Abstract
Pulmonary lymphangioleiomyomatosis (LAM) is a rare, low-grade neoplasm affecting almost exclusively women of childbearing age. LAM belongs to the family of perivascular epithelioid cell tumors, characterized by spindle and epithelioid cells with smooth muscle and melanocytic differentiation. LAM cells infiltrate the lungs, producing multiple, bilateral lesions rich in lymphatic channels and forming cysts, leading to respiratory insufficiency. Here we used antibodies against four lymphatic endothelial markers—podoplanin (detected by D2-40), prospero homeobox 1 (PROX1), vascular endothelial growth factor receptor 3 (VEGFR-3), and lymphatic vessel endothelial hyaluronan receptor 1 (LYVE1)—to determine whether LAM cells show lymphatic differentiation. Twelve of 12 diagnostic biopsy specimens (early-stage LAM) and 19 of 19 explants (late-stage LAM) showed immunopositivity for D2-40 in most neoplastic cells. PROX1, VEGFR-3, and LYVE1 immunoreactivity varied from scarce in the early stage to abundant in the late stage. Lymphatic endothelial, smooth muscle, and melanocytic markers were partially co-localized. These findings indicate that lymphatic endothelial differentiation is a feature of LAM and provide evidence of a previously unidentified third lineage of differentiation in this neoplasm. This study has implications for the histological diagnosis of LAM, the origin of the neoplastic cells, and potential future treatment with drugs targeting lymphangiogenesis.
Keywords: lymphangioleiomyomatosis, immunohistochemistry, lymphatic endothelium, lymphangiogenesis, D2-40, prospero homeobox 1, vascular endothelial growth factor receptor 3, lymphatic vessel endothelial hyaluronan receptor 1
Pulmonary lymphangioleiomyomatosis (LAM) is a rare, low-grade neoplasm involving primarily the lungs that is restricted predominantly to women of childbearing age, with a prevalence of approximately 2.6 cases per 1 million women aged 20 to 69 years (Cohen et al. 2005; Glasgow et al. 2010; Taveira-DaSilva et al. 2010). Pulmonary LAM is characterized by infiltration of the lung parenchyma by neoplastic spindle-shaped and epithelioid cells with combined myogenic and melanocytic differentiation (LAM cells) (Seyama et al. 2010). These cells spread throughout the lungs, developing scattered lesions associated with progressive cyst formation that eventually leads to respiratory insufficiency (Goncharova and Krymskaya 2008; Hohman et al. 2008; McCormack 2008; Cai et al. 2010). Lung transplant is currently the only validated treatment (Dilling et al. 2012).
The detection of LAM cells in blood, lymphatic channels, lymph nodes, the thoracic duct and its tributaries, pleural and peritoneal surfaces, and uterus provides strong evidence of their neoplastic character and metastatic potential (Sato et al. 2002; Karbowniczek et al. 2003; Crooks et al. 2004; Hayashi et al. 2011). Furthermore, LAM cells have been identified in transplanted lungs of some patients, providing additional confirmatory evidence of their metastatic spread (Karbowniczek et al. 2003).
LAM occurs either sporadically (S-LAM) or in association with tuberous sclerosis complex (TSC-LAM), an autosomal dominant genetic disorder characterized by tumors in the brain, heart, skin, kidneys, and other organs and often marked by mental retardation or seizures (Astrinidis and Henske 2005; Kwiatkowski and Manning 2005; Crino et al. 2006). TSC is caused by germline mutations in the tumor-suppressing genes TSC1 and TSC2, which code for hamartin and tuberin, respectively (Costello et al. 2000; Franz et al. 2001; Yu et al. 2001). These two proteins dimerize to inhibit the mammalian target of rapamycin (mTOR) pathway and suppress cell growth and proliferation (Tee et al. 2003; Goncharova and Krymskaya 2008; Hsu et al. 2011; Yu et al. 2011). In most S-LAM cases, the LAM cells have TSC2 mutations (Carsillo et al. 2000; Sato et al. 2002), suggesting that the latter play a pathogenic role in the development of LAM.
LAM is considered a member of the family of perivascular epithelioid cell tumors (PEComas), which are distinguished by their genetic background (mutations in TSC1 and/or TSC2) and dual phenotypic morphology (composed of both epithelioid cells and spindle cells) (Folpe and Kwiatkowski 2010). Histological diagnosis is made on the basis of immunohistochemical reaction to markers of smooth muscle and melanocytic differentiation, including smooth muscle actin (SMA) and the melanocytic protein gp100 (also called premelanosome protein or melanocyte protein PMEL), recognized by the antibody human melanoma black 45 (HMB45) (Folpe and Kwiatkowski 2010). Few PEComas, however, feature the rich lymphatic channel network seen in LAM as a mesh of thin-walled branching channels within the LAM lesions (Glasgow et al. 2012). LAM is also unique in the development of cysts, which are partially lined with lymphatic endothelium.
Intrigued by these peculiar features, we sought to determine whether LAM cells themselves exhibit a lymphatic lineage of differentiation. We performed immunohistochemical analyses of 31 cases of S-LAM (12 early-stage cases and 19 late-stage cases) with the monoclonal antibody D2-40, a lymphatic endothelial marker directed against the transmembrane mucoprotein podoplanin (Kalof and Cooper 2009), which is required for lymphatic capillary morphogenesis (Navarro et al. 2008). In all the cases studied (31 of 31), the majority of LAM cells immunoreacted positively for D2-40, with no qualitative or quantitative differences between early-stage and late-stage cases.
Additional immunohistochemical analyses showed that a number of LAM cells in all the cases were positive for the lymphatic-specific markers prospero homeobox 1 (PROX1), the master regulator of lymphatic endothelial cell differentiation (Hong et al. 2002; Johnson et al. 2008), and vascular endothelial growth factor receptor 3 (VEGFR-3, also called fms-related tyrosine kinase 4 [FLT4]), the first known lymphatic-specific marker (Kaipainen et al. 1995). Furthermore, all late-stage cases and four of eight early-stage cases showed LAM cells positive for lymphatic vessel endothelial hyaluronan receptor 1 (LYVE1) (Banerji et al. 1999). The quantity and intensity of PROX1, VEGFR-3, and LYVE1 immunostaining was significantly greater in late-stage than in early-stage cases. Double immunofluorescence followed by confocal microscopy demonstrated the presence of nuclear PROX1 in HMB45-positive cells, which are considered bona fide LAM cells. Our findings therefore provide evidence that LAM cells in S-LAM have an additional lineage of lymphatic endothelial differentiation.
Materials and Methods
Cases
Specimens from diagnostic open lung biopsy specimens (representing early-stage LAM) were obtained from 12 patients with S-LAM recruited through the LAM Foundation. De-identified specimens of lungs removed during lung transplantation (representing late-stage LAM) were obtained through the National Disease Research Interchange from 19 patients with S-LAM (representing late-stage LAM). Control specimens were obtained from the Human Tissue Resource Center of the University of Chicago Medical Center and comprised specimens of uterine leiomyoma (SMA positive control), melanoma (HMB45 positive control), liver (PROX1 positive control), 10 angiomyolipomas (AMLs), and lungs with usual interstitial pneumonia, emphysema, nonspecific fibrosis, and organizing pneumonia. This study was approved by the institutional review board of the University of Chicago Biological Sciences Division. All patients gave their informed consent.
Immunohistochemistry
Immunohistochemistry was performed on 4-µm-thick formalin-fixed, paraffin-embedded tissue sections using the following primary antibodies: monoclonal mouse anti–human D2-40 antibody (clone D2-40, code M3619; Dako, Glostrup, Denmark) at 1:25 dilution, monoclonal mouse anti–human melanosome antibody (clone HMB45, code M0634; Dako) at 1:50 dilution, monoclonal mouse anti–human CD31 endothelial cell antibody (clone JC70A, code M0823; Dako) at 1:500 dilution, monoclonal mouse anti–human SMA antibody (clone 1A4, code M0851; Dako) at 1:100 dilution, polyclonal rabbit anti–human PROX1 antibody (catalog no. 38692; Abcam, Cambridge, MA) at 1:200 dilution, polyclonal goat anti–human VEGFR-3 antibody (cat. AF349; R&D Systems, Minneapolis, MN) at 1:20 dilution, and polyclonal rabbit anti–human LYVE1 antibody (cat. 36993; Abcam) at 1:50 dilution.
Immunohistochemistry for D2-40, HMB45, and anti–CD31 antibodies was performed on a Bond-Max automated immunohistochemistry/in situ hybridization system (Leica Microsystems; Buffalo Grove, IL) using the Bond Polymer Refine detection system (Leica Microsystems), according to a modified manufacturer’s protocol. Briefly, following heat antigen retrieval in a high-pH buffer (ER2) for 20 min for D2-40 or in a low-pH buffer (ER1) for 30 min for anti-CD31 (no pretreatment for HMB45), the sections were incubated with the primary antibody for 25 min, followed by incubation with post–primary reagent for 15 min, Bond polymer horseradish peroxidase (HRP) for 25 min, and peroxidase block for 5 min. The peroxidase reaction was developed using 3,3′-diaminobenzidine (DAB) provided in the kit. Immunohistochemistry for SMA was performed on a Benchmark XT instrument using the UltraView HRP Universal detection kit (Ventana Medical Systems; Tucson, AZ) according to the manufacturer’s protocol, with the peroxidase reaction developed using DAB provided in the kit.
Immunohistochemistry for PROX1 was performed on a Bond 3 automated immunohistochemistry/in situ hybridization system (Leica Microsystems) using the Bond Polymer Refine detection system (Leica Microsystems), according to a modified manufacturer’s protocol. Following heat antigen retrieval in a high-pH buffer (ER2) for 20 min, sections were incubated with the primary antibody for 50 min followed by incubation with post–primary reagent for 15 min, Bond polymer HRP for 25 min, and peroxidase block for 5 minutes. The peroxidase reaction was developed using DAB provided in the kit.
For VEGFR-3 immunohistochemistry, endogenous peroxidase was blocked with Peroxidased 1 blocking reagent (Biocare Medical; Concord, CA) for 10 min followed by blocking with Background Sniper serum-free blocking reagent (Biocare Medical) for 15 min. Sections were incubated with primary antibody for 1 hr at room temperature followed by incubation with HRP-conjugated polyclonal rabbit anti–goat immunoglobulin (code P0449; Dako) at 1:150 dilution for 30 min at room temperature. The peroxidase reaction was developed using a Betazoid DAB chromogen kit (Biocare Medical).
For LYVE1 immunohistochemistry, endogenous peroxidase was blocked with EnVision+ blocking reagent (Dako) for 5 min followed by blocking with Background Sniper serum-free blocking reagent (Biocare Medical) for 15 min. Sections were incubated with primary antibody for 1 hr at room temperature, followed by incubation with Mach 3 rabbit probe (Biocare Medical) for 10 min at room temperature and then Mach 3 rabbit HRP-polymer (Biocare Medical) for 10 min at room temperature. The peroxidase reaction was developed using a Betazoid DAB chromogen kit (Biocare Medical).
Double immunohistochemistry was performed on a Bond 3 automated immunohistochemistry/in situ hybridization system (Leica Microsystems). In the first round, the sections were immunostained with anti–PROX1 antibody as described above, followed by a second round of antigen retrieval with a high-pH buffer (ER2) for 20 min; incubation with D2-40, HMB45, or anti–SMA antibody for 25 min; and processing with the Bond Polymer Red detection system (alkaline phosphatase; Leica Microsystems) according to the manufacturer’s protocol.
Immunofluorescence was performed on frozen tissue specimens cut into sections of 5 µm thickness and mounted onto Surgipath Snowcoat X-tra slides (Leica Biosystems). Sections were fixed in 2% paraformaldehyde for 15 min and permeabilized in 0.5% Igepal CA-630 (Sigma-Aldrich; St. Louis, MO) for 5 min. The sections were then incubated at room temperature for 3 hr with monoclonal mouse anti–human melanosome antibody (clone HMB45, code M0634; Dako) and polyclonal rabbit anti–human PROX1 antibody (cat. ab37128; Abcam) combined, each used at 1:50 dilution, followed by incubation for 1 hr with secondary antibodies (Alexa Fluor 488 goat anti–rabbit IgG H+L, cat. A-11008, and Alexa Fluor 594 goat anti–mouse IgG H+L, cat. A-11005; Molecular Probes, Life Technologies, Grand Island, NY), each used at 1:1000 dilution. Micrographs were obtained with a Zeiss spinning disk inverted confocal fluorescence microscope using SlideBook software (Intelligent Imaging Innovations; Denver, CO).
Data Analysis
All slides were examined by three board-certified pathologists (E.H., A.N.H., and L.S.). Cells were quantified using Spectrum and ImageScope software from Aperio Technologies (Vista, CA). Statistical analysis was performed using the Student’s t-test.
Results
The diagnosis of LAM was confirmed by microscopic examination of serial sections stained with hematoxylin and eosin (H&E), anti–SMA antibody, and HMB45 antibody. There were no morphological differences between early-stage and late-stage LAM lesions, but in some early-stage cases, the lesions were smaller and fewer. LAM lesions were composed of spindle and epithelioid cells, the majority of them positive for SMA. Cells positive for HMB45 were less numerous, with the percentage of positive cells varying from case to case (mean, 20%; range, 15%–95%). Variable numbers of lymphocytes, scant arterioles, and numerous endothelial-lined, slit-like channels devoid of smooth muscle were observed within the lesions (Fig. 1A–C; Suppl. Figs. S1A–C and S2A). Strongly positive immunoreactivity for D2-40 and absence of immunoreactivity for CD31 confirmed the lymphatic nature of the channels crisscrossing the LAM lesions (Fig. 1D, E; Suppl. Fig. S1D, E and S2B).
Figure 1.
Immunopositivity for lymphatic endothelial marker D2-40 (podoplanin) in early-stage sporadic lymphangioleiomyomatosis (LAM). Shown are serial histological sections of a lung specimen from a representative case with hematoxylin and eosin (H&E) staining (A), smooth muscle actin (SMA) immunostaining (B), melanocytic marker HMB45 immunostaining (C), D2-40 immunostaining (D), and immunostaining for CD31, a marker of blood vascular endothelium (E). Lymphatic vessels show strong immunoreactivity to D2-40 (arrows), but LAM cells also show positive immunoreaction. Scale bar, 20 µm.
In all cases (12 of 12 early-stage cases and 19 of 19 late-stage cases), the majority of LAM cells immunoreacted positively for D2-40 (mean, 88%; range, 70%–100%), without quantitative or qualitative differences between early and late stages of the disease (Fig. 1D; Suppl. Figs. S1D and 2B; Table 1). Immunoreaction for D2-40 was stronger in lymphatic endothelium than in the LAM cells (Fig. 1D; Suppl. Fig. S1D, arrows). In contrast, 10 of 10 specimens of renal AML were negative for D2-40, except for the lymphatic endothelium (Suppl. Fig. S3D, arrows), a finding consistent with previous studies (Fujii et al. 2008; Bonsib et al. 2009; Xian et al. 2011).
In all cases, variable percentages of LAM cells exhibited nuclear positivity for PROX1, with the percentage being significantly greater in late-stage LAM (mean, 62%; range, 10%–85%) than in early-stage LAM (mean, 12%; range, 5%–15%; p<0.0001) (Fig. 2D; Suppl. Fig. S4D; Table 1). Some cytoplasmic PROX1 staining was observed, but this is not an unusual occurrence (Duncan et al. 2002; Bosco et al. 2005; Laerm et al. 2007; Gill et al. 2009; Yamazaki et al. 2009; Dashkevich et al. 2010; da Cunha Castro and Galambos 2011; Skog et al. 2011). Similarly, VEGFR-3–positive LAM cells were detected in all cases, with greater percentages and more intense immunoreaction in late-stage LAM (mean, 76%; range, 61%–100%) than in early-stage LAM (mean, 50%; range, 40%–75%; p<0.0001) (Fig. 3A; Suppl. Fig. S5A; Table 1). Nuclear VEGFR-3 staining was observed, as previously documented in other neoplasms (Drescher et al. 2007; Mylona et al. 2007; Carrillo de Santa Pau et al. 2009). Immunopositivity for PROX1 and VEGFR-3 was stronger in the lymphatic endothelial cells than in the LAM cells (Figs. 2D and 3A; Suppl. Figs. S4D and S5A, arrows).
Figure 2.
Immunopositivity for lymphatic endothelial marker prospero homeobox 1 (PROX1) in late-stage sporadic lymphangioleiomyomatosis. Shown are serial histological sections of a lung specimen from a representative case with hematoxylin and eosin (H&E) staining (A), smooth muscle actin (SMA) immunostaining (B), melanocytic marker HMB45 immunostaining (C), and PROX1 immunostaining (D). Lymphatic endothelial cells show strong nuclear positivity for PROX1 (arrows), but LAM cells also show positive immunoreaction. Scale bar, 15 µm.
Figure 3.
Immunopositivity for lymphatic endothelial markers vascular endothelial growth factor receptor 3 (VEGFR-3) (A) and lymphatic vessel endothelial hyaluronan receptor 1 (LYVE1) (B) in late-stage sporadic lymphangioleiomyomatosis (LAM). Shown are sections of lung specimens from representative cases. Lymphatic endothelial cells show strong immunoreactivity to both markers (arrows), but LAM cells also show positive immunoreaction. Scale bar, 30 µm.
LAM cells with LYVE1 immunoreactivity were observed in all late-stage cases (mean, 68%; range, 40%–89%) (Fig. 3B; Table 1), with stronger immunostaining in the lymphatic endothelium (Fig. 3B, arrows). Only 8 of 12 early-stage cases were available for LYVE1 immunostaining; of these 8 cases, only 4 showed LYVE1-positive LAM cells, whereas there were no LYVE1-positive LAM cells in the other 4 cases. The LYVE1-positive LAM cells in the early-stage cases were fewer and showed weaker immunostaining than in the late-stage cases (mean, 10%; range, 0%–22%; p<0.0001) (Suppl. Fig. S5B; Table 1). Interestingly, three of the four early-stage cases with no LYVE-1–positive LAM cells showed no LYVE1 positivity in the lymphatic vessels associated with the LAM lesions, although the normal lung lymphatic vessels were LYVE1 positive (Suppl. Fig. S5D). The remaining one early-stage case without LYVE1-positive LAM cells did show LYVE1-positive lymphatic endothelial cells lining the lymphatic channels within the LAM lesions (Suppl. Fig. S5C).
Table 1.
Quantitative Analysis of Lymphatic Endothelial Markers in LAM Cells.
| Early-Stage LAM |
Late-Stage LAM |
|||
|---|---|---|---|---|
| IHC Stain | % of Positive LAM Cells, Mean (Range) | Positive LAM Cell Intensity | % of Positive LAM Cells, Mean (Range) | Positive LAM Cell Intensity |
| D2-40 | 89 (70–100) | +++ | 88 (70–100) | +++ |
| PROX1 | 12 (5–15) | + | 62 (10–85)a | ++ |
| VEGFR-3 | 50 (40–75) | ++ | 76 (61–100)a | +++ |
| LYVE1 | 10 (0–22) | + | 68 (40–89)a | ++ |
| PROX1 and D2-40 | 9 (1–12)b | NA | 46 (1–65)b | NA |
| PROX1 and HMB45 | 6 (1–10)b | NA | 32 (5–67)b | NA |
D2-40, monoclonal antibody that reacts with podoplanin; HMB45, monoclonal antibody that reacts with premelanosome protein (also called gp100); IHC, immunohistochemical; LAM, lymphangioleiomyomatosis; LYVE1, lymphatic vessel hyaluronan receptor 1; NA, not applicable; PROX1, prospero homeobox 1; VEGFR-3, vascular endothelial growth factor receptor 3.
Statistically significant difference from early-stage LAM (p<0.0001).
Percentage of LAM cells positive for both markers in double IHC analyses.
Double immunohistochemistry with D2-40 and anti–PROX1 antibodies revealed partial co-localization in LAM cells in both early-stage LAM (mean, 9%; range, 1%–12%) and late-stage LAM (mean, 46%; range, 1%–65%) (Suppl. Fig. S6D; Table 1). Double immunohistochemistry with HMB45 and anti–PROX1 antibodies showed partial co-localization in both early-stage LAM (mean, 6%; range, 1%–10%) and late-stage LAM (mean, 32%; range, 5%–67%) (Suppl. Fig. S7D; Table 1), whereas other LAM cells tended to be positive for either HMB45 or PROX1 (Suppl. Fig. S7E, F, respectively). Double immunofluorescence coupled with confocal microscopy confirmed that PROX1 and HMB45 were present in the same cells (Fig. 4C). Double immunohistochemistry with anti–SMA and anti–PROX1 antibodies showed that PROX1-positive LAM cells were generally positive for SMA (Suppl. Fig. S8D).
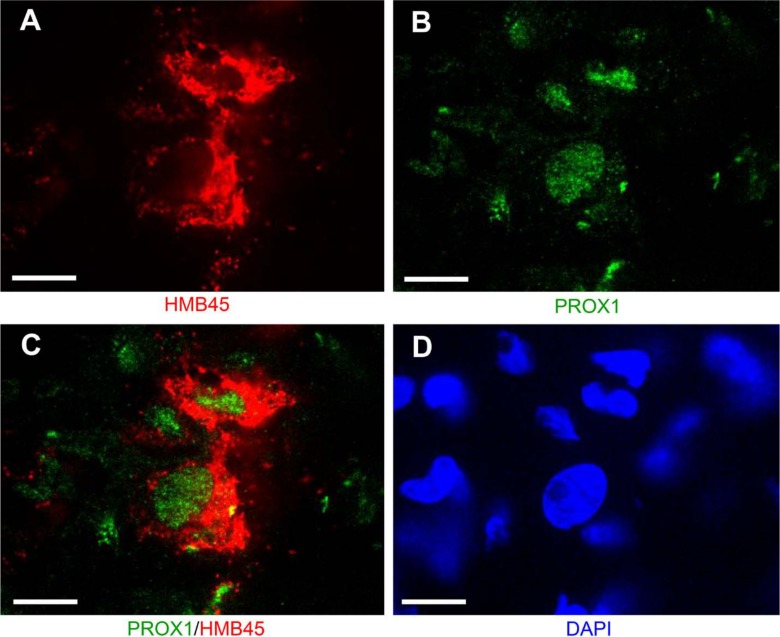
Figure 4.
Confocal microscopy of a lung specimen from a representative case of late-stage sporadic lymphangioleiomyomatosis confirming co-localization of melanocytic marker HMB45 (A) and lymphatic endothelial marker prospero homeobox 1 (PROX1) (B) in the same cells (C). Panel D shows 4′,6-diamidino-2-phenylindole (DAPI), a nuclear stain. Scale bar, 3 µm.
Lung alveoli in all cases of LAM, usual interstitial pneumonia, emphysema, nonspecific fibrosis, and organizing pneumonia were negative for all markers except for the myofibroblasts in usual interstitial fibrosis and organizing fibrosis, which were SMA positive, and the alveolar epithelial cells and the luminal surface of pulmonary airspaces, which were D2-40 positive (Sherman et al. 2012; Sozio et al. 2012) (data not shown). Bronchial and vascular smooth muscle were positive for SMA, and lymphatic vessels were positive for D2-40, PROX1, VEGFR-3, and LYVE1 in all control lung specimens (data not shown).
Discussion
Pulmonary LAM is a rare, low-grade neoplasm currently classified as a member of the PEComa family. PEComas are a group of neoplasms distinguished by their genetic background (mutations in TSC1 and/or TSC2), dual phenotypic morphology (composed of both epithelioid cells and spindle cells), and immunohistochemical positivity for SMA and HMB45 (Folpe and Kwiatkowski 2010). LAM is divided in two groups: TSC-associated (TSC-LAM) and sporadic (S-LAM). Reported cases of S-LAM outnumber those of TSC-LAM (Avila et al. 2010), accounting for 89% of LAM cases in the LAM Foundation registry.
Here we used immunohistochemical analysis to show that LAM cells in S-LAM are positive for four key lymphatic endothelial markers: D2-40 (which detects podoplanin), PROX1, VEGFR-3, and LYVE1. Immunoreactivity for these four markers was observed in diagnostic biopsy specimens (early-stage LAM) as well as in specimens from lungs removed at the time of transplant (late-stage LAM). Although the majority of LAM cells in both early-stage and late-stage cases showed intense D2-40 immunostaining, the intensity of PROX1, VEGFR-3, and LYVE1 immunostaining and the numbers of LAM cells positive for these markers were significantly greater in late-stage than in early-stage cases. The reason for this difference is currently unknown. Altogether, our studies indicated that expression of lymphatic endothelial markers is a feature of LAM cells on equal standing with the long-established myogenic and melanocytic differentiation.
To the best of our knowledge, this is the first systematic study of podoplanin (using D2-40), LYVE1, and PROX1 expression in LAM and the first to report a lymphatic endothelial lineage of differentiation in LAM cells. PROX1 expression had been examined in a diagnostic lung biopsy specimen from a patient with LAM by a previous group (Issaka et al. 2009), who regarded LAM cells as negative for PROX1. A possible explanation for this discrepancy is that the specimen may not have included PROX1-positive cells, which are scarce in early-stage LAM. Alternatively, since the antibody used by this group (Millipore; Billerica, MA) produces a significantly weaker signal than the one used in our study (E.H. and L.S., unpublished observation), it may have failed to detect PROX1 in LAM cells while detecting the stronger PROX1 positivity in lymphatic endothelium.
A previous immunohistochemical study examined D2-40 in lymphangioleiomyoma of the retroperitoneum and mediastinum, a PEComa with striking histological similarity to LAM, including the presence of a rich lymphatic channel network (Hansen et al. 2007). This study reported no immunoreactivity for D2-40 in the LAM-like cells. A plausible explanation for this discrepancy is that pulmonary LAM and lymphangioleiomyoma represent close but not identical entities, and unfortunately, we did not have access to specimens of lymphangioleiomyoma or extrapulmonary LAM for comparison. However, the discrepancy between this study and ours can be attributed to the interpretation of the results. Despite their use of D2-40 at a 100-fold lower concentration than in our study, the micrographs presented show faint but definite staining with D2-40 in most of the LAM-like cells (Hansen et al. 2007). Such mild immunopositivity was likely regarded as nonspecific staining, but D2-40 antibodies produce a clear background and are highly specific to lymphatic endothelium, as demonstrated by the complete negativity for D2-40 in the LAM-like cells of AMLs studied here and previously by others (Fujii et al. 2008; Bonsib et al. 2009; Xian et al. 2011). Moreover, the absence of D2-40 in all but the lymphatic endothelium of the AMLs in our study confirms that our experimental protocol did not result in overstaining of the LAM specimens.
The expression of VEGFR-3 in LAM was examined by two groups (Kumasaka et al. 2004; Kumasaka et al. 2005; Issaka et al. 2009; Hayashi et al. 2011). One group found LAM cells to express VEGFR-3 but interestingly did not refer to this result as indicative of lymphatic endothelial differentiation (Issaka et al. 2009). The other group reported only on the VEGFR-3 positivity of lymphatic endothelial cells without commenting on the status of the LAM cells (Kumasaka et al. 2004; Kumasaka et al. 2005; Hayashi et al. 2011), which appear to be weakly positive in some of the photomicrographs provided (Kumasaka et al. 2004).
LAM lesions might contain indiscernible stromal cells admixed with the LAM cells, as well as reactive myofibroblasts, which are SMA positive and partially resemble LAM cells. Therefore, we considered the possibility that the lymphatic-specific antibodies positively marked tumor-reactive stromal cells. Since LAM cells express melanocytic markers, a feature absent in a reactive process, we performed double immunohistochemistry with HMB45 and anti–PROX1 antibodies. The presence of multiple cells positive for both markers attested against a potential reactive process. Double immunofluorescence with anti–PROX1 and HMB45 antibodies coupled with confocal microscopy confirmed the presence of cells coexpressing both gp100 and PROX1 and therefore representing bona fide LAM cells with combined melanocytic and lymphatic endothelial differentiation.
Since our studies indicated that LAM cells have lymphatic differentiation, we considered whether the prominent lymphatic vessel development characteristic of LAM could be neoplastic rather than reactive in origin. However, careful screening of the lymphatic endothelium lining the channels by means of confocal microscopy did not reveal HMB45 positivity.
Although there is evidence suggesting that activation of the mTOR pathway stimulates lymphangiogenesis (Kobayashi et al. 2007; Patel et al. 2011; Luo et al. 2012) and hence may underlie LAM cell lymphatic differentiation, a recent study by our group stands against such a possibility (Badri et al. 2013). In that study, we examined the TSC1 and TSC2 genes by ultra-deep sequencing in 10 of the 19 late-stage LAM cases included here and found that 2 of those cases had neither TSC2 nor TSC1 mutations identified and no activation of the mTOR signaling cascade. Nevertheless, both cases showed D2-40, PROX1, VEGFR-3, and LYVE1 immunopositivity as well as SMA and HMB45 immunopositivity. Therefore, none of the three lineages of LAM cell differentiation seems to be dependent on, or affected by, the level of mTOR activation.
The origin and exact nature of LAM cells remain unknown, but the myogenic and melanocytic differentiation observed in LAM and other PEComas is consistent with an origin from neural crest cells (Fernandez-Flores 2011), a transitory population of vertebrate embryonic cells that differentiate into a variety of cell types, including smooth muscle and pigment cells (Sauka-Spengler and Bronner-Fraser 2008). Identification of the same TSC2 mutation in LAM and AML in patients bearing both lesions (Carsillo et al. 2000) also supports a neural crest origin of these neoplasms. The media (smooth muscular layer) portion of the anterior cardinal vein was found to be neural crest derived (Bergwerff et al. 1998; Adams and Alitalo 2007), and it is from this vein that PROX1-positive endothelial cells bud off to form rudimentary lymphatic vessels during embryonic development (Choi et al. 2012). However, no studies have been conducted showing derivation of the endothelium of blood or lymphatic vessels from neural crest cells. Hence, our immunohistochemical finding of lymphatic endothelial markers in LAM cells is still consistent with a neural crest origin of LAM.
In conclusion, we have found evidence of a third lineage of differentiation in S-LAM cells indicated by immunopositivity for lymphatic endothelial markers. Our findings have implications for the histological diagnosis of LAM, for elucidating the origin of LAM cells, and potentially in determining future therapeutic modalities with drugs that inhibit lymphangiogenesis (Wang et al. 2008; Tammela and Alitalo 2010; Zhou et al. 2010; Weckmann et al. 2012).
Supplementary Material
Acknowledgments
We thank the staff of the Human Tissue Resource Center, the Integrated Core Microscopy Facility, and the clinical immunohistochemistry laboratory for their kind assistance. We also thank the study volunteers for their participation.
Footnotes
Supplementary material for this article is available on the Journal of Histochemistry & Cytochemistry Web site at http://jhc.sagepub.com/supplemental.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institutes of Health [grant number HL-77514-05].
References
- Adams RH, Alitalo K. 2007. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol. 8(6):464–478 [DOI] [PubMed] [Google Scholar]
- Astrinidis A, Henske EP. 2005. Tuberous sclerosis complex: linking growth and energy signaling pathways with human disease. Oncogene. 24(50):7475–7481 [DOI] [PubMed] [Google Scholar]
- Avila NA, Dwyer AJ, Rabel A, Darling T, Hong CH, Moss J. 2010. CT of sclerotic bone lesions: imaging features differentiating tuberous sclerosis complex with lymphangioleiomyomatosis from sporadic lymphangioleiomymatosis. Radiology. 254(3):851–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badri KR, Gao L, Hyjek E, Schuger N, Schuger L, Qin W, Chekaluk Y, Kwiatkowski DJ, Zhe X. 2013. Exonic mutations of TSC2/TSC1 are common but not seen in all sporadic pulmonary lymphangioleiomyomatosis. Am J Respir Crit Care Med. 187(6):663–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerji S, Ni J, Wang SX, Clasper S, Su J, Tammi R, Jones M, Jackson DG. 1999. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J Cell Biol. 144(4):789–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergwerff M, Verberne ME, DeRuiter MC, Poelmann RE, Gittenberger-de Groot AC. 1998. Neural crest cell contribution to the developing circulatory system: implications for vascular morphology? Circ Res. 82(2):221–231 [DOI] [PubMed] [Google Scholar]
- Bonsib SM, Moghadamfalahi M, Bhalodia A. 2009. Lymphatic differentiation in renal angiomyolipomas. Hum Pathol. 40(3):374–380 [DOI] [PubMed] [Google Scholar]
- Bosco A, Cusato K, Nicchia GP, Frigeri A, Spray DC. 2005. A developmental switch in the expression of aquaporin-4 and Kir4.1 from horizontal to Muller cells in mouse retina. Invest Ophthalmol Vis Sci. 46(10):3869–3875 [DOI] [PubMed] [Google Scholar]
- Cai X, Pacheco-Rodriguez G, Fan QY, Haughey M, Samsel L, El-Chemaly S, Wu HP, McCoy JP, Steagall WK, Lin JP, et al. 2010. Phenotypic characterization of disseminated cells with TSC2 loss of heterozygosity in patients with lymphangioleiomyomatosis. Am J Respir Crit Care Med. 182(11):1410–1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrillo de Santa Pau E, Arias FC, Caso Peláez E, MuñozMolina GM, Sánchez Hernández I, Muguruza Trueba I, Moreno Balsalobre R, Sacristán López S, Gómez Pinillos A, del Val Toledo Lobo M. 2009. Prognostic significance of the expression of vascular endothelial growth factors A, B, C, and D and their receptors R1, R2, and R3 in patients with nonsmall cell lung cancer. Cancer. 115(8):1701–1712 [DOI] [PubMed] [Google Scholar]
- Carsillo T, Astrinidis A, Henske EP. 2000. Mutations in the tuberous sclerosis complex gene TSC2 are a cause of sporadic pulmonary lymphangioleiomyomatosis. Proc Natl Acad Sci U S A. 97(11):6085–6090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi I, Lee S, Hong YK. 2012. The new era of the lymphatic system: no longer secondary to the blood vascular system. Cold Spring Harb Perspect Med. 2(4):a006445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MM, Pollock-BarZiv S, Johnson SR. 2005. Emerging clinical picture of lymphangioleiomyomatosis. Thorax. 60(10):875–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello LC, Hartman TE, Ryu JH. 2000. High frequency of pulmonary lymphangioleiomyomatosis in women with tuberous sclerosis complex. Mayo Clin Proc. 75(6):591–594 [DOI] [PubMed] [Google Scholar]
- Crino PB, Nathanson KL, Henske EP. 2006. The tuberous sclerosis complex. N Engl J Med. 355(13):1345–1356 [DOI] [PubMed] [Google Scholar]
- Crooks DM, Pacheco-Rodriguez G, DeCastro RM, McCoy JP, Jr., Wang JA, Kumaki F, Darling T, Moss J. 2004. Molecular and genetic analysis of disseminated neoplastic cells in lymphangioleiomyomatosis. Proc Natl Acad Sci U S A. 101(50):17462–17467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Cunha Castro EC, Galambos C. 2011. Prox-1: a specific and sensitive marker for lymphatic endothelium in normal and diseased human tissues. Ann Thorac Surg. 92(1):407; author reply 407–408 [DOI] [PubMed] [Google Scholar]
- Dashkevich A, Heilmann C, Kayser G, Germann M, Beyersdorf F, Passlick B, Geissler HJ. 2010. Lymph angiogenesis after lung transplantation and relation to acute organ rejection in humans. Ann Thorac Surg. 90(2):406–411 [DOI] [PubMed] [Google Scholar]
- Dilling DF, Gilbert ER, Picken MM, Eby JM, Love RB, Le Poole IC. 2012. A current viewpoint of lymphangioleiomyomatosis supporting immunotherapeutic treatment options. Am J Respir Cell Mol Biol. 46(1):1–5 [DOI] [PubMed] [Google Scholar]
- Drescher D, Moehler M, Gockel I, Frerichs K, Müller A, Dünschede F, Borschitz T, Biesterfeld S, Holtmann M, Wehler T, et al. 2007. Coexpression of receptor-tyrosine-kinases in gastric adenocarcinoma—a rationale for a molecular targeting strategy? World J Gastroenterol. 13(26):3605–3609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan MK, Cui W, Oh DJ, Tomarev SI. 2002. Prox1 is differentially localized during lens development. Mech Dev. 112(1–2): 195–198 [DOI] [PubMed] [Google Scholar]
- Fernandez-Flores A. 2011. Evidence on the neural crest origin of PEComas. Rom J Morphol Embryol. 52(1):7–13 [PubMed] [Google Scholar]
- Folpe AL, Kwiatkowski DJ. 2010. Perivascular epithelioid cell neoplasms: pathology and pathogenesis. Hum Pathol. 41(1):1–15 [DOI] [PubMed] [Google Scholar]
- Franz DN, Brody A, Meyer C, Leonard J, Chuck G, Dabora S, Sethuraman G, Colby TV, Kwiatkowski DJ, McCormack FX. 2001. Mutational and radiographic analysis of pulmonary disease consistent with lymphangioleiomyomatosis and micronodular pneumocyte hyperplasia in women with tuberous sclerosis. Am J Respir Crit Care Med. 164(4):661–668 [DOI] [PubMed] [Google Scholar]
- Fujii T, Zen Y, Sato Y, Sasaki M, Enomae M, Minato H, Masuda S, Uehara T, Katsuyama T, Nakanuma Y. 2008. Podoplanin is a useful diagnostic marker for epithelioid hemangioendothelioma of the liver. Mod Pathol. 21(2):125–130 [DOI] [PubMed] [Google Scholar]
- Gill HK, Parsons SR, Spalluto C, Davies AF, Knorz VJ, Burlinson CE, Ng BL, Carter NP, Ogilvie CM, Wilson DI, et al. 2009. Separation of the PROX1 gene from upstream conserved elements in a complex inversion/translocation patient with hypoplastic left heart. Eur J Hum Genet. 17(11):1423–1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasgow CG, El-Chemaly S, Moss J. 2012. Lymphatics in lymphangioleiomyomatosis and idiopathic pulmonary fibrosis. Eur Respir Rev. 21(125):196–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasgow CG, Steagall WK, Taveira-Dasilva A, Pacheco-Rodriguez G, Cai X, El-Chemaly S, Moses M, Darling T, Moss J. 2010. Lymphangioleiomyomatosis (LAM): molecular insights lead to targeted therapies. Respir Med. 104(Suppl 1):S45–S58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncharova EA, Krymskaya VP. 2008. Pulmonary lymphangioleiomyomatosis (LAM): progress and current challenges.J Cell Biochem. 103(2):369–382 [DOI] [PubMed] [Google Scholar]
- Hansen T, Katenkamp K, Bittinger F, Kirkpatrick CJ, Katenkamp D. 2007. D2-40 labeling in lymphangiomyoma/lymphangiomyomatosis of the soft tissue: further evidence of lymphangiogenic tumor histogenesis. Virchows Arch. 450(4):449–453 [DOI] [PubMed] [Google Scholar]
- Hayashi T, Kumasaka T, Mitani K, Terao Y, Watanabe M, Oide T, Nakatani Y, Hebisawa A, Konno R, Takahashi K, et al. 2011. Prevalence of uterine and adnexal involvement in pulmonary lymphangioleiomyomatosis: a clinicopathologic study of 10 patients. Am J Surg Pathol. 35(12):1776–1785 [DOI] [PubMed] [Google Scholar]
- Hohman DW, Noghrehkar D, Ratnayake S. 2008. Lymphangioleiomyomatosis: a review. Eur J Intern Med. 19(5):319–324 [DOI] [PubMed] [Google Scholar]
- Hong YK, Harvey N, Noh YH, Schacht V, Hirakawa S, Detmar M, Oliver G. 2002. Prox1 is a master control gene in the program specifying lymphatic endothelial cell fate. Dev Dyn. 225(3):351–357 [DOI] [PubMed] [Google Scholar]
- Hsu PP, Kang SA, Rameseder J, Zhang Y, Ottina KA, Lim D, Peterson TR, Choi Y, Gray NS, Yaffe MB, et al. 2011. The mTOR-regulated phosphoproteome reveals a mechanism of mTORC1-mediated inhibition of growth factor signaling. Science. 332(6035):1317–1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issaka RB, Oommen S, Gupta SK, Liu G, Myers JL, Ryu JH, Vlahakis NE. 2009. Vascular endothelial growth factors C and D induces proliferation of lymphangioleiomyomatosis cells through autocrine crosstalk with endothelium. Am J Pathol. 175(4):1410–1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson NC, Dillard ME, Baluk P, McDonald DM, Harvey NL, Frase SL, Oliver G. 2008. Lymphatic endothelial cell identity is reversible and its maintenance requires Prox1 activity. Genes Dev. 22(23):3282–3291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaipainen A, Korhonen J, Mustonen T, van Hinsbergh VW, Fang GH, Dumont D, Breitman M, Alitalo K. 1995. Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc Natl Acad Sci U S A. 92(8):3566–3570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalof AN, Cooper K. 2009. D2-40 immunohistochemistry—so far! Adv Anat Pathol. 16(1):62–64 [DOI] [PubMed] [Google Scholar]
- Karbowniczek M, Astrinidis A, Balsara BR, Testa JR, Lium JH, Colby TV, McCormack FX, Henske EP. 2003. Recurrent lymphangiomyomatosis after transplantation: genetic analyses reveal a metastatic mechanism. Am J Respir Crit Care Med. 167(7):976–982 [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Kishimoto T, Kamata S, Otsuka M, Miyazaki M, Ishikura H. 2007. Rapamycin, a specific inhibitor of the mammalian target of rapamycin, suppresses lymphangiogenesis and lymphatic metastasis. Cancer Sci. 98(5):726–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumasaka T, Seyama K, Mitani K, Sato T, Souma S, Kondo T, Hayashi S, Minami M, Uekusa T, Fukuchi Y, Suda K. 2004. Lymphangiogenesis in lymphangioleiomyomatosis: its implication in the progression of lymphangioleiomyomatosis. Am J Surg Pathol. 28(8):1007–1016 [DOI] [PubMed] [Google Scholar]
- Kumasaka T, Seyama K, Mitani K, Souma S, Kashiwagi S, Hebisawa A, Sato T, Kubo H, Shibuya K, et al. 2005. Lymphangiogenesis-mediated shedding of LAM cell clusters as a mechanism for dissemination in lymphangioleiomyomatosis. Am J Surg Pathol. 29(10):1356–1366 [DOI] [PubMed] [Google Scholar]
- Kwiatkowski DJ, Manning BD. 2005. Tuberous sclerosis: a GAP at the crossroads of multiple signaling pathways. Hum Mol Genet. 14(Spec No. 2):R251–R258 [DOI] [PubMed] [Google Scholar]
- Laerm A, Helmbold P, Goldberg M, Dammann R, Holzhausen HJ, Ballhausen WG. 2007. Prospero-related homeobox 1 (PROX1) is frequently inactivated by genomic deletions and epigenetic silencing in carcinomas of the biliary system. J Hepatol. 46(1):89–97 [DOI] [PubMed] [Google Scholar]
- Luo Y, Liu L, Rogers D, Su W, Odaka Y, Zhou H, Chen W, Shen T, Alexander JS, Huang S. 2012. Rapamycin inhibits lymphatic endothelial cell tube formation by downregulating vascular endothelial growth factor receptor 3 protein expression. Neoplasia. 14(3):228–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack FX. 2008. Lymphangioleiomyomatosis: a clinical update. Chest. 133(2):507–516 [DOI] [PubMed] [Google Scholar]
- Mylona E, Alexandrou P, Giannopoulou I, Liapis G, Sofia M, Keramopoulos A, Nakopolou L. 2007. Clinicopathological and prognostic significance of vascular endothelial growth factors (VEGF)-C and -D and VEGF receptor 3 in invasive breast carcinoma. Eur J Surg Oncol. 33(3):294–300 [DOI] [PubMed] [Google Scholar]
- Navarro A, Perez RE, Rezaiekhaligh M, Mabry SM, Ekekezie II. 2008. T1alpha/podoplanin is essential for capillary morphogenesis in lymphatic endothelial cells. Am J Physiol Lung Cell Mol Physiol. 295(4):L543–L551 [DOI] [PubMed] [Google Scholar]
- Patel V, Marsh CA, Dorsam RT, Mikelis CM, Masedunskas A, Amornphimoltham P, Nathan CA, Singh B, Weigert R, Molinolo AA, et al. 2011. Decreased lymphangiogenesis and lymph node metastasis by mTOR inhibition in head and neck cancer. Cancer Res. 71(22):7103–7112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Seyama K, Fujii H, Maruyama H, Setoguchi Y, Iwakami S, Fukuchi Y, Hino O. 2002. Mutation analysis of the TSC1 and TSC2 genes in Japanese patients with pulmonary lymphangioleiomyomatosis. J Hum Genet. 47(1):20–28 [DOI] [PubMed] [Google Scholar]
- Sauka-Spengler T, Bronner-Fraser M. 2008. A gene regulatory network orchestrates neural crest formation. Nat Rev Mol Cell Biol. 9(7):557–568 [DOI] [PubMed] [Google Scholar]
- Seyama K, Kumasaka T, Kurihara M, Mitani K, Sato T. 2010. Lymphangioleiomyomatosis: a disease involving the lymphatic system. Lymphat Res Biol. 8(1):21–31 [DOI] [PubMed] [Google Scholar]
- Sherman CG, Jani P, Marks A, Kahn HJ. 2012. D2-40 is expressed on the luminal surface of pulmonary airspaces in normal developing and adult lung but is lost in conditions associated with intra-alveolar infiltrates. Pediatr Dev Pathol. 15(4):259–264 [DOI] [PubMed] [Google Scholar]
- Skog M, Bono P, Lundin M, Lundin J, Louhimo J, Linder N, Petrova TV, Andersson LC, Joensuu H, Alitalo K, et al. 2011. Expression and prognostic value of transcription factor PROX1 in colorectal cancer. Br J Cancer. 105(9):1346–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sozio F, Rossi A, Weber E, Abraham DJ, Nicholson AG, Wells AU, Renzoni EA, Sestini P. 2012. Morphometric analysis of intralobular, interlobular and pleural lymphatics in normal human lung. J Anat. 220(4):396–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tammela T, Alitalo K. 2010. Lymphangiogenesis: molecular mechanisms and future promise. Cell. 140(4):460–476 [DOI] [PubMed] [Google Scholar]
- Taveira-DaSilva AM, Pacheco-Rodriguez G, Moss J. 2010. The natural history of lymphangioleiomyomatosis: markers of severity, rate of progression and prognosis. Lymphat Res Biol. 8(1):9–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tee AR, Manning BD, Roux PP, Cantley LC, Blenis J. 2003. Tuberous sclerosis complex gene products, tuberin and hamartin, control mTOR signaling by acting as a GTPase-activating protein complex toward Rheb. Curr Biol. 13(15):1259–1268 [DOI] [PubMed] [Google Scholar]
- Wang J, Zhang B, Guo Y, Li G, Xie Q, Zhu B, Gao J, Chen Z. 2008. Artemisinin inhibits tumor lymphangiogenesis by suppression of vascular endothelial growth factor C. Pharmacology. 82(2):148–155 [DOI] [PubMed] [Google Scholar]
- Weckmann M, Moir LM, Heckman CA, Black JL, Oliver BG, Burgess JK. 2012. Lamstatin—a novel inhibitor of lymphangiogenesis derived from collagen IV. J Cell Mol Med. 16(12):3062–3073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xian ZH, Cong WM, Lu XY, Yu H, Wu MC. 2011. Angiogenesis and lymphangiogenesis in sporadic hepatic angiomyolipoma. Pathol Res Pract. 207(7):403–409 [DOI] [PubMed] [Google Scholar]
- Yamazaki T, Yoshimatsu Y, Morishita Y, Miyazono K, Watabe T. 2009. COUP-TFII regulates the functions of Prox1 in lymphatic endothelial cells through direct interaction. Genes Cells. 14(3):425–434 [DOI] [PubMed] [Google Scholar]
- Yu J, Astrinidis A, Henske EP. 2001. Chromosome 16 loss of heterozygosity in tuberous sclerosis and sporadic lymphangiomyomatosis. Am J Respir Crit Care Med. 164(8, Pt 1):1537–1540 [DOI] [PubMed] [Google Scholar]
- Yu Y, Yoon SO, oulogiannis G, Yang Q, Ma XM, Villén J, Kubica N, Hoffman GR, Cantley LC, Gygi SP, et al. 2011. Phosphoproteomic analysis identifies Grb10 as an mTORC1 substrate that negatively regulates insulin signaling. Science. 332(6035):1322–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Luo C, Wang X, Song X, Fu Y, Luo Y. 2010. Endostatin inhibits tumour lymphangiogenesis and lymphatic metastasis via cell surface nucleolin on lymphangiogenic endothelial cells. J Pathol. 222(3):249–260 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.