Abstract
Myoblast fusion is a critical process that contributes to the growth of muscle during development and to the regeneration of myofibers upon injury. Myoblasts fuse with each other as well as with multinucleated myotubes to enlarge the myofiber. Initial studies demonstrated that myoblast fusion requires extracellular calcium and changes in cell membrane topography and cytoskeletal organization. More recent studies have identified several cell-surface and intracellular proteins that mediate myoblast fusion. Furthermore, emerging evidence suggests that myoblast fusion is also regulated by the activation of specific cell-signaling pathways that lead to the expression of genes whose products are essential for the fusion process and for modulating the activity of molecules that are involved in cytoskeletal rearrangement. Here, we review the roles of the major signaling pathways in mammalian myoblast fusion.
Introduction
Skeletal muscle fibers are syncytia that arise from the fusion of myoblasts during development. In adults, fusion of myogenic cells is required to facilitate the growth and repair of myofibers after injury. At the cellular level, the fusion process is characterized by the alignment of myoblast and myotube membranes and rearrangements of actin cytoskeleton at contact sites followed by membrane fusion, which occurs in two stages. Initially, myoblast-myoblast fusion (which is referred to as “primary fusion”) results in the formation of nascent myotubes. In the second phase, myoblasts fuse with nascent myotubes (in a process henceforth known as “secondary fusion”), which results in nuclear accretion and growth of the myotube (1, 2). Most of the progress in understanding the process of myoblast fusion during development has been made by studies of the fruit fly Drosophila melanogaster in which genetic screening has identified the specific molecular machinery involved (1, 2). Research over the past decade has suggested several candidate gene products whose function might be conserved in mammals (1, 3, 4). The current understanding of myoblast fusion in mammals comes largely from experiments with a myoblast cell culture model in which the fusion steps can be recapitulated in vitro. These studies have suggested that myoblast fusion in mammals is regulated by various cell adhesion proteins, including the α3-, α9-, and β-integrin subunits, neogenin, M- and N-cadherin, CD36, and a disintegrin and metalloprotease12 (ADAM12); transmembrane lipids, including cholesterol and phosphatidylserine; and intracellular domain-associated signaling or adaptor proteins, including β-catenin, end binding 3 (EB3), kindlin-2, myoferlin, creatine kinase B, diacylglycerol kinase ξ, Rac1, focal adhesion kinase (FAK), and syntrophin, which accumulate at sites of contact between two myogenic cells either in a symmetrical or an asymmetrical manner (1, 3). Furthermore, it is now increasingly evident that an array of cell signaling pathways plays critical roles in myoblast fusion (Fig. 1). Some of these pathways are activated as a result of the recruitment of specific cell-surface proteins between fusion partners, whereas others are activated as part of the myogenic differentiation program, but they contribute to the fusion process (Fig. 1). Here, we review the roles of the major signaling pathways involved in mammalian myoblast fusion.
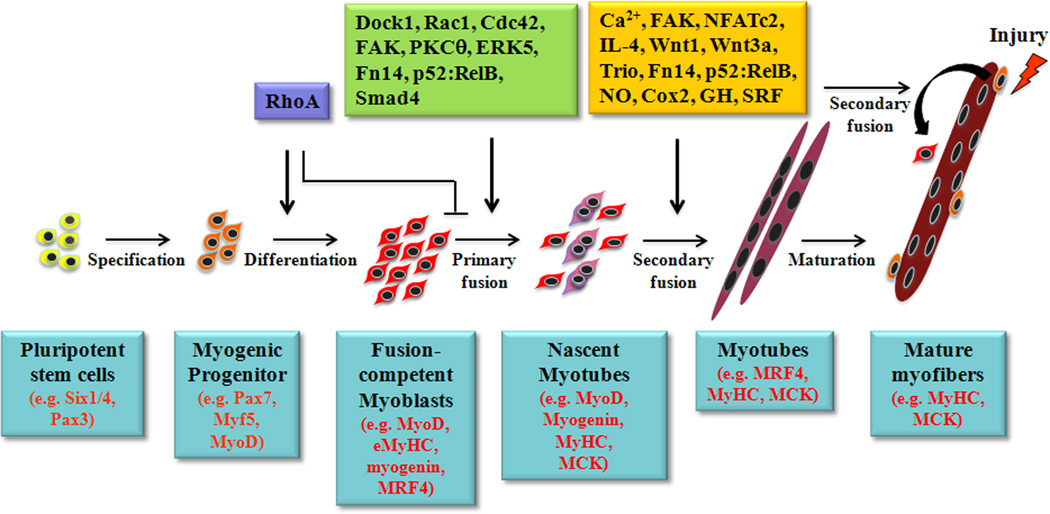
Fig. 1. The roles of different signaling molecules in primary and secondary myoblast fusion during myogenesis.
Muscle progenitor cells first undergo myogenic commitment and differentiation to become fusion-competent myoblasts. The initial commitment to differentiation requires the activity of the RhoA GTPase. Active RhoA interferes with myoblast fusion, and so it is deactivated before fusion occurs. A number of signaling molecules and pathways are activated in fusion-competent myoblasts that regulate primary myoblast fusion, which results in nascent myotubes. Additional signaling molecules are then recruited, which lead to fusion of additional mononucleated myoblasts with nascent myotubes. Secondary fusion also plays a critical role in the regeneration of injured myofibers. Major signaling molecules involved in primary and secondary myoblast fusion based on experimental evidence are depicted along the top. Specific myogenic markers expressed at different stages in cells of myogenic lineage during myogenesis are noted along the bottom.
Integrins and focal adhesion kinase
Integrins are hetrodimeric proteins consisting of α and β subunits that are found in the plasma membrane of mammalian cells. Integrins enable cells to adhere to the extracellular matrix and bind to various intracellular proteins, connecting the cell’s exterior and interior. Genetic studies have shown that the β1 integrin subunit in myogenic cells is an essential component for primary myoblast fusion and sarcomere assembly in mice (5). One of the molecules that mediate integrin signaling is the nonreceptor protein tyrosine kinase FAK (6). Binding of integrins to extracellular matrix ligands—such as fibronectin, vitronectin, vascular cell adhesion molecule 1 (VCAM1), collagen, and laminin—induces integrin clustering and the recruitment and autophosphorylation of FAK and its association with other signaling proteins, such as Src, Cas, and paxillin, which results in the activation of downstream signaling pathways (6).
FAK is involved in mammalian myoblast fusion both in vitro and in vivo (7). The phosphorylation of FAK is transiently increased during myogenic differentiation, and inhibition of this process blocks myoblast fusion without affecting the expression of differentiation-related genes, which suggests that FAK controls the morphological parameters of myogenesis (7). In addition, targeted deletion of FAK in satellite cells of adult mice attenuates myofiber regeneration in response to BaCl2-mediated injury. Although no difference was noticed in the numbers of regenerating myofibers in this study, the diameter of newly formed fibers was considerably smaller in mice with satellite cell–specific deletion of FAK 5 days after injury as compared with that in wild-type mice, potentially as a result of reduced myoblast fusion (7). Although FAK appears to mediate myoblast fusion in vitro and in response to muscle injury, it remains to be investigated whether FAK is also involved in fusion during embryogenesis.
Signaling through FAK affects the abundances of several molecules that are involved in the regulation of actin cytoskeletal, focal adhesion, Wnt, mitogen-activated protein kinase (MAPK), and insulin signaling pathways (7). Caveolin-3 and the β1D-integrin are some of the proteins that are involved in myoblast fusion whose amounts are increased as a result of FAK-mediated signaling. The β1D integrin subunit is upstream of FAK, which suggests that bidirectional signaling exists in the β1D-integrin–FAK pathway during myoblast fusion (7). The precise mechanisms that lead to the activation and recruitment of FAK to integrins remain enigmatic; however, this process may involve other second messengers. One such candidate is the protein kinase C (PKC) isoform PKCθ, which is highly abundant in cells of myogenic lineage. Forced activation of PKCθ enhances the phosphorylation of FAK, the amounts of caveolin-3 and the β1D integrin, as well as the extent of myoblast fusion (7, 8).
In addition to its interactions with integrins, FAK may also promote fusion through its interaction with other cell-surface receptors, such as neogenin (9). Neogenin (which is encoded by Neo1) and its ligands, the netrins, are present in dorsal somites and during the development of skeletal muscle of mice (10). Netrin2 causes the activation of FAK in cultured primary myoblasts in a neogenin-dependent manner (10). Although skeletal muscle develops normally, gene trap mutations in the Neo1 locus (Neo1Gt/Gt mice) result in the formation of thin myofibers. Moreover, phosphorylation of FAK is considerably reduced in the developing muscle of Neo1Gt/Gt mice compared with that in wild-type mice, which suggests that the netrin-neogenin dyad is a physiological stimulus of FAK during muscle development (10). Although these studies suggest that both integrin and neogenin signaling involves FAK, it remains to be determined whether FAK is activated independently in response to integrin and neogenin or whether the functional activation of FAK requires an interaction between these two receptors. The functions of FAK are not conserved between vertebrates and invertebrates. DFak56 is a Drosophila homolog of mammalian FAK. Unlike FAK-deficient mice, which are embryonically lethal (11), Drosophila lacking DFak56 are viable and fertile. Moreover, DFak56 is not required for integrin functions in cell adhesion, migration, or signaling in vivo (12).
Rho guanosine triphosphatases
The Rho family of guanosine triphosphatases (GTPases) plays crucial roles in various cellular processes, including cytoskeletal reorganization and the activation of downstream kinases. C2C12 is mouse myoblastic cell line that rapidly differentiates into myotubes under low-serum conditions (13, 14). These cells have been widely used to study myogenesis in vitro, although evidence suggests that they may not recapitulate all of the steps of myogenesis that occur in vivo during development or the differentiation of primary myoblasts in vitro. Studies of C2C12 myoblasts have identified RhoA, Rac1, and Cdc42 as important regulators of myogenesis (15–19). These GTPases function through modulating the activity of downstream kinases and transcription factors, such as p38 MAPK, c-Jun N-terminal kinases (JNKs), and serum response factor (SRF) (15, 16, 19). RhoA activity is rapidly and transiently increased in myoblasts upon their incubation in differentiation medium. Whereas RhoA is required for the initial induction of myogenesis (20), it must be deactivated before myoblast fusion. Active RhoA reduces the stability and perturbs the localization of M-cadherin, a cell adhesion molecule that has an essential role in myoblast fusion (21). RhoE (22) and a Rho GTPase-activating protein GRAF1 (GTPase regulator associated with focal adhesion kinase-1) suppress RhoA activity in differentiating myoblasts (23). The importance of GRAF1 in RhoA inhibition and myoblast fusion in vivo has been demonstrated in studies of Xenopus laevis. GRAF1-depleted embryos show increased RhoA activity and defective myofibrillogenesis that result in progressive muscle degeneration, defective motility, and embryonic lethality (23).
In contrast to the inhibition mediated by RhoA, other members of the Rho GTPase family—Rac1 and Cdc42—stimulate the fusion machinery both in vitro and in vivo (15). In Drosophilatwo closely related Rac GTPases, Rac1 and Rac2 (but not Cdc42), are activated in response to cellular adhesion and act redundantly to promote myoblast fusion (2, 24); however, the fusion of mammalian myoblasts requires both Rac1 and Cdc42 (25). Vasyutina et al. investigated the roles of Rac1 and Cdc42 in the fusion process in experiments with muscle precursor–specific conditional knockout mice (25). Analysis of muscle in mice at embryonic day 11.5 (E11.5) and E12.5 showed that deletion of either of these GTPases does not have any effect on the migration, proliferation, or differentiation of muscle progenitor cells. However, at later stages (for example, at E14.5 and E18.5), muscle development is impaired, as noted by the appearance of short- and thin-limb myofibers. Deletion of the genes encoding either Rac1 or Cdc42 substantially reduces the fusion index and the number of nuclei in developing muscle (25). Similarly, cultured primary myoblasts from Rac1- or Cdc42-deficient mice show a marked reduction in their fusion capacity. Moreover, the activities of both Rac1 and Cdc42 are required in both fusion partners (25). Although deficiency in Rac1 or Cdc42 does not affect myoblast adherence or the recruitment of α- and β-catenin to contact sites between fusion partners, the recruitment of vinculin, F-actin, and Vasp is substantially reduced at contact sites. Notably, the recruitment of Arp2/3, the actin polymerization-inducing complex, is reduced only in Rac1-deficient myoblasts, implying that Rac1 and Cdc42 may have nonredundant functions (25).
One of the first genes identified to regulate myoblast fusion in Drosophila was mbc (26, 27), which encodes a guanine nucleotide exchange factor (GEF) that acts upstream of the Rac GTPase in this process (28). Myoblast city (MBC) is the Drosophila ortholog of the mammalian protein Dock1. In mammals, Dock2 and Dock5 are additional members of the same subfamily of Dock1-related proteins (29). Although both Dock1 and Dock5 and their adaptor proteins Crk and Crk-like 1 (Crkl) are required for the fusion of fast-type myoblasts in Zebrafish (30), Dock1 is the primary GEF whose absence causes inhibition of skeletal muscle development because of a deficiency in primary myoblast fusion (31). Dock1−/− mice die within minutes after birth because of respiratory failure and have diaphragms that are strikingly thinner (and have impaired attachment to the intercostal muscle) than those of their wild-type counterparts.
A similar reduction in mass was noticeable in the deep back muscles, tongue, and limb muscles of Dock1−/− mice. This reduction in muscle mass correlates with a substantial reduction in fiber diameter at E18.5 in Dock1−/− mice. Further analysis of myogenesis defects in Dock1−/− mice showed that myosin heavy chain (MyHC)–containing fibers align with one another but remain mononucleated, which suggests that Dock1 plays an obligatory role in primary myoblast fusion (31). In contrast, the myotome develops normally in Dock5−/− and Dock1+/−Dock5+/− mice during embryogenesis. However, in Dock1−/−Dock5+/− mice, differentiated myoblasts remain mononucleated, similar to those in Dock1−/− mice, but show additional defects in MyHC organization, cell elongation, and alignment, indicating that Dock5 plays an important role in myofiber development at later stages (31). These findings also established that the role of Dock1 is conserved during myoblast fusion between vertebrates and invertebrates.
A previous study showed that Trio, a dual GEF for RhoA and Rac1, is essential for late embryonic development and that it plays a role in skeletal muscle formation (32). However, the myogenic defects in Trio−/− mice are different from those observed in Dock1−/− mice (31, 32). In addition, M-cadherin–dependent adhesion activates Rac1 through Trio during C2C12 myoblast fusion (33). These findings raise the possibility that although Dock1 is required for myoblast fusion during primary myogenesis, Trio may play a role during secondary myogenesis (Fig. 1). In contrast to its role in vertebrates, Trio appears to have no role in myoblast fusion during Drosophila development (24). Although more work is required to fully appreciate their roles, the available evidence suggests that different GEFs cause spatial and temporal regulation of Rac1 that promotes myoblast fusion during embryonic development and postnatal myogenesis through downstream regulation of actin cytoskeletal rearrangements at the sites of contact between myoblasts.
MAPKs
The signaling pathways of four MAPKs, extracellular signal–regulated kinase 1/2 (ERK1/2), JNK, p38, and ERK5 are among the most well-characterized cell-signaling pathways that are activated in response to various extracellular stimuli and regulate pluripotent cellular function (34). Although the stimulatory role of p38 MAPK in myogenesis has been established (35–37), both positive and negative roles for ERK1/2 and JNK have been suggested (15, 38–40). Further experimental evidence regarding the roles of ERK1/2, JNK, and p38 MAPK in myoblast fusion is still lacking. An early study demonstrated that the activity of ERK5 is increased within minutes upon exposure of C2C12 myoblasts to low-serum conditions and that it is sustained during terminal differentiation (41). Stimulation of ERK5 increases the activity of E-box–containing promoters, including those of Cdkn1a and Myl1 (41). Furthermore, ERK5 phosphorylates the proteins myoblast determination protein 1 (MyoD) and myocyte enhancer factor 2C (Mef2C) in vitro, and it synergistically increases the transactivation potential of MyoD (41). Blocking ERK5 through the expression of antisense mRNA abolishes myoblast fusion and the formation of multinucleated myotubes and moderately reduces the expression of differentiation-related genes, such as those that encode MyoD, myogenin, and p21 (41). Sunadome et al. evaluated the role of ERK1/2 and ERK5 in myoblast fusion and myogenic differentiation in experiments with both pharmacological and molecular approaches (42). Specific inhibition of ERK1/2 does not affect either the fusion or differentiation of C2C12 myoblasts (42). In contrast, ERK5 is critical for myoblast fusion, but not for the progression of myogenic differentiation (42). Overexpression of a dominant-negative mutant of ERK5 or short interfering RNA (siRNA)–mediated knockdown of ERK5 substantially reduces the extent of myoblast fusion, with resultant mononucleated myoblasts, which suggests that ERK5 is essential for primary myoblast fusion (42). However, in contrast to a previous study by Dinev et al. (31), Sunadome et al. reported that the inhibition of ERK5 has no effect on the expression of muscle-specific genes, such as those encoding MyoD, Mef2a, p21, MyHC, and muscle creatine kinase, although a slight reduction in the expression of genes encoding myogenin and Mef2c is observed at later stages of differentiation (32). These observed differences regarding the expression of muscle-specific genes in two studies could be attributed to the approaches that were used to inhibit ERK5 (41, 42). For example, it is possible that the overexpression of ERK5-specific antisense mRNA used in the first study (31) also led to its binding to other mRNAs that have homology to ERK5, resulting in more pronounced effects on the expression of muscle-specific genes than are seen with ERK5-specific siRNA.
The mechanisms by which ERK5 regulates myoblast fusion have also been studied. ERK5 promotes myoblast fusion through downstream activation of the transcription factor specificity protein 1 (SP1), which in turn binds to the promoters of genes encoding the transcription factors Kruppel-like factor (Klf)2 and Klf4, leading to their increased expression (42). The amounts of both Klf2 and Klf4 are increased in C2C12 myoblasts after serum withdrawal in an ERK5-dependent manner, and the inhibition or overexpression of Klf2 and Klf4 affects myoblast fusion in a similar fashion as observed with inhibition or overexpression of ERK5 (42). It is noteworthy that the increased activities of ERK5 or Klf2 and Klf4 alone are not sufficient to induce myoblast fusion; they promote myoblast fusion under conditions in which myogenic transcription factors, such as MyoD and Mef2C, are active and the myoblasts are undergoing differentiation (42). Nephronectin (which is encoded by Npnt), an extracellular matrix protein involved in cell-matrix adhesion, associates with the β1 integrin subunit in embryonic kidney (43). The abundance of nephronectin is increased during myogenic differentiation and stimulates primary myoblast fusion (42). The promoter region of Npnt contains multiple consensus Klf2- and Klf4-binding sites, and these transcription factors induce the expression of Npnt during myoblast fusion (42). Evidently, the MEK5-ERK5-SP1-Klf2/4-Npnt pathway represents one of the best characterized signaling pathways that promote mammalian myoblast fusion (Fig. 2). Future studies should focus on finding the stimuli and the proximal signaling events that lead to ERK5 activation during myogenesis and determining whether this pathway is also involved in myoblast fusion during embryonic development and the regeneration of injured myofibers.
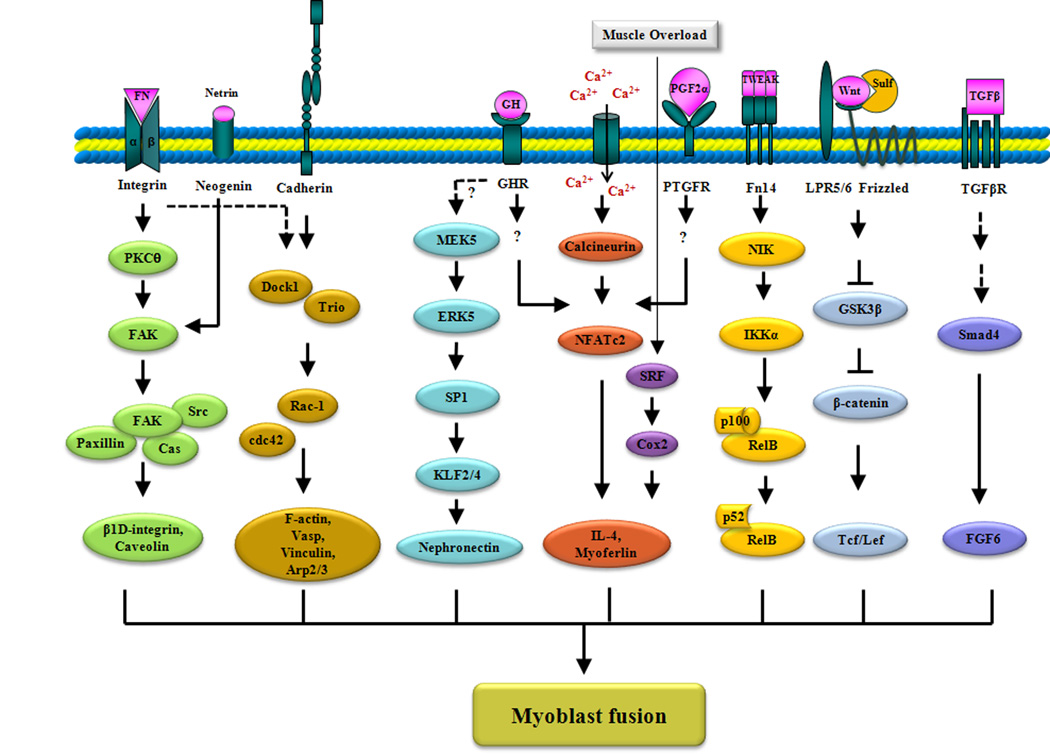
Fig. 2. The signaling mechanisms that stimulate myoblast fusion.
Emphasis is placed on ligands and receptors that activate pathways, the main effectors of each pathway, and their target molecules. The interactions of integrins with their ligands, such as fibronectin (FN), causes the autophosphorylation of FAK by PKC-θ, which leads to the activation of downstream signaling that results in the increased expression of the genes encoding caveolin-3 and the β1D integrin. Neogenin-netrin signaling also causes FAK activation. Ligation of M-cadherin activates Rac1 in a Trio- and Dock1-dependent manner. Cdc42 and Rac1 are major activators of vinculin, F-actin, Vasp, and the Arp2/3 complex for the cytoskeletal remodeling that occurs before myoblast fusion. The MEK5-dependent activation of ERK5 promotes binding of the transcription factor SP1 to the promoter of the genes encoding the transcription factors Klf2 and Klf4, leading to their increased abundance. Subsequently, Klf2 and Klf4 bind to the Npnt promoter and induce the production of nephronectin during myoblast fusion. Increases in intracellular calcium concentration lead to activation of the serine-threonine protein phosphatase calcineurin, which dephosphorylates and activates the transcription factor NFATc2. Activated NFATc2 stimulates myoblast fusion through the increased production of IL-4 and myoferlin. Possible stimuli for NFATc2 activation are PGF2α and GH. In response to mechanical overload, the transcription factor SRF becomes activated, which leads to the increased abundance of IL-4 though COX2-dependent mechanisms. Low amounts of the cytokine TWEAK activate the noncanonical NF-κB pathway through activation of NIK and IKKα. IKKα-mediated phosphorylation of p100 results in its proteolytic processing to generate the p52 subunit and the subsequent nuclear accumulation of p52-RelB heterodimers. Binding of Wnt to Frizzled and LRP5/6 disrupts the binding of GSK-3β to β-catenin, thus preventing the phosphorylation and degradation of β-catenin. β-catenin then translocates to the nucleus and acts as a transcriptional coactivator for Tcf/Lef target genes. Recruitment of TGF-β to its cell-surface receptor leads to the activation of Smad4, which translocates to the nucleus and recruits other transcription factors to collectively induce the expression of target genes, such as Fgf6. The dashed arrows indicate that direct interactions have not yet been shown.
Calcineurin-NFATc2
The fusion of myoblasts into multinucleated myotubes is regulated by calcium-dependent signaling (44–46). Among the downstream targets of calcium-induced signaling are the NFAT family of transcription factors (NFATc1 to -c4), which regulate gene transcription to coordinate proliferation, survival, and differentiation in a wide range of cell types (47, 48). Increases in the intracellular calcium concentration causes the activation of the serine and threonine phosphatase calcineurin, which dephosphorylates NFATc, enabling the nuclear translocation of NFATc and the regulation of gene transcription (48). Among the four isoforms, NFATc2 stimulates secondary myoblast fusion (49). NFATc2 translocates to the nucleus after primary fusion and is essential for subsequent myonuclear accretion. Although cultured primary myoblasts of Nfatc2−/− mice have no noticeable defect in their expression of differentiation markers, their myotubes are smaller in size and contain fewer myonuclei. Fusion defects in Nfatc2−/− myoblasts are rescued through the overexpression of NFATc2, which suggests that it plays a critical role in myoblast fusion (49). Consistent with the role of NFATc2 in secondary fusion, skeletal muscle mass and myofiber diameter are reduced in Nfatc2−/− mice compared with those in wild-type mice, without any change in number of myofibers. One of the mechanisms by which NFATc2 controls myoblast fusion is through regulating the production of the cytokine interleukin-4 (IL-4) (50). Whereas the IL-4 receptor (IL-4R) is present at all the stages of differentiation in myoblast cultures, IL-4 is produced only after primary fusion and in an NFATc2-dependent manner (50). The role of IL-4 in myoblast fusion is also evident by findings that myofiber diameter is smaller in mice deficient in either Il4 or Il4r than in wild-type mice; these mice show retardation in muscle growth after freeze injury, and primary myoblasts from Il4−/− or Il4r−/− mice have defects in secondary fusion similar to those observed in Nfatc2−/− myoblasts (50). The mechanisms by which IL-4 induces myoblast fusion remain poorly understood; however, based on its role in other cell types, such as macrophages, it is likely that IL-4 induces myoblast fusion by increasing the production of cell-adhesion molecules and by inducing chemotaxis (50). Another molecule regulated through NFATc2-dependent mechanisms is myoferlin (which is encoded by Myof), a membrane protein essential for myoblast fusion (51). The Myof promoter contains multiple NFAT-binding sites, and the production of myoferlin is regulated in a manner similar to that of NFATc2 in healthy and damaged myofibers (51).
In addition to increased amounts of calcium, several other stimuli may activate NFATc2 in fusing myoblasts. One such factor is prostaglandin F2α (PGF2α), which augments muscle growth (52). Whereas PGF2α induces myotube formation in IL-4–deficient cells, it does not increase the production of IL-4 by wild-type myoblasts, suggesting that NFATc2 might induce fusion through mechanisms independent of IL-4 (53). Furthermore, growth hormone (GH) also augments the activity of NFATc2 in cultured myoblasts (54). GH does not induce hypertrophy in NFATc2−/− myotubes, signifying the role of NFATc2 in GH-mediated signaling in myogenic cells (55). GH also induces the production of IL-4, which suggests that GH may be the stimulus for the activation of the NFATc2–IL-4 pathway during myoblast fusion (54).
Secondary myoblast fusion also plays an important role in muscle growth and hypertrophy in response to resistance exercise and mechanical loading. Guerci et al. investigated the mechanisms of overload-induced hypertrophy and discovered a role for the transcription factor SRF (56). Targeted deletion of Srf (which encodes SRF) in adult myofibers (but not in satellite cells) inhibits overload-induced hypertrophy and impairs the proliferation of satellite cells and of their fusion with preexisting myofibers. SRF is not required for the activation of insulin growth factor-like 1 (IGF1)–Akt signaling, which mediates overload-induced hypertrophy. In contrast, SRF promotes the proliferation and the fusion of myoblasts in a paracrine fashion. Specifically, activation of SRF in myofibers in response to overload leads to the increased production of IL-6 and IL-4, which enhance the proliferation of satellite cells and of their fusion to existing myofibers, respectively. SRF does not regulate Il4 expression directly; it does so through enhancing the expression of the gene encoding cyclooxygenase-2 (Cox2). This study also provided the initial evidence that satellite cell fusion is a major limiting cellular event in overload-induced muscle hypertrophy (56).
NF-κB
The NF-κB family contains five members: RelA (also known as p65), RelB, c-Rel, p105/p50, and p100/p52, which make homo- and heterodimers (57, 58). Depending on the type of stimulus, the activation of NF-κB occurs through canonical or noncanonical signaling pathways. Canonical NF-κB signaling involves the activation of inhibitor of κB (IκB) kinase-β (IKKβ) and the subsequent phosphorylation and degradation of the IκB protein. In contrast, activation of the noncanonical NF-κB pathway requires the activation of NF-κB–inducing kinase (NIK) and IKKα, leading to the phosphorylation and proteolytic processing of the p100 subunit to generate p52 (57). Cellular inhibitor of apoptosis 1 (cIAP1) and cIAP2 are some of the important upstream regulators of both the canonical and noncanonical arms of NF-κB signaling. The cIAPs are also E3 ubiquitin ligases that mediate both Lys48 (K48)– and (K63)–linked ubiquitination. In response to activating stimuli, the adaptor protein tumor necrosis factor (TNF) receptor–associated factor 2 (TRAF2) recruits cIAPs to a receptor-associated signaling complex, which causes K63-linked ubiquitination of receptor-interacting kinase (RIP), resulting in downstream activation of IKKβ. The cIAPs also inhibit the noncanonical branch of the NF-κB pathway by facilitating K48-linked ubiquitination of NIK, targeting it for proteasomal degradation (59). Under basal conditions, cIAP, NIK, TRAF2, and TRAF3 form a complex that leads to the constitutive degradation of NIK through a proteasome-dependent pathway. In response to activating stimuli, the TRAF2-TRAF3-cIAP complex is recruited to the receptor where TRAF2-mediated, K63-linked ubiquitination of cIAP1 and cIAP2 switches its K48 ubiquitin ligase activity from NIK to TRAF3. The degradation of TRAF3 destabilizes the TRAF-cIAP complex, thus enabling the accumulation of newly synthesized NIK and the downstream activation of IKKα and subsequent processing of p100 to generate p52 (60–62).
Several independent studies have shown that canonical NF-κB signaling inhibits myogenic differentiation (58, 63); however, the role of noncanonical NF-κB signaling in muscle formation has received less attention. Enwere and colleagues (64) reported that noncanonical NF-κB signaling promotes myoblast fusion. Cultured myoblasts from mice deficient in cIAP1 (which is encoded by Birc2) show an increased fusion capacity compared with that of wild-type mice when incubated in low-serum conditions. Although deletion of Birc2 enhances myoblast fusion and activates both the canonical and noncanonical pathways, it inhibits cell cycle withdrawal and the expression of various differentiation markers in myoblast cultures (64). The increased fusion of myoblasts is attributed to derepression of noncanonical NF-κB signaling, which is also supported by the findings that siRNA-mediated depletion of p100, IKKα, or RelB—all of which are components of the noncanonical pathway—diminishes nuclear accretion and hypertrophy in cultures of cIAP1–/– cells (64). Conversely, overexpression of p52 and RelB augments primary myoblast fusion in low-serum conditions. Together, these findings suggest that the loss of cIAP1 causes the activation of both the canonical and noncanonical NF-κB pathways; the former inhibits myogenic differentiation, whereas the later promotes myoblast fusion.
Bakkar et al. (63) previously investigated the role of noncanonical NF-κB signaling in myogenesis. They reported that the activity of the canonical pathway is reduced during C2C12 myoblast differentiation, with a gradual increase in noncanonical NF-κB signaling through upstream activation of IKKα. In experiments with IKKα−/− and RelB−/− mouse embryonic fibroblasts (MEFs), the authors found that MyoD-induced myogenic differentiation was somewhat reduced in these cells compared with that in wild-type MEFs. However, overexpression of IKKα does not enhance myogenic differentiation in C2C12 myoblasts or in MyoD-expressing fibroblasts. These somewhat contradictory findings in the two studies about the role of the NF-κB noncanonical pathway in myoblast fusion and myogenesis can be explained to some extent by the different experimental conditions used. Studies performed by Bakkar et al. (63) used cell lines for some of the experiments that may behave differently from the primary myoblasts that were used in the studies performed by Enwere et al. (64). Indeed, primary myoblasts have considerably higher fusion capacity than do C2C12 cells, and a small and transient increase in the activity of noncanonical NF-κB signaling may be sufficient to promote myoblast fusion. This is also evident by the findings that pharmacological inhibition of cIAP1 does not augment fusion in C2C12 cultures; instead, it attenuates fusion, further demonstrating differences in the fusion mechanisms between primary myoblasts and C2C12 myoblasts (64). Moreover, Bakkar et al. did not specifically evaluate fusion in IKKα-overexpressing C2C12 myoblasts or in primary myoblasts from IKKα−/− mice (63). Indeed, no noticeable muscle phenotype is evident between wild-type and IKKα−/− mice at the perinatal stage (63), indicating that the role of noncanonical NF-κB signaling may be compensated by other factors during embryonic development of skeletal muscle in vivo. Nevertheless, specific roles of IKKα should be further examined by studying the muscles of IKKα−/− mice during embryonic development as well as the fusion of cultured primary myoblasts from these mice. However, it is now evident that noncanonical NF-κB signaling improves mitochondrial biogenesis in differentiated myotubes through the induction of peroxisome proliferator-activated receptor-γ coactivator 1β (65).
The role of noncanonical NF-κB pathway in myoblast fusion has also been evaluated in a study of the cytokine TNF-like weak inducer of apoptosis (TWEAK). TWEAK activates both canonical and noncanonical NF-κB signaling in different cell types, including myogenic cells (66). Enwere et al. reported that low concentrations of TWEAK predominately activate noncanonical NF-κB signaling and augment the fusion of cultured primary myoblasts (64). These findings are consistent with published reports that the TWEAK receptor Fn14 is essential for myotube formation in cultures and for the regeneration of adult skeletal muscle in response to injury (67, 68). Previously, the effects of TWEAK in myogenesis were studied with the C2C12 cell line, which showed that large amounts of TWEAK activate the canonical NF-κB pathway and inhibit myogenic differentiation (69). Although the activation of the noncanonical NF-κB pathway was not specifically studied in undifferentiated C2C12 myoblasts, it has also been reported that large (but not small) amounts of TWEAK activate noncanonical NF-κB signaling in differentiated C2C12 myotube cultures (66, 70). These findings suggest that the C2C12 cell line and mouse primary myoblasts respond differentially to the same activating stimulus with respect to activation of the canonical and noncanonical branches of NF-κB signaling.
Whereas low doses of TWEAK may enhance myoblast fusion, it is noteworthy that the continued presence of TWEAK can lead to deleterious effects on nascent myotubes. A low concentration of TWEAK not only causes atrophy, its continuous presence can also reduce the survival of differentiated myotubes (70). Moreover, muscle regeneration after cardiotoxin-mediated injury is considerably reduced in TWEAK-transgenic mice, which is potentially a result of the chronic presence of increased concentrations of TWEAK in these mice (71). Another study showed that sporadic inclusion-body myositis (IBM) mesoangioblasts, which are defective in myogenic differentiation, produce increased amounts of TWEAK in differentiation medium, and that neutralization of TWEAK improves differentiation in IBM mesoangioblast cultures (72). Moreover, the membrane-bound form of TWEAK is more potent than its soluble variant with respect to activation of the canonical NF-κB pathway (73), which further explains why transgenic overexpression or increased production of membrane-bound TWEAK in disease conditions produces deleterious effects on muscle regeneration. Despite this recent progress, the mechanisms by which noncanonical NF-κB signaling drives myoblast fusion, and its roles in vivo remain unknown. More studies are required to further understand the role of noncanonical NF-κB signaling in skeletal muscle development and the regeneration of myofibers in response to injury. Nevertheless, the available literature provides initial evidence that the activation of noncanonical NF-κB signaling improves muscle health by augmenting myoblast fusion and improving muscle oxidative metabolism.
Wnt signaling
Wnt proteins are secreted ligands that transmit their signals across the plasma membrane by interacting with Frizzled receptors and their coreceptors low-density lipoprotein receptor-related protein 5 (LRP5) and LRP6 (74). Upon binding to their receptors, Wnt proteins induce a cascade of intracellular signaling events, involving proteins such as Disheveled, axin, adenomatosis polyposis coli (APC), and glycogen synthase kinase-3β (GSK-3β), which culminate in the stabilization of β-catenin and its translocation to the nucleus, where it binds to T-cell factor/lymphoid enhancer-binding factor (Tcf/Lef) to induce the transcription of Wnt target genes (74). In addition to this canonical pathway, certain Wnt proteins exert their effects by activation of the planar cell polarity (PCP) pathway (also known as the noncanonical Wnt pathway) and the calcium/calmodulin-dependent kinase pathway (74, 75).
Wnt signaling is essential both for embryonic muscle development and postnatal myogenesis (75). GSK-3β is a critical component of the classical Wnt signaling pathway because its inactivation leads to the stabilization and nuclear translocation of β-catenin (75). Initial evidence about the role of Wnt signaling in myoblast fusion came from the study by Rochat and colleagues (76) in which they found that the inhibition of GSK-3β with LiCl or coculturing with Wnt1-expressing 3T3 fibroblasts enhances the nuclear accumulation of β-catenin and the insulin-induced differentiation of quiescent C2C12 myoblasts (76). The addition of LiCl to the medium 36 to 48 hours after the induction of differentiation results in a marked increase in myotube diameter as well as in the number of nuclei in each myotube (76). Similarly, treatment with Wnt1 alone is sufficient to stimulate the fusion of myogenic cells (76). It was also reported that treatment with another Wnt ligand, Wnt3a, enhances C2C12 myoblast fusion (77), further suggesting that classical Wnt signaling promotes the fusion of cultured myoblasts.
The roles of canonical and noncanonical Wnt signaling in myoblast fusion in vivo have also been elucidated (78, 79). Canonical Wnt signaling is induced in muscle progenitor cells within 2 to 5 days of muscle injury (79). Injection of Wnt3a protein to injured muscle increases the size of regenerating myofibers without changing myofiber number, potentially by augmenting myoblast fusion (79). In addition, the Sulfs (6-O–endosulfatases) activate canonical but inhibit noncanonical Wnt signaling in injured myofibers (78). Sulf1 and Sulf2 selectively remove the 6-O-sulfate group from heparin sulfate (HS), which enables HS proteoglycan (HSPG)–mediated signaling. Sulf-mediated HS 6-O–desulfation represses fibroblast growth factor (FGF) signaling by disrupting the HS-FGF2-FGF receptor (FGFR) complex in satellite cells, which enables their differentiation and hence the formation of new myofibers (80). Muscle-specific deletion of both Sulf1 and Sulf2 considerably delays myofiber formation after injury because of defects in myoblast fusion (78). Similarly, cultured myoblasts from muscle-specific Sulf1−/−Sulf2−/−mice show reduced fusion after removal of FGF2 from the culture medium. This study also provided initial evidence that noncanonical Wnt signaling is activated in response to muscle injury and that it inhibits myoblast fusion (78). Notably, Sulfs repress noncanonical Wnt signaling during muscle regeneration by enhancing antagonistic canonical Wnt signaling (78). Because Wnt3a, but not Wnt7a, contains HS-binding Weintraub sequences, Sulfs might function by decreasing the extent of binding of Wnt3a to HS, increasing the availability of Wnt3a to activate canonical Wnt signaling. In addition, Sulfs stimulate the activation and membrane localization of FAK during myoblast fusion. In contrast, Wnt7a-mediated noncanonical Wnt signaling causes aberrant subcellular distribution of FAK (78). Together, these data provide new insights regarding the role of Sulfs and canonical and noncanonical Wnt signaling in myoblast fusion.
Other signaling pathways
Myoblast fusion also involves the activation of a number of other signaling molecules. For example, canonical transforming growth factor-β (TGF-β) signaling, which functions through the activation of Smad4 protein, inhibits both the proliferation and fusion of C2C12 myoblasts (81). Interestingly, Han et al. demonstrated that targeted ablation of Smad4 inhibits the terminal differentiation and fusion of myogenic cells during tongue development by inhibiting the expression of Fgf6 (82). Treatment with exogenous FGF6 protein partially rescues myoblast fusion defects in Smad4-deficient primary myoblasts (82). The opposing functions of TGF-β and Smad4 signaling in myoblast fusion observed in these two studies suggest that other members of the TGF-β superfamily are also involved and that they might function through the regulation of differential downstream target genes to control myoblast fusion. This could also be attributed to differences in the fusion mechanisms between primary myoblasts and C2C12 myoblasts. Nitric oxide (NO) is produced by the enzyme nitric oxide synthase in various cell types, including vascular endothelial cells, macrophages, fibroblasts, neurons, and injured myofibers. NO is one of the crucial messengers that promote myoblast fusion (83). Dahlman et al. have further shown that during development, the abundance of NOS within stromal fibroblasts is increased through NF-κB–dependent mechanisms to stimulate myoblast fusion and muscle hypertrophy (84).
Concluding remarks
Our understanding of the process of myoblast fusion has taken a quantum jump in recent years. It is increasingly clear that the fusion process is a highly complex and elaborate network involving the coordination and crosstalk of many signaling pathways and that it involves both extracellular and intracellular events. These signaling molecules and transcription factors act in synergy or antagonism in feed-back and feed-forward loops. Future investigations should focus on identifying the extracellular stimuli that lead to the activation of signaling pathways that stimulate myoblast fusion and whether the activation of some of these involved pathways is perturbed in various muscle-degenerative disorders, such as muscular dystrophy. Furthermore, fusion-related target genes regulated through the activation of these signaling pathways need to be identified. Recent technological advances are paving the way for further investigation of the signaling networks that control myoblast fusion on a proteome- and genome-wide scale.
Acknowledgments
We sincerely thank S. Kuang of Purdue University for critical reading and providing suggestions for this manuscript. We apologize to those authors whose work could not be cited because of space limitations or our oversight.
Funding: This work was supported by NIH grants R01AR059810 and RO1AG029623 to A.K.
Footnotes
Competing interests: The authors declare that they have no competing interests.
References
- 1.Abmayr SM, Pavlath GK. Myoblast fusion: Lessons from flies and mice. Development. 2012;139:641–656. doi: 10.1242/dev.068353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rochlin K, Yu S, Roy S, Baylies MK. Myoblast fusion: When it takes more to make one. Dev. Biol. 2010;341:66–83. doi: 10.1016/j.ydbio.2009.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pavlath GK. Spatial and functional restriction of regulatory molecules during mammalian myoblast fusion. Exp. Cell Res. 2010;316:3067–3072. doi: 10.1016/j.yexcr.2010.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krauss RS. Regulation of promyogenic signal transduction by cell-cell contact and adhesion. Exp. Cell Res. 2010;316:3042–3049. doi: 10.1016/j.yexcr.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwander M, Leu M, Stumm M, Dorchies OM, Ruegg UT, Schittny J, Müller U. Beta1 integrins regulate myoblast fusion and sarcomere assembly. Dev. Cell. 2003;4:673–685. doi: 10.1016/s1534-5807(03)00118-7. [DOI] [PubMed] [Google Scholar]
- 6.Schaller MD. Cellular functions of FAK kinases: Insight into molecular mechanisms and novel functions. J. Cell Sci. 2010;123:1007–1013. doi: 10.1242/jcs.045112. [DOI] [PubMed] [Google Scholar]
- 7.Quach NL, Biressi S, Reichardt LF, Keller C, Rando TA. Focal adhesion kinase signaling regulates the expression of caveolin 3 and beta1 integrin, genes essential for normal myoblast fusion. Mol. Biol. Cell. 2009;20:3422–3435. doi: 10.1091/mbc.E09-02-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Madaro L, Marrocco V, Fiore P, Aulino P, Smeriglio P, Adamo S, Molinaro M, Bouché M. PKCθ signaling is required for myoblast fusion by regulating the expression of caveolin-3 and β1D integrin upstream focal adhesion kinase. Mol. Biol. Cell. 2011;22:1409–1419. doi: 10.1091/mbc.E10-10-0821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Vries M, Cooper HM. Emerging roles for neogenin and its ligands in CNS development. J. Neurochem. 2008;106:1483–1492. doi: 10.1111/j.1471-4159.2008.05485.x. [DOI] [PubMed] [Google Scholar]
- 10.Bae GU, Yang YJ, Jiang G, Hong M, Lee HJ, Tessier-Lavigne M, Kang JS, Krauss RS. Neogenin regulates skeletal myofiber size and focal adhesion kinase and extracellular signal-regulated kinase activities in vivo and in vitro. Mol. Biol. Cell. 2009;20:4920–4931. doi: 10.1091/mbc.E09-06-0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ilić D, Furuta Y, Kanazawa S, Takeda N, Sobue K, Nakatsuji N, Nomura S, Fujimoto J, Okada M, Yamamoto T. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature. 1995;377:539–544. doi: 10.1038/377539a0. [DOI] [PubMed] [Google Scholar]
- 12.Grabbe C, Zervas CG, Hunter T, Brown NH, Palmer RH. Focal adhesion kinase is not required for integrin function or viability in Drosophila. Development. 2004;131:5795–5805. doi: 10.1242/dev.01462. [DOI] [PubMed] [Google Scholar]
- 13.Yaffe D, Saxel O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature. 1977;270:725–727. doi: 10.1038/270725a0. [DOI] [PubMed] [Google Scholar]
- 14.Blau HM, Chiu CP, Webster C. Cytoplasmic activation of human nuclear genes in stable heterocaryons. Cell. 1983;32:1171–1180. doi: 10.1016/0092-8674(83)90300-8. [DOI] [PubMed] [Google Scholar]
- 15.Meriane M, Roux P, Primig M, Fort P, Gauthier-Rouvière C. Critical activities of Rac1 and Cdc42Hs in skeletal myogenesis: Antagonistic effects of JNK and p38 pathways. Mol. Biol. Cell. 2000;11:2513–2528. doi: 10.1091/mbc.11.8.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Charrasse S, Meriane M, Comunale F, Blangy A, Gauthier-Rouvière C. N-cadherin-dependent cell-cell contact regulates Rho GTPases and beta-catenin localization in mouse C2C12 myoblasts. J. Cell Biol. 2002;158:953–965. doi: 10.1083/jcb.200202034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iwasaki K, Hayashi K, Fujioka T, Sobue K. Rho/Rho-associated kinase signal regulates myogenic differentiation via myocardin-related transcription factor-A/Smad-dependent transcription of the Id3 gene. J. Biol. Chem. 2008;283:21230–21241. doi: 10.1074/jbc.M710525200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang JS, Bae GU, Yi MJ, Yang YJ, Oh JE, Takaesu G, Zhou YT, Low BC, Krauss RS. A Cdo-Bnip-2-Cdc42 signaling pathway regulates p38alpha/beta MAPK activity and myogenic differentiation. J. Cell Biol. 2008;182:497–507. doi: 10.1083/jcb.200801119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bryan BA, Li D, Wu X, Liu M. The Rho family of small GTPases: crucial regulators of skeletal myogenesis. Cell. Mol. Life Sci. 2005;62:1547–1555. doi: 10.1007/s00018-005-5029-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wei L, Zhou W, Croissant JD, Johansen FE, Prywes R, Balasubramanyam A, Schwartz RJ. RhoA signaling via serum response factor plays an obligatory role in myogenic differentiation. J. Biol. Chem. 1998;273:30287–30294. doi: 10.1074/jbc.273.46.30287. [DOI] [PubMed] [Google Scholar]
- 21.Charrasse S, Comunale F, Grumbach Y, Poulat F, Blangy A, Gauthier-Rouvière C. RhoA GTPase regulates M-cadherin activity and myoblast fusion. Mol. Biol. Cell. 2006;17:749–759. doi: 10.1091/mbc.E05-04-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fortier M, Comunale F, Kucharczak J, Blangy A, Charrasse S, Gauthier-Rouvière C. RhoE controls myoblast alignment prior fusion through RhoA and ROCK. Cell Death Differ. 2008;15:1221–1231. doi: 10.1038/cdd.2008.34. [DOI] [PubMed] [Google Scholar]
- 23.Doherty JT, Lenhart KC, Cameron MV, Mack CP, Conlon FL, Taylor JM. Skeletal muscle differentiation and fusion are regulated by the BAR-containing Rho-GTPase-activating protein (Rho-GAP), GRAF1. J. Biol. Chem. 2011;286:25903–25921. doi: 10.1074/jbc.M111.243030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hakeda-Suzuki S, Ng J, Tzu J, Dietzl G, Sun Y, Harms M, Nardine T, Luo L, Dickson BJ. Rac function and regulation during Drosophila development. Nature. 2002;416:438–442. doi: 10.1038/416438a. [DOI] [PubMed] [Google Scholar]
- 25.Vasyutina E, Martarelli B, Brakebusch C, Wende H, Birchmeier C. The small G-proteins Rac1 and Cdc42 are essential for myoblast fusion in the mouse. Proc. Natl. Acad. Sci. U.S.A. 2009;106:8935–8940. doi: 10.1073/pnas.0902501106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rushton E, Drysdale R, Abmayr SM, Michelson AM, Bate M. Mutations in a novel gene, myoblast city, provide evidence in support of the founder cell hypothesis for Drosophila muscle development. Development. 1995;121:1979–1988. doi: 10.1242/dev.121.7.1979. [DOI] [PubMed] [Google Scholar]
- 27.Erickson MR, Galletta BJ, Abmayr SM. Drosophila myoblast city encodes a conserved protein that is essential for myoblast fusion, dorsal closure, and cytoskeletal organization. J. Cell Biol. 1997;138:589–603. doi: 10.1083/jcb.138.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nolan KM, Barrett K, Lu Y, Hu KQ, Vincent S, Settleman J. Myoblast city, the Drosophila homolog of DOCK180/CED-5, is required in a Rac signaling pathway utilized for multiple developmental processes. Genes Dev. 1998;12:3337–3342. doi: 10.1101/gad.12.21.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Côté JF, Vuori K. Identification of an evolutionarily conserved superfamily of DOCK180-related proteins with guanine nucleotide exchange activity. J. Cell Sci. 2002;115:4901–4913. doi: 10.1242/jcs.00219. [DOI] [PubMed] [Google Scholar]
- 30.Moore CA, Parkin CA, Bidet Y, Ingham PW. A role for the Myoblast city homologues Dock1 and Dock5 and the adaptor proteins Crk and Crk-like in zebrafish myoblast fusion. Development. 2007;134:3145–3153. doi: 10.1242/dev.001214. [DOI] [PubMed] [Google Scholar]
- 31.Laurin M, Fradet N, Blangy A, Hall A, Vuori K, Côté JF. The atypical Rac activator Dock180 (Dock1) regulates myoblast fusion in vivo. Proc. Natl. Acad. Sci. U.S.A. 2008;105:15446–15451. doi: 10.1073/pnas.0805546105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Brien SP, Seipel K, Medley QG, Bronson R, Segal R, Streuli M. Skeletal muscle deformity and neuronal disorder in Trio exchange factor-deficient mouse embryos. Proc. Natl. Acad. Sci. U.S.A. 2000;97:12074–12078. doi: 10.1073/pnas.97.22.12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Charrasse S, Comunale F, Fortier M, Portales-Casamar E, Debant A, Gauthier-Rouvière C. M-cadherin activates Rac1 GTPase through the Rho-GEF trio during myoblast fusion. Mol. Biol. Cell. 2007;18:1734–1743. doi: 10.1091/mbc.E06-08-0766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- 35.Wu Z, Woodring PJ, Bhakta KS, Tamura K, Wen F, Feramisco JR, Karin M, Wang JY, Puri PL. p38 and extracellular signal-regulated kinases regulate the myogenic program at multiple steps. Mol. Cell. Biol. 2000;20:3951–3964. doi: 10.1128/mcb.20.11.3951-3964.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perdiguero E, Ruiz-Bonilla V, Gresh L, Hui L, Ballestar E, Sousa-Victor P, Baeza-Raja B, Jardí M, Bosch-Comas A, Esteller M, Caelles C, Serrano AL, Wagner EF, Muñoz-Cánoves P. Genetic analysis of p38 MAP kinases in myogenesis: Fundamental role of p38alpha in abrogating myoblast proliferation. EMBO J. 2007;26:1245–1256. doi: 10.1038/sj.emboj.7601587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhatnagar S, Kumar A, Makonchuk DY, Li H, Kumar A. Transforming growth factor-beta-activated kinase 1 is an essential regulator of myogenic differentiation. J. Biol. Chem. 2010;285:6401–6411. doi: 10.1074/jbc.M109.064063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jones NC, Fedorov YV, Rosenthal RS, Olwin BB. ERK1/2 is required for myoblast proliferation but is dispensable for muscle gene expression and cell fusion. J. Cell. Physiol. 2001;186:104–115. doi: 10.1002/1097-4652(200101)186:1<104::AID-JCP1015>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 39.Tortorella LL, Milasincic DJ, Pilch PF. Critical proliferation-independent window for basic fibroblast growth factor repression of myogenesis via the p42/p44 MAPK signaling pathway. J. Biol. Chem. 2001;276:13709–13717. doi: 10.1074/jbc.M100091200. [DOI] [PubMed] [Google Scholar]
- 40.Gredinger E, Gerber AN, Tamir Y, Tapscott SJ, Bengal E. Mitogen-activated protein kinase pathway is involved in the differentiation of muscle cells. J. Biol. Chem. 1998;273:10436–10444. doi: 10.1074/jbc.273.17.10436. [DOI] [PubMed] [Google Scholar]
- 41.Dinev D, Jordan BW, Neufeld B, Lee JD, Lindemann D, Rapp UR, Ludwig S. Extracellular signal regulated kinase 5 (ERK5) is required for the differentiation of muscle cells. EMBO Rep. 2001;2:829–834. doi: 10.1093/embo-reports/kve177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sunadome K, Yamamoto T, Ebisuya M, Kondoh K, Sehara-Fujisawa A, Nishida E. ERK5 regulates muscle cell fusion through Klf transcription factors. Dev. Cell. 2011;20:192–205. doi: 10.1016/j.devcel.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 43.Brandenberger R, Schmidt A, Linton J, Wang D, Backus C, Denda S, Müller U, Reichardt LF. Identification and characterization of a novel extracellular matrix protein nephronectin that is associated with integrin alpha8beta1 in the embryonic kidney. J. Cell Biol. 2001;154:447–458. doi: 10.1083/jcb.200103069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Constantin B, Cognard C, Raymond G. Myoblast fusion requires cytosolic calcium elevation but not activation of voltage-dependent calcium channels. Cell Calcium. 1996;19:365–374. doi: 10.1016/s0143-4160(96)90109-8. [DOI] [PubMed] [Google Scholar]
- 45.Bijlenga P, Liu JH, Espinos E, Haenggeli CA, Fischer-Lougheed J, Bader CR, Bernheim L. T-type alpha 1H Ca2+ channels are involved in Ca2+ signaling during terminal differentiation (fusion) of human myoblasts. Proc. Natl. Acad. Sci. U.S.A. 2000;97:7627–7632. doi: 10.1073/pnas.97.13.7627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shin KS, Park JY, Ha DB, Chung CH, Kang MS. Involvement of K(Ca) channels and stretch-activated channels in calcium influx, triggering membrane fusion of chick embryonic myoblasts. Dev. Biol. 1996;175:14–23. doi: 10.1006/dbio.1996.0091. [DOI] [PubMed] [Google Scholar]
- 47.Wu H, Peisley A, Graef IA, Crabtree GR. NFAT signaling and the invention of vertebrates. Trends Cell Biol. 2007;17:251–260. doi: 10.1016/j.tcb.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 48.Horsley V, Pavlath GK. NFAT: Ubiquitous regulator of cell differentiation and adaptation. J. Cell Biol. 2002;156:771–774. doi: 10.1083/jcb.200111073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Horsley V, Friday BB, Matteson S, Kegley KM, Gephart J, Pavlath GK. Regulation of the growth of multinucleated muscle cells by an NFATC2-dependent pathway. J. Cell Biol. 2001;153:329–338. doi: 10.1083/jcb.153.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Horsley V, Jansen KM, Mills ST, Pavlath GK. IL-4 acts as a myoblast recruitment factor during mammalian muscle growth. Cell. 2003;113:483–494. doi: 10.1016/s0092-8674(03)00319-2. [DOI] [PubMed] [Google Scholar]
- 51.Demonbreun AR, Lapidos KA, Heretis K, Levin S, Dale R, Pytel P, Svensson EC, McNally EM. Myoferlin regulation by NFAT in muscle injury, regeneration and repair. J. Cell Sci. 2010;123:2413–2422. doi: 10.1242/jcs.065375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Horsley V, Pavlath GK. Prostaglandin F2(alpha) stimulates growth of skeletal muscle cells via an NFATC2-dependent pathway. J. Cell Biol. 2003;161:111–118. doi: 10.1083/jcb.200208085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pavlath GK, Horsley V. Cell fusion in skeletal muscle—Central role of NFATC2 in regulating muscle cell size. Cell Cycle. 2003;2:420–423. [PubMed] [Google Scholar]
- 54.Mavalli MD, DiGirolamo DJ, Fan Y, Riddle RC, Campbell KS, van Groen T, Frank SJ, Sperling MA, Esser KA, Bamman MM, Clemens TL. Distinct growth hormone receptor signaling modes regulate skeletal muscle development and insulin sensitivity in mice. J. Clin. Invest. 2010;120:4007–4020. doi: 10.1172/JCI42447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sotiropoulos A, Ohanna M, Kedzia C, Menon RK, Kopchick JJ, Kelly PA, Pende M. Growth hormone promotes skeletal muscle cell fusion independent of insulin-like growth factor 1 up-regulation. Proc. Natl. Acad. Sci. U.S.A. 2006;103:7315–7320. doi: 10.1073/pnas.0510033103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guerci A, Lahoute C, Hébrard S, Collard L, Graindorge D, Favier M, Cagnard N, Batonnet-Pichon S, Précigout G, Garcia L, Tuil D, Daegelen D, Sotiropoulos A. Srf-dependent paracrine signals produced by myofibers control satellite cell-mediated skeletal muscle hypertrophy. Cell Metab. 2012;15:25–37. doi: 10.1016/j.cmet.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 57.Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;18:2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 58.Li H, Malhotra S, Kumar A. Nuclear factor-kappa B signaling in skeletal muscle atrophy. J. Mol. Med. 2008;86:1113–1126. doi: 10.1007/s00109-008-0373-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Varfolomeev E, Blankenship JW, Wayson SM, Fedorova AV, Kayagaki N, Garg P, Zobel K, Dynek JN, Elliott LO, Wallweber HJ, Flygare JA, Fairbrother WJ, Deshayes K, Dixit VM, Vucic D. IAP antagonists induce autoubiquitination of c-IAPs, NF-kappaB activation, and TNFalpha-dependent apoptosis. Cell. 2007;131:669–681. doi: 10.1016/j.cell.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 60.Razani B, Reichardt AD, Cheng G. Non-canonical NF-κB signaling activation and regulation: Principles and perspectives. Immunol. Rev. 2011;244:44–54. doi: 10.1111/j.1600-065X.2011.01059.x. [DOI] [PubMed] [Google Scholar]
- 61.Zarnegar BJ, Wang Y, Mahoney DJ, Dempsey PW, Cheung HH, He J, Shiba T, Yang X, Yeh WC, Mak TW, Korneluk RG, Cheng G. Noncanonical NF-kappaB activation requires coordinated assembly of a regulatory complex of the adaptors cIAP1, cIAP2, TRAF2 and TRAF3 and the kinase NIK. Nat. Immunol. 2008;9:1371–1378. doi: 10.1038/ni.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vallabhapurapu S, Matsuzawa A, Zhang W, Tseng PH, Keats JJ, Wang H, Vignali DA, Bergsagel PL, Karin M. Nonredundant and complementary functions of TRAF2 and TRAF3 in a ubiquitination cascade that activates NIK-dependent alternative NF-kappaB signaling. Nat. Immunol. 2008;9:1364–1370. doi: 10.1038/ni.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bakkar N, Wang J, Ladner KJ, Wang H, Dahlman JM, Carathers M, Acharyya S, Rudnicki MA, Hollenbach AD, Guttridge DC. IKK/NF-kappaB regulates skeletal myogenesis via a signaling switch to inhibit differentiation and promote mitochondrial biogenesis. J. Cell Biol. 2008;180:787–802. doi: 10.1083/jcb.200707179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Enwere EK, Holbrook J, Lejmi-Mrad R, Vineham J, Timusk K, Sivaraj B, Isaac M, Uehling D, Al-awar R, LaCasse E, Korneluk RG. TWEAK and cIAP1 regulate myoblast fusion through the noncanonical NF-κB signaling pathway. Sci. Signal. 2012;5:ra75. doi: 10.1126/scisignal.2003086. [DOI] [PubMed] [Google Scholar]
- 65.Bakkar N, Ladner K, Canan BD, Liyanarachchi S, Bal NC, Pant M, Periasamy M, Li Q, Janssen PM, Guttridge DC. IKKα and alternative NF-κB regulate PGC-1β to promote oxidative muscle metabolism. J. Cell Biol. 2012;196:497–511. doi: 10.1083/jcb.201108118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li H, Mittal A, Paul PK, Kumar M, Srivastava DS, Tyagi SC, Kumar A. Tumor necrosis factor-related weak inducer of apoptosis augments matrix metalloproteinase 9 (MMP-9) production in skeletal muscle through the activation of nuclear factor-kappaB-inducing kinase and p38 mitogen-activated protein kinase: A potential role of MMP-9 in myopathy. J. Biol. Chem. 2009;284:4439–4450. doi: 10.1074/jbc.M805546200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Girgenrath M, Weng S, Kostek CA, Browning B, Wang M, Brown SA, Winkles JA, Michaelson JS, Allaire N, Schneider P, Scott ML, Hsu YM, Yagita H, Flavell RA, Miller JB, Burkly LC, Zheng TS. TWEAK, via its receptor Fn14, is a novel regulator of mesenchymal progenitor cells and skeletal muscle regeneration. EMBO J. 2006;25:5826–5839. doi: 10.1038/sj.emboj.7601441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dogra C, Hall SL, Wedhas N, Linkhart TA, Kumar A. Fibroblast growth factor inducible 14 (Fn14) is required for the expression of myogenic regulatory factors and differentiation of myoblasts into myotubes. Evidence for TWEAK-independent functions of Fn14 during myogenesis. J. Biol. Chem. 2007;282:15000–15010. doi: 10.1074/jbc.M608668200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dogra C, Changotra H, Mohan S, Kumar A. Tumor necrosis factor-like weak inducer of apoptosis inhibits skeletal myogenesis through sustained activation of nuclear factor-kappaB and degradation of MyoD protein. J. Biol. Chem. 2006;281:10327–10336. doi: 10.1074/jbc.M511131200. [DOI] [PubMed] [Google Scholar]
- 70.Dogra C, Changotra H, Wedhas N, Qin X, Wergedal JE, Kumar A. TNF-related weak inducer of apoptosis (TWEAK) is a potent skeletal muscle-wasting cytokine. FASEB J. 2007;21:1857–1869. doi: 10.1096/fj.06-7537com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mittal A, Bhatnagar S, Kumar A, Paul PK, Kuang S, Kumar A. Genetic ablation of TWEAK augments regeneration and post-injury growth of skeletal muscle in mice. Am. J. Pathol. 2010;177:1732–1742. doi: 10.2353/ajpath.2010.100335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Morosetti R, Gliubizzi C, Sancricca C, Broccolini A, Gidaro T, Lucchini M, Mirabella M. TWEAK in inclusion-body myositis muscle: Possible pathogenic role of a cytokine inhibiting myogenesis. Am. J. Pathol. 2012;180:1603–1613. doi: 10.1016/j.ajpath.2011.12.027. [DOI] [PubMed] [Google Scholar]
- 73.Roos C, Wicovsky A, Müller N, Salzmann S, Rosenthal T, Kalthoff H, Trauzold A, Seher A, Henkler F, Kneitz C, Wajant H. Soluble and transmembrane TNF-like weak inducer of apoptosis differentially activate the classical and noncanonical NF-kappa B pathway. J. Immunol. 2010;185:1593–1605. doi: 10.4049/jimmunol.0903555. [DOI] [PubMed] [Google Scholar]
- 74.Huang H, He X. Wnt/beta-catenin signaling: New (and old) players and new insights. Curr. Opin. Cell Biol. 2008;20:119–125. doi: 10.1016/j.ceb.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.von Maltzahn J, Chang NC, Bentzinger CF, Rudnicki MA. Wnt signaling in myogenesis. Trends Cell Biol. 2012;22:602–609. doi: 10.1016/j.tcb.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rochat A, Fernandez A, Vandromme M, Molès JP, Bouschet T, Carnac G, Lamb NJ. Insulin and wnt1 pathways cooperate to induce reserve cell activation in differentiation and myotube hypertrophy. Mol. Biol. Cell. 2004;15:4544–4555. doi: 10.1091/mbc.E03-11-0816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pansters NA, van der Velden JL, Kelders MC, Laeremans H, Schols AM, Langen RC. Segregation of myoblast fusion and muscle-specific gene expression by distinct ligand-dependent inactivation of GSK-3β. Cell. Mol. Life Sci. 2011;68:523–535. doi: 10.1007/s00018-010-0467-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tran TH, Shi X, Zaia J, Ai X. Heparan sulfate 6-O-endosulfatases (Sulfs) coordinate the Wnt signaling pathways to regulate myoblast fusion during skeletal muscle regeneration. J. Biol. Chem. 2012;287:32651–32664. doi: 10.1074/jbc.M112.353243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brack AS, Conboy IM, Conboy MJ, Shen J, Rando TA. A temporal switch from notch to Wnt signaling in muscle stem cells is necessary for normal adult myogenesis. Cell Stem Cell. 2008;2:50–59. doi: 10.1016/j.stem.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 80.Langsdorf A, Do AT, Kusche-Gullberg M, Emerson CP, Jr, Ai X. Sulfs are regulators of growth factor signaling for satellite cell differentiation and muscle regeneration. Dev. Biol. 2007;311:464–477. doi: 10.1016/j.ydbio.2007.08.053. [DOI] [PubMed] [Google Scholar]
- 81.Olson EN, Sternberg E, Hu JS, Spizz G, Wilcox C. Regulation of myogenic differentiation by type beta transforming growth factor. J. Cell Biol. 1986;103:1799–1805. doi: 10.1083/jcb.103.5.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Han D, Zhao H, Parada C, Hacia JG, Bringas P, Jr, Chai Y. A TGFβ-Smad4-Fgf6 signaling cascade controls myogenic differentiation and myoblast fusion during tongue development. Development. 2012;139:1640–1650. doi: 10.1242/dev.076653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee KH, Baek MY, Moon KY, Song WK, Chung CH, Ha DB, Kang MS. Nitric oxide as a messenger molecule for myoblast fusion. J. Biol. Chem. 1994;269:14371–14374. [PubMed] [Google Scholar]
- 84.Dahlman JM, Bakkar N, He W, Guttridge DC. NF-kappaB functions in stromal fibroblasts to regulate early postnatal muscle development. J. Biol. Chem. 2010;285:5479–5487. doi: 10.1074/jbc.M109.075606. [DOI] [PMC free article] [PubMed] [Google Scholar]