Abstract
The aberrant activation of the developmentally regulated anterior gradient protein 2 (AGR2) gene has been associated with a metastatic phenotype. However, its mechanism of action and its regulation in prostate cancer is unknown. We had previously found that the ErbB3 binding protein 1 (EBP1), whose expression is reduced in prostate cancer, is a putative repressor of AGR2. The current study demonstrates that overexpression of AGR2 promotes the motility and invasiveness of non-metastatic LNCaP cells, while silencing of AGR2 in metastatic LNCaP derivative C4–2B cells significantly reduced cell invasion, suggesting AGR2 is an important determinant of the invasive capacity in human prostate cancer. Further, we provide evidence that the anti-invasive effect of EBP1 occurs, at least in part, through its ability to inhibit the expression of AGR2. EBP1 suppresses the promoter activity of the AGR2 gene, downregulates Foxa1 & Foxa2-stimulated AGR2 transcription and decreases metastatic behavior. In contrast, ablation of EBP1 upregulates the expression of the AGR2 gene, enhances Foxa1 and Foxa2–stimulated AGR2 promoter activity, and results in a more metastatic phenotype. We also found a significant inverse correlation between EBP1 and AGR2 levels in prostate cancer cell lines and importantly, primary prostate tumors. Collectively, our results highlight a previously uncharacterized EBP1-Foxa-AGR2 signaling circuit that has potential mechanistic and functional significance in therapeutic management of metastatic prostate cancer.
Introduction
Metastasis is the major cause of death from prostate cancer. Interruption of early metastases is crucial for a majority of individuals with prostate cancer. Given the complex cascade of events in metastasis including invasion, intravasation, extravasation and colonization, defining the molecules and underlying mechanisms required for these steps may help identify therapeutic targets(1–3).
Aberrant or untimely activation of developmentally regulated genes contributes disproportionately to metastasis. Anterior gradient protein-2 (AGR2), isolated in a screen for differentially expressed genes in neural development of Xenopus laevis, plays an essential role in the formation of the forebrain and the mucus-secreting cement gland(4). In humans, AGR2 was first identified in breast cancers and forced expression of AGR2 cDNA confered a metastatic phenotype on benign nonmetastatic rat breast carcinoma cells(5). Aberrant AGR2 expression has been found in adenocarcinomas of breast(6–8), esophagus(9, 10), pancreas(11–13) and prostate (14, 15), but how AGR2 promotes the invasive phenotype in prostate cancer remains unknown.
Foxa (Foxa1, a2, and a3) proteins belong to a superfamily of forkhead transcription factors which act as genetic potentiators that facilitate the binding of other transcriptional factors during development and differentiation (16, 17). A recent report suggests that Foxa1 and Foxa2 positively regulate AGR2 promoter activity(18). In addition, Foxa proteins are important in prostate carcinogenesis. In particular, Foxa2 may be involved in progression of prostate cancer to androgen independence(19).
ErbB3 binding protein 1 (EBP1), cloned by our group, is the human homologue of a previously identified cell cycle regulated mouse protein p38–2G4(20). EBP1 is a conserved molecule with multiple roles in cell growth, differentiation and apoptosis(21). In particular, EBP1 has been characterized as a negative regulator of crosstalk between HRG-triggered signaling and the AR axis in prostate cancer(22–24). The expression of the EBP1 gene is reduced in clinical and preclinical models of prostate cancer(24, 25). Restoration of EBP1 expression ameliorates the hormone refractory phenotype both in vitro and in vivo(25). In conducting a microarray analysis of EBP1 transfected prostate cancer cells to determine the spectrum of differentially expressed genes contributing to the hormone refractory phenotype, we found that AGR2 is an EBP1-downregulated gene(25). Our current work identifies a previously uncharacterized signaling circuit which involves EBP1-Foxa-AGR2 that has potential mechanistic and functional significance in therapeutic management of metastatic prostate cancer.
Materials and Methods
Cell culture, gene transfection, and luciferase reporter assay
The LNCaP cell line was obtained from the American Type Culture Collection (Manassas, VA, USA). C81, C4–2, and C4–2B cells were gifts of Dr Yun Qiu (University of Maryland) per permission of Drs Lin(26) and Chung(27). C81 sublines stably transfected with EBP1 cDNA or vector control, EBP1-null C13 and control A16 cells were established previously in our lab(25). Cells were maintained at 37°C in a humidified atmosphere of 5% CO2 in air. Cell lines were routinely cultured in RPMI 1640 (LNCaP, C81) or T-medium supplemented (C4–2, C4–2B) with 10% fetal bovine serum (FBS) (Sigma, St Louis, MO, USA). pGL3B-AGR2, a reporter construct containing the AGR2 promoter was a gift from D. r J. Hampe(18). Foxa1 or Foxa2 expression plasmids were gifts of Dr. R. Matusik(19). Cells were seeded in 6-well plates in complete media for 24 hrs. Transfections were performed with Fugene 6 (Roche) with various amounts of plasmids as indicated. Luciferase activity was measured as previously described (18, 22).
Creation of stably transfected cell lines
LNCaP cells stably transfected with pcDNA3.1 or pcDNA3.1-hAGR2 plasmids (a gift from Dr. C. Young, Mayo Clinic) or C4–2B cells stably transfected with pEGFP-Ebp1(C4–2BE) or vector control(C4–2BG) were established as previously described (22). To generate an AGR2-silenced cell line, C4–2B cells were seeded into 96-well plates and transduced with lentiviral particles corresponding to different short hairpin RNA(shRNA) constructs targeting the AGR2 gene(Sigma, Mission shRNA, NM_006408) and the lentiviral transduction particles produced from the sequence-verified lentiviral plasmid, pKLO.1-puro control vector as a negative control (Sigma, SHC001V,03130808MN). The derivation of the EBP1 stably transfected C81 well line and the EBP1 deleted LNCaP C13 and A16 cell lines and their expression of EBP1 have previously been published (25).
GST-pull down assay, Immunoprecipitation, DNA affinity precipitation and Western blot analysis
For studies determining the interaction of EBP1 with Foxa, cell lysates were incubated with GST-EBP1, purified as previously described (22), for GST-pull down assay or GFP-conjugated GFP agarose (Millipore) for immunoprecipitation and analyzed by western blotting where indicated as described previously(22). The interaction of Foxa with the AGR2 promoter sequence was assessed by DNA affinity precipitation as previously described(28) using LNCaP cell extracts and biotinylated oligonucleotides GTGGGTTACTTGATTTGTATTTTTTTTCAT (Olig A) containing the Foxa binding consensus elements or attctgagct tttaaagactgcacacaact (Olig B) without any potential Foxa binding sites, all derived from the AGR2 promoter. The polyclonal AGR2 antibody was a gift from Dr. C Young (Mayo Clinic) and the EBP1 antibody was from Millipore. The monoclonal anti-β-actin antibody was from Sigma. Goat polyclonal antibodies Foxa1(C-20) and Foxa2 (P-19) were from Santa Cruz Biotechnology (Santa Cruz, CA).
Boyden chamber assay
A modified Boyden chamber assay as described previously (29–31) was used to determine cell migration and invasion. Basically, culture plate inserts (8-μm pore size and 12mm diameter, Millicell-PCF) were coated with 150μl PBS containing 10μg collagen and 1μg fibronectin (BD Bioscience) for 1h at room temperature. For invasion assays, collagen-coated filters were air dried, further coated with a total of 30μl of Matrigel matrix(BD Bioscience) diluted 1:5 in serum-free medium and allowed to gel in a 37°C incubator for 30min prior to adding the cells suspended in 450μl medium with 5% charcoal stripped serum. Bottom wells in the system were filled with 600 μl complete medium. After 40 hours of incubation, the inserts were fixed in 10% formalin for 20 min and after washing with water, stained with 0.5% crystal violet in 25% methanol for 30–60min. Non-migrating or invading cells on the top of the filters were removed with a cotton swab. Cells that had migrated or invaded to the undersurface of the filter were examined at 10 or 20 × magnifications. Three representative areas were photographed and number of cells counted. Each experiment was performed in triplicate.
Tissue Microarray and immunohistochemical analysis
An intermediate density tissue microarray of prostate cancer and benign prostate tissues was received from the CPCTR (NCI). Immunohistochemical analysis of AGR2 was performed as previously described (14). Immunostaining was evaluated as in (25).
Statistical analysis
Results for luciferase and Boyden chamber assays, and correlation between EBP1 and AGR2 protein levels in cell lines were analyzed using a two-tailed Student-t-test or Mann-Whitney U-test or one-way ANOVA as appropriate. The association between AGR2 expression and the nature of the prostate tissues (benign, hormone responsive, hormone refractory) was assessed by the Chi-Square test. The Pearson’s correlation coefficient test was used to determine the relationship between intensity of AGR2 staining and EBP1 levels. P ≤0.05 was considered statistically significant.
Results
EBP1 levels inversely correlate with AGR2 expression in prostate cancer cell lines
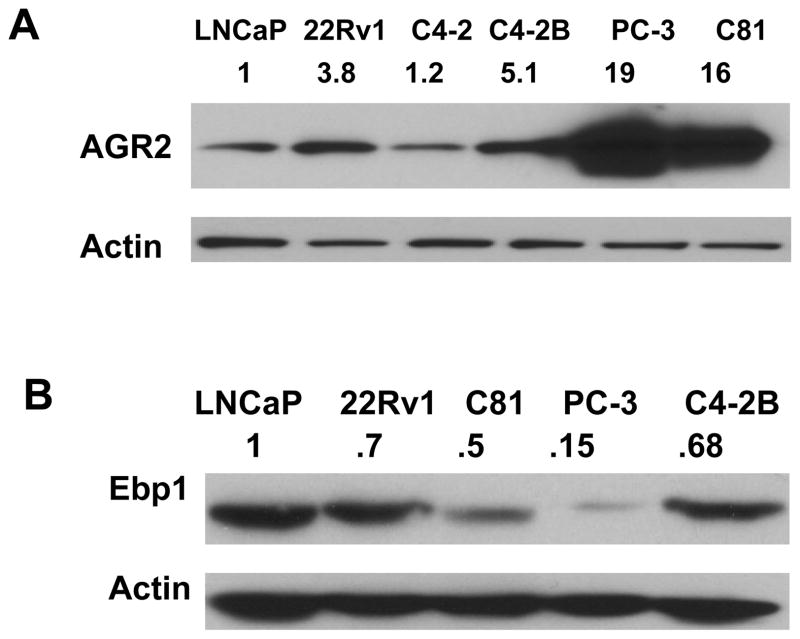
To establish a suitable cellular system to investigate how EBP1 regulates AGR2 expression, EBP1 and AGR2 expression levels were measured in a panel of prostate cancer cell lines. Examination focused on several preclinical models recapitulating the hormone refractory phenotype. The LNCaP subline C81 has been made androgen-independent by continuous long term passage of LNCaP cells in complete media (26). The LAPC-4 xenograft grows as an androgen dependent cancer in male SCID mice and regresses in response to androgen ablation, but eventually regrows as an androgen-independent tumor (32). The LNCaP derivative C4–2B exhibits two cardinal features of human prostate carcinoma progression: androgen independence and osseous metastasis(29). Intraosseous injection of androgen independent 22Rv1 cells generates osteolytic and osteoblastic responses, a subset of the pathology exhibited in patients with metastasis (33). AGR2 protein expression was much higher in androgen refractory 22Rv1, C81, metastatic C4–2B, and PC-3 cells compared with androgen-dependent nonmetastatic LNCaP cells (34, 35) (Fig. 1A), suggesting that AGR2 contributes to malignant progression in prostate cancer. Importantly, among these cell lines, there is a significant inverse correlation between EBP1 and AGR2 expression in prostate cancer cell lines (Fig. 1, B vs A, P<0.05, one-way ANOVA.), supporting our previous finding that EBP1 might be a negative regulator of AGR2 (25, 36). (is replaced by)
Figure 1. EBP1 levels inversely correlate with AGR2 expression in prostate cancer cell lines.
A & B, Immunoblots detecting AGR2 (A) and EBP1 (B) expression in prostate cancer cell lines. Values on top of the bands represent relative densities normalized to actin.
AGR2 promotes the motility and invasiveness of prostate cancer cells
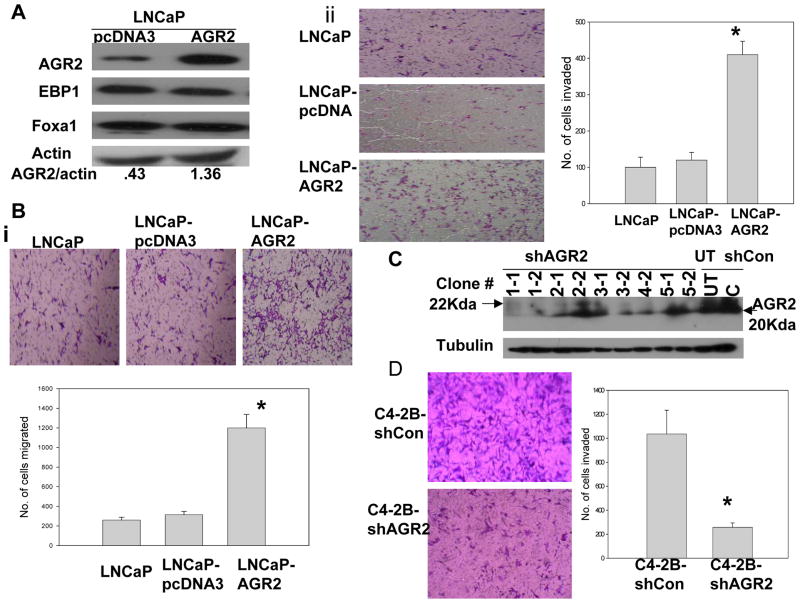
Nonmetastatic LNCaP cells expressing low levels of AGR2 (Fig. 1) were selected to test if forced expression of AGR2 cDNA promotes motility and invasiveness. LNCaP cells stably transfected with pcDNA3.1-hAGR2 cDNA have a 3- fold increase in AGR2 expression, but no changes in EBP1 and Foxa1 protein levels compared with the vector control transfected cells (Fig. 2A). Forced expression of AGR2 resulted in a marked (>10 fold) increase of motility in LNCaP cells as measured by migration assays (Fig. 2B, P<0.01versus control). Increased AGR2 expression also promoted the invasiveness of LNCaP cells through Matrgel, more than three fold over control transfectants (Fig. 2C, P<0.01). Of note, transfection of pcDNA has no effect on cell migration and invasiveness of LNCaP cells (Fig. 2B and 2C). In addition, at the 40 hour time point, there was no difference in numbers of LNCaP control and AGR2 transfected cells (data not shown). To further assess the effect of AGR2 in the metastatic process, we transduced metastatic LNCaP derivative C4–2B cells with five individual shRNA lentiviral particle constructs targeting AGR2 mRNA. Only one construct (NM_006408.2-252s1c1) FILL IN NT AND SEQUENCe, CGGCAAGACAAGCAACAAACCCTTCTCGAGAAGGGT TTGTTGCTTGTCTTGTTTTTTG targeted to NT 385--? Of the coding sequence) silenced AGR2 expression (Clones 1–1 and 1–2 in Fig. 2D). To ensure that our results hereafter were not specific to any individual subclone of transfected cells, we pooled clones 1–1 and 1–2 for biological analysis. Silencing of endogenous AGR2 in metastatic C4–2B cells significantly reduced invasiveness (Fig. 2D, P<0.01 versus control). No decreases in invasiveness were observed in cells transduced by control lentiviruses or by AGR2 targeted lentiviruses that did not decrease AGR2 expression (data not shown), suggesting that these biological changes were unlikely to be due to off-target effects. Silencing of AGR2 has no effect on cell proliferation at the 40 hour time point (data not shown) These results indicated that AGR2 expression is an important determinant of the invasive capacity of human prostate cancer cells.
Figure 2. AGR2 promotes non-metastatic prostate cancer cell migration and invasion.
A, Immunoblotting analysis of LNCaP cells stably transfected with pcDNA3 (LNCaP-pcDNA3) or pcDNA3-hAGR2 (LN-AGR2 or LNCaP-AGR2) using antibodies as indicated. Values under panel represent relative band densities normalized to actin. B, Migration ability was estimated using a Modified Boyden chamber assay. Left panel, representative image of three independent experiments showing cells that migrated through the transwell filters coated with collagen and fibronectin. LNCaP is the untransfected parental cell line. Right graph: Number of migrated cells in representative areas of each membrane that were examined microscopically at 10X magnification. Columns, mean number of cells migrated in three representative fields per well. Bars, SD (*, P<0.01 versus control, t test). Shown as one of three independent experiments. C, left panel, representative photomicrograph of invasion assay from three comparable experiments showing cells invading through Matrigel-coated membranes. Right graph, cells that had invaded in representative areas of each membrane were examined microscopically at 20X magnification. Columns, mean number of cells invaded in three representative fields per well. Bars, SD (*, P<0.01 versus control, t test). Shown as one of three independent experiments. D, left top panel, Western blot analysis of AGR2 expression in cells transduced with lentiviruses targeted to different portions of the AGR2 coding sequence. AGR2 was silenced in stable clones 1–1 and 1–2 using a viruses targeting XXXX of the coding sequence. UT-untransduced cells; shCON=empty lentivirus. Left lower panel, representative micrographs of effects of stable silencing of AGR2 on the invasiveness of C4–2B cells as described in Fig. 2B. Cells from clones 1–1 and 1–2 were pooled for the migration and invasion assays (shAGR2). Right panel, columns, mean number of cells migrated in three representative fields per well. Bars, SD (*, P<0.01 versus control, t test). Shown as one of three independent experiments.
EBP1 modulation of the expression of AGR2 is accompanied by changes in motility and invasiveness
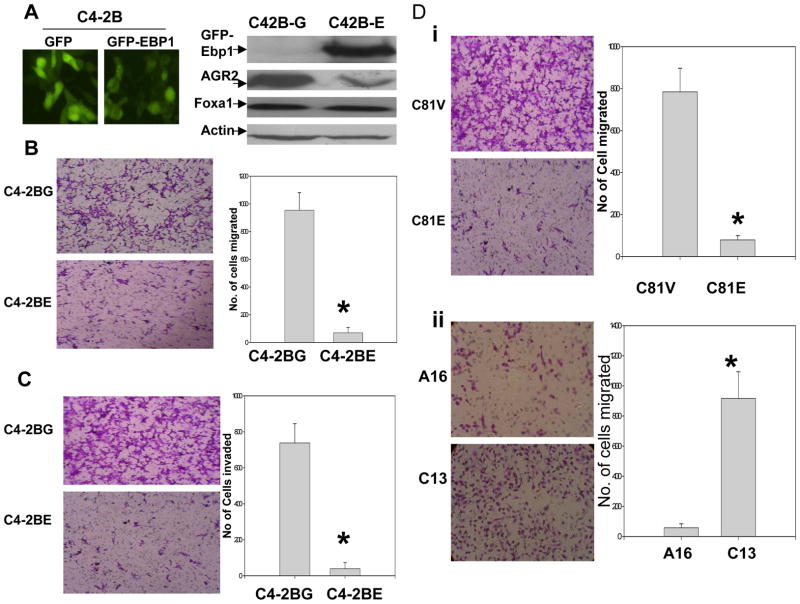
As AGR2 augments invasiveness of non metastatic prostate cancer LNCaP cells in vitro, we tested if overexpressing or knocking down EBP1, which alters AGR2 expression, could modulate motility and invasive ability. Metastatic C4–2B cells stably transfected with pEGFP-EBP1 cDNA (C4–2BE) expressed a GFP-EBP1 fusion protein (Fig. 3A, left panel). Endogenous EBP1 levels are not changed (not shown) Ectopic expression of EBP1 in C4–2B cells (C4–2BE) led to a significant reduction of AGR2 protein (Fig. 3A, right panel). Steady state levels of AGR2 mRNA were decreased 35% in EBP1 transfectants as compared with vector controls. Expression of Foxa1 remained the same (Fig. 3A, right panel), indicating that EBP1 inhibition of AGR2 is not cell-type specific (25). C4–2BE cells were less motile (Fig. 3B) and invasive (Fig. 3C) than C4–2BG controls (P<0.01 versus control), similar to C4–2B cells in which AGR2 was suppressed by shRNA (Fig. 2D). In addition, restoration of EBP1 in androgen independent C81 cells (C81E) leading to reduced AGR2 expression(25), decreased migration (Fig. 3D, P<0.01). Knocking down EBP1 in LNCaP cells(C13) leading to enhanced expression of AGR2(25), increased motility compared with A16 control cells(Fig. 3E, P<0.01). These data support the notion that EBP1 suppresses invasiveness in part via inhibition of expression of the AGR2 gene.
Figure 3. EBP1 modulates the expression of AGR2 accompanied by changes in motility and invasiveness in prostate cancer cells.
A, C4-2B cells stably expressing GFP(C4-2BG) or GFP-EBP1 fusion proteins(C4-2BE) were visualized by fluorescence microscopy(left panel) and confirmed by Western blotting assay using GFP antibody (right upper panel). AGR2 and Foxa1 levels were also analyzed in these cells and Actin was used as loading control (lower 3 panels). B, Left panel: Photomicrographs of Boyden Chamber membranes after migration assays showing that many C4-2BG but few C4-2BE cells traversed a coated membrane (left panel). Right panel: Quantification of the effects of EBP1 and AGR2 expression on migration. Columns= mean number of cells migrated in three representative fields per well. Bars=SD. Shown as one of three independent experiments. C. Invasion assay was described in Fig2. D & E, left panel, micrographs of Boyden chamber membranes after migration assays with C81V and C81E, A16 and C13. Right panel: relative quantification of the effects of EBP1 and AGR2 expression on the migration. Columns, mean number of cells migrated in three representative fields per well. Bars, SD (*, P<0.01 versus control, t test). Shown as one of three independent experiments.
EBP1 suppresses the AGR2 promoter
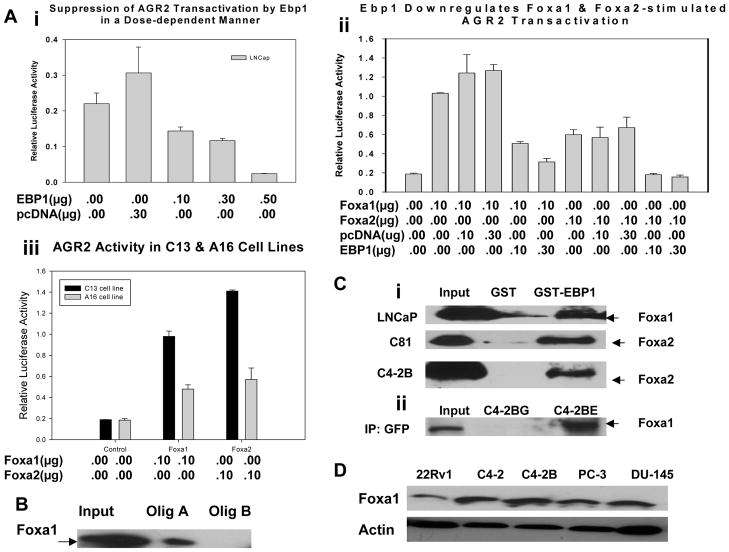
Our previous microarray and quantitative real-time PCR data suggested that EBP1 repression of AGR2 protein levels might be at the transcriptional level (25). To determine if the mechanism of AGR2 repression involves transcriptional regulation, we tested if EBP1 inhibits the promoter activity of the AGR2 gene using luciferase reporter assays. Transient transfection of exogenous EBP1 cDNA in LNCaP cells inhibited AGR2 promoter activity in a dose dependent manner (Fig. 4A, P<0.01, Mann-Whitney U-test), while levels of Foxa1 and Foxa2 protein remain the same (data not shown), suggesting that EBP1 might negatively regulate the expression of the AGR2 gene at the transcriptional level. Zheng, et al (18) recently reported that Foxa1 and Foxa2, implicated in prostate carcinogenesis, activate the human AGR2 promoter. We determined that EBP1 diminished Foxa1 and Foxa2-stimulated AGR2 transactivation (Fig. 4B, P<0.01, one-way ANOVA), indicating that EBP1 might interfere with the regulation of AGR2 promoter activity by Foxa1 and Foxa2.
Figure 4. EBP1 suppresses the promoter activity of the AGR2 gene by interfering with Foxa transcription factors and binds Foxa proteins.
A, B & C, LNCaP or its subline EBP-null C13 and control A16 cells were transfected with an AGR2 reporter (pGL3B-AGR2, 0.25μg/well) and other indicated plasmids. Forty-eight hours later, cells were harvested for luciferase measurement. Each bar represents mean±s.e of triplicate wells. Representative of three independent experiments. D, The interaction of Foxa with the AGR2 promoter sequence was assessed by DNA affinity precipitation as previously described(28) using LNCaP cell extracts and biotinylated olig A containing a Foxa consensus sequence or Olig B lacking potential Foxa binding sites, all derived from the AGR2 promoter as described in Materials and Methods. E, Association of EBP1 and Foxa proteins in vitro (three upper panels) and in vivo (lower panel). Upper three panels: Equal amounts of GST-EBP1 or GST proteins were incubated with lysates of LNCaP, C81, or C4-2B cells. Associated proteins were analyzed by Western blot analysis with the antibodies indicated. Lower panel: Lysates of stably expressing GFP (C4-2BG) or GFP-EBP1 (C4-2BE) cells were incubated with GFP agarose and immunopreciptation performed using a GFP antibody. Associated proteins were analyzed by Western blotting using Foxa1 and Foxa2 antibodies. F. Western blot analysis of Foxa1 expression in panel of prostate cancer cell lines.
To solidify this initial observation and definitively establish EBP1’s role in regulating AGR2 activity, we used EBP1-null LNCaP derived C13 cells which showed elevated AGR2 expression (25) to examine if AGR2 promoter activity is enhanced by Foxa in an EBP1 depleted environment. Both Foxa1 and Foxa2 induced greater AGR2 promoter activity in C13 cells as compared with control A16 cells (Fig. 4C, P<0.01, one-way ANOVA), suggesting the interaction of EBP1 and Foxa plays an important role in regulating AGR2 expression.
Foxa proteins share four distinct conserved regions (CRI-CRIV) with an almost identical DNA binding domain called the winged-helix/forkhead domain (CRI) (16, 17). CRII and CRIII in the C terminus and CRIV in the N terminus are capable of distinct interactions with other transcription factor(s) and/or cofactor(s) for the activity of Foxa (37). The DNA binding consensus sequence for Foxa proteins is VWWTRTTTRYTY or HWATTGAYTWWD with a 7 bp recognition core motif in common, whereas sequences flanking either side don’t share any obvious similarity (16, 17). However, the Foxa regulated element of the AGR2 promoter is not known. Based on these criteria, we identified several potential binding sites of Foxa in the AGR2 promoter (data not shown). Importantly, Foxa1 from LNCaP cell lysates bound specifically to the sequence containing Foxa consensus elements (Olig A, Fig. 4D), but not the sequence lacking a potential binding motif (Olig B, Fig. 4D). We also examined if EBP1 physically associates with Foxa by in vitro GST-pull down assays in several cells lines. GST-EBP1 associated with endogenous Foxa1 and Foxa2 (Fig. 4E, middle two panels). Finally, we tested if EBP1 and Foxa1 associate in vivo by immunoprecipitation analysis of GFP-EBP1 transfected cells. These results indicated that EBP1 binds Foxa1 in vivo (Fig. 4E). Finally,, last panel). Foxa1 expression has been shown to vary with in different cell lines (Matusik). To determine if a potential-EBP1 Foxa1 interaction may be important in different lines, we measured Foxa1 expression in several cell lines. We found, in agreement with previous reports (REF), that Foxa1 was expressed in all cell lines tested at approximately equal levels (Figs. 4F, 2A, 3A). Foxa2 was selectively expressed in LNCaP derivatives C81 and C4–2B cells(Fig. 4E) We hypothesize that EBP1 represses Foxa-mediated transactivation of the AGR2 gene by interference with the functions of Foxa DNA-binding and/or transactivation.
Expression of AGR2 increases with prostate cancer progression and inversely correlates with loss of EBP1 expression
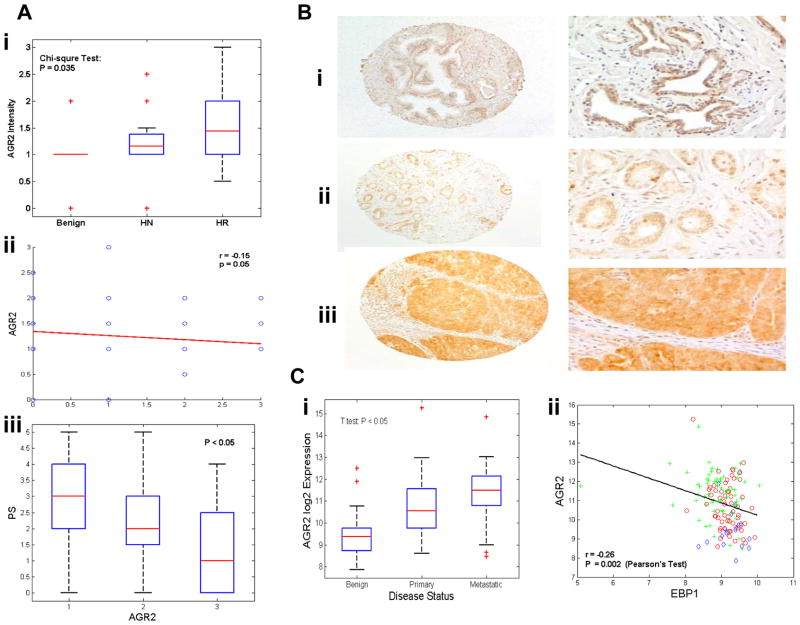
To extend our observations to the clinical setting, we next examined a possible correlation between EBP1 and AGR2 expression levels in primary tumors. A prostate cancer tissue microarray previously used for EBP1 staining (25) was used for immunohistochemical analysis of AGR2 (14). Immunohistochemical staining showed that among 17 benign cases, 5.88% were unstained, 52.94% stained weakly positive and 35.29% stained moderately positive for AGR2. Amongst 51 hormone responsive carcinoma cases, 60.78% stained weakly positive, 33.33% stained moderately positive and 5.88% stained strongly positive. Amongst 36 hormone refractory cases, 36.11% stained weakly positive, 41.67% stained moderately positive and 22.22% stained strongly positive. The difference in AGR2 staining among benign, hormone responsive and hormone refractory cases is statistically significant (Chi-square test, P=0.035). The differences in staining of hormone responsive and hormone refractory versus benign tissues are statistically significant (P < 0.05). Even though the difference in staining of hormone refractory versus hormone responsive tissues is not statistically significant (P = 0.12), the overall trend does indicate that AGR2 expression increases with the progression of prostate cancer (Fig. 5A, Left panel). This is shown by the non-zero correlation coefficient (r = 0.17, P =0.038) between AGR2 intensity and the progression status.. In the benign tissue, AGR2 immunostaining can be observed mainly in basal cells (Fig. 5B, left upper and lower panel), while in malignant tissues, enhanced intensity is seen in both the cytoplasm and nucleus of luminal epithelial cells(Fig. 5B, middle and right panel), similar to previous reports (14, 15). AGR2 was originally presumed to be a secreted protein. A recent analysis indicated that the KTEL sequence in the carboxyl-terminal of AGR2 was recognized by the endoplasmic reticulum(ER) retention receptor and served to localize AGR2 predominantly to the ER of transfected cells (38). Studies on different isoforms or variants of AGR2 and its interaction with different proteins may thus provide some suggestion on what controls the localization of AGR2 protein across species. We also found that increased AGR2 expression is significantly inversely correlated with EBP1 staining intensity (Fig. 5A, middle panel, r=−.15, P=0.05). In addition, increased AGR2 expression is significantly (p<0.05)correlated with a decrease in EBP1 labeling frequency(Fig. 5A, right panel).
Figure 5. Expression of AGR2 increases with prostate cancer progression and inversely correlates with EBP1 expression.
A, Left panel, box plots of immunohistochemical staining intensity of AGR2 in normal prostate tissue (n=42) or tissues from patients with prostate cancer (n=153). For all panels, the box represents the s.d. of the distribution, and the line through that box represents the mean of the distribution. The horizontal lines above and below the box represent the extreme values of the distribution. Middle panel, Correlation of AGR2 staining intensity with EBP1 staining intensity. Right panel, Correlation of AGR2 staining intensity with EBP1 proportion score (PS) (percentages of cells stained with EBP1: 1,<5%; 2, 5–30%; 3,31–70%; 4, >70%). B, representative staining of AGR2 in benign tissues (left panel), moderately malignant carcinomas (middle panel, Gleason 3+3) and highly malignant carcinomas (right panel, Gleason 4+5). C, Left panel, a public dataset ???? shows the average gene expression level at three different stages(benign n=23, primary n=64 and metastatic n=250) of prostate cancer. Right panel, scatter-plots of AGR2 versus EBP1 expression units in all these samples.
We further interrogated a publicly available cDNA microarray expression data set that shows a statistically significant decrease of EBP1 expression from with prostate cancer progression. We found a significant increase of AGR2 as prostate cancer progressed (Fig. 5C, left panel), indicating that AGR2 had a significant role in distant metastasis (P<0.05). To study the correlation between EBP1 and AGR2 expression, we directly quantified their expression relationship by plotting log-transformed expression units of EBP1 [Fig. 1B in Ref. (25)] against AGR2 staining and measured their expression similarity using Pearson’s correlation coefficients. As shown in Fig5C right panel, we observed a significant negative correlation between EBP1 and AGR2 across all samples with r=−0.26 and P-value=0.002. All together, these data suggest the possibility that EBP1 suppresses metastasis of prostate cancer cells by inhibiting the expression of the AGR2 gene; the loss of EBP1 activity may functionally enhance invasiveness of advanced prostate cancer by releasing the brake on AGR2 promoter activity.
Discussion
The role of the developmentally expressed gene AGR2 in metastasis is being increasingly studied in many cancer types. However, its action in prostate cancer specifically has not been analyzed extensively. This is the first report indicating that stable expression of mammalian AGR2 cDNA in nonmetastatic prostate cancer cells promotes motility and invasiveness, linking increases of AGR2 expression with a propensity for prostate cancer cells to undergo metastasis. A previous report showed that overexpression of AGR2 in benign, non-metastatic, rat mammary cells leads to metastasis (5). AGR2 expression also promotes tumor growth in esophageal adenocarcinoma cells (10). Silencing AGR2 significantly reduced cell proliferation and invasion of pancreatic cancer cells (13). The prognostic relevance of aberrant AGR2 expression has consistently been shown in primary breast (6–8), esophagus (9, 10), pancreas (11–13) and prostate (14, 15) carcinomas. Moreover, in the first global gene expression profiling of circulating tumor cells (CTCs), the AGR2 gene was expressed in the majority of the metastatic samples, regardless of the cancer type (39). These results are in keeping with our current findings that AGR2 serves a pro-invasive and pro-metastatic function. Notably, we indentified EBP1 as a novel potent suppressor of AGR2, in keeping with the demonstration that EBP1 is implicated in progression of prostate cancer (22, 24, 25). We hypothesize that EBP1 modulates the expression of AGR2 leading to changes in motility and invasiveness of prostate cancer cells. In addition, we observed a significant inverse correlation between EBP1 and AGR2 expression in prostate cancer cell lines and clinical samples of prostate cancer. These results strongly support our concept that EBP1 suppresses the invasive ability of cells by inhibiting the expression of AGR2.
The mechanisms underlying the deregulation of AGR2 in metastatic cancer remain elusive. A recent report suggested that genetic or epigenetic changes are not key to changes in AGR2 levels. Rather, AGR2 expression is controlled by a broad set of signaling and metabolic pathways (REF). In the prostate, regulation of AGR2 expression may be dependent or independent of the androgen receptor (14) which itself is subject to various upstream signals, such as aberrant growth factors and receptor tyrosine kinases(40). In this context, EBP1 negatively regulates crosstalk between the ErbB network and the AR signaling axis (22–24). On the other hand, Forkhead transcription factors regulate highly complex, multigenic processes in development and are directly involved in malignancies (16, 17), including that of prostate (19). Interestingly, AGR2 is positively modulated by Foxa1 and Foxa2 suggesting a potential Foxa-AGR2 axis. EBP1 down-regulated the expression of AGR2 by antagonizing Foxa activation of the AGR2 promoter leading to suppression of invasion and metastasis.
The detailed mechanisms underlying EBP1 mediated suppression of AGR2 is under investigation in our laboratory. The current study indicated a physical interaction between EBP1 and Foxa which bound to its consensus sequence in the AGR2 promoter. The crystal structure of EBP1 suggests that the conserved LxxLL motif in EBP1 interacts with structurally diverse binding sites, such as AR, Rb, mSIN3 and HDAC2(41). This motif might be well involved in the interaction between EBP1 and Foxa transcriptional factors, important in modulating AGR2 transactivation. Indeed, the three Foxa proteins share four distinct conserved regions (CRI-CRIV)(37, 42, 43). There is more than 90% homology among the Foxa proteins at CRI, which functions as the DNA binding domain. CRII and CRIII in the C terminus and CRIV in the N terminus are capable of distinct interactions with other transcription factor(s) and/or cofactor(s). Further investigation will clarify if EBP1 attenuates the transcriptional activity of Foxa by reducing the binding of Foxa proteins to the AGR2 promoter via its interaction with the Foxa DNA-binding domain and/or by blocking the FOXa transactivation domain via interaction with other conserved regions. Since Foxa1 and Foxa2 have been found to be expressed in a wide range in prostate cancer and prostate cancer cell lines (REF and current data), we suggest that Ebp1 may associate with Foxa1 and Foxa2 in a unique manner in different stages of prostate cancer.
Nevertheless, deregulation of both EBP1(25) and Foxa(19) may contribute to invasion and distant spread of cancer cells at least in part by promoting expression of AGR2. Further studies focusing on this previously uncharacterized EBP1-Foxa-AGR2 signaling circuit, especially in in vivo animal models, would identify new downstream targets with translational potential for early detection and therapy of prostate cancer.
Acknowledgments
This work was supported by Maryland Technology Corporation (June 6, 2006) and Department of Defense grant W81XWH-07-0267 (Y. Zhang), NIH grants R01 CA76047 and R21 088882-01, and a Department of Pathology grant (A.W. Hamburger). We also thank Dr. J. Hampe for the AGR2 promoter plasmid, Dr. R. Matuski for the Foxa expression plasmids and Dr. C. Young for the AGR2 antibody.
Contributor Information
Tehmina Z. Ali, Email: TALI@umm.edu.
Hua Zhou, Email: hzhou2@yahoo.com.
David R D’Souza, Email: ddsouza@som.umaryland.edu.
Yan Lu, Email: stonelyan@hotmail.com.
Jonathan Jaffe, Email: jjaffe@umd.edu.
Zhenqiu Liu, Email: zliu@umm.edu.
Antonino Passaniti, Email: apass001@umaryland.edu.
Anne W Hamburger, Email: ahamburg@som.umaryland.edu.
References
- 1.Rinker-Schaeffer CW, O’Keefe JP, Welch DR, Theodorescu D. Metastasis suppressor proteins: discovery, molecular mechanisms, and clinical application. Clin Cancer Res. 2006;12(13):3882–9. doi: 10.1158/1078-0432.CCR-06-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ye XC, Choueiri M, Tu SM, Lin SH. Biology and clinical management of prostate cancer bone metastasis. Front Biosci. 2007;12:3273–86. doi: 10.2741/2311. [DOI] [PubMed] [Google Scholar]
- 3.Steeg PS, Theodorescu D. Metastasis: a therapeutic target for cancer. Nature clinical practice. 2008;5(4):206–19. doi: 10.1038/ncponc1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aberger F, Weidinger G, Grunz H, Richter K. Anterior specification of embryonic ectoderm: the role of the Xenopus cement gland-specific gene XAG-2. Mechanisms of development. 1998;72(1–2):115–30. doi: 10.1016/s0925-4773(98)00021-5. [DOI] [PubMed] [Google Scholar]
- 5.Thompson DA, Weigel RJ. hAG-2, the human homologue of the Xenopus laevis cement gland gene XAG-2, is coexpressed with estrogen receptor in breast cancer cell lines. Biochemical and biophysical research communications. 1998;251(1):111–6. doi: 10.1006/bbrc.1998.9440. [DOI] [PubMed] [Google Scholar]
- 6.Liu D, Rudland PS, Sibson DR, Platt-Higgins A, Barraclough R. Human homologue of cement gland protein, a novel metastasis inducer associated with breast carcinomas. Cancer research. 2005;65(9):3796–805. doi: 10.1158/0008-5472.CAN-04-3823. [DOI] [PubMed] [Google Scholar]
- 7.Fritzsche FR, Dahl E, Pahl S, et al. Prognostic relevance of AGR2 expression in breast cancer. Clin Cancer Res. 2006;12(6):1728–34. doi: 10.1158/1078-0432.CCR-05-2057. [DOI] [PubMed] [Google Scholar]
- 8.Innes HE, Liu D, Barraclough R, et al. Significance of the metastasis-inducing protein AGR2 for outcome in hormonally treated breast cancer patients. British journal of cancer. 2006;94(7):1057–65. doi: 10.1038/sj.bjc.6603065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Groome M, Lindsay J, Ross PE, Cotton JP, Hupp TR, Dillon JF. Use of oesophageal stress response proteins as potential biomarkers in the screening for Barrett’s oesophagus. European journal of gastroenterology & hepatology. 2008;20(10):961–5. doi: 10.1097/MEG.0b013e3282ffd9bd. [DOI] [PubMed] [Google Scholar]
- 10.Wang Z, Hao Y, Lowe AW. The adenocarcinoma-associated antigen, AGR2, promotes tumor growth, cell migration, and cellular transformation. Cancer research. 2008;68(2):492–7. doi: 10.1158/0008-5472.CAN-07-2930. [DOI] [PubMed] [Google Scholar]
- 11.Iacobuzio-Donahue CA, Maitra A, Olsen M, et al. Exploration of global gene expression patterns in pancreatic adenocarcinoma using cDNA microarrays. The American journal of pathology. 2003;162(4):1151–62. doi: 10.1016/S0002-9440(10)63911-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lowe AW, Olsen M, Hao Y, et al. Gene expression patterns in pancreatic tumors, cells and tissues. PloS one. 2007;2(3):e323. doi: 10.1371/journal.pone.0000323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramachandran V, Arumugam T, Wang H, Logsdon CD. Anterior gradient 2 is expressed and secreted during the development of pancreatic cancer and promotes cancer cell survival. Cancer research. 2008;68(19):7811–8. doi: 10.1158/0008-5472.CAN-08-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang JS, Gong A, Cheville JC, Smith DI, Young CY. AGR2, an androgen-inducible secretory protein overexpressed in prostate cancer. Genes, chromosomes & cancer. 2005;43(3):249–59. doi: 10.1002/gcc.20188. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Forootan SS, Liu D, et al. Increased expression of anterior gradient-2 is significantly associated with poor survival of prostate cancer patients. Prostate cancer and prostatic diseases. 2007;10(3):293–300. doi: 10.1038/sj.pcan.4500960. [DOI] [PubMed] [Google Scholar]
- 16.Carlsson P, Mahlapuu M. Forkhead transcription factors: key players in development and metabolism. Developmental biology. 2002;250(1):1–23. doi: 10.1006/dbio.2002.0780. [DOI] [PubMed] [Google Scholar]
- 17.Costa RH, Kalinichenko VV, Lim L. Transcription factors in mouse lung development and function. American journal of physiology. 2001;280(5):L823–38. doi: 10.1152/ajplung.2001.280.5.L823. [DOI] [PubMed] [Google Scholar]
- 18.Zheng W, Rosenstiel P, Huse K, et al. Evaluation of AGR2 and AGR3 as candidate genes for inflammatory bowel disease. Genes and immunity. 2006;7(1):11–8. doi: 10.1038/sj.gene.6364263. [DOI] [PubMed] [Google Scholar]
- 19.Mirosevich J, Gao N, Gupta A, Shappell SB, Jove R, Matusik RJ. Expression and role of Foxa proteins in prostate cancer. The Prostate. 2006;66(10):1013–28. doi: 10.1002/pros.20299. [DOI] [PubMed] [Google Scholar]
- 20.Radomski N, Jost E. Molecular cloning of a murine cDNA encoding a novel protein, p38–2G4, which varies with the cell cycle. Experimental cell research. 1995;220(2):434–45. doi: 10.1006/excr.1995.1335. [DOI] [PubMed] [Google Scholar]
- 21.Sithanandam G, Anderson LM. The ERBB3 receptor in cancer and cancer gene therapy. Cancer gene therapy. 2008;15(7):413–48. doi: 10.1038/cgt.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y, Fondell JD, Wang Q, et al. Repression of androgen receptor mediated transcription by the ErbB-3 binding protein, Ebp1. Oncogene. 2002;21(36):5609–18. doi: 10.1038/sj.onc.1205638. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y, Hamburger AW. Specificity and heregulin regulation of Ebp1 (ErbB3 binding protein 1) mediated repression of androgen receptor signalling. British journal of cancer. 2005;92(1):140–6. doi: 10.1038/sj.bjc.6602257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y, Wang XW, Jelovac D, et al. The ErbB3-binding protein Ebp1 suppresses androgen receptor-mediated gene transcription and tumorigenesis of prostate cancer cells. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(28):9890–5. doi: 10.1073/pnas.0503829102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Y, Linn D, Liu Z, et al. EBP1, an ErbB3-binding protein, is decreased in prostate cancer and implicated in hormone resistance. Molecular cancer therapeutics. 2008;7(10):3176–86. doi: 10.1158/1535-7163.MCT-08-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Igawa T, Lin FF, Lee MS, Karan D, Batra SK, Lin MF. Establishment and characterization of androgen-independent human prostate cancer LNCaP cell model. The Prostate. 2002;50(4):222–35. doi: 10.1002/pros.10054. [DOI] [PubMed] [Google Scholar]
- 27.Thalmann GN, Anezinis PE, Chang SM, et al. Androgen-independent cancer progression and bone metastasis in the LNCaP model of human prostate cancer. Cancer research. 1994;54(10):2577–81. [PubMed] [Google Scholar]
- 28.Zhang Y, Hamburger AW. Heregulin regulates the ability of the ErbB3-binding protein Ebp1 to bind E2F promoter elements and repress E2F-mediated transcription. The Journal of biological chemistry. 2004;279(25):26126–33. doi: 10.1074/jbc.M314305200. [DOI] [PubMed] [Google Scholar]
- 29.Thalmann GN, Sikes RA, Wu TT, et al. LNCaP progression model of human prostate cancer: androgen-independence and osseous metastasis. The Prostate. 2000 Jul 1;44(2):91–103. doi: 10.1002/1097-0045(20000701)44:2<91::aid-pros1>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 30.Horak CE, Lee JH, Elkahloun AG, et al. Nm23-H1 suppresses tumor cell motility by down-regulating the lysophosphatidic acid receptor EDG2. Cancer research. 2007;67(15):7238–46. doi: 10.1158/0008-5472.CAN-07-0962. [DOI] [PubMed] [Google Scholar]
- 31.Xu J, Wang R, Xie ZH, et al. Prostate cancer metastasis: role of the host microenvironment in promoting epithelial to mesenchymal transition and increased bone and adrenal gland metastasis. The Prostate. 2006;66(15):1664–73. doi: 10.1002/pros.20488. [DOI] [PubMed] [Google Scholar]
- 32.Klein KA, Reiter RE, Redula J, et al. Progression of metastatic human prostate cancer to androgen independence in immunodeficient SCID mice. Nature medicine. 1997;3(4):402–8. doi: 10.1038/nm0497-402. [DOI] [PubMed] [Google Scholar]
- 33.Henry MD, Silva MD, Wen S, et al. Spiculated periosteal response induced by intraosseous injection of 22Rv1 prostate cancer cells resembles subset of bone metastases in prostate cancer patients. The Prostate. 2005;65(4):347–54. doi: 10.1002/pros.20300. [DOI] [PubMed] [Google Scholar]
- 34.Gleave ME, Hsieh JT, von Eschenbach AC, Chung LW. Prostate and bone fibroblasts induce human prostate cancer growth in vivo: implications for bidirectional tumor-stromal cell interaction in prostate carcinoma growth and metastasis. The Journal of urology. 1992;147(4):1151–9. doi: 10.1016/s0022-5347(17)37506-7. [DOI] [PubMed] [Google Scholar]
- 35.Wu HC, Hsieh JT, Gleave ME, Brown NM, Pathak S, Chung LW. Derivation of androgen-independent human LNCaP prostatic cancer cell sublines: role of bone stromal cells. International journal of cancer. 1994;57(3):406–12. doi: 10.1002/ijc.2910570319. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y, Lu Y, Zhou H, et al. Alterations in cell growth and signaling in ErbB3 binding protein -1 (Ebp1) Deficient Mice. BMC cell biology. 2008;9(1):69. doi: 10.1186/1471-2121-9-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qian X, Costa RH. Analysis of hepatocyte nuclear factor-3 beta protein domains required for transcriptional activation and nuclear targeting. Nucleic acids research. 1995;23(7):1184–91. doi: 10.1093/nar/23.7.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park SW, Zhen G, Verhaeghe C, et al. The protein disulfide isomerase AGR2 is essential for production of intestinal mucus. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(17):6950–5. doi: 10.1073/pnas.0808722106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smirnov DA, Zweitzig DR, Foulk BW, et al. Global gene expression profiling of circulating tumor cells. Cancer research. 2005;65(12):4993–7. doi: 10.1158/0008-5472.CAN-04-4330. [DOI] [PubMed] [Google Scholar]
- 40.Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nature reviews. 2001;1(1):34–45. doi: 10.1038/35094009. [DOI] [PubMed] [Google Scholar]
- 41.Kowalinski E, Bange G, Bradatsch B, Hurt E, Wild K, Sinning I. The crystal structure of Ebp1 reveals a methionine aminopeptidase fold as binding platform for multiple interactions. FEBS letters. 2007;581(23):4450–4. doi: 10.1016/j.febslet.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 42.Pani L, Overdier DG, Porcella A, Qian X, Lai E, Costa RH. Hepatocyte nuclear factor 3 beta contains two transcriptional activation domains, one of which is novel and conserved with the Drosophila fork head protein. Molecular and cellular biology. 1992;12(9):3723–32. doi: 10.1128/mcb.12.9.3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lai E, Prezioso VR, Tao WF, Chen WS, Darnell JE., Jr Hepatocyte nuclear factor 3 alpha belongs to a gene family in mammals that is homologous to the Drosophila homeotic gene fork head. Genes & development. 1991;5(3):416–27. doi: 10.1101/gad.5.3.416. [DOI] [PubMed] [Google Scholar]