Abstract
Purpose of review
One-third of the world’s population has hypertension and it is responsible for almost 50% of deaths from stroke or coronary heart disease. These statistics do not distinguish salt-sensitive from salt-resistant hypertension or include normotensives who are salt-sensitive even though salt sensitivity, independent of blood pressure, is a risk factor for cardiovascular and other diseases, including cancer. This review describes new personalized diagnostic tools for salt sensitivity.
Recent findings
The relationship between salt intake and cardiovascular risk is not linear, but rather fits a J-shaped curve relationship. Thus, a low-salt diet may not be beneficial to everyone and may paradoxically increase blood pressure in some individuals. Current surrogate markers of salt sensitivity are not adequately sensitive or specific. Tests in the urine that could be surrogate markers of salt sensitivity with a quick turn-around time include renal proximal tubule cells, exosomes, and microRNA shed in the urine.
Summary
Accurate testing of salt sensitivity is not only laborious but also expensive, and with low patient compliance. Patients who have normal blood pressure but are salt-sensitive cannot be diagnosed in an office setting and there are no laboratory tests for salt sensitivity. Urinary surrogate markers for salt sensitivity are being developed.
Keywords: renal proximal tubule cell, salt sensitivity, urine exosomes
INTRODUCTION
Methods for risk stratification and identification of patients at high risk for chronic disease are needed in the healthcare system in the future if costs are to be contained. Predictive genomics will assist disease management wellness programs. However, genetic tests are rarely highly predictive of disease [1,2] except in monogenic diseases, such as the rare monogenic forms of hypertension [3,4]. The need for screening and staging of chronic disease is borne out of the demographics that 90 million Americans have chronic medical conditions that are lifelong and progressive. The annual cost (billions) to the US health system is $300 for hypertension, $132 for diabetes per year, $82 for arthritis, and $30 for asthma (http://www.cdc.gov). Add to this fact the staggering observations that 75% of healthcare costs and 70% of deaths are because of chronic disease, and it becomes evident that a new model is necessary. About one-third of the world’s population has hypertension, which is responsible for almost 50% of deaths from stroke and coronary heart disease [5–7]. However, these statistics do not distinguish salt-sensitive from salt-resistant hypertension or include normotensive patients who are salt-sensitive. This distinction is important because salt sensitivity, independent of blood pressure, is a risk factor for cardiovascular morbidity and mortality [8,9] and other diseases, for example, asthma, gastric carcinoma, osteoporosis, and renal dysfunction [10]. Therefore, there are profound positive benefits that can be derived from developing diagnostic tests based on biomarkers for hypertension and salt sensitivity.
Hypertension and salt sensitivity are complex diseases as a result of genetic predisposition, coupled with environmental influences, such as excessive sodium consumption and sedentary lifestyles. Almost half of the US population has hypertension, salt sensitivity, or both (Fig. 1) [8,11].
FIGURE 1.
Venn diagram of the distribution of individuals with hypertension, salt sensitivity, or both in the US population [7,8,11]. The prevalence of hypertension increased from 23.9% in 1988–1994 to 28.5% in 1999–2000, but did not change significantly between 1999–2000 (28.1%) and 2007–2008 (30.9%) [7,11,12]. Women are slightly more likely than men to have hypertension, with 35 million women and 30 million men with the disease [12]. The prevalence of hypertension is highest in persons 65 years and older (69.7%), persons with Medicare coverage (68.1%), followed by those with less than high school education (42.1%), non-Hispanic Blacks (38.6%), non-Hispanic Whites (32.3%), Asian Americans (39%), and Mexican Americans (17.3%) [12–14]. However, the prevalence of hypertension in foreign-born African Americans and black persons living outside the United States is similar to other ethnic groups [14].
THE DIAGNOSIS OF HYPERTENSION
Hypertension is currently diagnosed by arm cuff sphygmomanometry, preferably with a mercury sphygmomanometer (JNCVII) [15]. Chronic ambulatory blood pressure monitoring is being advocated because it can distinguish sustained from white-coat, masked, nondipping, and overly dipping hypertension [16–18]. Blood pressure variability, an increasingly recognized cardiovascular risk, should be monitored, in addition to the actual level of blood pressure [19,20], using only validated instruments [21,22].
THE DIAGNOSIS OF SALT SENSITIVITY
Salt sensitivity is defined as a change in blood pressure (office measurement) of 5–10% or at least 5mmHg, in response to a change in NaCl intake [23]. Another definition of salt sensitivity is an increase in mean arterial blood pressure (MAP) of at least 4mmHg (24-h ambulatory blood pressure monitoring) with an increase in NaCl intake [24]. A salt sensitivity index (difference between MAP on low-salt and high-salt diets, divided by the MAP on low-salt diet) of at least 5% is also another definition of salt sensitivity [25]. An increase in MAP of at least 10 mmHg, after an infusion of 2l of normal saline over 4h relative to that measured the morning after 1 day of a low-sodium (10 mmol) diet and administration of a loop diuretic, is another definition of salt sensitivity [8] (Fig. 2). However, the most reliable method to measure salt sensitivity is the blood pressure response to a change in dietary salt intake [24,26▪].
FIGURE 2.
Diagrammatic representation of two protocols for testing for salt sensitivity. The first method requires 29 days to administer. A diagnosis of salt sensitivity is made if the individual has a 5–10% increase or decrease in blood pressure following an increase or decrease in salt intake, relative to blood pressure on a normal salt intake (data from [23–25,26▪,34▪▪]).
The salt sensitivity of patients with essential hypertension is not clinically documented and less so in salt-sensitive normotensive patients, who by definition have blood pressures less than 140/90 mmHg (systolic/diastolic) on random salt intake [15]. The failure to routinely test for salt sensitivity may be related to the methods and expense used to diagnose salt sensitivity, low compliance on restricted diets, and, critically, the lack of reimbursement from healthcare insurers. Nonetheless, the health and financial costs of not diagnosing salt sensitivity are enormous and mandate alternative approaches that are cheaper and easier.
SALT INTAKE AND HEALTH OUTCOME
The health risks, especially cardiovascular disease, associated with high-salt diet are well recognized [8–11]. Government and nonprofit health-based entities have generally recommended a significant reduction in NaCl consumption [27,28▪▪].
Salt is an irreplaceable preservative in many food products to prevent spoilage. Typical high sources of NaCl are bread, pizza, pasta dinners, cold cuts, ham, bacon, soups, and most fast food items. About 75% of an average individual’s consumption of NaCl comes from processed food, an additional 10–15% is added during cooking or at the table, and 10% occurs naturally in foods, especially meats. Fruits and vegetables are naturally low in NaCl and high in potassium. Low-sodium food should be no more than 40mg sodium (100mg NaCl) per 100 g of food product or 100 ml liquid. Reduced salt foods should have at least 25% less than the standard product. Salt-free or sodium-free should have no more than 5mg of sodium (1.25mg NaCl) per 100 g of food product or 100 ml of liquid (http://www.nutrition.org.uk/home.asp?siteId=43§ionId=836&parent Section=322&which=undefined, accessed 1 October 2012).
The adequate intake and upper limit of NaCl intake per day as defined by the Institute of Medicine is 3.7–5.8 g (1.5–2.3 g sodium) for young adults and 2.08 to less than 5.8 for older adults and elderly (≥60 years old) [27].
Although the dietary sodium recommendation by the Institute for Medicine is intended as a ‘one size fits all’ recommendation, it is becoming clear that each individual is genetically programmed with a ‘personal index of salt sensitivity’ and NaCl dietary guidelines should be personalized. More research is needed to understand the nonlinear effect of salt intake on cardiovascular disease or death [29]. Similar to the blood pressure following the treatment of hypertension [30], there is also a J-shaped curve relationship between sodium intake and mortality [31,32▪▪,33]. NaCl intakes above and below the range of 2.5–6.0g/day are associated with increased cardiovascular risk [31,32▪▪,33]. Furthermore, some individuals have a paradoxical increase in blood pressure on a low NaCl diet (inverse salt sensitivity) (Fig. 3) [34▪▪]. This complexity makes the ‘one-size-fits all’ approach not only ineffective, but also potentially dangerous.
FIGURE 3.
The distribution of salt-sensitive, salt-resistant, and inverse salt-sensitive individuals in a population of 183 individuals tested for salt sensitivity is shown. Elevated blood pressure occurs in salt-sensitive individuals on a high-salt diet and paradoxically in inverse salt-sensitive individuals on a low-salt diet [34▪▪]. Mean arterial pressure (MAP) increases over 7 mmHg on a high-salt diet were classified as salt-sensitive, whereas blood pressure decreases over 7mmHg were considered as inverse-salt-sensitive (data from [34▪▪]).
Because of the difficulty in measuring the responses to sodium intake, surrogate markers are often used. The absence of the 10–20% reduction in blood pressure with normal sleeping is associated with a higher incidence of salt sensitivity in some studies [35,36]. Low plasma renin activity (PRA) has also been associated with salt sensitivity in normotensive and hypertensive individuals [37–39]. Low PRA considered indicative of salt-sensitive hypertension [38,39] is limited by a lack of sensitivity and specificity. For example, in our Japanese hypertensive individuals who were grouped according to their response to a chronic oral salt loading protocol, 8% of the salt-sensitive individuals had normal PRA (n = 82), while 92% had low PRA (≤1ng/ml/h on a normal sodium diet) (n = 94). In contrast, 33% of salt-resistant individuals had low PRA, while 67% had normal PRA [39]. Thus, similar to studies from other investigators, PRA levels do not always distinguish salt-sensitive from salt-resistant individuals [37–39]. Other surrogate markers for salt sensitivity, such as circulating levels of atrial natriuretic peptide (ANP) [37], brain natriuretic peptide (BNP) [40,41], and endogenous ouabain [42], also suffer from the same limitations as PRA [39].
GENETIC SCREENING FOR HYPERTENSION AND SALT SENSITIVITY
The need for better surrogate markers for salt sensitivity other than PRA, ANP, BNP, and endogenous ouabain [37,38–42] has prompted the development of genetic screens for hypertension and salt sensitivity using large-scale microarrays and sensitive and specific biomarkers for salt sensitivity, including genetics [34▪▪], proteomics, and renal proximal tubule cells (RPTCs) [43], micro ribonucleic acid (miRNA) [44], and exosomes excreted into the urine [45]. The latter are also being evaluated as markers for chronic kidney and other diseases [46,47]. The use of limited screening arrays currently available has not yielded positive results. Furthermore, genomewide association studies have yielded gene variants that only account for a small percentage (2%) of blood pressure variability [48] and these studies do not include salt sensitivity.
DIAGNOSTIC OBJECTIVES FOR A GENETIC TEST
The diagnostic objectives for a genetic test would be to screen for the propensity to develop hypertension and salt sensitivity, especially in individuals with a positive family history. Patients with positive results would be encouraged to visit their physician more often and initiate or comply with in-home blood pressure monitoring. Testing for the genetic basis for salt sensitivity would encourage dietary salt restriction in individuals with increased likelihood for disease and serve as a basis for administering more extensive (and expensive) testing. Knowledge of increased genetic risk may provide motivational incentives for lifelong dietary modifications [28▪▪,49–51], while not subjecting those at low risk or even harmed from more restrictive diets.
GENE VARIANTS ASSOCIATED WITH SALT SENSITIVITY
Several gene variants have been associated with hypertension and/or salt sensitivity. These genes are usually associated with the regulation of vascular resistance and renal sodium transport.
-
Genes expressing proteins that increase renal sodium transport or vascular smooth muscle cell reactivity.
-
The renin–angiotensin–aldosterone system is an important regulator of renal sodium transport, especially under the conditions of sodium deficit [52]. Angiotensin II stimulation of the angiotensin type 1 receptor (AT1R) increases sodium retention [53,54], while decreased sodium retention involves angiotensin-(1–7) [55,56], angiotensin III, and the angiotensin type 2 receptor (AT2R) [57]. Dopamine produced by RPTs is critical in facilitating sodium excretion under the condition of moderate sodium excess acting through dopamine receptors interacting with the renin–angiotensin–aldosterone system and others [58]. Angiotensin-(1–7), angiotensin-(3–7), and angiotensin IV concentration dependently increase dopamine production [59] in the striatum, which is facilitated by AT1R blockade in the hypothalamus [60]. Whether or not a similar interaction occurs in the kidney remains to be determined. It is also not known whether polymorphisms of enzymes involved in dopamine synthesis are associated with salt sensitivity in humans.
Angiotensinogen (AGT – 6G–A [rs5051], 842T>C M235T [rs699], [chromosome 1q42.2]). Angiotensinogen is the precursor for the angiotensin peptides (e.g., angiotensin I, II, and III). Polymorphisms in AGT (rs2004776), T174M (rs4762), and –20C–A (rs5050) but not –6G–A or –217A–G (rs5049) have been associated with hypertension [61,62]. However, only one of seven studies has found an association between M235T (rs699 T>C) or –6G–A and salt sensitivity [63].
Angiotensin I converting enzyme (ACE) [chromosome 17q23.3]. An insertion/deletion polymorphism of a 287-base-pair fragment in intron 16 (rs1799752) of ACE has been associated with salt sensitivity in three studies (reviewed in [63]).
AGTR1 (rs5186 A>C) was not associated with salt sensitivity in two studies [63], but one study found an association of intronic polymorphism rs4524238 G>A and salt sensitivity [64].
Aldosterone synthase (CYP11B2) [chromosome 8q24.3]: The CYP11B2 gene encoding aldosterone synthase (P450c11AS) contains a single-nucleotide polymorphism (SNP) (–344C>T, rs1799998), associated with essential hypertension but not salt sensitivity (reviewed in [65]).
Solute carrier family (SLC). SLC8 sodium/ calcium exchanger, member 1 (SLC8A1) [chromosome 2p22.1], rs434082, G>A, and SLC24 sodium/potassium/calcium exchanger, member 3 (SLC24A3) [chromosome 20p11.13], rs3790261, G>A, were associated with an increase in systolic blood pressure in response to an acute salt load [66]. SLC8A1, which encodes NCX1, may be important in the pathogenesis of salt-sensitive hypertension [67].
-
Sodium transporters and channels.
Amiloride-sensitive epithelial sodium channel (ENaC), α subunit (SCNN1A) [chromosome12p13]. Mutations of ENaC cause monogenic, salt-sensitive hypertension [3], and some polymorphisms of ENaC (e.g., rs4073291 G>T) are associated with salt-sensitive polygenic hypertension [68]. Gene variants in two regulators of ENaC have also been associated with salt sensitivity. One is serum/glucocorticoid regulated kinase 1 (SGK1) [chromosome 6q23.2]; major allele carriers of SGK1 rs2758151 (C/T) and rs9402571 (T/G) are salt-sensitive [69]. The other is neural precursor cell-expressed developmentally downregulated 4-like (NEDD4L, chromosome 18q21.31) gene variants (NEDD4L rs4149601, A/G); GG genotype, and C-allele of NEDD4L (rs2288774, C/T) are also associated with salt sensitivity [70–72].
SLC4, sodium bicarbonate cotransporter, member 5 (SLC4A5) [chromosome 2p13.1]. SLC4A5 rs7571842 (intron 22–23) and rs10177833 (intron 25–26) are associated with salt sensitivity with large effect sizes replicated in two European American cohorts [34▪▪].
-
Genes expressing proteins that decrease vascular smooth muscle cell reactivity. Protein kinase cGMP-dependent, type 1 (PRKG1) [chromosome 10q11.2]: rs7897633 C>A was associated with an increase in diastolic blood pressure in response to an acute salt load [66].
-
Genes expressing proteins that do not normally decrease renal sodium transport:
Adducins are a family of cytoskeleton proteins encoded by three genes (ADD1, α [chromosome 4p16.3, ADD2, β [chromosome 2p 13.3], and ADD3, γ[chromosome 10 q25.2]). In expression studies, wildtype α-adducin minimally affects, whereas ADD1, rs4961 G460W, and rs4963 586C increase Na+K+-ATPase activity [73,74]. An increase in renal Na+K+-ATPase activity causes sodium retention and eventually an increase in blood pressure. The inconsistent reports on the association of ADD1 rs4961 with salt sensitivity could be related to the ethnic differences in the frequency of the minor allele: 8% in African Americans, 15–20% in whites, and 50% in Japanese [75,76], and differences in circulating ouabain levels, female sex, high body mass index, and interaction with other genes that regulate sodium transporters, WNK1–NEDD4L, in addition to Na+K+-ATPase [70–78].
-
Genes expressing proteins that normally decrease renal sodium transport:
As indicated above, the renal dopaminergic system, by itself or via its interaction with other hormonal or humoral factors that affect sodium transport: ANP/ANPA [79], AT1R [80–83], AT2R [57], eicosanoids [84,85], endothelin/endothelin type B receptor [86], gastrin [87,88], insulin [89,90], nitric oxide [91], prolactin [92], urodilatin [79], and even aldosterone [84], plays a critical role in the excretion of sodium under the conditions of sodium excess [58].
Deletion of any of the dopamine receptor genes in mice results in hypertension that may be observed only on a high-sodium diet [58,93]. However, except for DRD2 rs6276 (3′ UTR –A/G) [chromosome 11q23], variants of the dopamine receptor subtype genes have not been shown to be associated with salt sensitivity [34▪▪]. Instead, variants of G protein-coupled receptor kinase 4 (GRK4) [chromosome 4p.16.3], which regulates the dopamine D1 receptor (i.e., D1R), have been reported to be associated with salt sensitivity [58,94▪▪]. D1R, the protein product of DRD1, is important in the regulation of renal tubule ion transport, particularly evident in the proximal tubule [58]. Indeed, deletion of Ddc, the gene that expresses the enzyme that converts l-DOPA to dopamine in the RPT in mice, causes salt-sensitive hypertension [95▪▪]. The RPT and the thick ascending limb are the major sites of increased sodium transport in human essential hypertension [96,97]. GRK4 A486V, rs1801058, by itself [34▪▪,98] or in association with other GRK4 gene variants (GRK4 R65L, rs2960306; GRK4 A142V, rs1024323), is associated with salt sensitivity in a Japanese [39], Italian [98], and European American [34▪▪] population. Because ADD1 and GRK4 are within 40 kb of each other, we adjusted each SNP in the logistic regression analyses for the other SNPs in addition to sex, BMI, and age to assess the independence of these two SNPs. After adjustment for rs4961, rs1801058 remained statistically significant [P=0.016; odds ratio (OR), 0.545; 95% confidence interval (CI), 0.330–0.891], but the ADD1 rs4961 did not remain significant after adjusting for GRK4 genotypes (P=0.524; OR, 0.968; 95% CI, 0.877–1.07) [34▪▪].
Several criteria are needed to prove that a gene is causal of a complex trait [99], salt sensitivity, in this instance. Of all the genes that have been shown to be associated with salt sensitivity, depending on the genetic background, only GRK4 486V has been shown to produce hypertension in transgenic mice [100,101], recapitulating the association studies in humans [34▪▪,39,98].
DATA REDUCTION OF GENETIC TESTS
In order to use SNPs for diagnostic purposes, there must be a high degree of confidence that the genetic variation is associated with an increased risk of disease or the cause of the patient’s current condition. Sensitivity, specificity, positive and negative likelihood ratios, and diagnostics odds ratio (DOR) aid in the evaluation of the value of a diagnostic test. The power of these measures increases with ethnic homogeneity, and size and frequency of the polymorphisms in the study population. Allelic variants associated with increased risk of disease may be employed as useful diagnostic markers. However, some allelic variants that do not show such an association individually may still have significant diagnostic value, using novel multivariable or multigene analytical approaches. The multifactorial dimensionality reduction (MDR) test which examines all pair-wise combinations and then all three-way combinations for significant associations [102] is nonparametric and model independent. MDR has enough power to determine alleles that are strongly associated with disease even with relatively small datasets.
We calculated the traditional diagnostic statistical indices in our cohort of approximately 80 Japanese individuals, tested for salt sensitivity using a dietary protocol [39]. When a diagnostic threshold of three or more SNPs for GRK4 was used, this test yielded a sensitivity of 0.85 and a specificity of one. The positive likelihood ratio and DOR were infinite, as there were no false positives in this study of a small cohort. If these measures hold up under a larger cohort, then the genetic panel may provide significant diagnostic utility for physicians.
An alternative approach to the above analyses is to use the number of ‘risk’ alleles as a genetic score measurement for either hypertension or salt sensitivity. Using a Japanese dataset [39] that showed association of GRK4 SNPs with hypertension, we calculated a score based on the number of risk increasing alleles and demonstrated a monotonic increase in risk of hypertension (Table 1). We also used an alternative measure to calculate a quantitative genetic score measurement – the number of risk alleles divided by the total number of alleles for these loci, and a similar result was obtained (P< 0.001, logistic regression model adjusted for sex).
Table 1.
GRK4 variant association with essential hypertension
|
GRK4 variant modela |
Total (n)b |
HT controls (n)c |
HT cases (n)c |
Odds ratiod |
95% Confidence interval |
P value | Sensitivity | Specificity | Correctly classifiedf |
|---|---|---|---|---|---|---|---|---|---|
| 0 | 271 | 159 | 112 | Ref. | NA | NA | NA | NA | NA |
| 1 | 390 | 197 | 193 | 1.41 | 1.03–1.93 | 0.033 | 26.23% | 83.15% | 56.88% |
| 2 | 201 | 82 | 119 | 2.06 | 1.42–2.99 | <0.001 | 51.52% | 65.98% | 58.90% |
| 3 | 108 | 24 | 84 | 4.97 | 2.97–8.31 | <0.001 | 42.86% | 86.89% | 64.12% |
| 4 | 89 | 19 | 70 | 5.19 | 2.95–9.11 | <0.001 | 38.46% | 89.33% | 63.61% |
| ≥4e | 104 | 24 | 80 | 4.72 | 2.81–7.91 | <0.001 | 41.67% | 86.89% | 63.73% |
Genetic score or number of variant alleles at three GRK4 loci, GRK4142V, GRK4486V, GRK4R65L were counted in a Japanese human essential hypertension cohort; normotensive n = 486, hypertensive n = 588 (39 and unpublished data).
Total number of individuals in the study with the given number of GRK4 variant alleles.
Total number of hypertension cases or controls with the given number of GRK4 variant alleles.
Logistic Regression Model was adjusted for sex and is calculated relative to a genetic score of 0. Analysis performed using STATA 10.
Greater than four variants are not shown because of small sample size in each cell. Results were, however, highly statistically significant with ORs approaching 5.
Percentage of hypertension cases correctly predicted using the specified GRK4 variant logistic regression model adjusted for sex.
Unweighted genetic scores of GRK4 and SLC4A5 (in a salt-sensitive cohort [34▪▪]) using a sex and BMI adjusted logistic regression model (STATA10) also yielded a significant result (P = 0.003 for the genetic score as an independent predictor of salt sensitivity). The genetic score analyses, as presented above, represent simple models of genetic risk (additive or linear) that will require further validation. It is also possible that more complex genetic models will better explain risk and these can be elucidated by model-free methods, such as MDR, that will also require further study. Nonetheless, the results as presented are promising in that they do provide a reasonable estimate of the role that physiologically relevant gene variants can play in predicting both hypertension and salt sensitivity, and that multilocus models do perform better than simple, single variant assessments.
PHYSIOLOGICAL RESPONSES AND THEIR CORRELATION WITH GRK4 VARIANT ALLELES
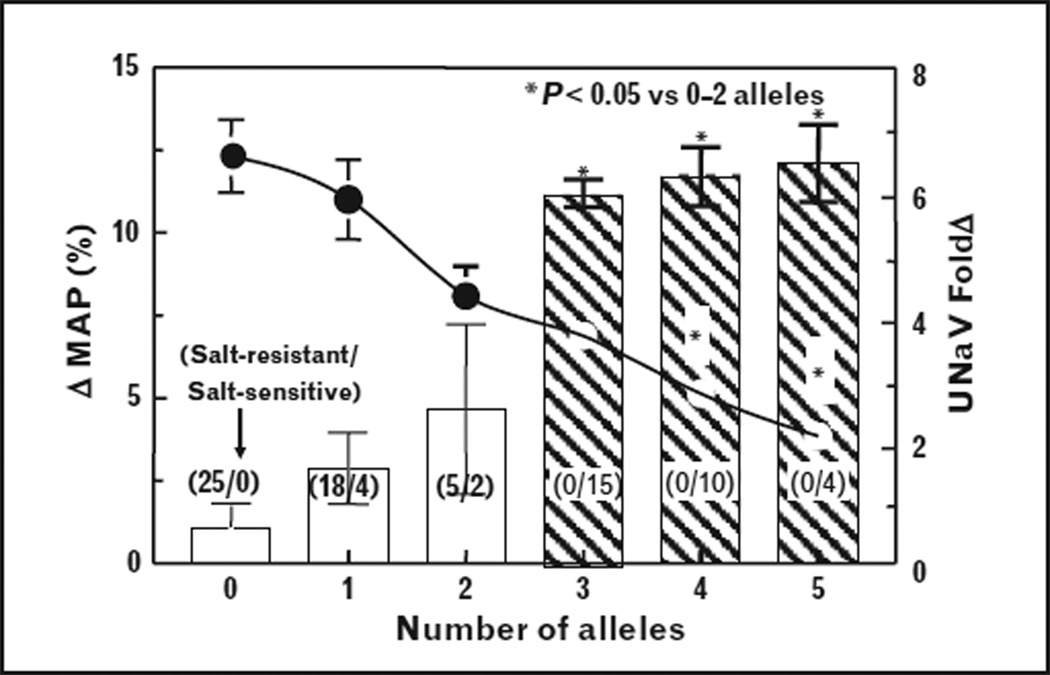
The value of genetic markers for disease increases if there is a correlation between the presence of SNPs and a physiological response [99]. In a study of 83 Japanese hypertensive individuals (protocol in Fig. 2), 35 being salt-sensitive and 48 salt-resistant [39], the presence of at least three GRK4 variant alleles was always associated with salt sensitivity (≥10% MAP, following the change from low to high salt diet), although some with one or two variant alleles were also salt-sensitive. Moreover, the change in sodium excretion with change in salt intake was inversely proportional to the number of GRK4 variant alleles (Fig. 4). Because salt sensitivity was always observed in hypertensive patients (54.8 ±0.8 years old, BMI = 23.1 ±0.2kg/m2) with three or more GRK4 variant alleles, we studied the effect of a dopamine prodrug, docarpamine, on sodium excretion in young normotensive individuals with no or three GRK4 variant alleles. Docarpamine increased urinary sodium in patients with less than three GRK4 SNPs [39], but not in individuals with three GRK4 SNPs. These data indicate a significant and physiologically relevant relationship between the presence of three GRK4 SNPs and the inability to excrete a sodium load. Speirs et al. [103] reported a gene dose effect of the GRK4 486 allele on systolic blood pressure in hypertensives, obese hypertensives, and male hypertensives but not normotensives (Fig. 5). These results are in accord with our genetic score analyses presented above. However, the relationship to salt sensitivity was not tested in these studies.
FIGURE 4.
All salt-sensitive hypertensive individuals have at least three GRK4 gene variants, but hypertensive individuals with no GRK4 gene variants were salt-resistant. There was an inverse relationship between the increase in mean arterial blood pressure (ΔMAP) and fold increase in sodium excretion (UNaV fold Δ) with the change in salt intake from low to high in hypertensive MAP classified according to the number of GRK4 variant alleles. The ΔMAP is shown by the bars, while the fold increase in sodium excretion is depicted by the circles connected by a continuous line and right-hand axis. The number of salt-resistant (SR) subjects/number of salt-sensitive (SS) individuals is shown in parenthesis for each bar in the bar graph. *P<0.05 vs. at least three alleles, factorial ANOVA, Newman-Keuls test. Data are mean±SE; there are no error bars in instances in which the symbol is bigger than the SE (modified with permission [39]).
FIGURE 5.
The GRK4 486V allele is associated with an increase in systolic blood pressure (SBP) in hypertensive patients (HT), regardless of sex (male HT, MHT) and obesity (obese HT, OHT) but not in normotensive patients (NT). The total allele effect on SBP was over 30mmHg (data from [103]).
CELL-BASED ASSAYS
RPTCs, isolated from urine, have provided important clues about the cause of disease [104–109]. The RPT is one site of increased sodium transport in polygenic hypertension [97] and therefore, living RPTCs isolated from urine (i.e., virtual renal biopsy) could serve as a convenient method to assess RPTC physiology, almost instantaneously. We hypothesized that the physiological consequences of stimulation of D1R and AT1R stimulation are recapitulated in freshly isolated RPTCs. Salt sensitivity was defined as an increase of at least 7 mmHg in MAP following the increase in sodium intake from low (10mmol/day for 5days) to high (300 mmol/day) (n = 4); inverse salt sensitivity as an increase of at least 7 mmHg in MAP following a change in sodium intake from high to low (n = 3), and salt resistance as less than 7 mmHg change in MAP from the change in salt intake (n = 5) (Table 1). We measured the intracellular sodium-induced recruitment of the D1R from cytosol to the plasma membrane and an increase in intracellular Ca++ with angiotensin II. There was a negative correlation between the degree of salt sensitivity and D1R plasma membrane recruitment (y= –0.0107x + 0.68 0.68 relative fluorescent units, R2 = 0.88, n=12, P< 0.0001) and angiotensin II-stimulated intracellular Ca++ (y= –0.0016x + 0.0336, R2 = 0.7112, n= 10, P< 0.001) [43]. There was also an inverse correlation between fenoldopam-mediated AT2R recruitment to the plasma membrane and the degree of salt sensitivity of blood pressure [110]. The natriuretic effect of the D1-like receptor agonist fenoldopam may require the stimulation of AT2R [57]. Therefore, the response of urine RPTCs to D1-like receptor and AT1R stimulation may provide a personalized cell-based diagnostic for salt sensitivity that offers advantages over a 2-week controlled diet with respect to rapidity of diagnosis in the doctor’s office, decreased cost, and enhanced patient compliance. Another advantage of using RPTCs is that they can be sequenced for genes that may cause salt sensitivity, as somatic mutations in RPTCs are unlikely to be detected by studying DNA obtained from blood samples, although sister chromatid exchange analysis may be informative ([111]; Scott M. Williams, unpublished observation).
URINE EXOSOMES AS BIOMARKERS
Exosomes are small 50–90 nm vesicles containing mRNA, proteins, and other subcellular components. Urine exosomes may contain cellular components that may be associated with aberrant nephron physiology in salt-sensitive individuals. The amounts of two proteins responsible for sodium transport in the distal nephron were not reliable markers of increased sodium reabsorption in hypertensive patients [112]. Therefore, we studied exosomes from immortalized human RPTCs treated with vehicle or fenoldopam (D1-like receptor agonist) [45]. Fenoldopam (1 µM/ 24 h) produced a 2.38 ± 0.053-fold increase in exosome release (n = 15, P < 0.04). Calcium-independent angiotensin II stimulation (1nM/24 h) also caused a 4.71 ± 0.35-fold increase in exosome release (n = 18, P<0.04). Lamp1-RFP or CD82-YFP (two proteins previously found to be contained in exosomes) electroporated into RPTCs were secreted into the culture medium and taken up by nontransfected cells. Proteomic and mRNA analyses demonstrated the presence of D1R and GRK4, which as discussed above can regulate renal tubular sodium transport, in exosomes derived from RPTCs. Thus, exosomes, which contain proteins, mRNA, and miRNA, could serve as an intranephron acellular signaling mechanism that may alter sodium homeostasis.
Total miRNA (miRNome) in urinary exosomes has not been previously evaluated as a disease bio-marker, particularly for salt sensitivity. Total RNA was collected from urinary exosomes (LC Sciences, miRBase Human version 18). Urinary exosomes (three samples per individual) were pooled from five humans previously phenotyped for salt sensitivity from a prior clinical study using a 2-week controlled sodium diet [45]. Patients were stratified according to salt sensitivity status: 306 miRNA targets out of 1898 probes were above background, providing the first data for urinary exosome miRNome in humans (n = 5). Bioinformatic analysis determined 20 significant differences in miRNome patterns between the salt-sensitive, salt-resistant, and inverse salt-sensitive individuals [45].
PHARMACOGENOMICS
Within the field of hypertension, there is a great need for pharmacogenomics to assist with instituting proper therapeutic strategies. Only 46% of hypertensive patients have their hypertension under adequate control [7]. Knowing specific genetic variants is critically important for appropriate personalized treatment, as current generic treatments are clearly inadequate.
A recent Harris poll indicated that 81% of individuals over the age of 18 would favor being tested for a genetic disease if an effective treatment existed. Substantial social and economic benefits to public health can be achieved also by encouraging individuals to adopt personal behaviors and related strategies that can improve their cardiovascular health. Positive predictive phenotypic genomics may provide increased incentives for positive lifestyle changes.
CONCLUSION
The statistics showing the common prevalence of essential hypertension do not distinguish salt-sensitive from salt-resistant hypertension or include normotensive patients who are salt-sensitive. This distinction is important because salt sensitivity, independent of blood pressure, is a risk factor for cardiovascular morbidity and mortality and other diseases. Patients who have normal blood pressure but are salt-sensitive cannot be diagnosed in an office setting and there are no laboratory tests for salt sensitivity. Urinary surrogate markers, including RPTCs, exosomes, and miRNA, hold promise for cost-effective methods to screen for salt sensitivity and inverse salt sensitivity.
KEY POINTS.
Salt sensitivity of blood pressure is best determined by measuring the blood pressure response to a chronic change in oral salt intake. Urinary surrogate markers, including renal proximal tubule cells, exosomes, and miRNA, hold promise for cost-effective methods to screen for salt sensitivity and inverse salt sensitivity.
The salt sensitivity of patients with or without essential hypertension is not clinically documented, especially in salt-sensitive normotensive patients, who by definition have blood pressures less than 140/90 mmHg (systolic/diastolic) on random salt intake. Almost half of the US population may be salt-sensitive.
The salt sensitivity of blood pressure in hypertensive and normotensive patients is not included in published statistics on the prevalence, awareness, treatment, or response to treatment. Salt sensitivity, independent of blood pressure, is a risk factor for cardiovascular morbidity and mortality and other diseases, for example, asthma, gastric carcinoma, osteoporosis, and renal dysfunction.
Sodium intake and cardiovascular morbidity and mortality have a J-shaped curve relationship. A low-salt diet may not be beneficial to everyone as a low salt intake, as with a high salt intake, has also been associated with increased cardiovascular risk. Some individuals have a paradoxical increase in blood pressure on a low-salt diet (inverse salt sensitivity).
Several gene variants are associated with salt sensitivity, but only variants of GRK4 have been shown to be highly associated with salt sensitivity in humans and cause salt-sensitive or salt-resistant hypertension when expressed in mice, that is dependent on the GRK4 variant and genetic background of the mice in which it is expressed.
Acknowledgements
This work was supported in part by the grants from the National Institutes of Health, HL068686, HL023081, HL074940, HL092196, DK039308, and DK090918.
Footnotes
Conflicts of interest
Drs. Robin A. Felder and Pedro A. Jose are co-owners of Hypogen Inc. and own the use patent for GRK4 (G protein-related kinase mutants in essential hypertension, U.S. Patent Number 6,660,474); and a U.S. Provisional Patent Application Serial No. 61,636,576 on Compositions and Methods for Identifying and Diagnosing Salt Sensitivity of Blood Pressure. Dr Scott M. Williams is a member of the External Advisory Board of Hypogen, Inc. and is co-owner of U.S. Provisional Patent Application Serial No. 61,636,576.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
Additional references related to this topic can also be found in the Current
- 1.Cardon LR, Palmer LJ. Population stratification and spurious allelic association. Lancet. 2003;361:598–604. doi: 10.1016/S0140-6736(03)12520-2. [DOI] [PubMed] [Google Scholar]
- 2.Li M, Reilly MP, Rader DJ, Wang LS. Correcting population stratification in genetic association studies using a phylogenetic approach. Bioinformatics. 2010;26:798–806. doi: 10.1093/bioinformatics/btq025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lifton RP. Genetic dissection of human blood pressure variation: common pathways from rare phenotypes. Harvey Lect. 2004–2005;100:71–101. [PubMed] [Google Scholar]
- 4.Boyden LM, Choi M, Choate KA, et al. Mutations in kelch-like 3 and cullin 3 cause hypertension and electrolyte abnormalities. Nature. 2012;482:98–102. doi: 10.1038/nature10814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alwan A. Global Status Report on noncommunicable diseases 2010. World Health Organization. 2011 ISBN 978-92-4-068645-8. [Google Scholar]
- 6.World health statistics. Switzerland: WHO Press, World Health Organization; 2012. [Google Scholar]
- 7.Centers for Disease Control and Prevention (CDC) Vital signs: awareness and treatment of uncontrolled hypertension among adults – United States, 2003–2010. MMWR Morb Mortal Wkly Rep. 2012;61:703–709. [PubMed] [Google Scholar]
- 8.Weinberger MH, Fineberg NS, Fineberg SE, Weinberger M. Salt sensitivity, pulse pressure, and death in normal and hypertensive humans. Hypertension. 2001;37(2 Part 2):429–432. doi: 10.1161/01.hyp.37.2.429. [DOI] [PubMed] [Google Scholar]
- 9.Frisoli TM, Schmieder RE, Grodzicki T, Messerli FH. Salt and hypertension: is salt dietary reduction worth the effort? Am J Med. 2012;125:433–439. doi: 10.1016/j.amjmed.2011.10.023. [DOI] [PubMed] [Google Scholar]
- 10.De Wardener HE, MacGregor GA. Harmful effects of dietary salt in addition to hypertension. J Hum Hypertens. 2002;16:213–223. doi: 10.1038/sj.jhh.1001374. [DOI] [PubMed] [Google Scholar]
- 11.Franco V, Oparil S. Salt sensitivity, a determinant of blood pressure, cardiovascular disease and survival. J Am Coll Nutr. 2006;25(3 Suppl):247S–255S. doi: 10.1080/07315724.2006.10719574. [DOI] [PubMed] [Google Scholar]
- 12.Cutler JA, Sorlie PD, Wolz M, et al. Trends in hypertension prevalence, awareness, treatment, and control rates in United States adults between 1988–1994 and 1999–2004. Hypertension. 2008;52:818–827. doi: 10.1161/HYPERTENSIONAHA.108.113357. [DOI] [PubMed] [Google Scholar]
- 13.Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA. 2010;303:2043–2050. doi: 10.1001/jama.2010.650. [DOI] [PubMed] [Google Scholar]
- 14.Hajjar I, Kotchen JM, Kotchen TA. Hypertension: trends in prevalence, incidence, and control. Annu Rev Public Health. 2006;27:465–490. doi: 10.1146/annurev.publhealth.27.021405.102132. [DOI] [PubMed] [Google Scholar]
- 15.Chobanian AV, Bakris GL, Black HR, et al. Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 16.Agarwal R, Bills JE, Hecht TJ, Light RP. Role of home blood pressure monitoring in overcoming therapeutic inertia and improving hypertension control: a systematic review and meta-analysis. Hypertension. 2011;57:29–38. doi: 10.1161/HYPERTENSIONAHA.110.160911. [DOI] [PubMed] [Google Scholar]
- 17.Ward AM, Takahashi O, Stevens R, Heneghan C. Home measurement of blood pressure and cardiovascular disease: systematic review and meta-analysis of prospective studies. J Hypertens. 2012;30:449–456. doi: 10.1097/HJH.0b013e32834e4aed. [DOI] [PubMed] [Google Scholar]
- 18.Viera AJ, Lin FC, Hinderliter AL, et al. Nighttime blood pressure dipping in young adults and coronary artery calcium 10–15 years later: the coronary artery risk development in young adults study. Hypertension. 2012;59:1157–1163. doi: 10.1161/HYPERTENSIONAHA.112.191536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muntner P, Shimbo D, Tonelli M, et al. The relationship between visit-to-visit variability in systolic blood pressure and all-cause mortality in the general population: findings from NHANES III, 1988 to 1994. Hypertension. 2011;57:160–166. doi: 10.1161/HYPERTENSIONAHA.110.162255. [DOI] [PubMed] [Google Scholar]
- 20.Johansson JK, Niiranen TJ, Puukka PJ, Jula AM. Prognostic value of the variability in home-measured blood pressure and heart rate: the Finn-Home Study. Hypertension. 2012;59:212–218. doi: 10.1161/HYPERTENSIONAHA.111.178657. [DOI] [PubMed] [Google Scholar]
- 21.O’Brien E, Atkins N, Stergiou G, et al. Working Group on Blood Pressure Monitoring of the European Society of Hypertension. European Society of Hypertension International Protocol revision 2010 for the validation of blood pressure measuring devices in adults. Blood Press Monit. 2010;15:23–38. doi: 10.1097/MBP.0b013e3283360e98. [DOI] [PubMed] [Google Scholar]
- 22.Alpert BS. Validation of the Welch Allyn SureBP (inflation) and StepBP (deflation) algorithms by AAMI standard testing and BHS data analysis. Blood Press Monit. 2011;16:96–98. doi: 10.1097/MBP.0b013e328345232f. [DOI] [PubMed] [Google Scholar]
- 23.Sullivan JM. Salt sensitivity. Definition, conception, methodology, and long-term issues. Hypertension. 1991;17(1 Suppl):I61–I68. doi: 10.1161/01.hyp.17.1_suppl.i61. [DOI] [PubMed] [Google Scholar]
- 24.De la Sierra A, Giner V, Bragulat E, Coca A. Lack of correlation between two methods for the assessment of salt sensitivity in essential hypertension. J Hum Hypertens. 2002;16:255–260. doi: 10.1038/sj.jhh.1001375. [DOI] [PubMed] [Google Scholar]
- 25.Yatabe MS, Yatabe J, Yoneda M, et al. Salt sensitivity is associated with insulin resistance, sympathetic overactivity, and decreased suppression of circulating renin activity in lean patients with essential hypertension. Am J Clin Nutr. 2010;92:77–82. doi: 10.3945/ajcn.2009.29028. [DOI] [PubMed] [Google Scholar]
- 26. Nichols J, Elijovich F, Laffer CL. Lack of validation of a same-day outpatient protocol for determination of salt sensitivity of blood pressure. Hypertension. 2012;59:390–394. doi: 10.1161/HYPERTENSIONAHA.111.185835. This report compares the different methods of diagnosing salt sensitivity.
- 27.Institute of Medicine. Strategies to reduce sodium intake in the United States. Washington, D.C.: The National Academic Press, U.S. Department of Agriculture, U.S. Department of Health and Human Services/Washington, D.C.: Government Publishing Office; 2010. [Google Scholar]
- 28. Ezzati M, Riboli E. Can noncommunicable diseases be prevented? Lessons from studies of populations and individuals. Science. 2012;337:1482–1487. doi: 10.1126/science.1227001. A review on the prevention of noncommunicable diseases, including hypertension.
- 29.Taylor RS, Ashton KE, Moxham T, et al. Reduced dietary salt for the prevention of cardiovascular disease. Cochrane Database Syst Rev. 2011;7:CD009217. doi: 10.1002/14651858.CD009217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dorresteijn JA, van der Graaf Y, Spiering W, et al. Secondary Manifestations of Arterial Disease Study Group. Relation between blood pressure and vascular events and mortality in patients with manifest vascular disease: J-curve revisited. Hypertension. 2012;59:14–21. doi: 10.1161/HYPERTENSIONAHA.111.179143. [DOI] [PubMed] [Google Scholar]
- 31.O’Donnell MJ, Yusuf S, Mente A, et al. Urinary sodium and potassium excretion and risk of cardiovascular events. JAMA. 2011;306:2229–2238. doi: 10.1001/jama.2011.1729. [DOI] [PubMed] [Google Scholar]
- 32. Alderman MH, Cohen HW. Dietary sodium intake and cardiovascular mortality: controversy resolved? Curr Hypertens Rep. 2012;14:193–201. doi: 10.1007/s11906-012-0275-6. A comprehensive review on the role of low and high dietary sodium intake on cardiovascular mortality.
- 33.Stolarz-Skrzypek K, Kuznetsova T, Thijs L, et al. European Project on Genes in Hypertension (EPOGH) Investigators. Fatal and nonfatal outcomes, incidence of hypertension, and blood pressure changes in relation to urinary sodium excretion. JAMA. 2011;305:1777–1785. doi: 10.1001/jama.2011.574. [DOI] [PubMed] [Google Scholar]
- 34. Carey RM, Schoeffel CD, Gildea JJ, et al. Salt sensitivity of blood pressure is associated with polymorphisms in the sodium-bicarbonate cotransporter. Hypertension. 2012;60:1359–1366. doi: 10.1161/HYPERTENSIONAHA.112.196071. Low-salt diet increases blood pressure in some patients with hypertension.
- 35.Uzu T, Kimura G, Yamauchi A, et al. Enhanced sodium sensitivity and disturbed circadian rhythm of blood pressure in essential hypertension. J Hypertens. 2006;24:1627–1632. doi: 10.1097/01.hjh.0000239299.71001.77. [DOI] [PubMed] [Google Scholar]
- 36.Simonetti GD, Farese S, Aregger F, et al. Nocturnal dipping behaviour in normotensive white children and young adults in response to changes in salt intake. J Hypertens. 2010;28:1027–1033. doi: 10.1097/HJH.0b013e328337854d. [DOI] [PubMed] [Google Scholar]
- 37.Kato N, Kanda T, Sagara M, et al. Proposition of a feasible protocol to evaluate salt sensitivity in a population-based setting. Hypertens Res. 2002;25:801–809. doi: 10.1291/hypres.25.801. [DOI] [PubMed] [Google Scholar]
- 38.Fisher ND, Hurwitz S, Jeunemaitre X, et al. Familial aggregation of low-renin hypertension. Hypertension. 2002;39:914–918. doi: 10.1161/01.hyp.0000013784.18175.51. [DOI] [PubMed] [Google Scholar]
- 39.Sanada H, Yatabe J, Midorikawa S, et al. Single-nucleotide polymorphisms for diagnosis of salt-sensitive hypertension. Clin Chem. 2006;52:352–360. doi: 10.1373/clinchem.2005.059139. [DOI] [PubMed] [Google Scholar]
- 40.Pimenta E, Gaddam KK, Oparil S, et al. Effects of dietary sodium reduction on blood pressure in subjects with resistant hypertension: results from a randomized trial. Hypertension. 2009;54:475–481. doi: 10.1161/HYPERTENSIONAHA.109.131235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yatabe MS, Yoneda M, Felder RA, et al. Hypertension-related gene polymorphisms of G-protein-coupled receptor kinase 4 are associated with NT-proBNP concentration in normotensive healthy adults. Int J Hypertens. 2012;2012:806810. doi: 10.1155/2012/806810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blaustein MP, Leenen FH, Chen L, et al. How NaCl raises blood pressure: a new paradigm for the pathogenesis of salt-dependent hypertension. Am J Physiol Heart Circ Physiol. 2012;302:H1031–H1049. doi: 10.1152/ajpheart.00899.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gildea JJ, Lahiff DT, Keene SA, et al. A rapid method for clinical diagnosis of salt sensitivity of blood pressure. High Blood Pressure Research, 2012 Scientific Sessions. :38–39. Abstract #32. [Google Scholar]
- 44.Carlson JM, Gildea JJ, Schoeffel CD, et al. Urinary exosome miRNome analysis and its application to salt sensitivity of blood pressure. High Blood Pressure Research. 2012:38. doi: 10.1016/j.clinbiochem.2013.05.052. Scientific Sessions. Abstract #30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carlson JM, Gildea JJ, Pettigrew AC, et al. Exosomes as potential acellular signaling mechanism in the kidney. High Blood Pressure Research, 2012. Scientific Sessions. :162. Abstract #481. [Google Scholar]
- 46.Alvarez ML, Khosroheidari M, Kanchi Ravi R, Distefano JK. Comparison of protein, microRNA, and mRNA yields using different methods of urinary exosome isolation for the discovery of kidney disease biomarkers. Kidney Int 2012. doi: 10.1038/ki.2012.256. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 47.Wang G, Tam LS, Li EK, et al. Serum and urinary cell-free MiR-146a and MiR-155 in patients with systemic lupus erythematosus. J Rheumatol. 2010;37:2516–2522. doi: 10.3899/jrheum.100308. [DOI] [PubMed] [Google Scholar]
- 48.Harrap SB. Blood pressure genetics: time to focus. J Am Soc Hypertens. 2009;3:231–237. doi: 10.1016/j.jash.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 49.King DE, Mainous AG, 3rd, Carnemolla M, Everett CJ. Adherence to healthy lifestyle habits in US adults, 1988–2006. Am J Med. 2009;122:528–534. doi: 10.1016/j.amjmed.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 50.Ohta Y, Tsuchihashi T, Onaka U, et al. Long-term compliance with salt restriction in Japanese hypertensive patients. Hypertens Res. 2005;28:953–957. doi: 10.1291/hypres.28.953. [DOI] [PubMed] [Google Scholar]
- 51.Mellen PB, Gao SK, Vitolins MZ, Goff DC., Jr Deteriorating dietary habits among adults with hypertension: DASH dietary accordance, NHANES 1988–1994 and 1999–2004. Arch Intern Med. 2008;168:308–314. doi: 10.1001/archinternmed.2007.119. [DOI] [PubMed] [Google Scholar]
- 52.Navar LG, Kobori H, Prieto MC, Gonzalez-Villalobos RA. Intratubular renin–angiotensin system in hypertension. Hypertension. 2011;57:355–362. doi: 10.1161/HYPERTENSIONAHA.110.163519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Luft FC. Renin–angiotensin system-related highlights from the High Blood Pressure Research Conference annual meeting. J Renin Angiotensin Aldosterone Syst. 2008;9:242–247. doi: 10.1177/1470320308100546. [DOI] [PubMed] [Google Scholar]
- 54.Huang J, Siragy HM. Sodium depletion enhances renal expression of (pro)-renin receptor via cyclic GMP-protein kinase G signaling pathway. Hypertension. 2012;59:317–323. doi: 10.1161/HYPERTENSIONAHA.111.186056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ferrario CM, Varagic J. The ANG-(1–7)/ACE2/mas axis in the regulation of nephron function. Am J Physiol Renal Physiol. 2010;298:F1297–F1305. doi: 10.1152/ajprenal.00110.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Garcia-Espinosa MA, Shaltout HA, Gallagher PE, et al. In vivo expression of angiotensin-(1–7) lowers blood pressure and improves baroreflex function in transgenic (mren2)27 rats. J Cardiovasc Pharmacol. 2012;60:150–157. doi: 10.1097/FJC.0b013e3182588b32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Padia SH, Carey RM. AT2 receptors: beneficial counter-regulatory role in cardiovascular and renal function. Pflugers Arch. 2012 doi: 10.1007/s00424-012-1146-3. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jose PA, Soares-da-Silva P, Eisner GM, Felder RA. Dopamine and G protein-coupled receptor kinase 4 in the kidney: role in blood pressure regulation. Biochim Biophys Acta. 2010;398:553–558. doi: 10.1016/j.bbadis.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stragier B, Hristova I, Sarre S, et al. In vivo characterization of the angio-tensin-(1–7)-induced dopamine and gamma-aminobutyric acid release in the striatum of the rat. Eur J Neurosci. 2005;22:658–664. doi: 10.1111/j.1460-9568.2005.04188.x. [DOI] [PubMed] [Google Scholar]
- 60.Pawlak R, Napiorkowska-Pawlak D, Takada Y, et al. The differential effect of angiotensin II and angiotensin 1–7 on norepinephrine, epinephrine, and dopamine concentrations in rat hypothalamus: the involvement of angiotensin receptors. Brain Res Bull. 2001;54:689–694. doi: 10.1016/s0361-9230(01)00489-0. [DOI] [PubMed] [Google Scholar]
- 61.Johnson AD, Newton-Cheh C, Chasman DI, et al. Cohorts for Heart and Aging Research in Genomic Epidemiology Consortium; Global BPgen Consortium; Women’s Genome Health Study. Association of hypertension drug target genes with blood pressure and hypertension in 86,588 individuals. Hypertension. 2011;57:903–910. doi: 10.1161/HYPERTENSIONAHA.110.158667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pereira TV, Nunes AC, Rudnicki M, et al. Meta-analysis of the association of 4 angiotensinogen polymorphisms with essential hypertension: a role beyond M235T? Hypertension. 2008;51:778–783. doi: 10.1161/HYPERTENSIONAHA.107.100370. [DOI] [PubMed] [Google Scholar]
- 63.Strazzullo P, Galletti F. Genetics of salt-sensitive hypertension. Curr Hyper-tens Rep. 2007;9:25–32. doi: 10.1007/s11906-007-0006-6. [DOI] [PubMed] [Google Scholar]
- 64.Gu D, Kelly TN, Hixson JE, et al. Genetic variants in the renin–angiotensin–aldosterone system and salt sensitivity of blood pressure. J Hypertens. 2010;28:1210–1220. [PMC free article] [PubMed] [Google Scholar]
- 65.Sanada H, Jones JE, Jose PA. Genetics of salt-sensitive hypertension. Curr Hypertens Rep. 2011;13:55–66. doi: 10.1007/s11906-010-0167-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Citterio L, Simonini M, Zagato L, et al. Genes involved in vasoconstriction and vasodilation system affect salt sensitive hypertension. PLoS One. 2011;6:e19620. doi: 10.1371/journal.pone.0019620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Blaustein MP, Zhang J, Chen L, et al. The pump, the exchanger, and endogenous ouabain: signaling mechanisms that link salt retention to hypertension. Hypertension. 2009;53:291–298. doi: 10.1161/HYPERTENSIONAHA.108.119974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhao Q, Gu D, Hixson JE, et al. Genetic Epidemiology Network of Salt Sensitivity Collaborative Research Group. Common variants in epithelial sodium channel genes contribute to salt sensitivity of blood pressure: the GenSalt study. Circ Cardiovasc Genet. 2011;4:375–380. doi: 10.1161/CIRCGENETICS.110.958629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rao AD, Sun B, Saxena A, et al. Polymorphisms in the serum- and gluco-corticoid-inducible kinase 1 gene are associated with blood pressure and renin response to dietary salt intake. J Hum Hypertens. 2012 doi: 10.1038/jhh.2012.22. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dahlberg J, Nilsson LO, von Wowern F, Melander O. Polymorphism in NEDD4L is associated with increased salt sensitivity, reduced levels of P-renin and increased levels of Nt-proANP. PLoS One. 2007;2:e432. doi: 10.1371/journal.pone.0000432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nicolas HA, Kulik R, Lamothe G, et al. Increased blood pressure in response to salt intake in hypertensive G carriers of the NEDD4L SNP rs4149601. High Blood Pressure Research, 2012 Scientific Sessions. :37. Abstract #28. [Google Scholar]
- 72.Manunta P, Lavery G, Lanzani C, et al. Physiological interaction between α-adducin and WNK1–NEDD4L pathways on sodium-related blood pressure regulation. Hypertension. 2008;52:366–372. doi: 10.1161/HYPERTENSIONAHA.108.113977. [DOI] [PubMed] [Google Scholar]
- 73.Torielli L, Tivodar S, Montella RC, et al. α-Adducin mutations increase Na/K pump activity in renal cells by affecting constitutive endocytosis: implications for tubular Na reabsorption. Am J Physiol Renal Physiol. 2008;295:F478–F487. doi: 10.1152/ajprenal.90226.2008. [DOI] [PubMed] [Google Scholar]
- 74.Ferrandi M, Molinari I, Torielli L, et al. Adducin- and ouabain-related gene variants predict the antihypertensive activity of rostafuroxin, part 1: experimental studies. Sci Transl Med. 2010;2:59ra86. doi: 10.1126/scitranslmed.3001815. [DOI] [PubMed] [Google Scholar]
- 75.Wang R, Zhong B, Liu Y, Wang C. Association between α-adducin gene polymorphism (Gly460Trp) and genetic predisposition to salt sensitivity: a meta-analysis. J Appl Genet. 2010;51:87–94. doi: 10.1007/BF03195715. [DOI] [PubMed] [Google Scholar]
- 76.Kelly TN, Rice TK, Gu D, et al. Novel genetic variants in the α-adducin and guanine nucleotide binding protein β-polypeptide 3 genes and salt sensitivity of blood pressure. Am J Hypertens. 2009;22:985–992. doi: 10.1038/ajh.2009.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Manunta P, Maillard M, Tantardini C, et al. Relationships among endogenous ouabain, α-adducin polymorphisms and renal sodium handling in primary hypertension. J Hypertens. 2008;26:914–920. doi: 10.1097/HJH.0b013e3282f5315f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fava C, Montagnana M, Almgren P, et al. Association between adducin-1 G460W variant and blood pressure in Swedes is dependent on interaction with body mass index and gender. Am J Hypertens. 2007;20:981–989. doi: 10.1016/j.amjhyper.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 79.Citarella MR, Choi MR, Gironacci MM, et al. Urodilatin and dopamine: a new interaction in the kidney. Regul Pept. 2009;153:19–24. doi: 10.1016/j.regpep.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 80.Zeng C, Luo Y, Asico LD, et al. Perturbation of D1 dopamine and AT1 receptor interaction in spontaneously hypertensive rats. Hypertension. 2003;42:787–792. doi: 10.1161/01.HYP.0000085334.34963.4E. [DOI] [PubMed] [Google Scholar]
- 81.Li H, Armando I, Yu P, et al. Dopamine 5 receptor mediates Ang II type 1 receptor degradation via a ubiquitin–proteasome pathway in mice and human cells. J Clin Invest. 2008;118:2180–2189. doi: 10.1172/JCI33637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li D, Scott L, Crambert S, et al. Binding of losartan to angiotensin AT1 receptors increases dopamine D1 receptor activation. J Am Soc Nephrol. 2012;23:421–428. doi: 10.1681/ASN.2011040344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Khan F, Spicarova´ Z, Zelenin S, et al. Negative reciprocity between angiotensin II type 1 and dopamine D1 receptors in rat renal proximal tubule cells. Am J Physiol Renal Physiol. 2008;295:F1110–F1116. doi: 10.1152/ajprenal.90336.2008. [DOI] [PubMed] [Google Scholar]
- 84.Yao B, Harris RC, Zhang MZ. Intrarenal dopamine attenuates deoxycorti-costerone acetate/high salt-induced blood pressure elevation in part through activation of a medullary cyclooxygenase 2 pathway. Hypertension. 2009;54:1077–1083. doi: 10.1161/HYPERTENSIONAHA.109.137174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kirchheimer C, Mendez CF, Acquier A, Nowicki S. Role of 20-HETE in D1/D2 dopamine receptor synergism resulting in the inhibition of Na+-K+-ATPase activity in the proximal tubule. Am J Physiol Renal Physiol. 2007;292:F1435–F1442. doi: 10.1152/ajprenal.00176.2006. [DOI] [PubMed] [Google Scholar]
- 86.Yu C, Yang Z, Ren H, et al. D3 dopamine receptor regulation of ETB receptors in renal proximal tubule cells from WKY and SHRs. Am J Hypertens. 2009;22:877–883. doi: 10.1038/ajh.2009.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jiang X, Wang W, Ning B, et al. Basal and postprandial serum levels of gastrin in normotensive and hypertensive adults. Clin Exp Hypertens. 2012 doi: 10.3109/10641963.2012.690474. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 88.Chen Y, Zhen S, Asico L, et al. Synergistic effects of renal dopamine and gastrin receptors in hypertension. High Blood Pressure Research. 2012:125. Scientific Sessions. Abstract #341. [Google Scholar]
- 89.Zeng C, Han Y, Huang H, et al. D1-like receptors inhibit insulin-induced vascular smooth muscle cell proliferation via down-regulation of insulin receptor expression. J Hypertens. 2009;27:1033–1041. doi: 10.1097/HJH.0b013e3283293c7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yang J, Cui Z, He D, et al. Insulin increases D5 dopamine receptor expression and function in renal proximal tubule cells from Wistar-Kyoto rats. Am J Hypertens. 2009;22:770–776. doi: 10.1038/ajh.2009.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Venkatakrishnan U, Chen C, Lokhandwala MF. The role of intrarenal nitric oxide in the natriuretic response to dopamine receptor activation. Clin Exp Hypertens. 2000;22:309–324. doi: 10.1081/ceh-100100080. [DOI] [PubMed] [Google Scholar]
- 92.Crambert S, Sjo¨berg A, Eklo¨f AC, et al. Prolactin and dopamine 1-like receptor interaction in renal proximal tubular cells. Am J Physiol Renal Physiol. 2010;299:F49–F54. doi: 10.1152/ajprenal.00582.2009. [DOI] [PubMed] [Google Scholar]
- 93.Ueda A, Ozono R, Oshima T, et al. Disruption of the type 2 dopamine receptor gene causes a sodium-dependent increase in blood pressure in mice. Am J Hypertens. 2003;16:853–858. doi: 10.1016/s0895-7061(03)01013-6. [DOI] [PubMed] [Google Scholar]
- 94. Harris RC. Abnormalities in renal dopamine signaling and hypertension: the role of GRK4. Curr Opin Nephrol Hypertens. 2012;21:61–65. doi: 10.1097/MNH.0b013e32834de2cb. This is an updated review of the role of dopamine, dopamine receptors, and GRK4 in the regulation of blood pressure.
- 95. Zhang MZ, Yao B, Wang S, et al. Intrarenal dopamine deficiency leads to hypertension and decreased longevity in mice. J Clin Invest. 2011;121:2845–2854. doi: 10.1172/JCI57324. This study shows the importance of the production of dopamine in the renal proximal tubule in the regulation of salt sensitivity of blood pressure.
- 96.Aviv A, Hollenberg NK, Weder A. Urinary potassium excretion and sodium sensitivity in blacks. Hypertension. 2004;43:707–713. doi: 10.1161/01.HYP.0000120155.48024.6f. [DOI] [PubMed] [Google Scholar]
- 97.Doris PA. Renal proximal tubule sodium transport and genetic mechanisms of essential hypertension. J Hypertens. 2000;18:509–519. doi: 10.1097/00004872-200018050-00002. [DOI] [PubMed] [Google Scholar]
- 98.Bengra C, Mifflin TE, Khripin Y, et al. Genotyping essential hypertension SNPs using a homogenous PCR method with universal energy transfer primers. Clin Chem. 2002;48:2131–2140. [PubMed] [Google Scholar]
- 99.Glazier AM, Nadeau JH, Aitman TJ. Finding genes that underlie complex traits. Science. 2002;298:2345–2349. doi: 10.1126/science.1076641. [DOI] [PubMed] [Google Scholar]
- 100.Wang Z, Asico LD, Escano CS, et al. Human G protein-coupled receptor kinase type 4γ (hGRK4γ) wild-type prevents salt sensitivity while its variant, hGRK4γ486V, promotes salt sensitivity in transgenic mice: role of genetic background. Hypertension. 2006;48:e27. [Google Scholar]
- 101.Wang Z, Escano C, Asico LD, Jose PA. Human GRK4 A486V gene promotes salt sensitivity by impairing D1R function and increasing renal proximal tubular sodium transport in transgenic mice. HBPR. 2010:232. AHA. [Google Scholar]
- 102.Moore JH, Williams SM. New strategies for identifying gene–gene interactions in hypertension. Ann Med. 2002;34:88–95. doi: 10.1080/07853890252953473. [DOI] [PubMed] [Google Scholar]
- 103.Speirs HJ, Katyk K, Kumar NN, et al. Association of G-protein-coupled receptor kinase 4 haplotypes, but not HSD3B1 or PTP1B polymorphisms, with essential hypertension. J Hypertens. 2004;22:931–936. doi: 10.1097/00004872-200405000-00014. [DOI] [PubMed] [Google Scholar]
- 104.Machnik A, Neuhofer W, Jantsch J, et al. Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C-dependent buffering mechanism. Nat Med. 2009;15:545–552. doi: 10.1038/nm.1960. [DOI] [PubMed] [Google Scholar]
- 105.Racusen LC, Fivush BA, Andersson H, Gahl WA. Culture of renal tubular cells from the urine of patients with nephropathic cystinosis. J Am Soc Nephrol. 1991;1:1028–1033. doi: 10.1681/ASN.V181028. [DOI] [PubMed] [Google Scholar]
- 106.Racusen LC, Wilson PD, Hartz PA, et al. Renal proximal tubular epithelium from patients with nephropathic cystinosis: Immortalized cell lines as in vitro model systems. Kidney Int. 1995;48:536–543. doi: 10.1038/ki.1995.324. [DOI] [PubMed] [Google Scholar]
- 107.Inoue CN, Kondo Y, Ohnuma S, et al. Use of cultured tubular cells isolated from human urine for investigation of renal transporter. Clin Nephrol. 2000;53:90–98. Erratum in Clin Nephrol 2000; 53(6):492. [PubMed] [Google Scholar]
- 108.Laube GF, Haq MR, van’t Hoff WG. Exfoliated human proximal tubular cells: a model of cystinosis and Fanconi syndrome. Pediatr Nephrol. 2005;20:136–140. doi: 10.1007/s00467-004-1703-x. [DOI] [PubMed] [Google Scholar]
- 109.Rahmoune H, Thompson PW, Ward JM, et al. Glucose transporters in human renal proximal tubular cells isolated from the urine of patients with noninsulin-dependent diabetes. Diabetes. 2005;54:3427–3434. doi: 10.2337/diabetes.54.12.3427. [DOI] [PubMed] [Google Scholar]
- 110.Gildea JJ, Keene SA, Lahiff DT, et al. A novel test for low salt sensitivity: Angiotensin type-II receptor recruitment after dopamine-1 receptor stimulation in urine-derived renal proximal tubule cells. High Blood Pressure Research. 2012:166. Scientific Sessions, Abstract #495. [Google Scholar]
- 111.Ross KA. Evidence for somatic gene conversion and deletion in bipolar disorder, Crohn’s disease, coronary artery disease, hypertension, rheumatoid arthritis, type-1 diabetes, and type-2 diabetes. BMC Med. 2011;9:12. doi: 10.1186/1741-7015-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Esteva-Font C, Wang X, Ars E, et al. Are sodium transporters in urinary exosomes reliable markers of tubular sodium reabsorption in hypertensive patients? Nephron Physiol. 2010;114:25–34. doi: 10.1159/000274468. [DOI] [PMC free article] [PubMed] [Google Scholar]