Abstract
Since its first discovery in an Iranian male in 1961, zinc deficiency in humans is now known to be an important malnutrition problem world-wide. It is more prevalent in areas of high cereal and low animal food consumption. The diet may not necessarily be low in zinc, but its bio-availability plays a major role in its absorption. Phytic acid is the main known inhibitor of zinc. Compared to adults, infants, children, adolescents, pregnant, and lactating women have increased requirements for zinc and thus, are at increased risk of zinc depletion. Zinc deficiency during growth periods results in growth failure. Epidermal, gastrointestinal, central nervous, immune, skeletal, and reproductive systems are the organs most affected clinically by zinc deficiency. Clinical diagnosis of marginal Zn deficiency in humans remains problematic. So far, blood plasma/serum zinc concentration, dietary intake, and stunting prevalence are the best known indicators of zinc deficiency. Four main intervention strategies for combating zinc deficiency include dietary modification/diversification, supplementation, fortification, and bio-fortification. The choice of each method depends on the availability of resources, technical feasibility, target group, and social acceptance. In this paper, we provide a review on zinc biochemical and physiological functions, metabolism including, absorption, excretion, and homeostasis, zinc bio-availability (inhibitors and enhancers), human requirement, groups at high-risk, consequences and causes of zinc deficiency, evaluation of zinc status, and prevention strategies of zinc deficiency.
Keywords: Zinc absorption, zinc bio-availability, zinc deficiency, zinc intervention, zinc nutrition, zinc requirement
INTRODUCTION
Zinc essentiality was established in 1869 for plants, in 1934 for experimental animals and in 1961 for humans.[1] A syndrome of anemia, hypogonadism and dwarfism was reported in a 21-year-old Iranian farmer in 1961 who was subsisting on a diet of unrefined flat bread, potatoes, and milk.[2] Shortly after, a similar syndrome was observed in Egyptian adolescents who had similar dietary history to that of the Iranians, mainly subsisting on bread and beans.[3] Administration of supplemental zinc or diets containing adequate animal-protein foods improved growth and corrected the hypogonadism, while anemia responded to oral iron treatment. Subsequent studies showed that the syndrome was primarily the result of low dietary zinc intake in the diet. Since the discovery of zinc deficiency as a human health problem in 1961,[1] interest in the biochemical and clinical aspects of zinc nutrition has increased markedly. In this paper, we review zinc biochemical and physiological functions, metabolism including, absorption, excretion, and homeostasis, zinc bioavailability (inhibitors and enhancers), human requirement, groups at high-risk, consequences and causes of zinc deficiency, evaluation of zinc status, and prevention strategies of zinc deficiency.
Bio-chemical and physiologic functions
Although, zinc-dependent biochemical mechanisms in physiologic functions have received extensive study, clear relationships have not been fully established. Zinc is ubiquitous within cells in contrast to iron, which is contained in defined cellular components and has defined physiological roles. The role of zinc in biology can be grouped into three general functional classes, namely catalytic, structural and regulatory functions.[4]
METABOLISM
Absorption
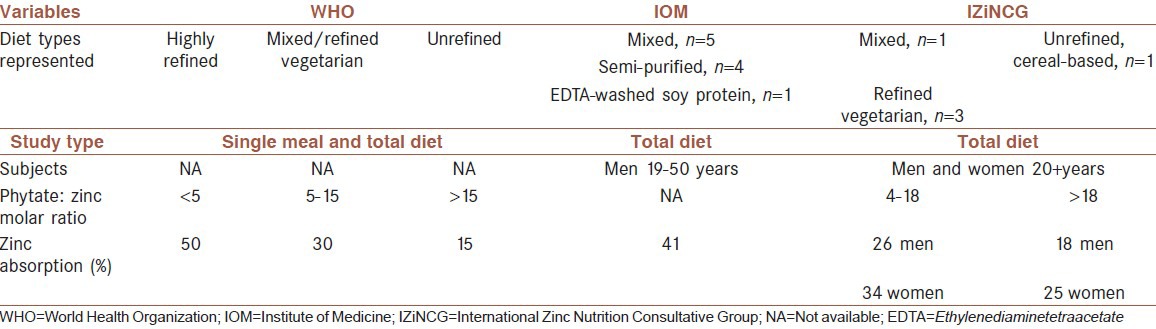
Zinc is absorbed in the small intestine by a carrier-mediated mechanism.[5] Under normal physiologic conditions, transport processes of uptake are not saturated. The fraction of zinc absorbed is difficult to determine because zinc is also secreted into the gut. Zinc administered in aqueous solutions to fasting subjects is absorbed efficiently (60-70%), whereas absorption from solid diets is less efficient and varies depending on zinc content and diet composition.[6] Generally, 33% is accepted as the average zinc absorption in humans).[5,7] More recent studies have suggested different absorption rates for different population groups based on their type of diet and phytate: Zinc molar ratio [Table 1]. Zinc absorption is concentration dependent and increases with increasing dietary zinc up to a maximum rate.[6,8] In addition, zinc status may influence zinc absorption. Zinc-deprived humans absorb this element with increased efficiency, whereas humans on a high-zinc diet show a reduced efficiency of absorption.[9]
Table 1.
Estimates of dietary zinc absorption, as developed by world health organization, food and nutrition board/Institute of medicine, and international Zinc nutrition consultative group, and summaries of the data used to derive them
Zinc is released from food as free ions during digestion. These liberated ions may then bind to endogenously secreted ligands before their transport into the enterocytes in the duodenum and jejunum.[6,10] Specific transport proteins may facilitate the passage of zinc across the cell membrane into the portal circulation. With high intakes, zinc is also absorbed through a passive paracellular route. The portal system carries absorbed zinc directly to the liver, and then released into systemic circulation for delivery to other tissues. About 70% of the zinc in circulation is bound to albumin, and any condition that alters serum albumin concentration can have a secondary effect on serum zinc levels. Although, serum zinc represents only 0.1% of the whole body zinc, the circulating zinc turns over rapidly to meet tissue needs.[6,10,11]
Zinc transporters (ZnTs)
There are at least 10 ZnTs and 15 zip transporters in human cells.[12] They appear to have opposite roles in cellular zinc homeostasis. The expression and cellular distribution of the ZnTs is highly regulated by changes in zinc level.[13] ZnTs reduce intracellular zinc availability by promoting zinc efflux from cells or into intracellular vesicles, while zip transporters increase intracellular zinc availability by promoting extracellular zinc uptake and perhaps, vesicular zinc release into the cytoplasm.[14] Both the ZnT and zip transporter families exhibit unique tissue-specific expression, differential responsiveness to dietary zinc deficiency and excess, and differential responsiveness to physiologic stimuli via hormones and cytokines.[15]
The recently characterized ZnTs have significantly increased understanding of the interrelationships of cellular zinc uptake and efflux but do not yet account for observations at the whole body level. ZnTs-1 is a ubiquitously expressed protein that has been found to be present in the villi of the proximal small bowel.[16] In response to manipulation of dietary zinc, however, expression in rats was increased in response to zinc supplementation but not to zinc restriction.[17] These and other observations have led to a current consensus that ZnTs-1 functions mainly as a zinc exporter and may play a role in zinc homeostasis as a mechanism for zinc acquisition and elimination under conditions of excess of zinc.[17]
The role of metallothionein (MT), an intracellular metal binding protein, in the regulation of zinc absorption, particularly in conjunction with the ZnTs, also remains unclear. Hepatic and inte stinal MT synthesis is stimulated by dietary zinc supplementation, by intraperitoneal zinc injection and by inflammation and the acute phase response. Dietary restriction also results in diminished MT synthesis. In experiments with knockout and transgenic mice, the rise in serum zinc after a single dose of zinc was much greater in the MT knockouts than in the control animals. In contrast, the serum zinc response of the MT transgenic animals was blunted compared with that of the control animals. The expression of ZnTs-1 was also measured and found to be directly related to serum zinc levels but unaffected by MT levels.[18] Thus, MT may function in cellular responses to limit free zinc concentrations within quite narrow ranges[4] and function as a zinc pool.[18]
Another transporter potentially involved in zinc and other metal uptake is divalent cation transporter 1 (DCT 1), a transmembrane polypeptide that is found in the duodenum in the crypts and lower villi and may be available for the uptake of several metal ions.[17]
As these transport proteins are identified and characterized, investigations in the whole animal, under conditions of a range of dietary intake, will be needed. Animal and human studies indicate considerable ability to enhance efficiency of absorption in response to low dietary zinc intake or increased physiologic demand; as yet, the subcellular correlates of these observations are lacking. Observations relating the amount of absorbed zinc to the amount of excreted zinc and to exchangeable pool sizes also await corroboration with the subcellular processes.
Ion gradients are generated by two main mechanisms: (1) A primary pump, utilizing the energy of Adenosine triphosphate (ATP)-hydrolysis; or (2) a secondary active mechanism that uses an ion gradient, such as Na+, for generating Zn2+ gradients.[14] A Zn2+ pump has been demonstrated in bacteria, where several forms of p-type ATPases have been shown to catalyze active Zn2+ transport.[19] Recently, a similar ATPase, which transports Zn2+ and Cd2+ and to a lesser extent other heavy metals, has been discovered in Arabidopsis.[20] Surprisingly, there is still no evidence for a Zn2+ pump in either yeast or mammalian cells, though a Cu pump has been identified that is linked to heavy metal ion transport.[21]
A Na+-dependent secondary active mechanism has, however, been suggested to facilitate formation of the transmembrane Zn2+ gradient in neurons.[14] Early studies suggested that the neuronal Na+/Ca2+ exchanger mediates Zn2+ extrusion,[22] however, more recent findings seem to support the existence of a distinct Na+/Zn2+ exchanger. These studies have indicated that a putative Na+/Zn2+ exchanger, probably a member of the Na+/Ca2+ exchanger superfamily, operates with a stoichiometry of 3Na+/Zn2+, promoting Zn2+ efflux against a 500-fold transmembrane gradient.[23] This mechanism is pharmacologically and molecularly distinct from the classical Na+/Ca2+ exchangers. Whether this exchanger is the principle plasma membrane extruder of Zn2+ or is accompanied by an as yet unidentified Zn2+ pump, is an open and intriguing question.[22]
Homeostasis
Maintaining a constant state of cellular zinc, or homeostasis, is essential for survival. In animals and humans, adjustments in total zinc absorption and endogenous intestinal excretion are the primary means of maintaining zinc homeostasis.[24] The adjustments in gastrointestinal zinc absorption and endogenous excretion are synergistic. Shifts in the endogenous excretion appear to occur quickly with changes in intake just above or below optimal intake while the absorption of zinc responds more slowly, but it has the capacity to cope with large fluctuations in intake.[25] With extremely low zinc intakes or with prolonged marginal intakes, secondary homeostatic adjustments may augment the gastrointestinal changes. These secondary adjustments include changes in urinary zinc excretion, a shift in plasma zinc turnover rates and possibly, an avid retention of zinc released from selected tissues, such as bone, in other tissues to maintain function.[11,26]
Excretion
Loss of zinc through gastrointestinal tract accounts for approximately half of all zinc eliminated from the body. Considerable amount of zinc is secreted through the biliary and intestinal secretions, but most of it is reabsorbed. This is an important process in the regulation of zinc balance. Other routes of zinc excretion include urine and surface losses (desquamated skin, hair, sweat).[6,10,11] Measurements in humans of endogenous intestinal zinc have primarily been made as fecal excretion; these indicate that amounts excreted are responsive to zinc intake, absorbed zinc and physiologic need.[9]
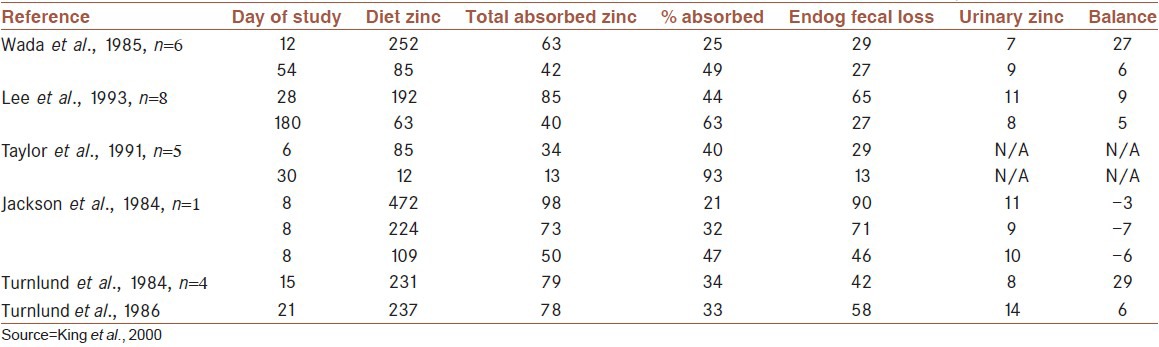
Typically, human zinc intakes range from 107 μmol/day to 231 μmol/day (equivalent to 14-30 mg/kg). These intakes support crude zinc balance (i.e., replace fecal and urinary losses) in healthy adults, but balance can be achieved when as little as 22 μmol/day (2.8 mg/kg) or as much as 306 μmol/day (40 mg/kg) is fed.[27] With these extreme reductions or increases in zinc intake, zinc losses either fell or increased during the first 6-12 d after the dietary change so that balance was achieved.[25] Thus, humans appear to have the capacity to regulate whole body zinc content over a 10-fold change in intake, as has been observed in experimental animals. Body zinc content is regulated, by homeostatic mechanisms, over a wide range of intake by changes in fractional absorption (normally 20-40%) and urinary (0.5 mg/day) and intestinal (1-3 mg/day) excretion.[28] A number of fractional zinc absorption studies in humans are summarized in Table 2 to provide some numbers regarding zinc intake, zinc excretion and retention. All of the studies were performed with healthy adults who consumed diets with adequate amounts of zinc before implementation of the study diet.
Table 2.
Zinc absorption and endogenous losses with different levels of zinc intake in adult men (μmol/day)
BIOAVAILABILITY
Bio-availability refers to the fraction of intake that can be absorbed into the blood system and used for physiologic functions of the body. For zinc, in healthy individuals, it is determined by three factors: the individual's zinc status, the total zinc content of the diet, and the availability of soluble zinc from the diet's food components.[29] If the individual's zinc status is discounted, zinc absorption is largely determined by its solubility in the intestinal lumen, which in turn is affected by the chemical form of zinc and the presence of specific inhibitors and enhancers of zinc absorption.
Long-term zinc intake, i.e., zinc status, can also affect absorption of dietary zinc. Although, the long-term use of zinc supplements does not appear to cause any down-regulation of zinc absorption compared with normal, healthy subjects not taking any supplements,[30] low zinc intake and zinc status do affect zinc absorption. Istfan et al.[31] fed young men a formula diet containing either 1.5 or 15 mg zinc/day and measured zinc absorption using the method of stable tracer isotope neutron activation analysis in a fasted state after 6 days. Zinc absorption was 92% from the low zinc diet and 81% from the high zinc diet. Wada et al.[32] performed similar stable isotope studies in young men and found that zinc absorption from the diet was 53% when the zinc intake was 5.5 mg/day and that it decreased to 25% when 16.5 mg/day was fed. Similarly, August et al.[33] found that young adult subjects absorbed 64 ± 5% of zinc from the diet when it contained 2.8-5 mg/day but only 39 ± 3% when it contained 12.8-15 mg/day. Differences were also found in elderly subjects (43 ± 7% vs. 21 ± 1%), but as can be seen, the extent of zinc absorption was lower in this age group. Thus, it appears that feeding low zinc diets increases zinc absorption in all age groups and homeostatic mechanisms up-regulate zinc absorption and retention. Previous zinc intake may therefore, have an effect on studies on zinc bioavailability.[29]
Inhibitors and enhancers
Various dietary factors can influence zinc absorption. Phytic acid (inositol hexa- and penta-phosphate) is the principal dietary factor known to limit zinc bio-availability by strongly binding zinc in the gastrointestinal tract.[34] It is the major phosphorus (P) storage compound in plant seeds, especially, cereals and legumes, and can account for up to 80% of seed total P.[1] Because of its high-density of negatively charged phosphate groups, phytate forms mixed salts with mineral cations, which are assumed to play an important role in mineral storage.[35] The inhibitory effects of phytic acid (PA) on zinc can be predicted by the molar ratios of phytate: Zinc in the diet. Molar ratios in excess of 15: 1 according to World Health Organization (WHO),[36] or 18:1 according to International Zinc Nutrition Consultative Group (IZiNCG)[11] progressively inhibit zinc absorption and have been associated with suboptimal zinc status in humans. It appears unlikely that calcium per se has a negative effect on zinc absorption.[29] As calcium has the propensity to form complexes with phytic acid and zinc that are insoluble, it has been proposed that the phytate: Zinc molar ratio should be multiplied by the dietary calcium concentration to improve the prediction of zinc bio-availability.[29,35] However, the interactions between zinc and calcium are complex and not all studies have shown that calcium further increases the impact of phytic acid on zinc absorption.[29,37] Techniques such as soaking, germination, and fermentation promote enzymatic hydrolysis of PA in whole grain cereals and legumes by enhancing the activity of endogenous or exogenous phytase enzyme.[38] Furthermore, non-enzymatic methods such as milling have been successful in reducing phytic acid content in plant-based staples.[39] Thermal processing and extrusion cooking may cause only modest phytate losses.[38]
The potential interaction between iron and zinc has been a cause of concern. Solomons and Jacob[40] found that high doses of inorganic iron decreased zinc uptake as measured by changes in plasma zinc over the next 4 h after an oral dose. Human adults were administered 25 mg of zinc (as Zinc sulfate [ZnSO4]) in water solution, and iron was added at 25, 50 or 75 mg. Plasma zinc was reduced significantly with increasing dose of iron. Lonnerdal[29] used a dose of zinc similar to that obtained from most meals and studied zinc absorption by using radiolabeled zinc and whole-body counting. He found a significant reduction in zinc absorption in the fasting state when iron was added to the zinc dose in water solution at a 25:1 molar ratio but not at a 2.5:1 ratio, which is similar to the ratio used in the study by Solomons and Jacob.[40] Thus, the interaction appears much less pronounced when zinc intake is closer to a “physiological” level. He concluded that the effect of iron on zinc is exerted only at a very high ratio of iron to zinc and in water solution. This suggests that iron fortification will not affect zinc absorption. Some inhibitory effects would be seen only if very high iron to zinc ratio is administered apart from a meal. It was demonstrated by Davidsson et al.[41] that iron fortification of foods is unlikely to affect zinc absorption. They examined the effect of iron fortification of bread (65 mg/kg), weaning cereal (500 mg/kg) and infant formula (12 mg/L) in human adults with the use of stable isotopes. No significant negative effect on zinc absorption was found compared with the same foods without iron fortification. Similar results were obtained by Fairweather-Tait et al.,[42] who studied the effect of iron fortification of a weaning food on zinc absorption in infants with the use of stable isotopes.
Proteins generally have positive influence on zinc absorption, because zinc absorption tends to increase with protein intake.[29,43,44] Consumption of animal proteins (e.g., beef, eggs and cheese) improve the bioavailability of zinc from plant food sources possibly because amino acids released from the animal protein keep zinc in solution[29] or the protein binds the phytate. Generally, binding of zinc to soluble ligands or chelators has a positive effect on zinc absorption as they increase the zinc solubility.[29,45]
HUMAN REQUIREMENTS
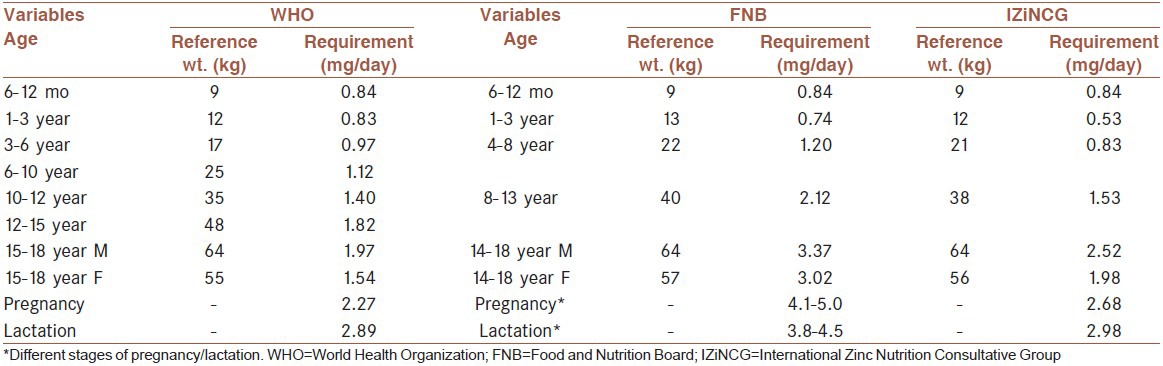
Since the mid-1990s, the WHO, the Food and Agriculture Organization, the International Atomic Energy Association and the Food and Nutrition Board (FNB) of the Institute of Medicine (IOM) have convened expert committees to develop estimates of human zinc requirements and dietary intakes needed to satisfy these requirements.[46,47] For most age groups the committees used a factorial method to estimate the average physiological requirement, which is defined as the amount of zinc that must be absorbed to offset the amount of zinc lost through both intestinal and non-intestinal pathways. For growing children and pregnant women the amount of zinc retained in newly accrued tissues is added to the requirements, and for lactating women the zinc secreted in breast milk is added. More recently, the IZiNCG reported revised estimates of zinc requirement and recommended dietary intake as given in [Table 3].[11]
Table 3.
Estimated physiological requirements for absorbed zinc by age group and sex
The estimates of zinc physiological requirements by the IZiNCG in 2004, however, were conspicuously low in comparison with those estimated by the IOM in 2001 Hambidge et al.[34] explained that this difference was due to an error in zinc menstrual losses, as well as a minor error in the linear regression of endogenous fecal zinc (EFZ) versus total daily zinc absorption (TAZ) by IOM. The review also revealed an error by IZiNCG in selecting two data points for the linear regression of EFZ on TAZ. A second major reason for the “gap” was attributable to weighting of the data in the regression analysis by number of subjects per study by IZiNCG. Adjusting for these factors, together with use of the same reference data for body weights, resulted in satisfactory agreement between the two estimates of physiological requirements.[34]
Different types of dietary reference intakes are derived depending on whether they are being used to assess the intakes of individuals or populations. Methods for calculating these reference intake values and their uses have been described by the FNB/IOM Dietary Reference Intake Committees,[47] and the same terminology and methods were used by IZiNCG. The estimated average requirement (EAR) represents the mean dietary requirement, or the dietary intake level at which 50% of individuals would meet their physiological requirement. The EAR is thus derived by dividing the mean physiological requirement for absorbed zinc by the estimated average absorption of zinc. For example, the EAR for adult women (55 kg) consuming unrefined, cereal-based diets would be calculated as: 1.86 mg absorbed zinc/day/0.25 = 7.4 mg zinc/day, and rounded to 7 mg/day.
GROUPS AT HIGH RISK
Compared to adults, infants, children, adolescents, pregnant and lactating women have increased requirements for zinc and thus, are at increased risk of zinc depletion (King and Cousins, 2006[1]).
Infants and children
Young children are at greater risk of zinc deficiency because of increased zinc requirements during growth. Exclusively breast-fed infants of mothers with adequate zinc nutriture obtain sufficient zinc for the 1st 5-6 months of their life.[11] After this age, complementary foods containing absorbable zinc are required to satisfy their requirements. In many low-income countries, complementary feeding is delayed and cereal foods are then used for feeding. These foods have low content of total and absorbable zinc and thus, fail to meet the needs for zinc. Conversely, early introduction of such foods may interfere with the absorption of zinc from breast milk due to their high phytate content.[47]
Zinc requirements of malnourished children are estimated to be between 2 mg/kg and 4 mg/kg body weight.[48] These requirements are much higher than those for healthy children (0.17 mg/kg at 1-3 years), presumably because of prior zinc depletion and reduced zinc absorption due to changes in the intestinal tract.
Adolescents
The physiological requirements for zinc peak during adolescence at the time of the pubertal growth spurt, which generally occurs in girls between 10 years and 15 years and in boys between 12 years and 15 years. Even after the growth spurt has ceased, adolescents may require additional zinc to replenish depleted tissue zinc pools.[49]
Pregnant and lactating women
Increased nutritional demands during pregnancy and lactation predispose women to zinc deficiency.[26] These demands are greater during lactation, although, physiological adjustments in zinc absorption help to meet the needs for lactation. A number of studies have demonstrated a negative impact of therapeutic supplemental iron on zinc absorption during pregnancy[50] and lactation.[51] In pregnant women where dietary intakes of zinc were low, supplemental iron, in dosages as low as 60 mg/day prevented them from meeting their needs for zinc.[50] Situations that seem most likely to encounter problematic interactions are those in which the iron is administered in solution or as a separate supplement rather than incorporated into a meal.[52]
Elderly
Diet surveys indicate that zinc intakes by elderly persons are often inadequate, even in rich countries.[53] Several factors may contribute to poor zinc nutrition among the elderly, in particular, reduced consumption of zinc-rich foods such as red meat. In addition, there is some evidence that the efficiency of zinc absorption may decrease with age.[53]
CONSEQUENCES AND CAUSES OF ZINC DEFICIENCY
Consequences of zinc deficiency
Due to the multitude of basic biochemical functions of zinc in the cells of human body, there is a broad range of physiological signs of zinc deficiency. These signs vary depending on the severity of the condition. Organ systems known to be affected clinically by zinc deficiency states include the epidermal, gastrointestinal, central nervous, immune, skeletal, and reproductive systems.[54]
Clinical signs of severe zinc deficiency were identified in industrialized countries notably in persons suffering from acrodermatitis enteropathica, a rare genetic disorder that specifically affects zinc absorption.[55] Severe zinc deficiency resulting from other causes such as prolonged parenteral nutrition with inadequate zinc content produced similar clinical signs as in acrodermatitis enteropathica.[56]
Although, less impressive in their clinical presentation, milder zinc deficiency is of numerically much greater importance. Moreover, most of the clinical features of acrodermatitis enteropathica were documented also in milder forms of zinc deficiency. Functional impairments identified in community-based trials may be more representative of mild or moderate deficiency. Some of these functional impairments are as follows:
Growth and development
One of the most studied clinical features related to zinc deficiency is the impairment of physical growth and development.[57,58] The mechanisms involved, however, are not well understood. This effect is of most significance during the periods of rapid growth such as pregnancy, infancy and puberty during which zinc requirements are highest.[11]
Risk of infections
Diarrhea: Diarrhea is characteristically, although not inevitably, a prominent feature of acrodermatitis enteropathica.[59] Plausible explanations for a link between zinc deficiency and diarrhea include impairment of the immune system and of intestinal mucosal cell transport.[60] A causal relationship between zinc deficiency and diarrhea is indicated by the beneficial effects of zinc supplements and concurrent increase in growth velocity.[61]
Pneumonia: Community zinc supplementation studies in children have demonstrated a substantial and statistically significant reduction in the prevalence of pneumonia in developing countries.[62]
Malari: It is uncertain to what extent oral supplementation with zinc can reduce episodes of malaria in endemic areas. According to some studies, malaria also appears to be reduced by zinc supplementation.[63] However, there are studies showing no effect of zinc supplementation against malaria.[64] Further studies are required to establish this effect.
Relationship between zinc deficiency and age
Furthermore, degenerative changes associated with aging may partly be due to zinc deficiency, including a decline in immunocompetence, delayed wound healing and certain neurological and psychological changes.[52]
In general, clinical manifestations of zinc deficiency vary with age. In early infancy, diarrhea is a prominent symptom. Zinc deficiency also leads to impaired cognitive function, behavioral problems, impaired memory, learning disability, and neuronal atrophy.[11,45] Skin problems become more frequent as the child grows older. Alopecia, growth retardation and recurrent infections are common in school-age children. Chronic non-healing skin ulcers and also recurrent infections are common among the elderly. These effects have been observed in controlled clinical trials showing positive response to supplemental zinc.[11]
Infectious diseases and malnutrition are the principal causes of childhood morbidity and mortality globally. Providing adequate zinc nutriture is perhaps one of the effective preventive measures to decrease the rates of morbidity and mortality in children of the developing world when vitamin A or iron are not deficient.[48,56]
Childhood obesity and its co-morbidities as insulin resistance and metabolic syndrome are becoming a major health problem. Zinc supplementation might be useful in controlling some of these metabolic aspects.[65,66]
Moreover, serum zinc level is found to be lower in children of parents with premature atherosclerosis, and this might be an evidence for the protective role of zinc in the process of inflammation and atherosclerosis.[67]
Causes of zinc deficiency
The general causes of zinc deficiency include inadequate intake, increased requirements, malabsorption, increased losses and impaired utilization.[1] Inadequate dietary intake of absorbable zinc is the primary cause of zinc deficiency in most situations.[29] This may result from low dietary intake or heavy reliance on foods with little or poorly absorbable zinc. Inadequate dietary zinc intake is common in many parts of the world. It is often exacerbated by physiologic conditions associated with elevated zinc requirements.[1,53]
Malabsorption of zinc may occur in a number of situations for example, acrodermatitis enteropathica.[55] Malabsorption syndromes and inflammatory diseases of the bowel, resulting in poor absorption and loss of zinc, may lead to secondary zinc deficiency particularly in the presence of marginal dietary intakes.[1] Utilization of zinc is impaired in the presence of infection as decreased circulation of zinc reduces the availability of zinc to the tissues.
Conditions of impaired intestinal integrity not only reduce absorption, but also result in increased endogenous losses of zinc. Fecal excretion of zinc is increased during acute diarrhea.[61] It is not clear to what extent this represents unabsorbed zinc or zinc of endogenous origin.[11] Diarrheal diseases are common in many low-income countries. The fact that zinc deficiency increases the susceptibility to childhood diarrhea while increased losses of endogenous zinc associated with diarrhea further deplete body zinc, results in a vicious cycle that merits further study.[68]
EVALUATION OF ZINC STATUS
Assessing the nutritional status of a population is critical in developing intervention programs. Regrettably, there are no simple markers of marginal, mild or moderate zinc deficiency in individuals. Nevertheless, there is sufficient evidence to suggest that zinc deficiency is common in many low-income countries. For example, animal foods that are particularly rich sources of zinc are not easily accessible to many of the world's poorer population. Diets based on cereals and legumes and poor in animal products make it difficult to meet the zinc requirements because their high phytate content reduces the bioavailability of zinc. Evidence for widespread zinc deficiency in developing countries also results from intervention trials in children, which showed that zinc supplementation improved growth among stunted children. Although, other nutritional and environmental factors can also cause stunting, an elevated prevalence of this condition is considered as suggestive evidence of zinc deficiency in a population.
In recent years, efforts have been made to derive more precise and reliable indicators of zinc deficiency using direct measures of zinc status. These include assessment of dietary zinc intakes and biochemical markers.
Blood plasma or serum zinc concentration
The concentration of zinc in blood plasma or serum is currently the best available biomarker of the risk of zinc deficiency in a population. Suggested lower cut-offs for serum zinc concentration are based on data collected in the 2nd National Health and Nutrition Examination Survey in US population and are given in [Table 4].[69] Although, serum zinc concentrations may have limitations in validity and reliability for identification of mild or moderate zinc deficiency in individuals, this index is useful for assessing zinc status at the population level. The risk of zinc deficiency is considered to be elevated and of public health concern when low serum zinc concentration is prevalent in >20% of the population.
Table 4.
Suggested lower cutoffs for assessment of serum zinc concentration (μg/dl) in population studies
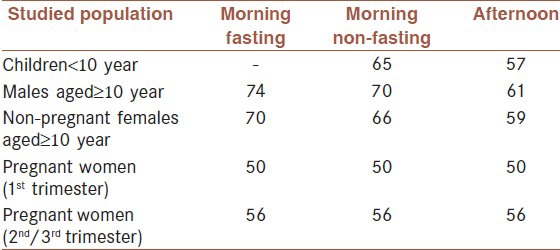
Serum zinc concentrations fluctuate by as much as 20% during a 24-h period,[70] largely due to the effects of food ingestion. Following a meal, there is an immediate initial increase, after which the concentration declines progressively for the next 4 h and then rises until food is eaten again. During an overnight fast, the concentration of serum zinc increases slightly, so the highest levels of the day are generally seen in the morning.[71,72] However, diurnal variations in serum zinc concentration among fasted individuals have also been observed, whereby serum zinc decreased from morning to mid-afternoon and then began to rise again to morning levels.[73]
Low serum zinc concentrations can occur in the presence of several conditions, representing a normal physiologic response and not necessarily indicative of low zinc status. Serum zinc concentrations are reduced during acute infections and inflammation, which is likely due to the redistribution of zinc from the plasma to the liver;[74] cytokines released during the acute phase response activate hepatic MT synthesis,[75] a metal-binding protein which appears to alter the hepatic uptake of zinc.[76] Elevated concentrations of C-reactive protein or other markers of the acute phase response can be used to indicate the presence of infection and should be considered in the interpretation of results. Stress and myocardial infarction also reduce serum zinc levels.[77] Because zinc is transported in plasma bound to albumin, diseases, such as cirrhosis and protein-energy malnutrition, that produce hypoalbuminemia result in lower serum zinc concentrations.[78] Hemodilution, as observed during pregnancy, oral contraceptive use, and other hormonal treatments, also results in a lower serum zinc concentration.[79] On the other hand, conditions resulting in intrinsic or extrinsic hemolysis of blood cells can result in extremely high serum zinc levels because the concentration of intracellular zinc is considerably greater than in serum.
Dietary zinc intake
Given that chronic inadequate dietary intake of zinc is the most likely cause of zinc deficiency, quantitative dietary intake surveys are useful to evaluate zinc intake and the risk of zinc deficiency in populations. Based on the type and bioavailability of the diets, the risk of zinc deficiency is estimated by comparing the intakes with the respective EAR values. The risk of zinc deficiency is considered to be elevated and of public health concern when the prevalence of inadequate intake is >25%.
Stunting prevalence
Height-for-age, a measure of nutritional stunting, is the best known and easiest to measure of the adverse outcomes associated with zinc deficiency in populations. Stunting prevalence is expressed as the percentage of children under 5 years of age with height-for-age below the expected range of a reference population (i.e., <2.0 standard deviations with respect to the reference median). WHO considers a prevalence of stunting greater than 20% of the population to indicate a public health concern.[11] As zinc deficiency is not the only factor affecting children's growth, assessment of dietary zinc intake and serum zinc levels can be used to confirm the risk of zinc deficiency in these high-risk countries.[11]
The causes and etiology of stunting include the following: (1) Nutrition (energy, macronutrients, micronutrients and toxic factors); (2) infection (injury to gastrointestinal mucosa, systemic effects and immunostimulation); and 3) mother-infant interaction (maternal nutrition and stores at birth, and behavioral interactions).[80]
Growth stunting could be the consequence of deficiency of one or several nutrients. In communities in which stunting is prevalent, it is highly likely that several nutrient deficiencies occur simultaneously in the stunted children. In a study in a rural community in Mexico, 82% of children 18-36 months of age were deficient in at least two micronutrients out of five that were determined (iron, zinc, vitamin A, vitamin B-12, and riboflavin).[81] Results of studies with single nutrient supplementation are conflicting; thus, that there is no consistent evidence for any nutrient that its use for supplementation will promote linear growth. In the case of Mexican preschoolers in whom deficiencies of multiple micronutrients were demonstrated, no effect on linear growth was found after 1 year of supplementation with zinc and/or iron. Although, supplementation with multiple micronutrients produced a significant increment in linear growth, the actual increment in height was much less than the potential increment expected.[81]
Other bio-chemical indicators of zinc status
MT
Several zinc-dependent enzymes have been shown to be affected by zinc intake or zinc status in experimental animal models and human populations. MT is a metal storage protein that is present in serum at a low concentration; the circulating concentration of MT appears to correlate with zinc intake. However, similar to several of the enzymes and to serum zinc, MT may be affected by other factors, such as infection and stress, although this has not been confirmed by direct studies. Because of these limitations and the relative difficulty of performing these assays outside the research laboratory, it is presently unlikely that they will be useful for assessment of zinc status at the population level.[82]
Exchangeable zinc pools (EZPS)
The zinc that is available for maintaining zinc-dependent functions is thought to be mobilized from small, rapidly exchanging zinc pools found primarily in the plasma and liver.[83] The size of this pool can be estimated from the tracer-tracee disappearance curves using kinetic modeling software. The total exchangeable mass varies with the length of time over which the decay curves are measured. For example, if tracer disappearance is followed for 3 h, the EZP mass is approximately 18 mg in healthy men; if the tracer disappearance is followed for 192 h, or 8 days, it is approximately 150 mg or about 10% of the whole-body zinc pool. A decline in one or more of the EZPs could be associated with a reduction in the zinc available for zinc-dependent functions, especially among rapidly turning over proteins.[84] If so, then EZP mass would provide a good indication of tissue zinc status.
EZP mass has been measured in individuals freely selecting their diets,[83] in men fed zinc-depleted diets,[85,86] and in populations with chronically low zinc intakes. Among individuals freely selecting their zinc intake, EZP mass varied directly with dietary zinc, both in individuals[83] and populations.[87] Furthermore, experimental acute, severe zinc depletion induced in adults by feeding a diet providing 0.23 mg zinc/day for 5 weeks[85] lowered total EZP by 36%. Plasma zinc concentrations declined 65% in that study suggesting that plasma zinc is more sensitive to severe zinc depletion than is EZP mass. When dietary zinc was reduced to a marginal level (4.6 mg/day) in a group of healthy men, EZP mass did not change.[86] Thus total EZP mass does not appear to be a good indicator of modest short-term changes in zinc intake. However, longer-term low intakes or acute zinc depletion causing a reduction in whole-body zinc content appears to cause a concomitant reduction in EZP.
PREVENTION OF ZINC DEFICIENCY (INTERVENTION STRATEGIES)
Numerous zinc supplementation trials have shown that a wide range of health benefits can be realized by increasing the intake of zinc where diets are inadequate in this micronutrient.[49,63] The results of these trials strongly argue for the development of programs to improve zinc status in high-risk populations. To give best results such efforts should be integrated into existing health and nutrition programs. The major intervention strategies are dietary diversification/modification, supplementation, fortification, and bio-fortification. These strategies are not mutually exclusive but can be used in a complementary way. Their choice depends upon the available resources and technical feasibility.
Dietary diversification/modification
Dietary diversification or modification is a sustainable long-term approach to improving the intake of several nutrients simultaneously. Dietary diversification or modification strategies at the community or household level have the potential to increase the intake of bio-available zinc. Such strategies include (1) Agricultural interventions (2) Production and promotion of animal-source foods through animal husbandry or aquaculture (3) Processing strategies at the commercial or household level to enhance zinc absorption from plant-based diets.[88] Agricultural interventions focused on plant-based foods may have little impact on intake of bio-available zinc. Some benefit may be realized if accompanied by processing strategies to reduce the levels of substances that inhibit zinc absorption, such as phytate, however, this is likely to be insufficient to meet zinc needs of infants and young children. Animal husbandry efforts that increase red meat or liver consumption by infants and young children can have a positive impact. Milk and cheese are also important sources of dietary zinc.[89] These foods generally have a higher content of readily absorbed zinc than poultry, eggs, or fish. Nutrition education to promote dietary diversification or modification can lead to greater intakes of animal-source foods and thus bioavailable zinc. Care must be taken to avoid potential adverse effects of the above strategies, such as aflatoxin contamination of germinated cereals, loss of water-soluble nutrients from soaking cereal flours, and displacement of breast milk by increased intakes of other foods.[88] However, more information is required on zinc content and zinc absorption modifiers in local foods to identify suitable sources of absorbable zinc. Although, dietary modification and diversification is the most sustainable approach, change of the dietary practices and preferences is difficult and foods that provide highly bio-available zinc (such as red meat) are generally expensive.
Supplementation
Supplementation programs are useful for targeting vulnerable population subgroups, which are at a particular high-risk of micronutrient deficiencies. The easiest way to supplement zinc could be to include it in programs already delivering daily or weekly nutrient supplements for the prevention of iron deficiency anemia and other micronutrient deficiencies. The recommended zinc dosages are 5 mg/day for children between 7 months and 3 years and 10 mg/day for older children.[48,57] When formulating multi-nutrient supplements, it is recommended that salts providing readily absorbable zinc, like ZnSO4, zinc gluconate or zinc acetate are used because they are absorbed more efficiently.[11]
Supplemental zinc is also recommended as an adjunct therapy during the treatment of diarrhea in children.[90] The recommended daily dosage is twice the age-specific RDA per day for 14 days; that is 10 mg/day for children under 3 years and 20 mg for older children. Several clinical trials have demonstrated that zinc supplements reduce the severity and duration of acute and persistent diarrhea.[48,63]
Fortification
Food fortification is a more cost-effective and sustainable strategy to overcome micronutrient malnutrition than supplementation. Where micronutrient deficiency is widely distributed in a population and dietary modification or diversification is difficult to achieve, fortification of centrally processed foods is an appropriate alternative. Mexico provides an example of a country with a nationwide zinc fortification program. Apart from zinc, other micronutrients are added to wheat and corn flours that are used in preparing bread and tortilla, the two principal staple foods in the country. For such multiple interventions synergistic and antagonistic interactions between micronutrients have to be taken into account during the development of appropriate formulations.[6]
Fortification programs can also be specifically targeted to increase the intake of zinc in groups of high-risk such as infants and young children who consume particular type of food. In many countries, infant formulas and complementary foods are currently fortified with zinc and other micronutrients. Commercially available standard infant formulas contain zinc in concentrations of around 1 mg/L, following current recommendations. In general, the food selected for fortification should be one that is widely consumed in stable and predictable amounts. Among several zinc compounds that are available for fortification, zinc oxide and ZnSO4 are least expensive and most commonly used by the food industry. Suggested levels for fortification of flour are 30-70 mg zinc/kg.[91] ZnSO4 theoretically provides more absorbable zinc because of its greater solubility,[92] but it is more expensive.[11] However, there are studies in humans that do not show a difference in the absorption of zinc from zinc oxide and ZnSO4.[93] Further information is required on the bio-availability of zinc, acceptability and cost of fortifying food products with different chemical forms of zinc.
Bio-fortification
Recently, plant breeding or genetic engineering strategies that either increase the level of zinc, reduce the content of inhibitors (e.g., phytate), or increase the expression of compounds that enhance zinc absorption (e.g., amino acids) have been considered to improve the bio-availability of zinc from plant foods.[94] Bio-fortification differs from ordinary fortification because it focuses on intrinsic enrichment of micronutrients in plant parts that are used for food while the plants are still growing, rather than having nutrients from external resources added to the foods when they are being processed.[95] This is an improvement on ordinary fortification when it comes to providing nutrients for the rural poor, who rarely have access to commercially fortified foods.[95] As such, bio-fortification is seen as an upcoming strategy for dealing with deficiencies of micronutrients in the developing world. Its additional benefits include higher yield where micronutrients are limiting plant growth and increased vitality of seedlings emerging from zinc-enriched seeds.
Methods of zinc bio-fortification
Bio-fortification is an agricultural strategy that aims to increase the content of select micronutrients, including zinc, in staple food crops such as rice, wheat, maize, pearl millet, and others.[96] Bio-fortification strategies include the application of zinc fertilizers and the development of crop genotypes that acquire more zinc from the soil and accumulate it in edible portions.[97] The bio-fortification strategy seeks to take advantage of the consistent daily consumption of large amounts of food staples by all family members, including, women and children as they are most at risk for micronutrient malnutrition. As a consequence of the predominance of food staples in the diets of the poor, this strategy implicitly targets low-income households.[98]
Dietary zinc intakes can be increased through a variety of interventions.[99] These include both agronomic and genetic bio-fortification of edible crops.[100,101] Agronomic bio-fortification can be achieved by increasing soil zinc phytoavailability or by applying zinc fertilizers. This requires appropriate infrastructures, however, can be very successful in regions where mineral fertilizers are used to increase crop yields and zinc is added to these at the point of manufacture or distribution.[102] Genetic bio-fortification is predicated on increasing zinc acquisition from the soil and its accumulation in edible portions. In most agricultural soils there is sufficient zinc to produce bio-fortified crops for many years, provided it becomes phytoavailable.[103] Genetic bio-fortification strategies are, of course, ineffective if there is insufficient zinc present in the soil. Most economic analyses suggest that genetic strategies toward zinc bio-fortification are more practical, enduring, and cost effective than dietary diversification, supplementation, or food fortification programs for increasing dietary zinc intakes of vulnerable populations.[99,100,101]
Application of zinc fertilizers to soil and/or foliar seems to be a practical approach to improving grain zinc concentration (e.g., agronomic bio-fortification). Very recently, a global zinc fertilizer project has been initiated, so called HarvestZinc project (www.harvestzinc.org) under HarvestPlus program. This project aims at evaluating the potential of zinc containing fertilizers for increasing zinc concentration of cereal grains (e.g., wheat, rice and wheat) and improving crop production in different target countries (e.g., India, China, Pakistan, Thailand, Turkey, Mozambique, Zimbabwe, and Brazil). The zinc fertilizer strategy represents an important complementary approach to ongoing breeding programs for developing new genotypes with high zinc density in grain. As described in HarvestZinc project (www.harvestzinc.org), bio-fortification of cereal grains through use of zinc fertilizers (e.g., agronomic bio-fortification) is required for (i) keeping sufficient amount of available zinc in soil solution, (ii) maintaining adequate zinc transport to the seeds during reproductive growth stage and (iii) optimizing the success of bio-fortification of staple food crops with zinc through use of breeding tools.
Increasing evidence is available indicating that soil and/or foliar applications of zinc fertilizers greatly contribute to grain zinc concentrations.[104] In the past, numerous studies have been published on the role of soil- and foliar-applied zinc fertilizers in order to correct zinc deficiency and increase yield. However, there are only few studies that investigated the effects of zinc fertilizers on grain zinc concentrations (or in other edible parts). ZnSO4 is the widely applied source of zinc because of its high solubility and low cost. In Central Anatolia, application of ZnSO4 fertilizers was very effective in increasing grain zinc concentration of wheat. Applying zinc fertilizer into soil doubled grain zinc concentrations[105] [Figure 1]. As presented in Figure 2, foliar-applied zinc resulted in much greater increase in grain zinc concentration than the soil application of zinc.[106] It seems that combined application of soil and foliar zinc fertilizers is the most effective way to maximize grain zinc accumulation. Besides improving grain zinc concentrations, these soil or foliar zinc applications resulted also in significant increases in plant growth [Figure 2] and grain yield in various locations in Central Anatolia.[106]
Figure 1.
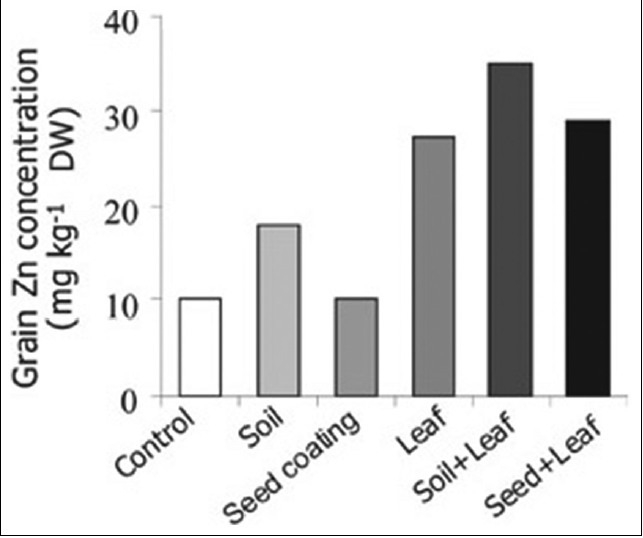
Effect of various zinc application methods on grain zinc concentration of wheat grown in Central Anatolia[105]
Figure 2.

Effect of foliar applied zinc on growth of barley plants in Central Anatolia[106]
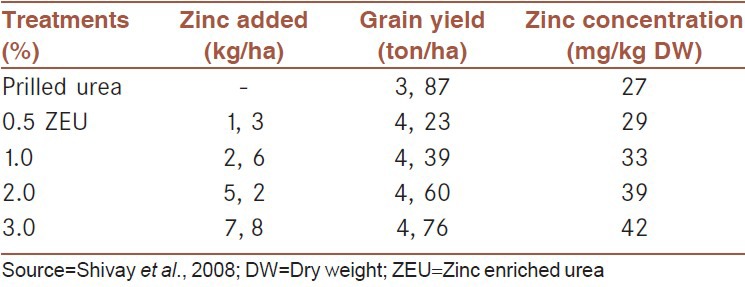
Due to significant effects of zinc fertilizers on grain yield production in Central Anatolia, farmers showed a growing interest in zinc containing fertilizers in Turkey since the mid of 1990s. In the past 10-15 years increasing amount of zinc supplemented fertilizers has been produced and applied in Turkey, especially in Central Anatolia. The total amount of zinc containing compound fertilizers applied in Turkey increased from zero in 1994 to a record level of 400,000 tons per annum. Use of such high amounts of zinc containing fertilizers increases in grain zinc concentration, and obviously contributes to human health and nutrition in Turkey, especially, in rural areas where wheat provides more than 50% of the daily calorie intake.[104] Little information is, however, available about the effectiveness of zinc containing compound fertilizers in improving grain zinc concentrations in other countries. In India, zinc enriched urea fertilizers are becoming an important source for zinc application to wheat and rice. Applying zinc coated urea fertilizers (up to 3% zinc) increased both grain yield and grain zinc concentration in rice[107] [Table 5].
Table 5.
Effect of zinc enriched urea (up to 3% zinc in urea) on grain yield and grain zinc concentrations of aromatic rice grown in India. data show average values of 2-year field trials
Recent studies also indicate that intercropping systems contribute to grain zinc and iron concentrations. Various field tests in China with peanut/maize and chickpea/wheat intercropping systems showed that gramineaceous species are highly beneficial in biofortfying dicots with micronutrients. In the case of chickpea/wheat intercropping, zinc concentration of the wheat grains was 2.8-fold higher than those of wheat under mono-cropping.[108] In many wheat-cultivated countries, continuous wheat cropping is a widely used cropping system. Inclusion of legumes in the crop rotation system may contribute to grain concentrations of wheat plants. Elevated soil organic matter content of soils up to a certain level improves solubility and root uptake of zinc, especially in alkaline soils. There are several reports on combined applications of zinc fertilizers together with organic materials (like farmyard manure and green manures) being particularly effective in facilitating zinc uptake by roots and correcting zinc deficiency.[104]
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.King JC, Cousins RJ. Zinc. In: Shils ME, Shike M, Ross AC, Caballero B, Cousins RJ, editors. Modern Nutrition in Health and Disease. 10th ed. Baltimore: Lippincott Williams and Wilkins; 2006. pp. 271–85. [Google Scholar]
- 2.Prasad AS, Miale A, Jr, Farid Z, Sandstead HH, Schulert AR. Zinc metabolism in patients with the syndrome of iron deficiency anemia, hepatosplenomegaly, dwarfism, and hypognadism. J Lab Clin Med. 1963;61:537–49. [PubMed] [Google Scholar]
- 3.Sandstead HH, Prasad AS, Schulert AR, Farid Z, Miale A, Jr, Bassilly S, et al. Human zinc deficiency, endocrine manifestations and response to treatment. Am J Clin Nutr. 1967;20:422–42. doi: 10.1093/ajcn/20.5.422. [DOI] [PubMed] [Google Scholar]
- 4.Cousins RJ. Zinc. In: Filer LJ, Ziegler EE, editors. Present Knowledge in Nutrition. 7th ed. Washington DC: International Life Science Institute Nutrition Foundation; 1996. pp. 293–306. [Google Scholar]
- 5.Cousins RJ. Absorption, transport, and hepatic metabolism of copper and zinc: Special reference to metallothionein and ceruloplasmin. Physiol Rev. 1985;65:238–309. doi: 10.1152/physrev.1985.65.2.238. [DOI] [PubMed] [Google Scholar]
- 6.2nd ed. Bangkok, Thailand: 2004. FAO/WHO. Expert Consultation on Human Vitamin and Mineral Requirements, Vitamin and mineral requirements in human nutrition: Report of joint FAO/WHO expert consolation; p. 341. [Google Scholar]
- 7.Turnlund JR, King JC, Keyes WR, Gong B, Michel MC. A stable isotope study of zinc absorption in young men: Effects of phytate and alpha-cellulose. Am J Clin Nutr. 1984;40:1071–7. doi: 10.1093/ajcn/40.5.1071. [DOI] [PubMed] [Google Scholar]
- 8.Steel L, Cousins RJ. Kinetics of zinc absorption by luminally and vascularly perfused rat intestine. Am J Physiol. 1985;248:G46–53. doi: 10.1152/ajpgi.1985.248.1.G46. [DOI] [PubMed] [Google Scholar]
- 9.Krebs NF. Overview of zinc absorption and excretion in the human gastrointestinal tract. J Nutr. 2000;130:1374S–7S. doi: 10.1093/jn/130.5.1374S. [DOI] [PubMed] [Google Scholar]
- 10.Tubek S. Selected zinc metabolism parameters in premenopausal and postmenopausal women with moderate and severe primary arterial hypertension. Biol Trace Elem Res. 2007;116:249–56. doi: 10.1007/BF02698009. [DOI] [PubMed] [Google Scholar]
- 11.International Zinc Nutrition Consultative Group (IZiNCG) Brown KH, Rivera JA, Bhutta Z, Gibson RS, King JC, et al. International Zinc Nutrition Consultative Group (IZiNCG) technical document #1. Assessment of the risk of zinc deficiency in populations and options for its control. Food Nutr Bull. 2004;25:S99–203. [PubMed] [Google Scholar]
- 12.Cousins RJ, Liuzzi JP, Lichten LA. Mammalian zinc transport, trafficking, and signals. J Biol Chem. 2006;281:24085–9. doi: 10.1074/jbc.R600011200. [DOI] [PubMed] [Google Scholar]
- 13.Devergnas S, Chimienti F, Naud N, Pennequin A, Coquerel Y, Chantegrel J, et al. Differential regulation of zinc efflux transporters ZnT-1, ZnT-5 and ZnT-7 gene expression by zinc levels: A real-time RT-PCR study. Biochem Pharmacol. 2004;68:699–709. doi: 10.1016/j.bcp.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 14.Sekler I, Sensi SL, Hershfinkel M, Silverman WF. Mechanism and regulation of cellular zinc transport. Mol Med. 2007;13:337–43. doi: 10.2119/2007-00037.Sekler. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liuzzi JP, Cousins RJ. Mammalian zinc transporters. Annu Rev Nutr. 2004;24:151–72. doi: 10.1146/annurev.nutr.24.012003.132402. [DOI] [PubMed] [Google Scholar]
- 16.McMahon RJ, Cousins RJ. Mammalian zinc transporters. J Nutr. 1998;128:667–70. doi: 10.1093/jn/128.4.667. [DOI] [PubMed] [Google Scholar]
- 17.McMahon RJ, Cousins RJ. Regulation of the zinc transporter ZnT-1 by dietary zinc. Proc Natl Acad Sci U S A. 1998;95:4841–6. doi: 10.1073/pnas.95.9.4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davis SR, McMahon RJ, Cousins RJ. Metallothionein knockout and transgenic mice exhibit altered intestinal processing of zinc with uniform zinc-dependent zinc transporter-1 expression. J Nutr. 1998;128:825–31. doi: 10.1093/jn/128.5.825. [DOI] [PubMed] [Google Scholar]
- 19.Banci L, Bertini I, Ciofi-Baffoni S, Finney LA, Outten CE, O’Halloran TV. A new zinc-protein coordination site in intracellular metal trafficking: Solution structure of the Apo and Zn (II) forms of ZntA (46-118) J Mol Biol. 2002;323:883–97. doi: 10.1016/s0022-2836(02)01007-0. [DOI] [PubMed] [Google Scholar]
- 20.Eren E, Kennedy DC, Maroney MJ, Argüello JM. A novel regulatory metal binding domain is present in the C terminus of Arabidopsis Zn2+-ATPase HMA2. J Biol Chem. 2006;281:33881–91. doi: 10.1074/jbc.M605218200. [DOI] [PubMed] [Google Scholar]
- 21.Petrukhin K, Lutsenko S, Chernov I, Ross BM, Kaplan JH, Gilliam TC. Characterization of the Wilson disease gene encoding a P-type copper transporting ATPase: Genomic organization, alternative splicing, and structure/function predictions. Hum Mol Genet. 1994;3:1647–56. doi: 10.1093/hmg/3.9.1647. [DOI] [PubMed] [Google Scholar]
- 22.Sensi SL, Canzoniero LM, Yu SP, Ying HS, Koh JY, Kerchner GA, et al. Measurement of intracellular free zinc in living cortical neurons: Routes of entry. J Neurosci. 1997;17:9554–64. doi: 10.1523/JNEUROSCI.17-24-09554.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohana E, Segal D, Palty R, Ton-That D, Moran A, Sensi SL, et al. A sodium zinc exchange mechanism is mediating extrusion of zinc in mammalian cells. J Biol Chem. 2004;279:4278–84. doi: 10.1074/jbc.M309229200. [DOI] [PubMed] [Google Scholar]
- 24.Hambidge M, Krebs NF. Interrelationships of key variables of human zinc homeostasis: Relevance to dietary zinc requirements. Annu Rev Nutr. 2001;21:429–52. doi: 10.1146/annurev.nutr.21.1.429. [DOI] [PubMed] [Google Scholar]
- 25.King JC, Shames DM, Woodhouse LR. Zinc homeostasis in humans. J Nutr. 2000;130:1360S–6S. doi: 10.1093/jn/130.5.1360S. [DOI] [PubMed] [Google Scholar]
- 26.King JC. Determinants of maternal zinc status during pregnancy. Am J Clin Nutr. 2000;71:1334S–43S. doi: 10.1093/ajcn/71.5.1334s. [DOI] [PubMed] [Google Scholar]
- 27.Johnson PE, Hunt CD, Milne DB, Mullen LK. Homeostatic control of zinc metabolism in men: Zinc excretion and balance in men fed diets low in zinc. Am J Clin Nutr. 1993;57:557–65. doi: 10.1093/ajcn/57.4.557. [DOI] [PubMed] [Google Scholar]
- 28.Fairweather-Tait S, Hurrell RF. Bioavailability of minerals and trace elements. Nutr Res Rev. 1996;9:295–324. doi: 10.1079/NRR19960016. [DOI] [PubMed] [Google Scholar]
- 29.Lonnerdal B. Dietary factors influencing zinc absorption. J Nutr. 2000;130:S1378–83. doi: 10.1093/jn/130.5.1378S. [DOI] [PubMed] [Google Scholar]
- 30.Sandström B, Davidsson L, Eriksson R, Alpsten M. Effect of long-term trace element supplementation on blood trace element levels and absorption of (75Se), (54Mn) and (65Zn) J Trace Elem Electrolytes Health Dis. 1990;4:65–72. [PubMed] [Google Scholar]
- 31.Istfan NW, Janghorbani M, Young VR. Absorption of stable 70 Zn in healthy young men in relation to zinc intake. Am J Clin Nutr. 1983;38:187–94. doi: 10.1093/ajcn/38.2.187. [DOI] [PubMed] [Google Scholar]
- 32.Wada L, Turnlund JR, King JC. Zinc utilization in young men fed adequate and low zinc intakes. J Nutr. 1985;115:1345–54. doi: 10.1093/jn/115.10.1345. [DOI] [PubMed] [Google Scholar]
- 33.August D, Janghorbani M, Young VR. Determination of zinc and copper absorption at three dietary Zn-Cu ratios by using stable isotope methods in young adult and elderly subjects. Am J Clin Nutr. 1989;50:1457–63. doi: 10.1093/ajcn/50.6.1457. [DOI] [PubMed] [Google Scholar]
- 34.Hambidge KM, Miller LV, Krebs NF. Physiological requirements for zinc. Int J Vitam Nutr Res. 2011;81:72–8. doi: 10.1024/0300-9831/a00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lopez HW, Leenhardt F, Coudray C, Remesy C. Minerals and phytic acid interactions: Is it a real problem for human nutrition? Int J Food Sci Tech. 2002;37:727–39. [Google Scholar]
- 36.Allen L, Benoist B, Dary O, Hurrell R. Guidelines on Food Fortification with Micronutrients. In: Allen L, de Benoist B, Dary O, Hurrell R, editors. Geneva: WHO and FAO; 2006. p. 376. [Google Scholar]
- 37.Hunt JR, Beiseigel JM. Dietary calcium does not exacerbate phytate inhibition of zinc absorption by women from conventional diets. Am J Clin Nutr. 2009;89:839–43. doi: 10.3945/ajcn.2008.27175. [DOI] [PubMed] [Google Scholar]
- 38.Hurrell RF. Phytic acid degradation as a means of improving iron absorption. Int J Vitam Nutr Res. 2004;74:445–52. doi: 10.1024/0300-9831.74.6.445. [DOI] [PubMed] [Google Scholar]
- 39.Schlemmer U, Frølich W, Prieto RM, Grases F. Phytate in foods and significance for humans: Food sources, intake, processing, bioavailability, protective role and analysis. Mol Nutr Food Res. 2009;53:S330–75. doi: 10.1002/mnfr.200900099. [DOI] [PubMed] [Google Scholar]
- 40.Solomons NW, Jacob RA. Studies on the bioavailability of zinc in humans: Effects of heme and nonheme iron on the absorption of zinc. Am J Clin Nutr. 1981;34:475–82. doi: 10.1093/ajcn/34.4.475. [DOI] [PubMed] [Google Scholar]
- 41.Davidsson L, Almgren A, Sandström B, Hurrell RF. Zinc absorption in adult humans: The effect of iron fortification. Br J Nutr. 1995;74:417–25. doi: 10.1079/bjn19950145. [DOI] [PubMed] [Google Scholar]
- 42.Fairweather-Tait SJ, Wharf SG, Fox TE. Zinc absorption in infants fed iron-fortified weaning food. Am J Clin Nutr. 1995;62:785–9. doi: 10.1093/ajcn/62.4.785. [DOI] [PubMed] [Google Scholar]
- 43.Sandström B, Almgren A, Kivistö B, Cederblad A. Effect of protein level and protein source on zinc absorption in humans. J Nutr. 1989;119:48–53. doi: 10.1093/jn/119.1.48. [DOI] [PubMed] [Google Scholar]
- 44.McDowell LR. 2nd ed. Amsterdam: Elsevier Science; 2003. Zinc.Minerals in Animal and Human Nutrition; p. 660. [Google Scholar]
- 45.Hambidge KM, Casey CE, Kreps NF. Zinc. In: Mez W, editor. Trace Elements in Human and Animal Nutrition. Orlando: Academic Press; 1986. pp. 1–37. [Google Scholar]
- 46.Geneva: WHO; 2002. WHO, FAO, IAEA. Trace Elements in Human Health and Nutrition; pp. 230–45. [Google Scholar]
- 47.Food and Nutrition Board/Institute of Medicine. Washington DC: National Academy Press; 2002. Dietary Reference Intakes of Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon and Zinc; p. ???. [Google Scholar]
- 48.Müller O, Becher H, van Zweeden AB, Ye Y, Diallo DA, Konate AT, et al. Effect of zinc supplementation on malaria and other causes of morbidity in west African children: Randomised double blind placebo controlled trial. BMJ. 2001;322:1567. doi: 10.1136/bmj.322.7302.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maret W, Sandstead HH. Zinc requirements and the risks and benefits of zinc supplementation. J Trace Elem Med Biol. 2006;20:3–18. doi: 10.1016/j.jtemb.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 50.O’Brien KO, Zavaleta N, Caulfield LE, Wen J, Abrams SA. Prenatal iron supplements impair zinc absorption in pregnant Peruvian women. J Nutr. 2000;130:2251–5. doi: 10.1093/jn/130.9.2251. [DOI] [PubMed] [Google Scholar]
- 51.Fung EB, Ritchie LD, Woodhouse LR, Roehl R, King JC. Zinc absorption in women during pregnancy and lactation: A longitudinal study. Am J Clin Nutr. 1997;66:80–8. doi: 10.1093/ajcn/66.1.80. [DOI] [PubMed] [Google Scholar]
- 52.Whittaker P. Iron and zinc interactions in humans. Am J Clin Nutr. 1998;68:S442–6. doi: 10.1093/ajcn/68.2.442S. [DOI] [PubMed] [Google Scholar]
- 53.Andriollo-Sanchez M, Hininger-Favier I, Meunier N, Toti E, Zaccaria M, Brandolini-Bunlon M, et al. Zinc intake and status in middle-aged and older European subjects: The ZENITH study. Eur J Clin Nutr. 2005;59:S37–41. doi: 10.1038/sj.ejcn.1602296. [DOI] [PubMed] [Google Scholar]
- 54.Hambidge KM, Walravens PA. Disorders of mineral metabolism. Clin Gastroenterol. 1982;11:87–117. [PubMed] [Google Scholar]
- 55.Van Wouwe JP. Clinical and laboratory diagnosis of acrodermatitis enteropathica. Eur J Pediatr. 1989;149:2–8. doi: 10.1007/BF02024322. [DOI] [PubMed] [Google Scholar]
- 56.Hambidge KM. Human zinc deficiency. J Nutr. 2000;130:S1344–9. doi: 10.1093/jn/130.5.1344S. [DOI] [PubMed] [Google Scholar]
- 57.Brown KH, Peerson JM, Rivera J, Allen LH. Effect of supplemental zinc on the growth and serum zinc concentrations of prepubertal children: A meta-analysis of randomized controlled trials. Am J Clin Nutr. 2002;75:1062–71. doi: 10.1093/ajcn/75.6.1062. [DOI] [PubMed] [Google Scholar]
- 58.Anderson JJ. Minerals. In: Mahan LK, Escott-stump S, editors. Krause's Food, Nutrition and Diet Therapy. USA: WB Saunders Co; 2004. pp. 120–63. [Google Scholar]
- 59.Hambidge KM. Zinc and diarrhea. Acta Paediatr Suppl. 1992;381:82–6. doi: 10.1111/j.1651-2227.1992.tb12377.x. [DOI] [PubMed] [Google Scholar]
- 60.Ghishan FK. Transport of electrolytes, water, and glucose in zinc deficiency. J Pediatr Gastroenterol Nutr. 1984;3:608–12. doi: 10.1097/00005176-198409000-00022. [DOI] [PubMed] [Google Scholar]
- 61.Brown KH, Peerson JM, Allen LH. Effect of zinc supplementation on children's growth: A meta-analysis of intervention trials. In: Sandström B, Walter P, editors. Role of Trace Elements for Health Promotion and Disease Prevention. California: S Karger Pub; 1998. pp. 76–83. [DOI] [PubMed] [Google Scholar]
- 62.Bhutta ZA, Black RE, Brown KH, Gardner JM, Gore S, Hidayat A, et al. Prevention of diarrhea and pneumonia by zinc supplementation in children in developing countries: Pooled analysis of randomized controlled trials. Zinc Investigators’ Collaborative Group. J Pediatr. 1999;135:689–97. doi: 10.1016/s0022-3476(99)70086-7. [DOI] [PubMed] [Google Scholar]
- 63.Shankar AH, Genton B, Baisor M, Paino J, Tamja S, Adiguma T, et al. The influence of zinc supplementation on morbidity due to Plasmodium falciparum: A randomized trial in preschool children in Papua New Guinea. Am J Trop Med Hyg. 2000;62:663–9. doi: 10.4269/ajtmh.2000.62.663. [DOI] [PubMed] [Google Scholar]
- 64.Veenemans J, Milligan P, Prentice AM, Schouten LR, Inja N, van der Heijden AC, et al. Effect of supplementation with zinc and other micronutrients on malaria in Tanzanian children: A randomised trial. PLoS Med. 2011;8:e1001125. doi: 10.1371/journal.pmed.1001125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kelishadi R, Hashemipour M, Adeli K, Tavakoli N, Movahedian-Attar A, Shapouri J, et al. Effect of zinc supplementation on markers of insulin resistance, oxidative stress, and inflammation among prepubescent children with metabolic syndrome. Metab Syndr Relat Disord. 2010;8:505–10. doi: 10.1089/met.2010.0020. [DOI] [PubMed] [Google Scholar]
- 66.Hashemipour M, Kelishadi R, Shapouri J, Sarrafzadegan N, Amini M, Tavakoli N, et al. Effect of zinc supplementation on insulin resistance and components of the metabolic syndrome in prepubertal obese children. Hormones (Athens) 2009;8:279–85. doi: 10.14310/horm.2002.1244. [DOI] [PubMed] [Google Scholar]
- 67.Kelishadi R, Alikhassy H, Amiri M. Zinc and copper status in children with high family risk of premature cardiovascular disease. Ann Saudi Med. 2002;22:291–4. doi: 10.5144/0256-4947.2002.291. [DOI] [PubMed] [Google Scholar]
- 68.Maret W. Zinc biochemistry, physiology, and homeostasis: Recent insights and current trends. BioMetals. 2001;14:187–90. [Google Scholar]
- 69.Hotz C, Peerson JM, Brown KH. Suggested lower cutoffs of serum zinc concentrations for assessing zinc status: Reanalysis of the second National Health and Nutrition Examination Survey data (1976-1980) Am J Clin Nutr. 2003;78:756–64. doi: 10.1093/ajcn/78.4.756. [DOI] [PubMed] [Google Scholar]
- 70.Hambidge KM, Goodall MJ, Stall C, Pritts J. Post-prandial and daily changes in plasma zinc. J Trace Elem Electrolytes Health Dis. 1989;3:55–7. [PubMed] [Google Scholar]
- 71.Couturier E, van Onderbergen A, Bosson D, Neve J. Circadian variations in plasma zinc and cortisol in man. J Trace Elem Electrolytes Health Dis. 1988;2:245–9. [PubMed] [Google Scholar]
- 72.Goode HF, Robertson DA, Kelleher J, Walker BE. Effect of fasting, self-selected and isocaloric glucose and fat meals and intravenous feeding on plasma zinc concentrations. Ann Clin Biochem. 1991;28:442–5. doi: 10.1177/000456329102800503. [DOI] [PubMed] [Google Scholar]
- 73.Guillard O, Piriou A, Gombert J, Reiss D. Diurnal variations of zinc, copper and magnesium in the serum of normal fasting adults. Biomedicine. 1979;31:193–4. [PubMed] [Google Scholar]
- 74.Singh A, Smoak BL, Patterson KY, LeMay LG, Veillon C, Deuster PA. Biochemical indices of selected trace minerals in men: Effect of stress. Am J Clin Nutr. 1991;53:126–31. doi: 10.1093/ajcn/53.1.126. [DOI] [PubMed] [Google Scholar]
- 75.Schroeder JJ, Cousins RJ. Interleukin 6 regulates metallothionein gene expression and zinc metabolism in hepatocyte monolayer cultures. Proc Natl Acad Sci U S A. 1990;87:3137–41. doi: 10.1073/pnas.87.8.3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rofe AM, Philcox JC, Coyle P. Trace metal, acute phase and metabolic response to endotoxin in metallothionein-null mice. Biochem J. 1996;314:793–7. doi: 10.1042/bj3140793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Prasad AS. Laboratory diagnosis of zinc deficiency. J Am Coll Nutr. 1985;4:591–8. doi: 10.1080/07315724.1985.10720101. [DOI] [PubMed] [Google Scholar]
- 78.Solomons NW. On the assessment of zinc and copper nutriture in man. Am J Clin Nutr. 1979;32:856–71. doi: 10.1093/ajcn/32.4.856. [DOI] [PubMed] [Google Scholar]
- 79.Hobisch-Hagen P, Mörtl M, Schobersberger W. Hemostatic disorders in pregnancy and the peripartum period. Acta Anaesthesiol Scand Suppl. 1997;111:216–7. [PubMed] [Google Scholar]
- 80.Frongillo EA., Jr Symposium: Causes and Etiology of Stunting. Introduction. J Nutr. 1999;129:529S–30S. doi: 10.1093/jn/129.2.529S. [DOI] [PubMed] [Google Scholar]
- 81.Rosado JL. Separate and joint effects of micronutrient deficiencies on linear growth. J Nutr. 1999;129:531S–3S. doi: 10.1093/jn/129.2.531S. [DOI] [PubMed] [Google Scholar]
- 82.Lowe NM, Fekete K, Decsi T. Methods of assessment of zinc status in humans: A systematic review. Am J Clin Nutr. 2009;89:2040S–51S. doi: 10.3945/ajcn.2009.27230G. [DOI] [PubMed] [Google Scholar]
- 83.Miller LV, Hambidge KM, Naake VL, Hong Z, Westcott JL, Fennessey PV. Size of the zinc pools that exchange rapidly with plasma zinc in humans: Alternative techniques for measuring and relation to dietary zinc intake. J Nutr. 1994;124:268–76. doi: 10.1093/jn/124.2.268. [DOI] [PubMed] [Google Scholar]
- 84.Golden MH. The diagnosis of zinc deficiency. In: Mills CF, editor. Zinc in Human Biology. London: Springer-Verlag; 1989. pp. 323–33. [Google Scholar]
- 85.King JC, Shames DM, Lowe NM, Woodhouse LR, Sutherland B, Abrams SA, et al. Effect of acute zinc depletion on zinc homeostasis and plasma zinc kinetics in men. Am J Clin Nutr. 2001;74:116–24. doi: 10.1093/ajcn/74.1.116. [DOI] [PubMed] [Google Scholar]
- 86.Pinna K, Woodhouse LR, Sutherland B, Shames DM, King JC. Exchangeable zinc pool masses and turnover are maintained in healthy men with low zinc intakes. J Nutr. 2001;131:2288–94. doi: 10.1093/jn/131.9.2288. [DOI] [PubMed] [Google Scholar]
- 87.Sian L, Mingyan X, Miller LV, Tong L, Krebs NF, Hambidge KM. Zinc absorption and intestinal losses of endogenous zinc in young Chinese women with marginal zinc intakes. Am J Clin Nutr. 1996;63:348–53. doi: 10.1093/ajcn/63.3.348. [DOI] [PubMed] [Google Scholar]
- 88.Gibson RS, Anderson VP. A review of interventions based on dietary diversification or modification strategies with the potential to enhance intakes of total and absorbable zinc. Food Nutr Bull. 2009;30:S108–43. doi: 10.1177/15648265090301S107. [DOI] [PubMed] [Google Scholar]
- 89.Roohani N, Hurrell R, Wegmueller R, Schulin R. Zinc and phytic acid in major foods consumed by a rural and a suburban population in central Iran. J Food Comp Anal. 2012;28:8–15. [Google Scholar]
- 90.Fontaine O. Effect of zinc supplementation on clinical course of acute diarrhoea. J Health Popul Nutr. 2001;19:339–46. [PubMed] [Google Scholar]
- 91.Geneva: World Health Organization; 2009. [Last accessed 2012 Oct 11]. WHO. Recommendations on wheat and maize flour fortification. Meeting Report: Interim Consensus Statement (WHO, FAO, UNICEF, GAIN, MI, FFI) Available from: http://www.who.int/nutrition/publications/micronutrients/wheat_maize_fort.pdf . [PubMed] [Google Scholar]
- 92.Wolfe SA, Gibson RS, Gadowsky SL, O’Connor DL. Zinc status of a group of pregnant adolescents at 36 weeks gestation living in southern Ontario. J Am Coll Nutr. 1994;13:154–64. doi: 10.1080/07315724.1994.10718389. [DOI] [PubMed] [Google Scholar]
- 93.Hotz C, DeHaene J, Woodhouse LR, Villalpando S, Rivera JA, King JC. Zinc absorption from zinc oxide, zinc sulfate, zinc oxide+EDTA, or sodium-zinc EDTA does not differ when added as fortificants to maize tortillas. J Nutr. 2005;135:1102–5. doi: 10.1093/jn/135.5.1102. [DOI] [PubMed] [Google Scholar]
- 94.Lönnerdal B. Genetically modified plants for improved trace element nutrition. J Nutr. 2003;133:1490S–3S. doi: 10.1093/jn/133.5.1490S. [DOI] [PubMed] [Google Scholar]
- 95.Banuelos G, Lin ZQ. Florida: CRC Press, Boca Raton; 2009. Phytoremediation of selenium-contaminated soil and water produces biofortified products and new agricultural byproducts. Development and Uses of Biofortified Agricultural Products; pp. 57–70. [Google Scholar]
- 96.Hotz C. The potential to improve zinc status through biofortification of staple food crops with zinc. Food Nutr Bull. 2009;30:S172–8. doi: 10.1177/15648265090301S109. [DOI] [PubMed] [Google Scholar]
- 97.White PJ, Broadley MR. Physiological limits to zinc biofortification of edible crops. Front Plant Sci. 2011;2:80. doi: 10.3389/fpls.2011.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bouis HE. Micronutrient fortification of plants through plant breeding: Can it improve nutrition in man at low cost? Proc Nutr Soc. 2003;62:403–11. doi: 10.1079/pns2003262. [DOI] [PubMed] [Google Scholar]
- 99.Stein AJ. Global impacts of human mineral malnutrition. Plant Soil. 2010;335:133–54. [Google Scholar]
- 100.Graham RD, Welch RM, Saunders DA, Ortiz-Monasterio I, Bouis HE, Bonierbale M, et al. Nutritious subsistence food systems. Adv Agron. 2007;92:1–74. [Google Scholar]
- 101.Bouis HE, Welch RM. Biofortification: A sustainable agricultural strategy for reducing micronutrient malnutrition in the global south. Crop Sci. 2010;50:S20–32. [Google Scholar]
- 102.Cakmak I. Enrichment of fertilizers with zinc: An excellent investment for humanity and crop production in India. J Trace Elem Med Biol. 2009;23:281–9. doi: 10.1016/j.jtemb.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 103.Graham R, Senadhira D, Beebe S, Iglesias C, Monasterio I. Breeding for micronutrient density in edible portions of staple food crops: Conventional approaches. Field Crops Res. 1999;60:57–80. [Google Scholar]
- 104.Cakmak I. Enrichment of cereal grains with zinc: Agronomic or genetic biofortification? Plant Soil. 2008;302:1–17. [Google Scholar]
- 105.Yilmaz A, Ekiz H, Torun B, Gultekin I, Karanlik S, Bagci SA, et al. Effect of different zinc application methods on grain yield and zinc concentration in wheat grown on zinc-deficient calcareous soils in Central Anatolia. J Plant Nutr. 1997;20:461–71. [Google Scholar]
- 106.Cakmak I, Yilmaz A, Ekiz H, Torun B, Erenoglu B, Braun HJ. Zinc deficiency a critical nutritional problem in wheat production in central Anatolia. Plant Soil. 1996;180:165–72. [Google Scholar]
- 107.Shivay YS, Kumar D, Prasad R. Effect of zinc-enriched urea on productivity, zinc uptake and efficiency of an aromatic rice-wheat cropping system. Nutr Cycl Agroecosyst. 2008;81:229–43. [Google Scholar]
- 108.Zuo Y, Zhang F. Iron and zinc biofortification strategies in dicot plants by intercropping with gramineous species. A review. Agron Sust Dev. 2009;29:63–71. [Google Scholar]


