Abstract
Background:
This randomized clinical trial compared rates of stroke or transient ischemic attack recurrence or death in patients with cryptogenic stroke and patent foramen ovale (PFO) who received medical treatment with aspirin or warfarin.
Materials and Methods:
Forty-four Iranian patients with cryptogenic stroke and patent foramen ovale participated in this randomized, single-blind trial between July 2007 and June 2010. All patients underwent transesophageal echocardiography and contrast-transcranial Doppler sonography to confirm the presence of patent foramen ovale. The patients were randomly assigned to receive aspirin or warfarin and were followed for 18 months for the recurrence of ischemic events or death. The principal investigator was blind to the group assignment. This trial is registered under number IRCT138805192323N1.
Results:
Five (11.4%) patients had a stroke, 2 (4.5%) had a transient ischemic attack and 2 (4.5%) died. There was no difference in the rate of ischemic events or death between the aspirin- and warfarin-treated groups (hazard ratio: 0.45; 95% CI: 0.1-1.8; P = 0.259).
Conclusion:
There was no difference in ischemic event recurrence, death rates or side-effects between patients with cryptogenic stroke and patent foramen ovale who were treated with aspirin vs. warfarin.
Keywords: Aspirin, cryptogenic stroke, patent foramen ovale, warfarin
INTRODUCTION
The etiology of ischemic stroke remains unidentified despite standard diagnostic work-ups, and in 30.6-42.1% of patients the cause is recorded as cryptogenic stroke.[1,2] Patent foramen ovale (PFO) is a hemodynamically insignificant inter-atrial communication which has been suggested to be the cause of emboli and cryptogenic stroke.[2,3,4] However, the causal relationship of PFO with cryptogenic stroke is still a matter of debate, and PFO can be found incidentally without any clinical implication in this situation.[5]
The best preventive medical treatment for co-occurrence of PFO with cryptogenic stroke remains to be elucidated. Previous studies failed to show any superiority for any drug in the treatment of this situation. To our knowledge no such study had been conducted to evaluate Iranian patients with cryptogenic stroke and PFO. Stroke is a major health issue in Iran. According to one population-based study, the incidence of stroke (particularly ischemic stroke) in Iran is greater than in most Western countries.[6] In addition, there are important inter-ethnic differences in the metabolic capacity to clear aspirin and warfarin between Iranian people and people in other countries.-[7] These considerations led us to evaluate the effects of two medical treatments in a sample of Iranian patients and to compare them to the results in industrialized countries.
Here we studied the rate of stroke or transient ischemic attack recurrence, or death, in cryptogenic stroke patients with PFO with regard to the treatment in a single-blind design in an Iranian population. We hypothesized that the rates of death or recurrence of stroke and transient ischemic attack would be different in the two groups.
MATERIALS AND METHODS
This single-center, single-blind, non-placebo-controlled, two parallel-group, prospective study was conducted at the neurology and cardiology departments of Nemazee Hospital, a tertiary hospital affiliated to the Shiraz University of Medical Sciences in Shiraz, Iran, from July 2007 to June 2010.
Inclusion criteria were adult age (≥18 years), single recent (within 30 days of enrollment) transient ischemic attack or stroke which fulfilled the criteria for unknown subgroup of undetermined causes of stroke according to the Causative classification of stroke modified Trial of Org 10172 in Acute Stroke Treatment criteria (CCS-TOAST) classification,[8] and presence of PFO confirmed by both transesophageal echocardiographic (TEE) and contrast-transcranial Doppler sonography (c-TCD) examination.
The main criterion for exclusion was the presence of any determined cause for ischemic stroke according to the CCS-TOAST classification. These included: (1) Evident large-artery atherosclerosis defined as >50% stenosis or occlusion of a major brain artery or branch cortical artery; (2) unequivocal cardiac source of embolism defined as chronic or paroxysmal atrial fibrillation, mitral stenosis, mechanical heart valve, endocarditis, intracardiac clot or vegetation, myocardial infarction within 3 months, dilated cardiomyopathy, and ejection fraction less than 30%; (3) small-vessel disease defined as cortical, cerebellar, brainstem or subcortical infarct <1.5 cm; (4) other determined cause of stroke defined as any known vasculitis, any known thrombophilic disease, any infectious vasculopathy, arterial dissection, Moya Moya disease, radiation-induced vasculopathy, fibromuscular dysplasia, sickle cell disease, neurofibromatosis, reversible cerebral vasoconstriction syndrome, vasospasm after subarachnoid hemorrhage, and cerebral sinus venous thrombosis. Patients without a suitable temporal window for performance of c-TCD or with poor clinical condition were excluded. In addition, patients with severe aphasia (impaired comprehension), severe disabling stroke (score of 4 or 5 on the modified Rankin scale), and known dementia were excluded.
The study was approved by the Institutional Review Board of the Shiraz University of Medical Sciences (No. 88-4639) and is registered with the Iranian registry for clinical trials (www.irct.ir, registration number: IRCT138805192323N1). The study and possible outcomes were explained to all participants or their first-degree relatives and written informed consent was obtained for each participant.
Consecutive patients fulfilling all inclusion and exclusion criteria were enrolled by the principal investigators. Eligible patients who agreed to participate in the study were randomized to two treatment groups according to a computer-generated random number list and simple randomization procedure [Figure 1]. Eligible patients were referred by the principal investigators to a research assistant for randomization and allocation. The principal investigators and statistician were blind to the therapeutic interventions, but patients and the research assistant who allocated the patients and monitored the drug effects and adverse drug reactions were not blinded.
Figure 1.
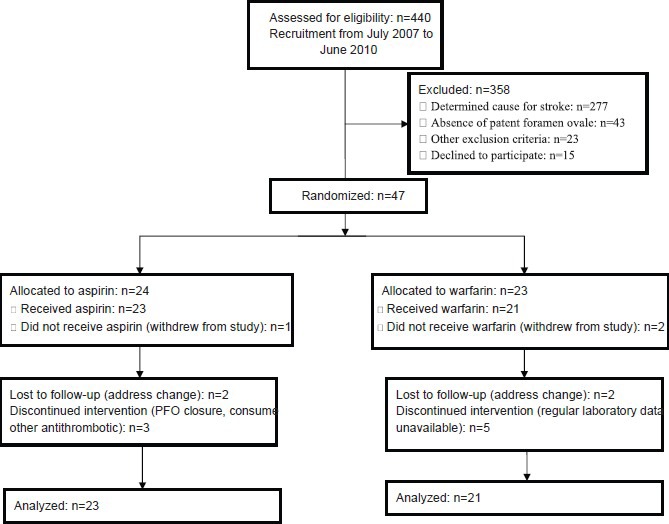
Flowchart for a single-center, single-blind, non-placebo-controlled, parallel-group randomized trial of aspirin versus warfarin in patients with cryptogenic stroke and patent foramen ovale in Iran, 2007-2010
For each participant a questionnaire was completed that included items on demographic characteristics, a checklist of inclusion and exclusion criteria, the results of basic laboratory tests and findings on neurological imaging studies. Transesophageal echocardiography and c-TCD were done for all eligible patients.
A Vivid 3 Echo machine with a 5-MHz TEE probe was used (GE Medical Systems, Oslo, Norway). Contrast (agitated saline) was injected in mid-esophageal four-chamber view during the Valsalva maneuver to detect any inter-atrial right-to-left shunt across the PFO. Agitated saline was generated by agitating a mixture of 9 mL normal saline and 1 mL air in two 10-mL syringes connected by a 3-way stopcock. Once the contrast became milky, 10 mL of the contrast was rapidly injected as a bolus into a cubital vein that had previously been cannulated with a large-gauge indwelling intravenous catheter. The diagnosis of PFO was based on the appearance of bubbles in the left atrium during five cardiac cycles after opacification of the right atrium with contrast bubbles. The maximal diameter of the PFO was measured in the same view. PFOs with a diameter < 4 mm were considered small and those ≥ 4 mm were considered large.
A Sunray version FD-T98II transcranial Doppler ultrasound device (Guangzhou Doppler Electronic Technologies, Guangzhou, China) was used for c-TCD examinations. While the patient was lying supine, both middle cerebral arteries were insonated simultaneously. Two 2-MHz transducers were fixed on the temporal windows with a helmet. The device was set to a small sample volume of 10 mm in length and minimum possible gain to provide an optimal setting for microembolic signal discrimination from the background spectrum. We defined microembolic signal as a typical visible and audible signal (click, chirp or whistle) of short duration and high intensity within the Doppler flow spectrum. The same protocol was used for contrast preparation and injection and the Valsalva maneuver as for TEE.
Patients were randomly assigned to aspirin (acetylsalicylic acid, Jalinous, Teheran, Iran), 80 mg orally 3 times daily, or warfarin (Marevan, Orion Pharma, Espoo, Finland), started at 2.5 mg orally once daily, and then adjusted to achieve an international normalized ratio target of 2 to 3. The interventions lasted for 18 months, after which the patients were free to select any therapeutic option. In the case of adverse drug reactions, the study drug was withheld temporarily and the patient was encouraged to consult a relevant specialist.
Suspected adverse drug reactions of aspirin and warfarin were followed up scrupulously by phone calls (weekly during the first month and monthly thereafter). The patients were specifically questioned regarding major hemorrhage (gastrointestinal bleeding or intracranial hemorrhage). In the warfarin-treated group, international normalized ratio was evaluated weekly during the first month, biweekly during months 2 and 3, and monthly thereafter. Patients who did not have regular blood tests for international normalized ratio for any reason during the study period were excluded from the final analysis.
The primary endpoint was recurrence of ischemic event (transient ischemic attack or stroke) or death due to any cause. The secondary endpoints were recurrence of myocardial infarction, and death due to vascular causes (stroke, myocardial infarction, and intracranial hemorrhages). Information about the occurrence of endpoints was sought monthly by phone, and the research assistant visited the patient (or next of kin) if an endpoint occurred.
All statistical analyses were done with SPSS v. 15.0 software (SPSS, Chicago, IL, USA). The population comprised all patients admitted to our hospital during one year with cryptogenic stroke. The results are expressed as absolute frequencies and percentages where appropriate. Descriptive results are presented as the mean value ± standard deviation. The Chi-square test and Mann-Whitney U test were used to compare variables between groups. Kaplan-Meier survival analysis was used to compare cumulative survival in the treatment groups. Patients were censored if they were lost to follow-up, in the case of PFO closure and at the end of follow-up. The log-rank and Cox proportional-hazards models were used to assess the predictive value of treatment with respect to primary and secondary endpoints, and the models were adjusted for age, sex, hypertension, diabetes mellitus, hyperlipidemia and smoking. A P value < 0.05 was considered as significant.
RESULTS
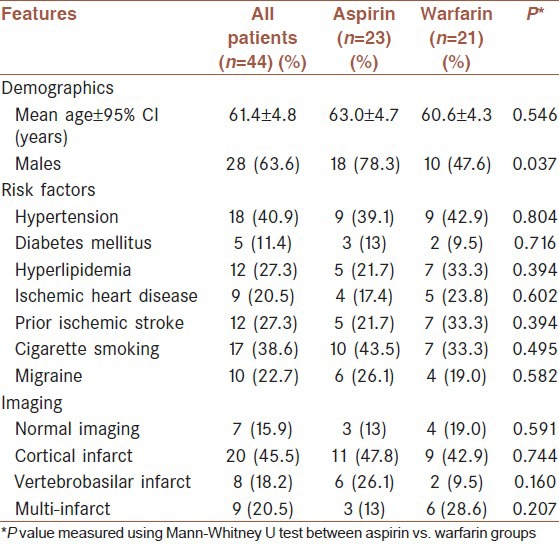
Of the 440 patients with ischemic stroke or transient ischemic attack admitted to our stroke center during July 2007 to January 2009, 29.1% (128/440) had stroke of undetermined cause according to the CCS-TOAST classification. Based on the TEE and c-TCD examinations, 19.3% (85/440) of patients were diagnosed as having a PFO. Of these, 44.7% (38/85) were excluded due to the presence of at least one exclusion criterion or because they declined to participate in the study. Of the intent-to-treat population (47 patients), 23 patients were initially treated with warfarin and 24 patients with aspirin. Three patients withdrew from the study. Therefore, the per-protocol population consisted of 44 patients. Of these, 52.3% (23/44) received aspirin and 47.7% (21/44) received warfarin. The demographic and clinical characteristics of the per-protocol patients are shown in Table 1. Most patients (84.0%, 37/44) had an ischemic stroke and 15.9% (7/44) had a transient ischemic attack. The mean duration of follow-up was 14.6 ± 3.7 months in the aspirin group and 13.5 ± 4.0 months in the warfarin group (P = 0.220). The mean international normalized ratio in patients who received warfarin was 2.25 ± 0.86. Fourteen patients (31.7%) had large PFOs, of which 6 were in the aspirin group and 8 were in the warfarin group. There were no significant differences between the size of PFO between the two groups (P = 0.398).
Table 1.
Demographic and clinical characteristics of patients with cryptogenic stroke and patent foramen ovale in iran, 2007-2010
The patients were followed until June 2010. In this study, none of the patients had a myocardial infarction or died due to vascular causes during follow-up; therefore, only recurrence of ischemic events was included in the analysis as a secondary endpoint. The incidence of the overall primary endpoint was 20.5% (9/44): 11.4% (5/44) patients had a stroke, 4.5% (2/44) had a transient ischemic attack and 4.5% (2/44) died. Both deaths were unrelated to vascular causes (multiple myeloma and renal failure). Of the patients who were treated with aspirin, 8.7% (2/23) had an ischemic event and 4.3% (1/23) died. In the warfarin group, 23.8% (5/21) had an ischemic event and 4.8% (1/21) died.
There were no significant differences in the time to primary endpoint (hazard ratio: 0.45; 95% CI: 0.1-1.8; P = 0.259) and ischemic event recurrence (hazard ratio: 0.33; 95% CI: 0.06-1.7; P = 0.183) between patients treated with aspirin vs. warfarin. The mean event-free time to death or ischemic event was 17.0 months (95% CI: 15.8-18.2) in the aspirin group and 14.8 months (95% CI: 12.4-17.3) in the warfarin group. Figures 2 and 3 show the probability of an ischemic event or death in the two treatment groups.
Figure 2.
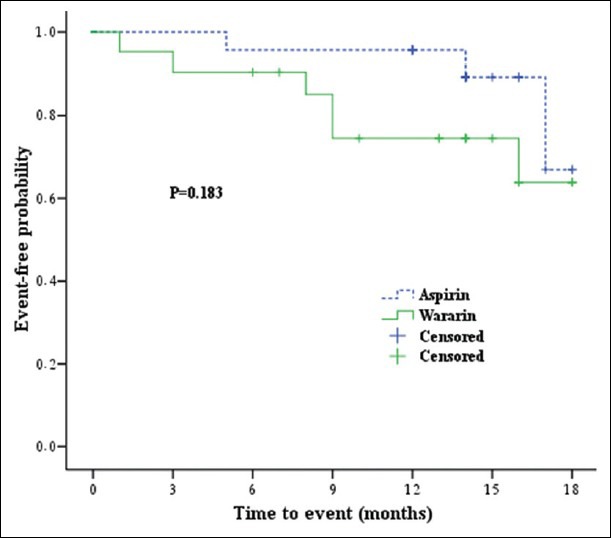
Kaplan-Meier cumulative survival curve for ischemic event or death in patients treated with warfarin or aspirin in Iran, 2007-2010
Figure 3.
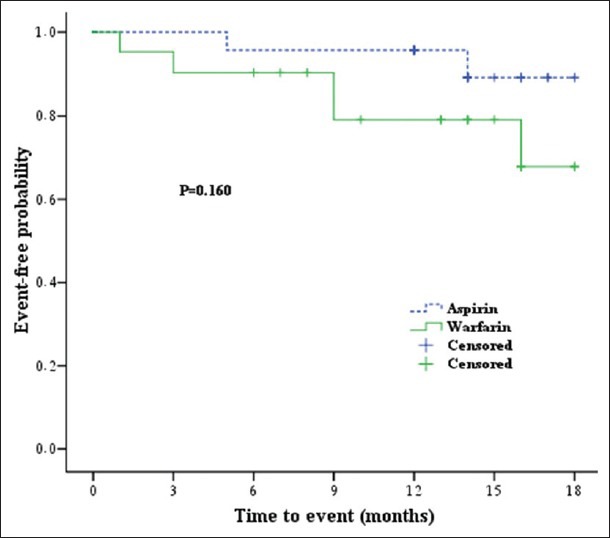
Kaplan-Meier cumulative survival curve for ischemic event in patients treated with warfarin or aspirin in Iran, 2007-2010
Major bleeding (upper gastrointestinal hemorrhage) occurred in 4.3% (1/23) of the patients in the aspirin group and in 9.5% (2/21) of those in the warfarin group (P = 0.501). The medication was withheld temporarily and the patients were seen by a gastroenterologist. Among the patients who were taking aspirin, one developed melena (4.3%) and one developed epistaxis (4.3%). None of the patients developed intracranial hemorrhage. Dyspepsia occurred in 26.1% (6/23) of the patients in the aspirin group and 14.3% (3/21) of those in the warfarin group (P = 0.338). Omeprazol (a protein pump inhibitor) was started to treat dyspepsia. None of the patients reported skin rash.
DISCUSSION
We found no significant difference in the time to recurrence of ischemic event or time to death between patients with cryptogenic stroke and PFO treated with aspirin vs. warfarin. In addition, these treatments did not show any difference regarding their complications. Previous studies which had been conducted in the industrialized countries showed the same results.
In the Patent Foramen Ovale in Cryptogenic Stroke Study (PICSS), 630 patients with stroke were randomly assigned to aspirin or warfarin, and PFO was present in 33.8% of the sample. As in the present study, there was no difference in the time to stroke or death between patients who received aspirin or warfarin.[2] According to some reports, some morphological features of PFO and the co-occurrence of PFO with atrial septal aneurysm substantially increased the risk of further stroke.[9,10,11,12,13] This situation may warrant more vigorous treatment. Other prospective studies likewise failed to document an association between risk of stroke and these clinical characteristics. In a multicenter study of right to left in cryptogenic stroke (CODICIA), 486 patients with cryptogenic stroke were treated with either aspirin or warfarin, and the effects of treatment on stroke recurrence were compared. Of their sample, 61.1% had PFO. Like us, these authors found no statistically significant treatment-related difference in stroke recurrence in their patients.[14]
In our study, although there was no difference in the rate of complications between the aspirin and warfarin groups, we noted a non-significantly lower rate of recurrence in patients who received aspirin. Considering the potential costs and risks of anticoagulation, which requires regular laboratory checks and involves an increased probability of intracranial hemorrhage, we propose that anticoagulation has no benefits over antiplatelet therapy in patients with cryptogenic stroke and PFO. This is consistent with American Heart Association/American Stroke Association guidelines, which state that antiplatelet therapy seems to be reasonable in patients with an ischemic stroke or transient ischemic attack with a PFO.[15] The indifference between medical treatments in cryptogenic stroke with PFO may be due to the fact that PFO may be the cause of first stroke but does not have any causal role in stroke recurrence.[16]
Our study had some limitations. We evaluated a limited number of cryptogenic stroke patients with PFO at a single center with a relatively short follow-up period and with a relatively high number of missing cases. Accordingly, attempts to generalize from our conclusions should be made with caution. The main advantage of our study is the purity of our per-protocol population. Unlike the PICCS study, our patients had exclusively “cryptogenic stroke” and unlike both the previous studies, all the patients had PFO.
CONCLUSION
There was no difference in the rate of ischemic event recurrence or death in a sample of Iranian patients with cryptogenic stroke and PFO who were treated with either aspirin or warfarin. The prescription of more vigorous treatments seems to be not necessary in the co-occurrence of PFO with cryptogenic stroke.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Yaghoubi E, Nemati R, Aghasadeghi K, Borhani Haghighi A. The diagnostic efficacy of transesophageal compared to transthoracic echocardiographic findings from 405 patients with ischemic stroke. J Clin Neurosci. 2011;18:1846–9. doi: 10.1016/j.jocn.2011.02.034. [DOI] [PubMed] [Google Scholar]
- 2.Homma S, Sacco RR, Di Tullio M, Sciacca RR, Mohr JP. Effect of medical treatment in stroke patients with patent foramen ovale. Circulation. 2002;105:2625–31. doi: 10.1161/01.cir.0000017498.88393.44. [DOI] [PubMed] [Google Scholar]
- 3.Handke M, Harlof A, Olschewski M, Hetzel A, Geibel A. Patent Foramen Ovale and cryptogenic stroke in older patients. N Engl J Med. 2007;257:2262–8. doi: 10.1056/NEJMoa071422. [DOI] [PubMed] [Google Scholar]
- 4.Overell JR, Bone I, Lees KR. Interatrial septal abnormalities and stroke: A meta-analysis of case-control studies. Neurology. 2000;55:1172–9. doi: 10.1212/wnl.55.8.1172. [DOI] [PubMed] [Google Scholar]
- 5.Alsheikh-Ali AA, Thaler DE, Kent DM. Patent foramen ovale in cryptogenic stroke: Incidental or pathogenic? Stroke. 2009;40:2349–55. doi: 10.1161/STROKEAHA.109.547828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Azarpazhooh MR, Etemadi MM, Donnan GA, Mokhber N, Majidi MR, Ghayour-Mobarhan M, et al. Excessive incidence of stroke in Iran: Evidence from the Mashhad Stroke Incidence Study (MSIS), a population-based study of stroke in the Middle East. Stroke. 2010;41:e3–10. doi: 10.1161/STROKEAHA.109.559708. [DOI] [PubMed] [Google Scholar]
- 7.Azarpira N, Namazi S, Hendijani F, Banan M, Darai M. Investigation of allele and genotype frequencies of CYP2C9, CYP2C19 and VKORC1 in Iran. Pharmacol Rep. 2010;62:740–6. doi: 10.1016/s1734-1140(10)70332-7. [DOI] [PubMed] [Google Scholar]
- 8.Ay H, Benner T, Arsava EM, Furie KL, Singhal AB, Jensen MB, et al. A computerized algorithm for etiologic classification of ischemic stroke: The Causative Classification of Stroke System. Stroke. 2007;38:2979–84. doi: 10.1161/STROKEAHA.107.490896. [DOI] [PubMed] [Google Scholar]
- 9.Goel SS, Tuzcu EM, Shishehbor MH, de Oliveira DI, Borek PP, Krasuski RR, et al. Morphology of the patent foramen ovale in asymptomatic versus symptomatic (stroke or transient ischemic attack) patients. Am J Cardiol. 2009;103:124–9. doi: 10.1016/j.amjcard.2008.08.036. [DOI] [PubMed] [Google Scholar]
- 10.Mas JL, Arquizan C, Lamy C, Zuber M, Cabanes L, Derumeaux G, et al. Recurrent cerebrovascular events associated with patent foramen ovale, atrial septal aneurysm, or both for the Patent Foramen Ovale and Atrial Septal Aneurysm Study Group. N Engl J Med. 2001;345:1740–6. doi: 10.1056/NEJMoa011503. [DOI] [PubMed] [Google Scholar]
- 11.Serena J, Segura T, Perez-Ayuso MJ, Bassaganyas J, Molins A, Davalos A. The need to quantify right-to-left shunt in acute ischemic stroke: A case-control study. Stroke. 1998;29:1322–8. doi: 10.1161/01.str.29.7.1322. [DOI] [PubMed] [Google Scholar]
- 12.Shariat A, Yaghoubi E, Nemati R, Moaref AR, Aghasadeghi K, Borhani Haghighi AB. Microembolic signals in patients with cryptogenic stroke with or without patent foramen ovale. J Stroke Cerebrovas Dis. 2012;21:662–6. doi: 10.1016/j.jstrokecerebrovasdis.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 13.Schuchlenz HW, Weihs W, Horner S, Quehenberg F. The association between the diameter of a patent foramen ovale and the risk of embolic cerebrovascular events. Am J Med. 2000;109:456–62. doi: 10.1016/s0002-9343(00)00530-1. [DOI] [PubMed] [Google Scholar]
- 14.Serena J, Marti-Fabregas J, Santamarina E, Rodriquezz JJ, Perez-Ayuso MJ, Masjuan J, et al. Recurrent stroke and massive right-to-left shunt: Results from the prospective Spanish multicenter (CODICIA) study. Stroke. 2008;39:3131–6. doi: 10.1161/STROKEAHA.108.521427. [DOI] [PubMed] [Google Scholar]
- 15.Furie KL, Kasner SE, Adams RJ, Albers GW, Bush RL, Fagan SC, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack. A guideline for healthcare professionals from the American heart association/American stroke association. Stroke. 2011;42:227–76. doi: 10.1161/STR.0b013e3181f7d043. [DOI] [PubMed] [Google Scholar]
- 16.Mono ML, Geister L, Galimanis A, Jung S, Praz F, Arnold M, et al. Patent foramen ovale may be causal for the first stroke but unrelated to subsequent ischemic events. Stroke. 2011;10:2891–5. doi: 10.1161/STROKEAHA.111.619577. [DOI] [PubMed] [Google Scholar]


