Abstract
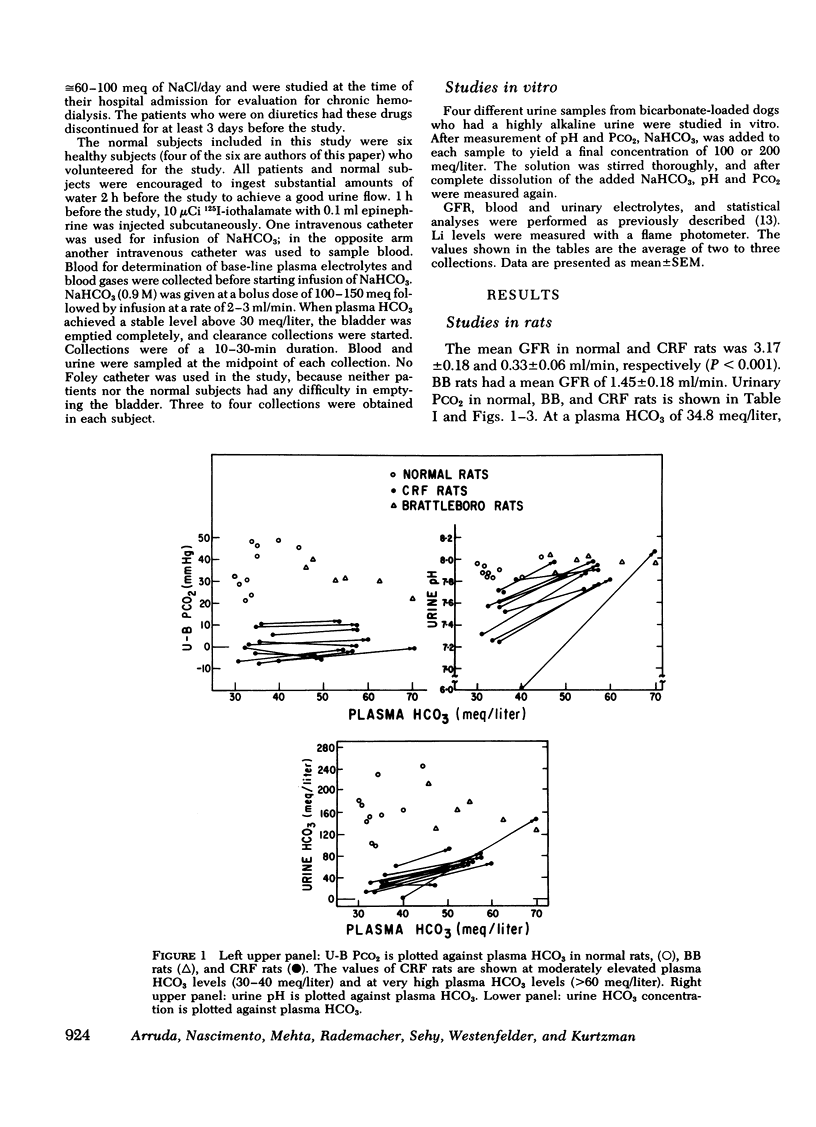
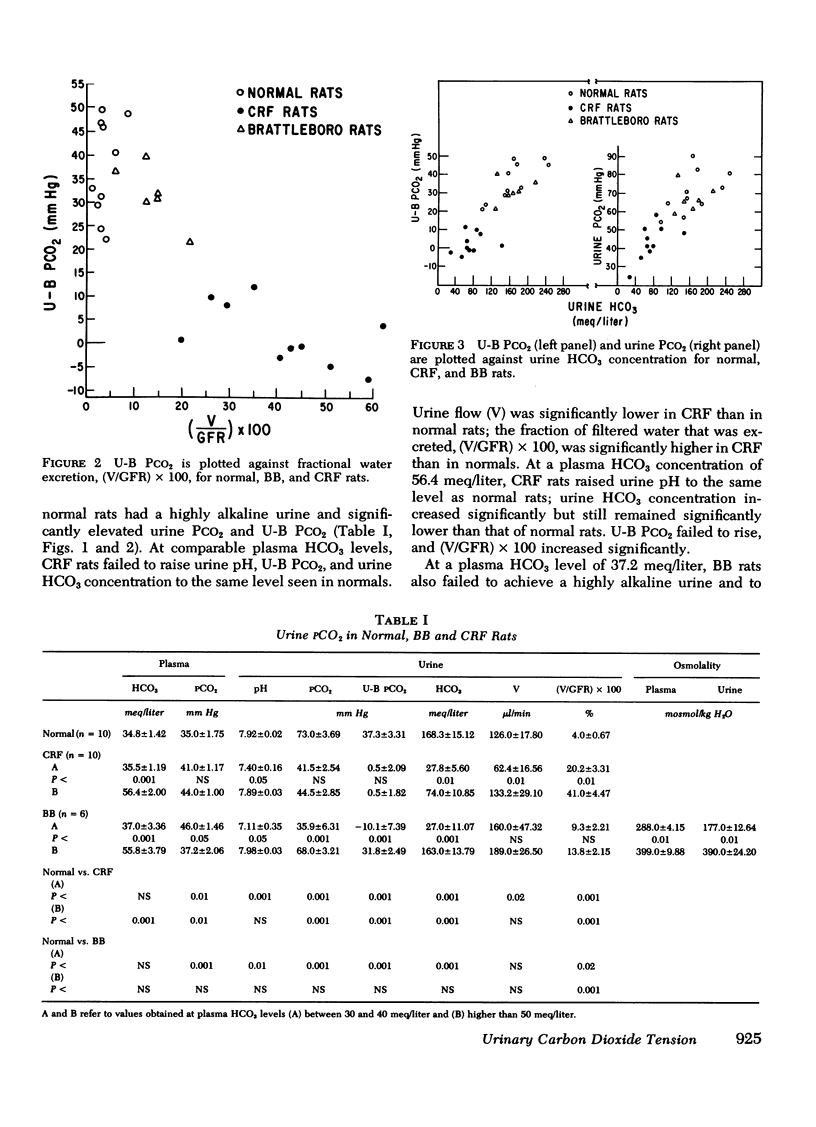
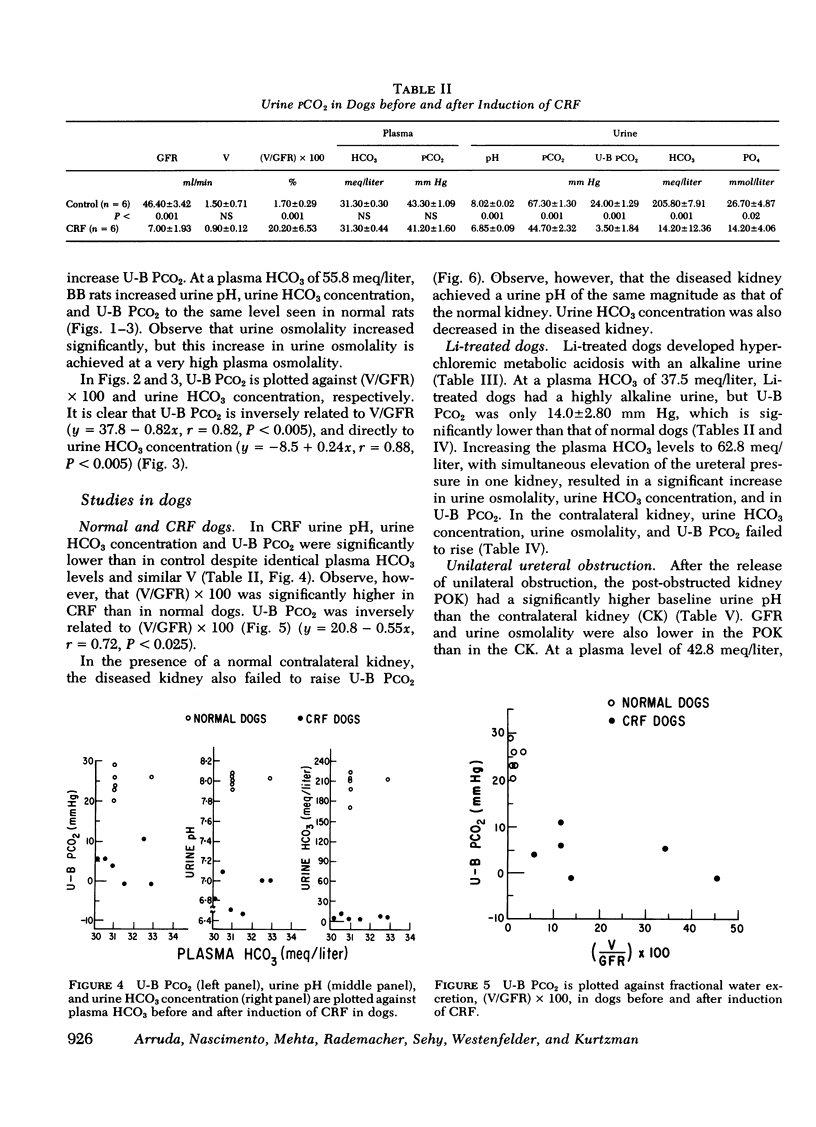
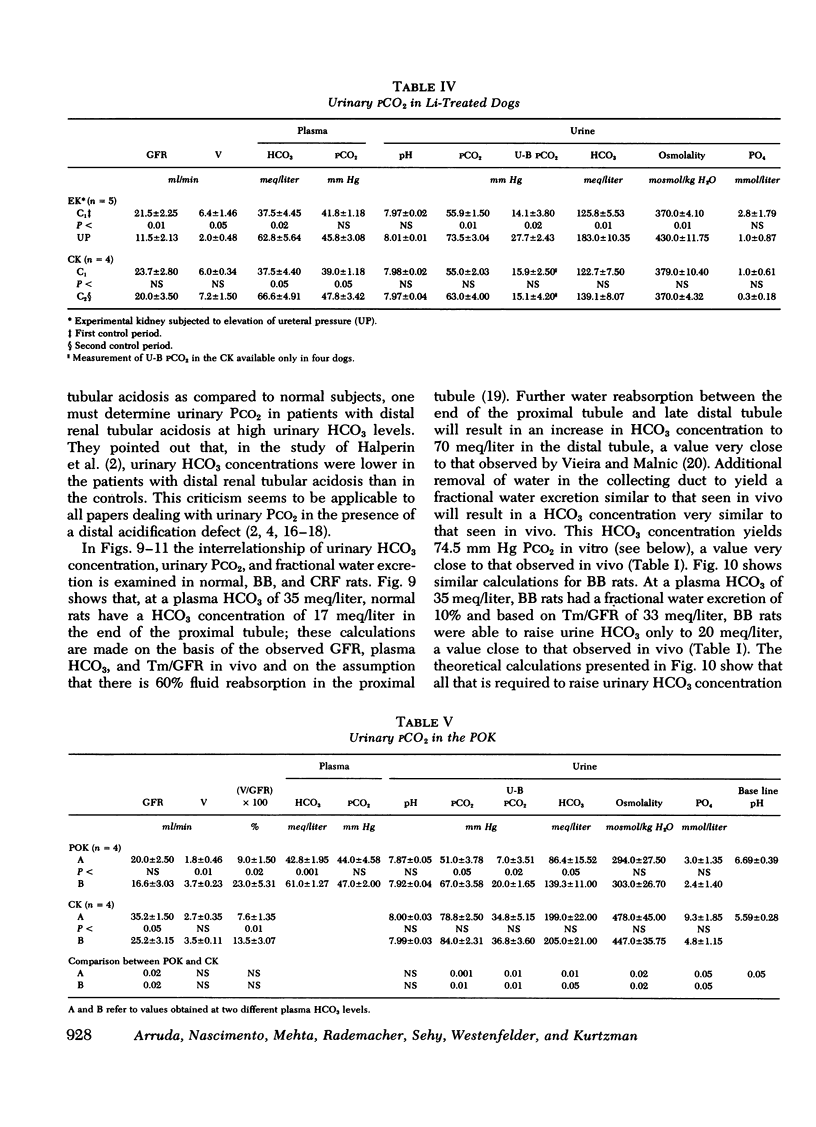
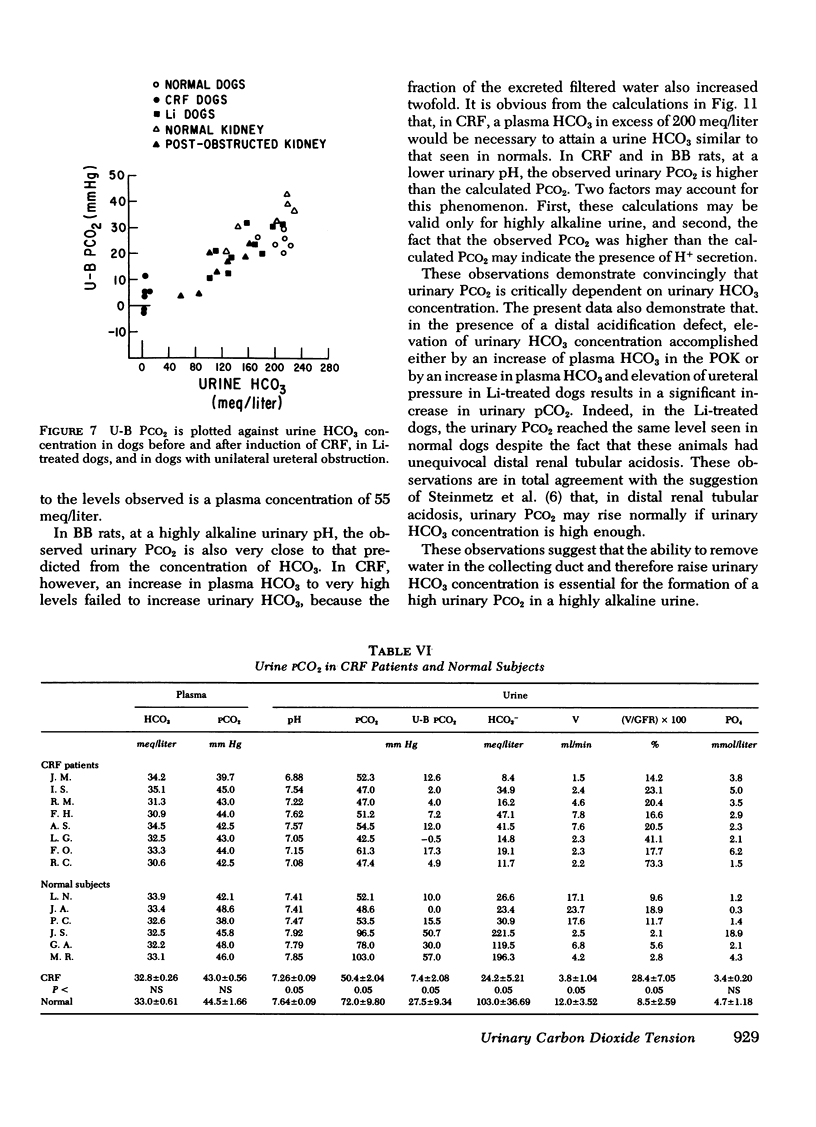
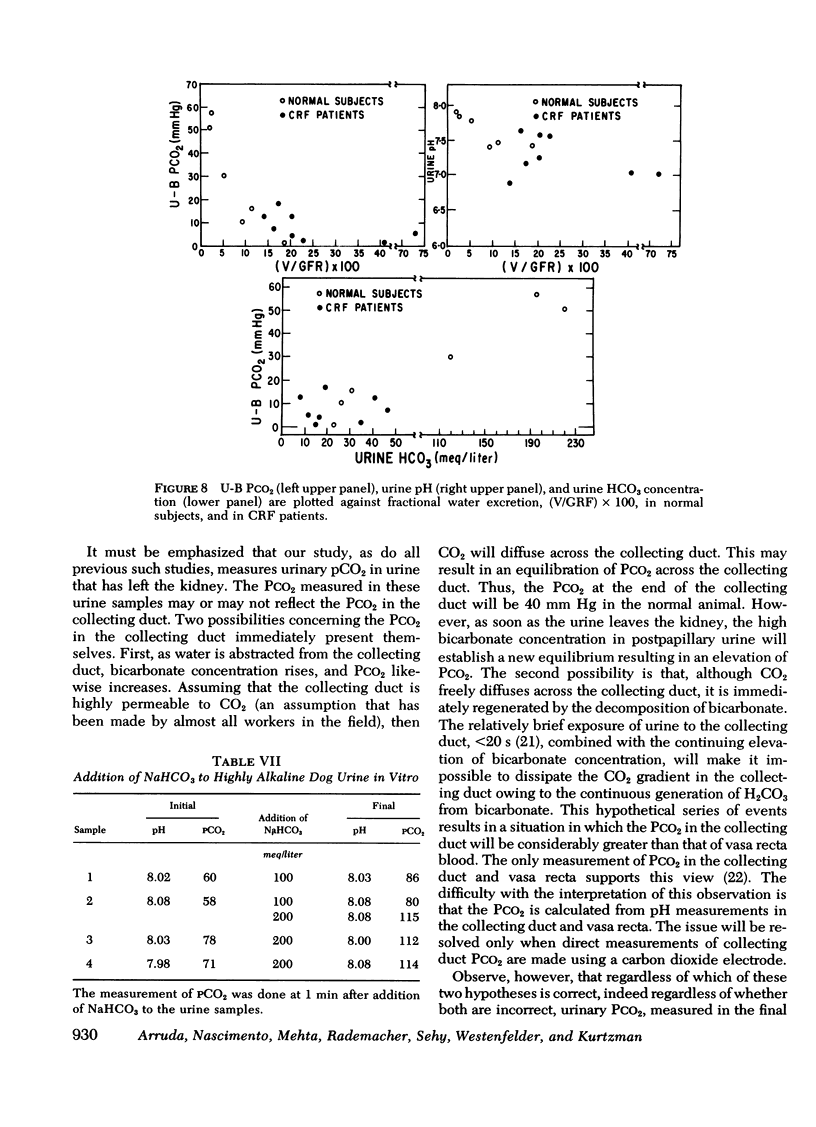
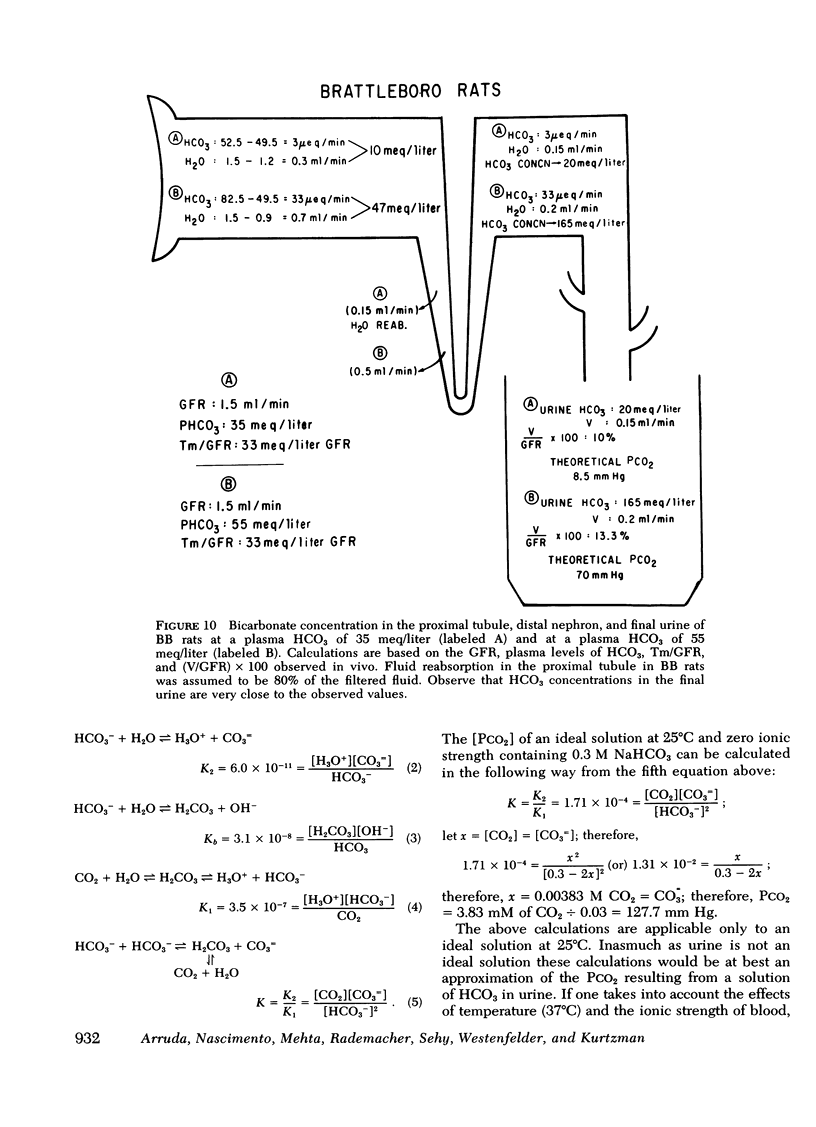
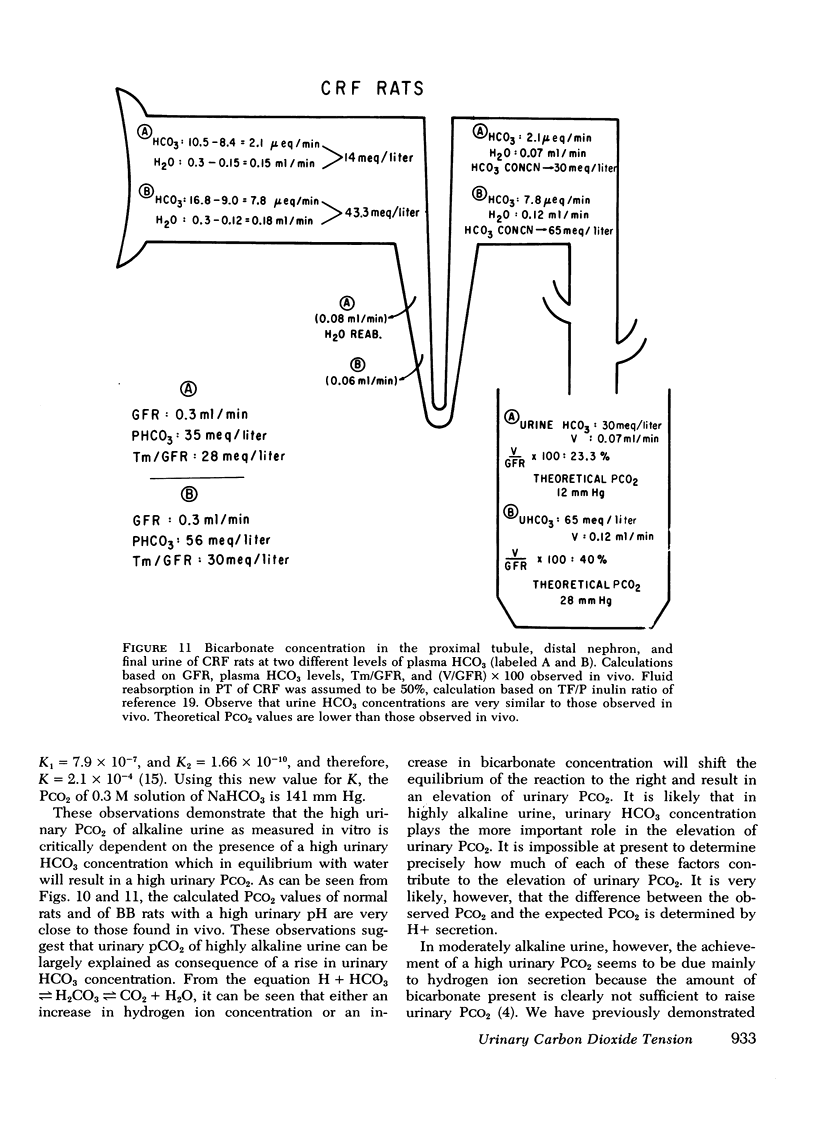
Measurement of urine to blood (U-B) carbon dioxide tension (PCO2) gradient during alkalinization of the urine has been suggested to assess distal H+ secretion. A fact that has not been considered in previous studies dealing with urinary PCO2 is that dissolution of HCO3 in water results in elevation of PCO2 which is directly proportional to the HCO3 concentration. To investigate the interrelationship of urinary HCO3 and urinary acidification, we measured U-B PCO2 in (a) the presence of enhanced H+ secretion and decreased concentrating ability i.e., chronic renal failure (CRF), (b) animals with normal H+ secretion and decreased concentrating ability, Brattleboro (BB) rats, and (c) the presence of both impaired H+ secretion and concentrating ability (LiCl treatment and after release of unilateral ureteral obstruction). At moderately elevated plasma HCO3 levels (30-40 meq/liter), normal rats achieved a highly alkaline urine (urine pH > 7.8) and raised urine HCO3 concentration and U-B PCO2. At similar plasma HCO3 levels, BB rats had a much higher fractional water excretion and failed to raise urine pH, urine HCO3 concentration, and U-B PCO2 normally. At a very high plasma HCO3 (>50 meq/liter), BB rats raised urine pH, urine HCO3 concentration, and U-B PCO2 to the same levels seen in normals. CRF rats failed to raise urine pH, urine HCO3, and U-B PCO2 normally at moderately elevated plasma HCO3 levels; at very high plasma HCO3 levels, CRF rats achieved a highly alkaline urine but failed to raise U-B PCO2. Dogs and patients with CRF were also unable to raise urine pH, urine HCO3 concentration, and U-B PCO2 normally at moderately elevated plasma HCO3 levels. In rats, dogs, and man, U-B PCO2 was directly related to urine HCO3 concentration and inversely related to fractional water excretion. At moderately elevated plasma HCO3 levels, animals with a distal acidification defect failed to raise U-B PCO2; increasing the plasma HCO3 to very high levels resulted in a significant increase in urine HCO3 concentration and U-B PCO2. The observed urinary PCO2 was very close to the PCO2 which would be expected by simple dissolution of a comparable amount of HCO3 in water. These data demonstrate that, in highly alkaline urine, urinary PCO2 is largely determined by concentration of urinary HCO3 and cannot be used as solely indicating distal H+ secretion.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arruda J. A., Nascimento L., Kumar S. K., Kurtzman N. A. Factors influencing the formation of urinary carbon dioxide tension. Kidney Int. 1977 May;11(5):307–317. doi: 10.1038/ki.1977.48. [DOI] [PubMed] [Google Scholar]
- Arruda J. A., Nascimento L., Westenfelder C., Kurtzman N. A. Effect of parathyroid hormone on urinary acidification. Am J Physiol. 1977 May;232(5):F429–F433. doi: 10.1152/ajprenal.1977.232.5.F429. [DOI] [PubMed] [Google Scholar]
- Dorhout-Mees E. J., Machado M., Slatopolsky E., Klahr S., Bricker N. S. The functional adaptation of the diseased kidney. 3. Ammonium excretion. J Clin Invest. 1966 Mar;45(3):289–296. doi: 10.1172/JCI105342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillastre J. P., Ardaillou R., Richet G. pH et P CO2 urinaires en réponse à une surcharge alcaline au cours de l'insuffisance rénale chronique. Nephron. 1969;6(2):91–101. doi: 10.1159/000179717. [DOI] [PubMed] [Google Scholar]
- Halperin M. L., Goldstein M. B., Haig A., Johnson M. D., Stinebaugh B. J. Studies on the pathogenesis of type I (distal) renal tubular acidosis as revealed by the urinary PCO2 tensions. J Clin Invest. 1974 Mar;53(3):669–677. doi: 10.1172/JCI107604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KENNEDY T. J., Jr, ORLOFF J., BERLINER R. W. Significance of carbon dioxide tension in urine. Am J Physiol. 1952 Jun;169(3):596–608. doi: 10.1152/ajplegacy.1952.169.3.596. [DOI] [PubMed] [Google Scholar]
- Kurtzman N. A. Regulation of renal bicarbonate reabsorption by extracellular volume. J Clin Invest. 1970 Mar;49(3):586–595. doi: 10.1172/JCI106269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOREL F., MYLLE M., GOTTSCHALK C. W. TRACER MICROINJECTION STUDIES OF EFFECT OF ADH ON RENAL TUBULAR DIFFUSION OF WATER. Am J Physiol. 1965 Jul;209:179–187. doi: 10.1152/ajplegacy.1965.209.1.179. [DOI] [PubMed] [Google Scholar]
- Nascimento L., Rademacher D. R., Hamburger R., Arruda J. A., Kurtzman A. On the mechanism of lithium-induced renal tubular acidosis. J Lab Clin Med. 1977 Mar;89(3):455–462. [PubMed] [Google Scholar]
- OCHWADT B. K., PITTS R. F. Effects of intravenous infusion of carbonic anhydrase on carbon dioxide tension of alkaline urine. Am J Physiol. 1956 May;185(2):426–429. doi: 10.1152/ajplegacy.1956.185.2.426. [DOI] [PubMed] [Google Scholar]
- PORTWOOD R. M., SELDIN D. W., RECTOR F. C., Jr, CADE R. The relation of urinary CO2 tension to bicarbonate excretion. J Clin Invest. 1959 May;38(5):770–776. doi: 10.1172/JCI103858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POY R. K., WRONG O. The urinary pCO2 in renal disease. Clin Sci. 1960 Nov;19:631–639. [PubMed] [Google Scholar]
- RECTOR F. C., Jr, CARTER N. W., SELDIN D. W. THE MECHANISM OF BICARBONATE REABSORPTION IN THE PROXIMAL AND DISTAL TUBULES OF THE KIDNEY. J Clin Invest. 1965 Feb;44:278–290. doi: 10.1172/JCI105142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RECTOR F. C., Jr, PORTWOOD R. M., SELDIN D. W. Examination of the mixing hypothesis as an explanation for elevated urinary carbon dioxide tensions. Am J Physiol. 1959 Oct;197:861–864. doi: 10.1152/ajplegacy.1959.197.4.861. [DOI] [PubMed] [Google Scholar]
- REID E. L., HILLS A. G. DIFFUSION OF CARBON DIOXIDE OUT OF THE DISTAL NEPHRON IN MAN DURING ANTIDIURESIS. Clin Sci. 1965 Feb;28:15–28. [PubMed] [Google Scholar]
- Roscoe J. M., Goldstein M. B., Halperin M. L., Wilson D. R., Stinebaugh B. J. Lithium-induced impairment of urine acidification. Kidney Int. 1976 Apr;9(4):344–350. doi: 10.1038/ki.1976.40. [DOI] [PubMed] [Google Scholar]
- Schultze R. G., Taggart D. D., Shapiro H., Pennell J. P., Caglar S., Bricker N. S. On the adaptation in potassium excretion associated with nephron reduction in the dog. J Clin Invest. 1971 May;50(5):1061–1068. doi: 10.1172/JCI106577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seldin D. W., Coleman A. J., Carter N. W., Rector F. C., Jr The effect of Na2SO4 on urinary acidification in chronic renal disease. J Lab Clin Med. 1967 Jun;69(6):893–903. [PubMed] [Google Scholar]
- Thirakomen K., Kozlov N., Arruda J. A., Kurtzman N. A. Renal hydrogen ion secretion after release of unilateral ureteral obstruction. Am J Physiol. 1976 Oct;231(4):1233–1239. doi: 10.1152/ajplegacy.1976.231.4.1233. [DOI] [PubMed] [Google Scholar]
- Uhlich E., Baldamus C. A., Ullrich K. J. Verhalten von CO2-Druck und Bicarbonat im Gegenstromysystem des Nierenmarks. Pflugers Arch. 1968;303(1):31–48. doi: 10.1007/BF00586825. [DOI] [PubMed] [Google Scholar]
- Vieira F. L., Malnic G. Hydrogen ion secretion by rat renal cortical tubules as studied by an antimony microelectrode. Am J Physiol. 1968 Apr;214(4):710–718. doi: 10.1152/ajplegacy.1968.214.4.710. [DOI] [PubMed] [Google Scholar]



