMed] [Google Scholar]
- 146.Saito O, Ando Y, Kusano E, Asano Y. Functional characterization of basolateral and luminal dopamine receptors in rabbit CCD. Am J Physiol Renal Physiol. 2001;281:F114–F122. doi: 10.1152/ajprenal.2001.281.1.F114. [DOI] [PubMed] [Google Scholar]
- 147.Chen CJ, Lokhandwala MF. An impairment of renal tubular DA-1 receptor function as the causative factor for diminished natriuresis to volume expansion in spontaneously hypertensive rats. Clin Exp Hypertens. 1992;14:615–628. doi: 10.3109/10641969209036211. [DOI] [PubMed] [Google Scholar]
- 148.O’Connell DP, Ragsdale NV, Boyd DG, Felder RA, Carey RM. Differential human renal tubular responses to dopamine type 1 receptor stimulation are determined by blood pressure status. Hypertension. 1997;29:115–122. doi: 10.1161/01.hyp.29.1.115. [DOI] [PubMed] [Google Scholar]
- 149.Nielsen CB, Pedersen EB. Abnormal distal tubular sodium reabsorption during dopamine infusion in patients with essential hypertension evaluated by the lithium clearance methods. Clin Nephrol. 1997;47:304–309. [PubMed] [Google Scholar]
- 150.Chen C, Beach RE, Lokhandwala MF. Dopamine fails to inhibit renal tubular sodium pump in hypertensive rats. Hypertension. 1993;21:364–372. doi: 10.1161/01.hyp.21.3.364. [DOI] [PubMed] [Google Scholar]
- 151.Felder CC, Jose PA, Axelrod J. The dopamine-1 agonist, SKF 82526, stimulates phospholipase-C activity independent of adenylate cyclase. J Pharmacol Exp Ther. 1989;248:171–175. [PubMed] [Google Scholar]
- 152.Albrecht FE, Xu J, Moe OW, Hopfer U, Simonds WF, Orlowski J, Jose PA. Regulation of NHE3 activity by G protein subunits in renal brush-border membranes. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1064–1073. doi: 10.1152/ajpregu.2000.278.4.R1064. [DOI] [PubMed] [Google Scholar]
- 153.Kinoshita S, Sidhu A, Felder RA. Defective dopamine-1 receptor adenylate cyclase coupling in the proximal convoluted tubule from the spontaneously hypertensive rat. J Clin Invest. 1989;84:1849–1856. doi: 10.1172/JCI114371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ohbu K, Felder RA. Nephron specificity of dopamine receptor-adenylyl cyclase defect in spontaneous hypertension. Am J Physiol. 1993;264:F274–F279. doi: 10.1152/ajprenal.1993.264.2.F274. [DOI] [PubMed] [Google Scholar]
- 155.Hussain T, Lokhandwala MF. Renal dopamine DA1 receptor coupling with GS and Gq/11 proteins in spontaneously hypertensive rats. Am J Physiol. 1997;272:F339–F346. doi: 10.1152/ajprenal.1997.272.3.F339. [DOI] [PubMed] [Google Scholar]
- 156.Jose PA, Eisner GM, Felder RA. Dopaminergic defect in hypertension. Pediatr Nephrol. 1993;7:859–864. doi: 10.1007/BF01213374. [DOI] [PubMed] [Google Scholar]
- 157.Michel MC, Siepmann F, Buscher R, Philipp T, Brodde OE. Ontogenesis of sympathetic responsiveness in spontaneously hypertensive rats. I. Renal alpha 1-, alpha 2-, and beta-adrenergic receptors and their signaling. Hypertension. 1993;22:169–177. doi: 10.1161/01.hyp.22.2.169. [DOI] [PubMed] [Google Scholar]
- 158.Sanada H, Jose PA, Hazen-Martin D, Yu PY, Xu J, Bruns DE, Phipps J, Carey RM, Felder RA. Dopamine-1 receptor coupling defect in renal proximal tubule cells in hypertension. Hypertension. 1999;33:1036–1042. doi: 10.1161/01.hyp.33.4.1036. [DOI] [PubMed] [Google Scholar]
- 159.Lao YS, Hendley ED, Felder RA, Jose PA. Elevated renal cortical calmodulin- dependent protein kinase activity and blood pressure. Clin Exp Hypertens. 2002;24:289–300. doi: 10.1081/ceh-120004232. [DOI] [PubMed] [Google Scholar]
- 160.Ohbu K, Hendley ED, Yamaguchi I, Felder RA. Renal dopamine-1 receptors in hypertensive inbred rat strains with and without hyperactivity. Hypertension. 1993;21:485–490. doi: 10.1161/01.hyp.21.4.485. [DOI] [PubMed] [Google Scholar]
- 161.Krushkal J, Xiong M, Ferrell R, Sing CF, Turner ST, Boerwinkle E. Linkage and association of adrenergic and dopamine receptor genes in the distal portion of the long arm of chromosome 5 with systolic blood pressure variation. Hum Mol Genet. 1998;7:1379–1383. doi: 10.1093/hmg/7.9.1379. [DOI] [PubMed] [Google Scholar]
- 162.Sato M, Soma M, Nakayama T, Kanmatsuse K. Dopamine D1 receptor gene polymorphism is associated with essential hypertension. Hypertension. 2000;36:183–186. doi: 10.1161/01.hyp.36.2.183. [DOI] [PubMed] [Google Scholar]
- 163.Beige J, Bellmann A, Sharma AM, Gessner R. Ethnic origin determines the impact of genetic variants in dopamine receptor gene (DRD1) concerning essential hypertension. Am J Hypertens. 2004;17:1184–1187. doi: 10.1016/j.amjhyper.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 164.Rao F, Wessel J, Wen G, Zhang L, Rana BK, Kennedy BP, Greenwood TA, Salem RM, Chen Y, Khandrika S, Hamilton BA, Smith DW, Holstein-Rathlou NH, Ziegler MG, Schork NJ, O’Connor DT. Renal albumin excretion: twin studies identify influences of heredity, environment, and adrenergic pathway polymorphism. Hypertension. 2007;49:1015–1031. doi: 10.1161/HYPERTENSIONAHA.106.081679. [DOI] [PubMed] [Google Scholar]
- 165.Allayee H, de Bruin TW, Michelle Dominguez K, Cheng LS, Ipp E, Cantor RM, Krass KL, Keulen ET, Aouizerat BE, Lusis AJ, Rotter JI. Genome scan for blood pressure in Dutch dyslipidemic families reveals linkage to a locus on chromosome 4p. Hypertension. 2001;38:773–778. doi: 10.1161/hy1001.092617. [DOI] [PubMed] [Google Scholar]
- 166.Cravchik A, Gejman PV. Functional analysis of the human D5 dopamine receptor missense and nonsense variants: differences in dopamine binding affinities. Pharmacogenetics. 1999;9:199–206. [PubMed] [Google Scholar]
- 167.Holmes A, Hollon TR, Gleason TC, Liu Z, Dreiling J, Sibley DR, Crawley JN. Behavioral characterization of dopamine D5 receptor null mutant mice. Behav Neurosci. 2001;115:1129–1144. [PubMed] [Google Scholar]
- 168.Zeng C, Yang Z, Asico LD, Jose PA. Regulation of blood pressure by D5 dopamine receptors. Cardiovasc Hematol Agents Med Chem. 2007;5:241–248. doi: 10.2174/187152507781058708. [DOI] [PubMed] [Google Scholar]
- 169.Yang Z, Asico LD, Yu P, Wang Z, Jones JE, Escano CS, Wang X, Quinn MT, Sibley DR, Romero GG, Felder RA, Jose PA. D5 dopamine receptor regulation of reactive oxygen species production, NADPH oxidase, and blood pressure. Am J Physiol Regul Integr Comp Physiol. 2006;290:R96–R104. doi: 10.1152/ajpregu.00434.2005. [DOI] [PubMed] [Google Scholar]
- 170.Wang Z, Armando I, Asico LD, Escano C, Wang X, Lu Q, Felder RA, Schnackenberg CG, Sibley DR, Eisner GM, Jose PA. The elevated blood pressure of human GRK4gamma A142V transgenic mice is not associated with increased ROS production. Am J Physiol Heart Circ Physiol. 2007;292:H2083–H2092. doi: 10.1152/ajpheart.00944.2006. [DOI] [PubMed] [Google Scholar]
- 171.Thomas GN, Critchley JA, Tomlinson B, Cockram CS, Chan JC. Relationships between the taqI polymorphism of the dopamine D2 receptor and blood pressure in hyperglycaemic and normoglycaemic Chinese subjects. Clin Endocrinol (Oxf) 2001;55:605–611. doi: 10.1046/j.1365-2265.2001.01404.x. [DOI] [PubMed] [Google Scholar]
- 172.Thomas GN, Tomlinson B, Critchley JA. Modulation of blood pressure and obesity with the dopamine D2 receptor gene TaqI polymorphism. Hypertension. 2000;36:177–182. doi: 10.1161/01.hyp.36.2.177. [DOI] [PubMed] [Google Scholar]
- 173.Kren V, Pravenec M, Lu S, Krenova D, Wang JM, Wang N, Merriouns T, Wong A, St Lezin E, Lau D, Szpirer C, Szpirer J, Kurtz TW. Genetic isolation of a region of chromosome 8 that exerts major effects on blood pressure and cardiac mass in the spontaneously hypertensive rat. J Clin Invest. 1997;99:577–581. doi: 10.1172/JCI119198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hellstrand M, Danielsen EA, Steen VM, Ekman A, Eriksson E, Nilsson CL. The ser9gly SNP in the dopamine D3 receptor causes a shift from cAMP related to PGE2 related signal transduction mechanisms in transfected CHO cells. J Med Genet. 2004;41:867–871. doi: 10.1136/jmg.2004.020941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Soma M, Nakayama K, Rahmutula D, Uwabo J, Sato M, Kunimoto M, Aoi N, Kosuge K, Kanmatsuse K. Ser9Gly polymorphism in the dopamine D3 receptor gene is not associated with essential hypertension in the Japanese. Med Sci Monit. 2002;8:CR1–CR4. [PubMed] [Google Scholar]
- 176.James K, Weitzel LR, Engelman CD, Zerbe G, Norris JM Framingham Heart Study. Genome scan linkage results for longitudinal systolic blood pressure phenotypes in subjects from the Framingham Heart Study. BMC Genet. 2003;4(Suppl 1):S83. doi: 10.1186/1471-2156-4-S1-S83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Rice T, Rankinen T, Province MA, Chagnon YC, Perusse L, Borecki IB, Bouchard C, Rao DC. Genome-wide linkage analysis of systolic and diastolic blood pressure: the Quebec Family Study. Circulation. 2000;102:1956–1963. doi: 10.1161/01.cir.102.16.1956. [DOI] [PubMed] [Google Scholar]
- 178.Luippold G, Zimmermann C, Mai M, Kloor D, Starck D, Gross G, Muhlbauer B. Dopamine D3 receptors and salt-dependent hypertension. J Am Soc Nephrol. 2001;12:2272–2279. doi: 10.1681/ASN.V12112272. [DOI] [PubMed] [Google Scholar]
- 179.Luippold G, Piesch C, Osswald H, Muhlbauer B. Dopamine D3 receptor mRNA and renal response to D3 receptor activation in spontaneously hypertensive rats. Hypertens Res. 2003;26:855–861. doi: 10.1291/hypres.26.855. [DOI] [PubMed] [Google Scholar]
- 180.Zeng C, Liu Y, Wang Z, He D, Huang L, Yu P, Zheng S, Jones JE, Asico LD, Hopfer U, Eisner GM, Felder RA, Jose PA. Activation of D3 dopamine receptor decreases AT1 angiotensin receptor expression in rat renal proximal tubule cells. Circ Res. 2006;99:494–500. doi: 10.1161/01.RES.0000240500.96746.ec. [DOI] [PubMed] [Google Scholar]
- 181.Sanada H, Yatabe J, Midorikawa S, Katoh T, Hashimoto S, Watanabe T, Xu J, Luo Y, Wang X, Zeng C, Armando I, Felder RA, Jose PA. Amelioration of genetic hypertension by suppression of renal G protein-coupled receptor kinase type 4 expression. Hypertension. 2006;47:1131–1139. doi: 10.1161/01.HYP.0000222004.74872.17. [DOI] [PubMed] [Google Scholar]
- 182.Van Tol HHM, Wu CM, Guan H-C, Ohara K, Bunzow JR, Civelli O, Kennedy J, Seeman P, Niznik HB, Jovanovic V. Multiple dopamine D4 receptor variants in the human population. Nature. 1992;358:149–152. doi: 10.1038/358149a0. [DOI] [PubMed] [Google Scholar]
- 183.Sen S, Nesse R, Sheng L, Stoltenberg SF, Gleiberman L, Burmeister M, Weder AB. Association between a Dopamine-4 receptor polymorphism and blood pressure. Am J Hypertens. 2005;18:1206–1210. doi: 10.1016/j.amjhyper.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 184.Armando I, Wang X, Villar VA, Jones JE, Asico LD, Escano C, Jose PA. Reactive oxygen species-dependent hypertension in dopamine D2 receptor-deficient mice. Hypertension. 2007;49:672–678. doi: 10.1161/01.HYP.0000254486.00883.3d. [DOI] [PubMed] [Google Scholar]
- 185.Armando I, Wang X, Pascua AM, Villar VAM, Luo Y, Jones JE, Asico L, Escano C, Jose PA. Regulation of renal NADPH oxidase and inflammation by dopamine D-2 receptor (abstract) Circulation. 2007;116(Suppl):124. [Google Scholar]
- 186.Staudacher T, Pech B, Tappe M, Gross G, Muhlbauer B, Luippold G. Arterial blood pressure and renal sodium excretion in dopamine D3 receptor knockout mice. Hypertens Res. 2007;30:93–101. doi: 10.1291/hypres.30.93. [DOI] [PubMed] [Google Scholar]
- 187.Sugiyama F, Churchill GA, Higgins DC, Johns C, Makaritsis KP, Gavras H, Paigen B. Concordance of murine quantitative trait loci for salt-induced hypertension with rat and human loci. Genomics. 2001;71:70–77. doi: 10.1006/geno.2000.6401. [DOI] [PubMed] [Google Scholar]
- 188.Goldstein DS, Grossman E, Armando I, Wolfovitz E, Folio CJ, Holmes C, Keiser HR. Correlates of urinary excretion of catechols in humans. Biog Amines. 1993;10:3–17. [Google Scholar]
- 189.Gerlo EAM, Schoors DF, Dupont MG. Age- and sexrelated differences for the urinary excretion of norepinephrine, epinephrine, and dopamine in adults. Clin Chem. 1991;37:875–878. [PubMed] [Google Scholar]
- 190.Lakatua DJ, Nicolau GY, Bogdan C, Plinga L, Jachimowicz A, Sackett-Lundeen L, Petrescu E, Ungureanu E, Haus E. Chronobiology of catecholamine excretion in different age groups. Prog Clin Biol Res. 1987;227B:31–50. [PubMed] [Google Scholar]
- 191.Vanpée M, Herin P, Lagercrantz H, Aperia A. Effect of extreme prematurity on renal dopamine and norepinephrine excretion during the neonatal period. Pediatr Nephrol. 1997;11:46–8. doi: 10.1007/s004670050231. [DOI] [PubMed] [Google Scholar]
- 192.Sulyok E, Gyódi G, Ertl T, Bódis J, Hartmann G. The influence of NaCl supplementation on the postnatal development of urinary excretion of noradrenaline, dopamine, and serotonin in premature infants. Pediatr Res. 1985;19:5–8. doi: 10.1203/00006450-198501000-00002. [DOI] [PubMed] [Google Scholar]
- 193.Meister B, Fried G, Holgert H, Aperia A, Hökfelt T. Ontogeny of aromatic L-amino acid decarboxylase-containing tubule cells in rat kidney. Kidney Int. 1992;42:617–623. doi: 10.1038/ki.1992.326. [DOI] [PubMed] [Google Scholar]
- 194.Armando I, Nowicki S, Aguirre J, Barontini M. A decreased tubular uptake of DOPA results in defective dopamine production in aged rats. Am J Physiol. 1995;268:F1087–F1092. doi: 10.1152/ajprenal.1995.268.6.F1087. [DOI] [PubMed] [Google Scholar]
- 195.Soares da Silva P, Fernandes MH. A study of the renal synthesis of dopamine in aged-rats. Acta Physiol Scand. 1991;143:287–293. doi: 10.1111/j.1748-1716.1991.tb09234.x. [DOI] [PubMed] [Google Scholar]
- 196.Soares-da-Silva P, Vieira-Coelho MA, Pestana M, Fernandes MH, Guimarães JT. Ontogeny of the cell outward dopamine transporter in canine renal tissues. Fundam Clin Pharmacol. 1995;9:255–262. doi: 10.1111/j.1472-8206.1995.tb00293.x. [DOI] [PubMed] [Google Scholar]
- 197.Tenore G, Barili P, Sabbatini M, Tayebati SK, Amenta F. Postnatal development of dopamine D1-like and D2-like receptors in the rat kidney: a radioligand binding study. Mech Ageing Dev. 1997;95:1–11. doi: 10.1016/s0047-6374(97)01864-2. [DOI] [PubMed] [Google Scholar]
- 198.Kinoshita S, Felder RA. Ontogeny of DA1 receptor-adenylate cyclase coupling in proximal convoluted tubules. Am J Physiol. 1990;259:F971–F976. doi: 10.1152/ajprenal.1990.259.6.F971. [DOI] [PubMed] [Google Scholar]
- 199.Svennilson J, Aperia A. Dopamine in the developing kidney. Int J Dev Biol. 1999;43:441–443. [PubMed] [Google Scholar]
- 200.Massmann GA, Zhang J, Rose JC, Figueroa JP. Acute and long-term effects of clinical doses of antenatal glucocorticoids in the developing fetal sheep kidney. J Soc Gynecol Invest. 2006;13:174–180. doi: 10.1016/j.jsgi.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 201.Felder RA, Nakamura KT, Robillard JE, Kanadjian M, Jose PA. Dopamine receptors in the developing sheep kidney. Pediatr Nephrol. 1988;2:156–162. doi: 10.1007/BF00870397. [DOI] [PubMed] [Google Scholar]
- 202.Cantalamessa F, Barili P, Cavagna R, Sabbatini M, Tenore G, Amenta F. Influence of neonatal treatment with the pyrethroid insecticide cypermethrin on the development of dopamine receptors in the rat kidney. Mech Ageing Dev. 1998;103:165–178. doi: 10.1016/s0047-6374(98)00039-6. [DOI] [PubMed] [Google Scholar]
- 203.Fryckstedt J, Svensson LB, Lindén M, Aperia A. The effect of dopamine on adenylate cyclase and Na+,K+-ATPase activity in the developing rat renal cortical and medullary tubule cells. Pediatr Res. 1993;34:308–311. doi: 10.1203/00006450-199309000-00014. [DOI] [PubMed] [Google Scholar]
- 204.Hussain T, Kansra V, Lokhandwala MF. Renal dopamine receptor signaling mechanisms in spontaneously hypertensive and Fischer 344 old rats. Clin Exp Hypertens. 1999;21:25–36. doi: 10.3109/10641969909068646. [DOI] [PubMed] [Google Scholar]
- 205.Kansra V, Hussain T, Lokhandwala MF. Alterations in dopamine DA1 receptor and G proteins in renal proximal tubules of old rats. Am J Physiol. 1997;273:F53–F59. doi: 10.1152/ajprenal.1997.273.1.F53. [DOI] [PubMed] [Google Scholar]
- 206.Li XX, Xu J, Zheng S, Albrecht FE, Robillard JE, Eisner GM, Jose PA. D1 dopamine receptor regulation of NHE3 during development in spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1650–R1660. doi: 10.1152/ajpregu.2001.280.6.R1650. [DOI] [PubMed] [Google Scholar]
- 207.Kaneko S, Albrecht F, Asico LD, Eisner GM, Robillard JE, Jose PA. Ontogeny of DA1 receptor-mediated natriuresis in the rat: in vivo and in vitro correlations. Am J Physiol. 1992;263:R631–R638. doi: 10.1152/ajpregu.1992.263.3.R631. [DOI] [PubMed] [Google Scholar]
- 208.Li XX, Albrecht FE, Robillard JE, Eisner GM, Jose PA. Gβ regulation of Na/H exchanger-3 activity in rat renal proximal tubules during development. Am J Physiol Regul Integr Comp Physiol. 2000;278:R931–R936. doi: 10.1152/ajpregu.2000.278.4.R931. [DOI] [PubMed] [Google Scholar]
- 209.Buckely NM, Brazeau P, Frazier ID. Cardiovascular effects of dopamine in developing swine. Biol Neonate. 1983;43:50–60. doi: 10.1159/000241637. [DOI] [PubMed] [Google Scholar]
- 210.Fiser DH, Fewell JE, Hill DE, Brown AL. Cardiovascular and renal effects of dopamine and dobutamine in healthy, conscious piglets. Crit Care Med. 1988;16:340–345. doi: 10.1097/00003246-198804000-00007. [DOI] [PubMed] [Google Scholar]
- 211.Gootman NB, Buckley BJ, Gootman PM, Nagelberg JS. Age-related effects of single injections of dopamine on cardiovascular function in developing swine. Dev Pharmacol Ther. 1982;4:139–150. doi: 10.1159/000457403. [DOI] [PubMed] [Google Scholar]
- 212.Vane DW, Weber TR, Caresky J, Grosfeld JL. Systemic and renal effects of dopamine in the infant pig. J Surg Res. 1982;32:477–483. doi: 10.1016/0022-4804(82)90129-9. [DOI] [PubMed] [Google Scholar]
- 213.Nakamura KT, Felder RA, Jose PA, Robillard JE. Effects of dopamine in the renal vascular bed of fetal, newborn, and adult sheep. Am J Physiol. 1987;252:R490–R497. doi: 10.1152/ajpregu.1987.252.3.R490. [DOI] [PubMed] [Google Scholar]
- 214.Kohli JD, Goldberf LI. Dopamine receptors: a classification based on physiological studies. In: Creese I, Fraser CM, editors. Dopamine receptors. New York: Liss; 1987. pp. 97–114. [Google Scholar]
- 215.Pelayo JC, Fildes RD, Jose PA. Age-dependent renal effects of intrarenal dopamine infusion. Am J Physiol. 1984;247:R212–R216. doi: 10.1152/ajpregu.1984.247.1.R212. [DOI] [PubMed] [Google Scholar]
- 216.Segal JL, Smith FG, Guillery EN, Jose PA, Robillard JE. Ontogeny of renal response to specific dopamine DA1-receptor stimulation in sheep. Am J Physiol. 1992;263:R868–R873. doi: 10.1152/ajpregu.1992.263.4.R868. [DOI] [PubMed] [Google Scholar]
- 217.Seri I, Aperia A. Contribution of dopamine2 receptors to dopamine-induced increase in glomerular filtration rate. Am J Physiol. 1988;254:F196–F201. doi: 10.1152/ajprenal.1988.254.2.F196. [DOI] [PubMed] [Google Scholar]
- 218.Sulyok E. Dopaminergic control of neonatal salt and water metabolism. Pediatr Nephrol. 1988;2:163–165. doi: 10.1007/BF00870398. [DOI] [PubMed] [Google Scholar]
- 219.Holtback U, Aperia A. Molecular determinants of sodium and water balance during early human development. Semin Neonatol. 2003;8:291–299. doi: 10.1016/S1084-2756(03)00042-3. [DOI] [PubMed] [Google Scholar]
- 220.Cheung PY, Barrington KJ. Renal dopamine receptors: mechanisms of action and developmental aspects. Cardiovasc Res. 1996;31:2–6. [PubMed] [Google Scholar]
- 221.Vieira-Coelho MA, Hussain T, Kansra V, Serrao MP, Guimaraes JT, Pestana M, Soares-Da-Silva P, Lokhandwala MF. Aging, high salt intake, and renal dopaminergic activity in Fischer 344 rats. Hypertension. 1999;34:666–672. doi: 10.1161/01.hyp.34.4.666. [DOI] [PubMed] [Google Scholar]