Abstract
RNA editing by adenosine deamination is a process used to diversify the proteome. The expression of ADARs, the editing enzymes, is ubiquitous among true metazoans, and so adenosine deamination is thought to be universal. By changing codons at the level of mRNA, protein function can be altered, perhaps in response to physiological demand. Although the number of editing sites identified in recent years has been rising exponentially, their effects on protein function, in general, are less well understood. This review assesses the state of the field and highlights particular cases where the biophysical alterations and functional effects caused by RNA editing have been studied in detail.
Introduction
RNA editing by adenosine-to-inosine conversion (A-to-I editing) can introduce codon changes in mRNAs and hence generate structurally and functionally different isoforms of proteins. These isoforms cannot be divined from the genomic sequences. The extent to which the population of isoforms differs from the original exon-encoded protein should be proportional to the extent of editing, which differs widely between different edits, and in most cases is known only as an average percentage in tissue(s), rather than on a cellular level. Excellent reviews have informed us on the principal enzymes that catalyze the adenosine deamination underlying the A-to-I conversion (Hogg et al., 2011), about mechanistic aspects of editing (Rieder and Reenan, 2011), and an ever-growing list of RNA targets (Eisenberg et al., 2010; Wulff et al., 2011). Most targets in invertebrates and vertebrates, including mammals, are found in the nervous system, but the biophysical and physiological changes that A-to-I editing evokes are nearly completely unknown. In invertebrates, hundreds of recoding events have been identified. In humans, the story is different. Although thousands of editing sites have been reported by large-scale screens, the vast majority occur in non-coding sequence. In the present perspective, we focus only on a few editing sites in mRNAs encoding AMPA receptors in mammals, voltage-dependent potassium channels in mammals and invertebrates, and the sodium pump in squid. We end the review by highlighting a recent article that draws a link between RNA editing and the physical environment and speculate on the plasticity of the process.
RNA Editing in Mammals: Transmitter and Voltage-Gated Ion Channels
AMPA Receptors Feature an Edit Critical for Survival
We begin our description of important edits in the nervous system and the functional consequences editing provides with a particular one in AMPA receptors of the mammalian brain, that is distinguished from all others by being present in virtually 100% of the cognate mRNAs. AMPA receptors are glutamate-activated cation channels and mediate the bulk of fast synaptic excitatory neurotransmission in the mammalian/vertebrate brain. These receptors are assembled from subunits named GluA1–4 (formerly GluR-A to -D or GluR1–4), encoded by four related genes, into tetramers configured as a rule from two different subunits (e.g., GluA1/A2). Primary transcripts of the gene for the GluA2 subunit undergo A-to-I editing at a CAG codon for glutamine (Q; Figure 1). This particular glutamine participates in lining the ion channel’s pore and is conserved across the subunits GluA1, 3, 4. Only GluA2 carries the edited codon CIG, with GluA2 thus contributing an arginine (R) instead of glutamine to the channel lining in hetero-oligomeric AMPA receptor channels that include GluA2. Having an arginine at this critical position renders the channel impermeable to Ca2+ and decreases the single-channel conductance of the activated ion channel approximately ten-fold relative to GluA2-less AMPA receptors.
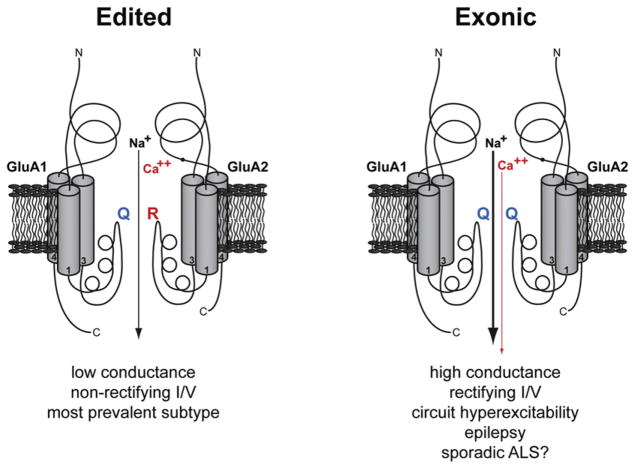
Figure 1. Edited Mammalian AMPA Receptors Are Impermeable to Ca2+.
Depicted are two versions of a heteromeric AMPA receptor, each showing two of the four subunits that make up a functional receptor. The transmembrane regions of the subunits are shown as cylinders, the re-entrant channel loop with the typical α-helical segment and the functionally critical Q/R site line the ion channel. Extracellular and intracellular subunit portions are sketched. The filled dot in the extracellular region of GluA2, between transmembrane segments 3 and 4, denotes the R/G edit (see text). The receptor version depicted on the left corresponds to the most prevalent AMPA channel in the brain, composed of the subunits GluA1 and GluA2, the latter edited in the Q/R site of the channel segment M2. The version on the right is the same channel except that the Q/R site of GluA2 is unedited, thus having the exonically encoded GluA2 sequence. This receptor is probably never expressed normally and can only be generated by gene manipulation. The characteristic property differences of the two AMPA receptor versions are listed below the channels, along with consequences on circuits and CNS disease for the unedited receptor. A role for the unedited form in sporadic ALS is presently an attractive hypothesis under debate.
The Q/R site is positioned toward the 3′-end of the Gria2 (the gene encoding GluA2) exon 11. In primary transcripts, this region forms an imperfect double-stranded structure with a short downstream sequence that is essential for Q/R site editing, located a few hundred nucleotides into intron 11. Such cis-acting exon-complementary sequences (ECS) have been found surrounding many other edits in diverse species and can occur as far as thousands of nucleotides up- or downstream of a particular edit. When one of the GluA2 alleles in the mouse has its ECS deleted to prevent Q/R site editing of its transcripts, severe epilepsy and premature death ensues, thus revealing the critical importance of this edit (Brusa et al., 1995). The phenotype is not only linked to developmental problems, as epilepsy can also be induced in the adult mouse if a GluA2 allele lacking the ECS but silenced via a large floxed insert within intron 11 becomes expression-activated by Cre-mediated recombination in all principal forebrain neurons (Krestel et al., 2004). Moreover, distinct neurological dysfunctions, ranging from lethargy to hyperexcitability, are generated in mice expressing different levels of Q/R site-unedited GluA2 (Feldmeyer et al., 1999).
The circuit alterations in the forebrain causing epilepsy may be related to elevated Ca2+ influx through receptors containing unedited GluA2 subunits. The severity of the phenotype is surprising, given that lack of the ECS causes transcripts to undergo attenuated intron 11 splicing, resulting in normally edited mRNAs from the wild-type allele outnumbering unedited ones from the mutant allele by at least three to one (Brusa et al., 1995). Hence, a postulated increase in Ca2+ influx through an unedited AMPA channel population should be modest at best, and indeed, no cell death could be observed in the brains of such mice. A plausible mechanistic link between the introduced mutation in a single Gria2 allele and the resulting mouse phenotype may be the greater tetramerization and trafficking potential of Q/R site-unedited GluA2 subunits (Greger et al., 2002, 2003). The specific impact of Q/R site editing on protein function is reminiscent of edits in the tetramerization domain of Kv channels of cephalopods (see below).
Intriguingly, a potential role for Q/R site-under-edited GluA2 in causing cell death has been postulated for motoneurons, based on a postmortem analysis of individuals with sporadic amyotrophic lateral sclerosis (Kawahara et al., 2004). A more recent study (Hideyama et al., 2012), also on deceased ALS patients, traced this underediting to downregulation of ADAR2 (but not ADAR1 and 3) in all motoneurons. Indeed, an ALS-like phenotype could be induced in mice carrying floxed ADAR2 alleles by selective Cre-mediated ADAR2 knockout in motoneurons, and no such phenotype developed when the mice expressed pre-edited Gria2 alleles (Hideyama et al., 2010). Thus, Q/R site underediting of GluA2 appears to induce in motoneurons a profound pathological change with relevance to ALS.
As anticipated from the importance of AMPA editing, global (different from cell population selective) knockout of ADAR2, the enzyme responsible for Q/R site editing of GluA2 transcripts, results in early postnatal death of the mice. This fate can be prevented by making the mice homozygous for Gria2 alleles that carry a codon for arginine instead of glutamine for the Q/R site. The normal life span and unimpaired home cage phenotype of ADAR2-lacking mice that carry only the “pre-edited Gria2 alleles” was unexpected: ADAR2, which is widely expressed beyond the brain, is known to edit many messages besides GluA2. A more detailed phenotypic examination covering approximately 320 parameters on a large cohort of mice pre-edited in Gria2 alleles and lacking all ADAR2 indeed revealed a few conspicuous features, which included a change in hearing ability (Horsch et al., 2011). While this study did not investigate higher brain functions such as task learning, one is led to surmise that all ADAR2-mediated edits other than the Q/R site in GluA2 are used to fine-tune particular physiological functions.
Mammalian Kv1.1 Editing Fine Tunes Channel Inactivation
For voltage-gated K+ channels, timing is critical. It’s long been known that their opening kinetics, just a shade slower than those of Na+ channels, help set the action potential’s duration. For other physiological processes, like repetitive firing, the speed at which they shut down is just as important. So much so that nature has developed elaborate strategies to turn ion channels off in the face of a voltage signal telling them to stay open. Collectively, these processes are known as inactivation. Fast inactivation, which occurs over milliseconds, is well understood. In 1977, Armstrong and Bezanilla, while looking at ionic currents in squid axons, postulated that inactivation was caused by a tethered intracellular particle that could physically plug a channel’s pore only after it opened (Armstrong and Bezanilla, 1977). Aldrich and colleagues gave structural reality to this idea by showing that the N terminus of the shaker K+ channel acts as a functional inactivation unit or “ball and chain” (Hoshi et al., 1990). K+ channels are tetramers, always composed of four pore-forming α subunits, which are sometimes joined by four accessory cytoplasmic β subunits. In some K+ channels, the ball and chain resides at the beginning of the α subunit, and in others it’s attached to the β subunit, but in either case its mechanism of action is similar. After the channel opens in response to depolarization, the inactivation particle diffuses through one of four large cytoplasmic portals, past the now-open gate, and then docks in a spacious internal vestibule. Once bound immediately below the selectivity filter, it presumably blocks ion flow, temporarily removing that channel from the equation. After the membrane returns to rest, the inactivation particle is free to unbind and return to the cytoplasm. After the inactivation particle unbinds, the channel passes through the open state where it briefly continues to conduct ions before the gate closes with the normal deactivation process, allowing the channel to be recruited into action during the next depolarization. The inactivation particle’s binding kinetics are determined by access to its receptor; its unbinding kinetics are determined by how tightly it binds. Slow unbinding rates tend to exaggerate the action potential’s afterhyperpolarization phase due to the transient passage through the open state before closing. This has the effect of limiting repetitive firing.
Mammals find the process of K+ channel fast inactivation an attractive target for regulation by RNA editing. While performing a bulk screen for editing sites in mouse brain mRNAs, Robert Reenan and colleagues identified a new candidate in mRNAs encoding Kv1.1 (Hoopengardner et al., 2003). This edit changes an isoleucine to a valine at codon 400, a highly conserved position in the sixth transmembrane span that lies along the ion conduction pathway. Intriguingly, this site is also edited in the human brain (Figure 2). Past studies on how organic compounds block K+ currents hinted at why the I400V edit might be important: mutations at this position reduced block by quaternary amines by close to 400-fold (Zhou et al., 2001). It was reasonable to speculate that I400V may have a similar effect on block by its endogenous “ball and chain,” which for human Kv1.1 is attached to the Kvβ1.1 subunit. As hypothesized, I400V had a profound effect on fast inactivation, specifically targeting the rate of recovery (Bhalla et al., 2004). While the onset of inactivation was largely unchanged, recovery from inactivation was ~20 times faster, an outcome best explained by an increase in the inactivation particle’s rate of release from its receptor. These results raised some intriguing questions. The I400V edit removes a single methyl group. Are the faster kinetics due to a reduction in hydrophobicity at position 400 and is this position within the receptor?
Figure 2. Edited Mammalian Potassium Channels Recover More Quickly from Inactivation.

Fast inactivation in voltage-dependent potassium channels is caused by a tethered inactivation particle, which enters the channel’s inner vestibule after opening and plugs the ion conduction pathway by binding to a receptor through a hydrophobic interaction. In the case of human Kv1.1, the inactivation particle is attached to a β subunit. RNA editing reduces the hydrophobicity of the inactivation particle’s receptor, allowing the particle to unbind more rapidly. The dashed arrow indicates a slower rate. The channel’s gate, in the open position, is shown in black.
Miguel Holmgren and colleagues provided answers to these questions with exceptional clarity (Gonzalez et al., 2011). By substituting a cysteine at position 400, they were able to independently modify either the hydrophobicity or bulk at this site by direct chemical modification. In doing so, they showed that hydrophobicity at position 400 was the principal determinant of recovery. Further, by also substituting a cysteine at the very tip of the inactivation particle at codon 2, they were able to lock it to position 400 through the formation of a disulfide bond. These data carry important structural implications. First of all, position 400 is located at the top of a large inner vestibule of the channel, right under the selectivity filter. Accordingly, to block current, the inactivation particle must reach deeply into the vestibule, where it’s very tip makes contact with the residue affected by the editing site. Interestingly, this mechanism may bear relevance to more than block by traditional inactivation particles. For quite some time it has been known that highly unsaturated fatty acids like arachidonic acid, which are commonly found in the mammalian brain, can block in an analogous manner, converting non-inactivating K+ currents into A currents (Oliver et al., 2004). A recent report shows that the I400V edit affects block by polyunsaturated fatty acids in a similar fashion (Decher et al., 2010). Although the I400V edit is now very well understood on a mechanistic level, its importance to higher order physiology is just beginning to be explored. Unlike the Q/R site in AMPA receptors, editing at codon 400 in Kv1.1 varies substantially between different parts of the brain (Hoopengardner et al., 2003). A recent study showed that the frequency of the I400V edit in the entorhinal cortex was four times higher in a rat model for chronic epilepsy, suggesting this site’s importance on brain function (Streit et al., 2011). Specifically how this edit affects neuronal excitability, and behavior, are the clear next questions.
Other Targets in Mammals
Many mRNAs besides GluA2 and Kv1.1 are edited in mammals, most of nervous tissue origin, prominently including functionally relevant sites in most AMPA and kainate receptor subunit transcripts. A functionally intriguing example centers on a second editing site in AMPA receptor subunit GluA2, termed the R/G site (Lomeli et al., 1994), which immediately precedes the alternatively spliced flip and flop modules within S2 of the bipartite ligand binding domain (Figure 1). The edit is also found in subunits GluA3 and 4. AMPA receptors containing subunits with edited R/G site (“G-form” subunits) possess faster recovery rates from desensitization than receptors containing unedited “R-form” subunits. This physiologically relevant functional distinction can be interpreted with the help of high-resolution structural data for the edited (Armstrong and Gouaux, 2000) and unedited (Greger et al., 2006) forms. It appears that the arginines at the unedited R/G site stabilize a subunit interphase, thus facilitating GluA2 receptor assembly and slowing entry into desensitization.
Curiously, the enzyme ADAR2 edits its own primary transcripts, thereby producing an alternative splicing event (Rueter et al., 1999), which regulates ADAR2 levels (Feng et al., 2006). A survey of the human brain transcriptome uncovered 38 recoding events (Li et al., 2009), many of which have been previously reported, and more recent screens suggest the number may be even higher (Li et al., 2011). For some of these targets, the effects of editing on protein function have been explored. For example, editing of the serotonin 5-HT2c receptor reduces the receptor’s affinity for its G protein (Burns et al., 1997), and editing of the GABA-gated Cl− channel subunit α3 affects gating kinetics, rectification, and trafficking (Daniel et al., 2011; Ohlson et al., 2007; Rula et al., 2008). At present, the mechanistic details behind these effects are largely unknown and certainly provide fertile ground for further studies, as do the many yet to be explored editing sites.
RNA Editing in Invertebrates
Unlike the case for mammals, where relatively few edited codons have been uncovered, recoding by RNA editing appears to be a surprisingly common event in higher invertebrates. As will be described in the upcoming sections, this assertion is based on two groups: fruit flies and squid. It should be noted that editing has been examined in detail in the relatively primitive C. elegans and, as far as we know, no recoding events have been found. Nevertheless, the prevalence of editing in higher invertebrates prompts us to speculate on its biological use. As poikilotherms, invertebrates are at the mercy of the temperature environment. This must place constraints on their machinery for excitability: a collection of ion pumps, ion channels, and neurotransmitter release components, all with slightly different temperature sensitivities. Being able to fine tune the individual components could prove highly useful.
RNA Editing in Fruit Flies: A Panoply of Recoding Events
More genetic recoding by A-to-I editing has been uncovered in Drosophila melanogaster than in any other organism to date. This is because a comparatively large effort has been spent on looking for editing sites in this model and the fact that, quite simply, Drosophila appears to edit a great deal. As with all systems, the first sites were discovered through serendipitous encounters, when astute investigators noticed guanosine residues in cDNA sequences at positions occupied by adenosine in the genome. One often wonders how many editing sites escaped discovery when such discrepancies were attributed to sequencing artifacts. Most of the early sites discovered in flies were in mRNAs encoding voltage-gated or ligand-gated ion channels. The completion of the Drosophila genome in 2000 enabled more sophisticated screens to be undertaken and soon close to 50 new transcripts were found to be edited, many at multiple sites (Hoopengardner et al., 2003; Stapleton et al., 2006). Intriguingly, most of these transcripts encode proteins involved in electrical signaling. A recent effort by Graveley and colleagues, using high throughput sequencing to examine complete transcriptomes of 32 developmental stages from embryos to adults, has probably uncovered the majority of editing sites (Graveley et al., 2011). In it, they report 972 editing sites in 597 mRNAs, representing ~4% of all transcripts. Remarkably, about two-thirds of the sites change codons. Of the rest, 201 are silent and 141 are in UTRs. Such a high bias toward recoding events suggests that editing is under selective pressure and therefore being used to actively regulate physiological processes. Close to a quarter of the editing sites are within mRNAs encoding proteins involved in the machinery for excitability (defined here as ion channels, ion transporters, and proteins involved in neurotransmitter release and recycling). However, mRNAs encoding proteins involved in other cellular functions (e.g., cytoskeletal architecture and protein phosphorylation) are edited as well. This study is an exceptionally important addition to the field of RNA editing. It should enable the investigator to move beyond editing site identification and begin to address fundamental questions on the biology of the process.
In spite of the abundance of editing sites that have been identified in Drosophila, very little is known about how they affect protein function, and in no cases are the mechanistic underpinnings of their effects understood. In two studies on the voltage-dependent K+ channels Shaker (Kv1 subfamily) and ShaB (Kv2 subfamily), each edited at multiple sites, editing was found to affect the kinetics of opening, closing, fast inactivation, and the voltage dependence of activation (Ingleby et al., 2009; Ryan et al., 2008). Some of the effects were substantial, particularly those in ShaB. Interestingly, the overall effect of editing at multiple sites within the same transcript could not be predicted from the effects of the individual sites, a phenomenon known as functional epistasis. Thus the functional outcomes of editing can be exceptionally complex. In a different study, editing was shown to decrease the sensitivity of a GABA-gated Cl− channel to GABA, an effect predicted to increase excitability (Jones et al., 2009). With hundreds of editing sites in Drosophila yet to be investigated, these studies are obviously just the beginning.
On the other end of the physiological spectrum from molecular structure-function studies, there have been several investigations into how RNA editing affects Drosophila behavior. These have been aided by the fact that Drosophila contains a single ADAR locus and its removal results in viable flies, although just barely (Palladino et al., 2000a, 2000b). The Drosophila ADAR locus resides at the tip of the X chromosome, and the protein that it encodes closely resembles vertebrate ADAR2. Null mutants for Drosophila ADAR (dADAR) appear morphologically normal, have a normal life-span and, when maintained under favorable conditions, can be coaxed into reproducing. However, adult flies are obviously compromised (Palladino et al., 2000b). Problems include seizures, whose severity increase with age, poorly coordinated locomotion, compulsive preening, abnormal posture, tremors, and a reluctance to jump and fly. On a morphological level, conspicuous neurodegeneration is evident in the brain and retinas. Although dADAR is expressed outside of the nervous system, and has activities beyond editing mRNAs, it has been demonstrated that much of the dADAR null phenotype results from a lack of editing of brain messages (Jepson and Reenan, 2009). Because a complete dADAR knockout results in such a severe phenotype, it is difficult to assess the importance of editing for complex behaviors using these flies. To address this problem, Reenan and colleagues engineered flies in which dADAR expression was greatly reduced but not abolished (Jepson et al., 2011). Interestingly, although the severe locomotor phenotypes of the null mutants were not evident, defects in courtship and circadian behavior were evident and a knockdown of editing in a specific neuronal subset was sufficient to alter the male courtship song. Now that we know the more or less complete set of edited targets in Drosophila, due to the genetic manipulations that are possible in this system, we can begin to design experiments that link the mechanistic changes caused by RNA editing with the complex behaviors that these changes regulate.
RNA Editing in Squid Regulates Multiple Aspects of Excitability
As a mechanism, RNA editing generates diversity. Cephalopods, which are by far the most sophisticated invertebrates in terms of learning and complexity of behaviors, edit extensively, apparently exploiting this mechanism to a far greater extent than complex vertebrates. By examining only a handful of messages, studies on cephalopods have uncovered close to 100 editing sites, mostly in voltage-dependent ion channels, ion transporters, and RNA editing enzymes (Colina et al., 2010; Palavicini et al., 2009; Patton et al., 1997; Rosenthal and Bezanilla, 2002b). In fact, thus far only a Na+/K+ ATPase β subunit was found not to be edited. Another interesting feature of cephalopod editing is that most of the editing events alter codons. Admittedly, these results are based on few mRNAs, most of which encode proteins involved in excitability, a class of messages known to be edited in other systems. However, in the entire human brain transcriptome only 38 sites that recode amino acids have been found (Li et al., 2009). The rich variety of edited targets in cephalopods allows us to better understand the biological significance of RNA editing. In a few cases, detailed biophysical investigations have already uncovered how editing sites affect function.
RNA editing sites have turned up in mRNAs encoding the historically most intensively studied K+ channels. In their seminal papers using the squid giant axon, Hodgkin and Huxley provided a model for how voltage dependent conductances operate to create action potentials (Hodgkin and Huxley, 1952). In their model, the delayed rectifier K+ conductance was given a dimensionless variable termed “n” that implied a single entity generated the conductance. From the standpoint of parsimony toward their data, and the resolution offered by the available experimental tools, their model was a revelation. However, molecular work on squid K+ channels began to suggest that the picture was not quite so simple. First, the cloning of a Kv2 subfamily member from squid brain revealed 18 RNA editing sites within a 380 nucleotide span centered on sequence encoding the channel’s pore domain (Patton et al., 1997). Two of the sites were shown to create slight alterations in the rates of channel closure and slow inactivation. In a subsequent study on the Kv1 channel thought to contribute to the delayed recitifier K+ conductance of the giant axon, 14 editing sites were identified within the entire open reading frame (Rosenthal and Bezanilla, 2002b; Rosenthal et al., 1996). The sites were clustered in sequence encoding two regions of the channel: transmembrane spans 1 and 3, and the tetramerization domain which regulates the oligomerization of the α-subunit monomers into tetramers. As with squid Kv2, many of the sites had subtle effects on gating. More robust effects were encountered with several of the tetramerization domain edits, which dramatically reduced the affinity of one tetramerization domain for another, as measured through direct biochemical analysis. Because these effects were mirrored in heterologous channel expression in Xenopus oocytes, one potential reason for editing would be to regulate overall K+ conductance in the axon. It is known that different species of squid within the genus Loligo tightly control K+ conductance in their giant axons in order to regulate action potential duration in response to their thermal environment (Rosenthal and Bezanilla, 2002a). Another untested possibility is that RNA editing regulates the composition of heteromultimers between different α-subunits.
Much as it has for K+ channels, the squid giant axon has served as an important model for our present understanding of Na+/K+ ATPase function. The importance of this pump for neurophysiology cannot be overstated. By creating the Na+ and K+ ion gradients, it provides the driving force for action potentials, synaptic potentials, and solute transport across the plasma membrane. It does so at a cost: far more ATP is consumed by it than any other molecule. Work on squid axon taught us much about how the Na+/K+ ATPase operates. For example, its ion transport rate is voltage dependent, becoming significantly inhibited at negative voltages and reaching a maximum at voltages greater than ~0 mV. The origin of this voltage dependence is thought to arise from the process of Na+ ion release to the outside (Gadsby et al., 1993). Na+ ions are thought to unbind deep within the Na+/K+ ATPase. To gain the extracellular medium they must traverse an access channel, much like that of an ion channel, that spans a portion of the membrane’s electric field. At negative voltage, they must move against both an electrical and chemical gradient, both of which cause inhibition. Another important finding was that the three Na+ ions are released from the Na+/K+ ATPase sequentially, in three successive steps, each of which can be tracked by kinetically distinct transient electrical currents (Holmgren et al., 2000).
A recent report shows the mRNAs for the squid giant axon Na+/K+ ATPase are edited in three codons (Colina et al., 2010). One site, located in the seventh transmembrane span (I877V), reduced the voltage-dependent inhibition, thereby causing an increase in the transport rate over the physiological range (Figure 3). Past work on squid axons gave the investigators an idea of where to look for mechanistic interpretations. By directly examining the transient currents generated from the release of the three individual Na+ ions there was an apparent change in the occupancy of the underlying states. The I877V RNA edit shifted the equilibrium toward those states favoring Na+ release, and away from those favoring occlusion. In particular, occupancy of the state immediately preceding the release of the last Na+ ion was increased. The efficiency of editing at I877V was tightly regulated between tissues, suggesting that RNA editing is playing an active role regulating ion homeostasis, perhaps in response to the metabolic demands of different neurons.
Figure 3. Edited Squid Na+/K+ Pumps Release Na+ to the Outside More Quickly.
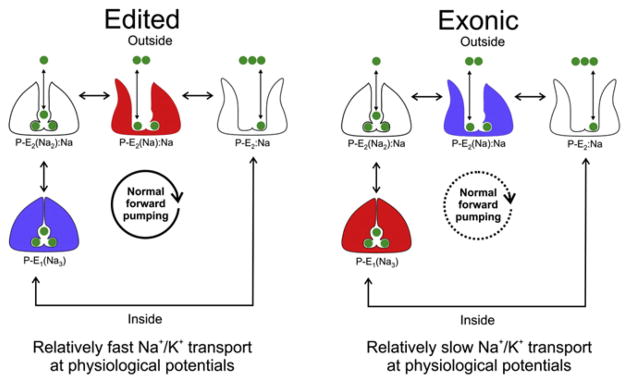
States involved in the sequential release of three Na+ ions to the outside are depicted. State names are written below each cartoon where P indicates phosphorylation, E1 indicates ion binding sites facing inward, E2 indicates ion binding sites facing outwards, and parentheses indicate occlusion. States that involve K+ movement, or ion binding/release to the inside, have been left out. States colored in red indicate a relatively high occupancy, and those in blue a relatively low occupancy. Na+ ions are shown in green. The overall effect of editing is to reduce the pumps inhibition from high concentrations of extracellular Na+ and negative voltages, leading to faster Na+ release and turnover over the physiological range of voltages. The dashed arrow indicates a slower rate.
Perspectives
Thus far, we have described edits in vertebrates and invertebrates with a special focus on their profound effects on nervous system function. As such, each edit is characterized by its very own idiosyncrasies. We now wish to turn our attention to the commonalities of edits, more precisely of all edits where A-to-I RNA editing generates amino acid substitutions relative to the exon-encoded protein sequences. Such edits clearly expand the protein sequence space normally constrained by the exonic DNA sequence and widen the functional range that can be accessed by a single protein product. Differently put, edits are seen to occur at functionally critical protein positions, thereby expanding the operant scope within which the editing-generated protein isoforms can interact with their effectors. As most known edits occur in nervous tissue, the expanded functionality prominently includes that of particular ion channels and pumps, which are likely to occupy a central position in systems and circuit physiology. This view is exemplified by the AMPA receptor for fast excitatory neurotransmission in vertebrates, the potassium channel Kv1 subfamily, which tune various aspects of excitability, in both vertebrates and invertebrates, and the Na+/K+ ATPase in invertebrates. In the latter two examples (potassium channel and Na+/K+ pump), the edited protein versions occur side-by-side with the unedited ones, in cellular ratios presently undetermined. This situation holds true for most A-to-I generated recoding, which typically results in isoform populations, in particular when several edits occur within the same gene product. The only known exception is the Q/R site within the AMPA receptor subunit GluA2, which is always fully edited. Even a moderate decrease in global Q/R site-editing causes epilepsy and a shortened life span in mice.
Recoding by RNA editing thus allows for the expression of heterogeneous isoform populations for key proteins involved in excitability where the functional properties shift depending on the precise isoform composition. Accordingly, organisms can regulate functionality in a graded manner merely by regulating the extent of editing. It is well known that editing generally increases with development, in both vertebrates and invertebrates (Graveley et al., 2011; Palladino et al., 2000b; Wahlstedt et al., 2009). An attractive proposition is that organisms can use editing to change isoform composition in response to environmental factors to keep neurophysiological signaling operating in an optimal state. This might be especially important for invertebrates, which have no temperature control. Editing might provide a means of changing neurophysiological parameters in response to heat or cold, perhaps within a matter of hours.
A recent report on RNA editing in octopus potassium channels provides some substance to this idea (Garrett and Rosenthal, 2012). As encoded by their genes, orthologous Kv1 channels cloned from an Antarctic and a tropical species were virtually identical, both in terms of their primary sequences and the properties of the potassium currents that they produced. However, the mRNAs for these channels were extensively edited, and some of the sites were edited to much higher extents, or exclusively, in one species or the other. In fact, far more functional diversity was created by editing than by changes in the genes. One site in particular, which recodes an isoleucine to a valine in the fifth transmembrane span (I321V), is particularly interesting for several reasons (Figure 4). First, it alters a position near the channel’s gate, and on an electrophysiological level, selectively accelerates the closing rate, a property important for repetitive firing. Mechanistically this is accomplished by destabilizing the open state in order to poise the channel for rapid closure. Second, the efficiency of editing makes sense; the site is highly edited in the Antarctic species, which would need to offset the effects of the extreme cold on closing kinetics, but mostly unedited in the tropical species, which live in a stable warm environment. Examining I321V in other octopus species lends further support to the idea that it is an adaptation to the cold. Arctic species also edit it at a high level, temperate species edit it at an intermediate level, and other tropical species also edit it at a low level. Thus, in octopus, editing appears to be responding to an external factor.
Figure 4. Edited Octopus Potassium Channels Close More Quickly.
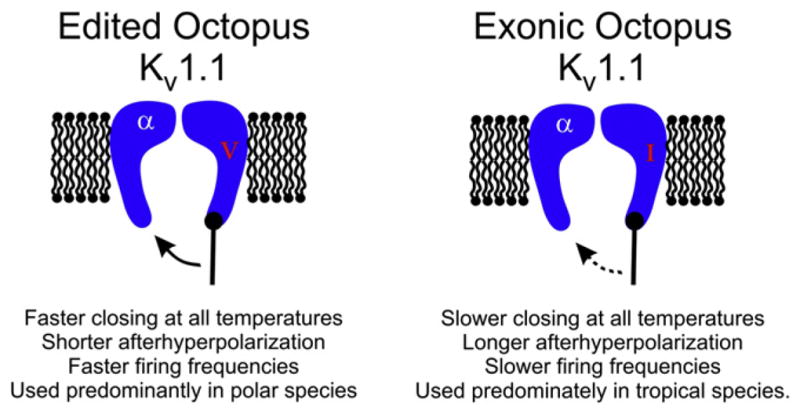
Editing of octopus Kv1.1 at position I321V in the fifth transmembrane span destabilizes the open state, allowing the channels to close rapidly upon repolarization. The overall physiological effect would be to reduce the length of the afterhyperpolarization, allowing higher firing frequencies. Because this site is highly edited in polar species and scarcely edited in tropical species, it is hypothesized to be an adaptation to temperature. The dashed arrow indicates a slower rate.
Results from octopus lead to intriguing questions, particularly with regard to the speed of the response. Is editing at I321V a slow adaptation to temperature, or can it be used as a rapid acclimation to temperature variation? In each case, we would expect the underlying biochemical mechanism to be quite different. For adaptation, we could envision that the ADARs, or the RNA structures that they recognize, have evolved to promote more efficient editing in the cold species. The fine scale evolution of an RNA structure that promotes editing has already been tracked among different species of Drosophila and other insects (Reenan, 2005). For acclimation, perhaps as-yet-unidentified cellular factors could regulate ADAR’s access to an editing site, or the RNA structures surrounding an editing site are themselves stabilized by the cold. Past studies on messages encoding the G protein coupled serotonin receptor 5HT2C in mouse brain and human glioblastoma cells support the idea that acclimation is possible. In these studies, editing frequency responded rapidly to the application of a receptor agonist or interferon (Gurevich et al., 2002; Yang et al., 2004). Clearly, the idea of editing in response to the environment is relevant beyond octopus. ADAR expression is universal in true metazoans (Keegan et al., 2011). Even in vertebrates, most taxa have not developed the ability to regulate their body temperatures, and next to nothing is known about editing in fish, reptiles, and amphibians. In mammals, RNA editing could be used to respond to external factors besides temperature. Knowing why an organism chooses to edit a specific adenosine within an mRNA is fundamental for truly understanding the editing event. As a first step, having a detailed understanding of how the edit alters protein function is critical.
Acknowledgments
The authors acknowledge support by the National Institutes of Health R01 NS064259NIH and RCMI G12 RR 03051 for J.J.C.R. and the Max Planck Society for P.H.S.
References
- Armstrong CM, Bezanilla F. Inactivation of the sodium channel. II Gating current experiments. J Gen Physiol. 1977;70:567–590. doi: 10.1085/jgp.70.5.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong N, Gouaux E. Mechanisms for activation and antagonism of an AMPA-sensitive glutamate receptor: crystal structures of the GluR2 ligand binding core. Neuron. 2000;28:165–181. doi: 10.1016/s0896-6273(00)00094-5. [DOI] [PubMed] [Google Scholar]
- Bhalla T, Rosenthal JJ, Holmgren M, Reenan R. Control of human potassium channel inactivation by editing of a small mRNA hairpin. Nat Struct Mol Biol. 2004;11:950–956. doi: 10.1038/nsmb825. [DOI] [PubMed] [Google Scholar]
- Brusa R, Zimmermann F, Koh DS, Feldmeyer D, Gass P, Seeburg PH, Sprengel R. Early-onset epilepsy and postnatal lethality associated with an editing-deficient GluR-B allele in mice. Science. 1995;270:1677–1680. doi: 10.1126/science.270.5242.1677. [DOI] [PubMed] [Google Scholar]
- Burns CM, Chu H, Rueter SM, Hutchinson LK, Canton H, Sanders- Bush E, Emeson RB. Regulation of serotonin-2C receptor G-protein coupling by RNA editing. Nature. 1997;387:303–308. doi: 10.1038/387303a0. [DOI] [PubMed] [Google Scholar]
- Colina C, Palavicini JP, Srikumar D, Holmgren M, Rosenthal JJ. Regulation of Na+/K+ ATPase transport velocity by RNA editing. PLoS Biol. 2010;8:e1000540. doi: 10.1371/journal.pbio.1000540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel C, Wahlstedt H, Ohlson J, Björk P, Ohman M. Adenosine- to-inosine RNA editing affects trafficking of the gamma-aminobutyric acid type A (GABA(A)) receptor. J Biol Chem. 2011;286:2031–2040. doi: 10.1074/jbc.M110.130096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decher N, Streit AK, Rapedius M, Netter MF, Marzian S, Ehling P, Schlichthörl G, Craan T, Renigunta V, Köhler A, et al. RNA editing modulates the binding of drugs and highly unsaturated fatty acids to the open pore of Kv potassium channels. EMBO J. 2010;29:2101–2113. doi: 10.1038/emboj.2010.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg E, Li JB, Levanon EY. Sequence based identification of RNA editing sites. RNA Biol. 2010;7:248–252. doi: 10.4161/rna.7.2.11565. [DOI] [PubMed] [Google Scholar]
- Feldmeyer D, Kask K, Brusa R, Kornau HC, Kolhekar R, Rozov A, Burnashev N, Jensen V, Hvalby O, Sprengel R, Seeburg PH. Neurological dysfunctions in mice expressing different levels of the Q/R site-unedited AMPAR subunit GluR-B. Nat Neurosci. 1999;2:57–64. doi: 10.1038/4561. [DOI] [PubMed] [Google Scholar]
- Feng Y, Sansam CL, Singh M, Emeson RB. Altered RNA editing in mice lacking ADAR2 autoregulation. Mol Cell Biol. 2006;26:480–488. doi: 10.1128/MCB.26.2.480-488.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadsby DC, Rakowski RF, De Weer P. Extracellular access to the Na,K pump: pathway similar to ion channel. Science. 1993;260:100–103. doi: 10.1126/science.7682009. [DOI] [PubMed] [Google Scholar]
- Garrett S, Rosenthal JJ. RNA editing underlies temperature adaptation in K+ channels from polar octopuses. Science. 2012;335:848–851. doi: 10.1126/science.1212795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez C, Lopez-Rodriguez A, Srikumar D, Rosenthal JJ, Holmgren M. Editing of human K(V)1.1 channel mRNAs disrupts binding of the N-terminus tip at the intracellular cavity. Nat Commun. 2011;2:436. doi: 10.1038/ncomms1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graveley BR, Brooks AN, Carlson JW, Duff MO, Landolin JM, Yang L, Artieri CG, van Baren MJ, Boley N, Booth BW, et al. The developmental transcriptome of Drosophila melanogaster. Nature. 2011;471:473–479. doi: 10.1038/nature09715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greger IH, Khatri L, Ziff EB. RNA editing at arg607 controls AMPA receptor exit from the endoplasmic reticulum. Neuron. 2002;34:759–772. doi: 10.1016/s0896-6273(02)00693-1. [DOI] [PubMed] [Google Scholar]
- Greger IH, Khatri L, Kong X, Ziff EB. AMPA receptor tetramerization is mediated by Q/R editing. Neuron. 2003;40:763–774. doi: 10.1016/s0896-6273(03)00668-8. [DOI] [PubMed] [Google Scholar]
- Greger IH, Akamine P, Khatri L, Ziff EB. Developmentally regulated, combinatorial RNA processing modulates AMPA receptor biogenesis. Neuron. 2006;51:85–97. doi: 10.1016/j.neuron.2006.05.020. [DOI] [PubMed] [Google Scholar]
- Gurevich I, Englander MT, Adlersberg M, Siegal NB, Schmauss C. Modulation of serotonin 2C receptor editing by sustained changes in serotonergic neurotransmission. J Neurosci. 2002;22:10529–10532. doi: 10.1523/JNEUROSCI.22-24-10529.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hideyama T, Yamashita T, Suzuki T, Tsuji S, Higuchi M, Seeburg PH, Takahashi R, Misawa H, Kwak S. Induced loss of ADAR2 engenders slow death of motor neurons from Q/R site-unedited GluR2. J Neurosci. 2010;30:11917–11925. doi: 10.1523/JNEUROSCI.2021-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hideyama T, Yamashita T, Aizawa H, Tsuji S, Kakita A, Takahashi H, Kwak S. Profound downregulation of the RNA editing enzyme ADAR2 in ALS spinal motor neurons. Neurobiol Dis. 2012;45:1121–1128. doi: 10.1016/j.nbd.2011.12.033. [DOI] [PubMed] [Google Scholar]
- Hogg M, Paro S, Keegan LP, O’Connell MA. RNA editing by mammalian ADARs. Adv Genet. 2011;73:87–120. doi: 10.1016/B978-0-12-380860-8.00003-3. [DOI] [PubMed] [Google Scholar]
- Holmgren M, Wagg J, Bezanilla F, Rakowski RF, De Weer P, Gadsby DC. Three distinct and sequential steps in the release of sodium ions by the Na+/K+-ATPase. Nature. 2000;403:898–901. doi: 10.1038/35002599. [DOI] [PubMed] [Google Scholar]
- Hoopengardner B, Bhalla T, Staber C, Reenan R. Nervous system targets of RNA editing identified by comparative genomics. Science. 2003;301:832–836. doi: 10.1126/science.1086763. [DOI] [PubMed] [Google Scholar]
- Horsch M, Seeburg PH, Adler T, Aguilar-Pimentel JA, Becker L, Calzada- Wack J, Garrett L, Götz A, Hans W, Higuchi M, et al. Requirement of the RNA-editing enzyme ADAR2 for normal physiology in mice. J Biol Chem. 2011;286:18614–18622. doi: 10.1074/jbc.M110.200881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi T, Zagotta WN, Aldrich RW. Biophysical and molecular mechanisms of Shaker potassium channel inactivation. Science. 1990;250:533–538. doi: 10.1126/science.2122519. [DOI] [PubMed] [Google Scholar]
- Hodgkin AF, Huxley AL. A quantitative description of membrane current and its application to conduction and excitiation in nerve. J Physiol. 1952;1:500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingleby L, Maloney R, Jepson J, Horn R, Reenan R. Regulated RNA editing and functional epistasis in Shaker potassium channels. J Gen Physiol. 2009;133:17–27. doi: 10.1085/jgp.200810133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepson JE, Reenan RA. Adenosine-to-inosine genetic recoding is required in the adult stage nervous system for coordinated behavior in Drosophila. J Biol Chem. 2009;284:31391–31400. doi: 10.1074/jbc.M109.035048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepson JE, Savva YA, Yokose C, Sugden AU, Sahin A, Reenan RA. Engineered alterations in RNA editing modulate complex behavior in Drosophila: regulatory diversity of adenosine deaminase acting on RNA (ADAR) targets. J Biol Chem. 2011;286:8325–8337. doi: 10.1074/jbc.M110.186817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AK, Buckingham SD, Papadaki M, Yokota M, Sattelle BM, Matsuda K, Sattelle DB. Splice-variant- and stage-specific RNA editing of the Drosophila GABA receptor modulates agonist potency. J Neurosci. 2009;29:4287–4292. doi: 10.1523/JNEUROSCI.5251-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara Y, Ito K, Sun H, Aizawa H, Kanazawa I, Kwak S. Glutamate receptors: RNA editing and death of motor neurons. Nature. 2004;427:801. doi: 10.1038/427801a. [DOI] [PubMed] [Google Scholar]
- Keegan LP, McGurk L, Palavicini JP, Brindle J, Paro S, Li X, Rosenthal JJ, O’Connell MA. Functional conservation in human and Drosophila of Metazoan ADAR2 involved in RNA editing: loss of ADAR1 in insects. Nucleic Acids Res. 2011;39:7249–7262. doi: 10.1093/nar/gkr423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krestel HE, Shimshek DR, Jensen V, Nevian T, Kim J, Geng Y, Bast T, Depaulis A, Schonig K, Schwenk F, et al. A genetic switch for epilepsy in adult mice. J Neurosci. 2004;24:10568–10578. doi: 10.1523/JNEUROSCI.4579-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JB, Levanon EY, Yoon JK, Aach J, Xie B, Leproust E, Zhang K, Gao Y, Church GM. Genome-wide identification of human RNA editing sites by parallel DNA capturing and sequencing. Science. 2009;324:1210–1213. doi: 10.1126/science.1170995. [DOI] [PubMed] [Google Scholar]
- Li M, Wang IX, Li Y, Bruzel A, Richards AL, Toung JM, Cheung VG. Widespread RNA and DNA sequence differences in the human transcriptome. Science. 2011;333:53–58. doi: 10.1126/science.1207018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomeli H, Mosbacher J, Melcher T, Höger T, Geiger JR, Kuner T, Monyer H, Higuchi M, Bach A, Seeburg PH. Control of kinetic properties of AMPA receptor channels by nuclear RNA editing. Science. 1994;266:1709–1713. doi: 10.1126/science.7992055. [DOI] [PubMed] [Google Scholar]
- Ohlson J, Pedersen JS, Haussler D, Ohman M. Editing modifies the GABA(A) receptor subunit alpha3. RNA. 2007;13:698–703. doi: 10.1261/rna.349107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver D, Lien CC, Soom M, Baukrowitz T, Jonas P, Fakler B. Functional conversion between A-type and delayed rectifier K+ channels by membrane lipids. Science. 2004;304:265–270. doi: 10.1126/science.1094113. [DOI] [PubMed] [Google Scholar]
- Palavicini JP, O’Connell MA, Rosenthal JJ. An extra double-stranded RNA binding domain confers high activity to a squid RNA editing enzyme. RNA. 2009;15:1208–1218. doi: 10.1261/rna.1471209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palladino MJ, Keegan LP, O’Connell MA, Reenan RA. dADAR, a Drosophila double-stranded RNA-specific adenosine deaminase is highly developmentally regulated and is itself a target for RNA editing. RNA. 2000a;6:1004–1018. doi: 10.1017/s1355838200000248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palladino MJ, Keegan LP, O’Connell MA, Reenan RA. A-to-I pre-mRNA editing in Drosophila is primarily involved in adult nervous system function and integrity. Cell. 2000b;102:437–449. doi: 10.1016/s0092-8674(00)00049-0. [DOI] [PubMed] [Google Scholar]
- Patton DE, Silva T, Bezanilla F. RNA editing generates a diverse array of transcripts encoding squid Kv2 K+ channels with altered functional properties. Neuron. 1997;19:711–722. doi: 10.1016/s0896-6273(00)80383-9. [DOI] [PubMed] [Google Scholar]
- Reenan RA. Molecular determinants and guided evolution of species-specific RNA editing. Nature. 2005;434:409–413. doi: 10.1038/nature03364. [DOI] [PubMed] [Google Scholar]
- Rieder LE, Reenan RA. The intricate relationship between RNA structure, editing, and splicing. Semin Cell Dev Biol. 2011 doi: 10.1016/j.semcdb.2011.11.004. Published online December 8, 2011. [DOI] [PubMed] [Google Scholar]
- Rosenthal JJ, Bezanilla F. A comparison of propagated action potentials from tropical and temperate squid axons: different durations and conduction velocities correlate with ionic conductance levels. J Exp Biol. 2002a;205:1819–1830. doi: 10.1242/jeb.205.12.1819. [DOI] [PubMed] [Google Scholar]
- Rosenthal JJ, Bezanilla F. Extensive editing of mRNAs for the squid delayed rectifier K+ channel regulates subunit tetramerization. Neuron. 2002b;34:743–757. doi: 10.1016/s0896-6273(02)00701-8. [DOI] [PubMed] [Google Scholar]
- Rosenthal JJ, Vickery RG, Gilly WF. Molecular identification of SqKv1A. A candidate for the delayed rectifier K channel in squid giant axon. J Gen Physiol. 1996;108:207–219. doi: 10.1085/jgp.108.3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueter SM, Dawson TR, Emeson RB. Regulation of alternative splicing by RNA editing. Nature. 1999;399:75–80. doi: 10.1038/19992. [DOI] [PubMed] [Google Scholar]
- Rula EY, Lagrange AH, Jacobs MM, Hu N, Macdonald RL, Emeson RB. Developmental modulation of GABA(A) receptor function by RNA editing. J Neurosci. 2008;28:6196–6201. doi: 10.1523/JNEUROSCI.0443-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan MY, Maloney R, Reenan R, Horn R. Characterization of five RNA editing sites in Shab potassium channels. Channels (Austin) 2008;2:202–209. doi: 10.4161/chan.2.3.6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton M, Carlson JW, Celniker SE. RNA editing in Drosophila melanogaster: New targets and functional consequences. RNA. 2006;12:1922–1932. doi: 10.1261/rna.254306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit AK, Derst C, Wegner S, Heinemann U, Zahn RK, Decher N. RNA editing of Kv1.1 channels may account for reduced ictogenic potential of 4-aminopyridine in chronic epileptic rats. Epilepsia. 2011;52:645–648. doi: 10.1111/j.1528-1167.2011.02986.x. [DOI] [PubMed] [Google Scholar]
- Wahlstedt H, Daniel C, Ensterö M, Ohman M. Large-scale mRNA sequencing determines global regulation of RNA editing during brain development. Genome Res. 2009;19:978–986. doi: 10.1101/gr.089409.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulff BE, Sakurai M, Nishikura K. Elucidating the inosinome: global approaches to adenosine-to-inosine RNA editing. Nat Rev Genet. 2011;12:81–85. doi: 10.1038/nrg2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Wang Q, Kanes SJ, Murray JM, Nishikura K. Altered RNA editing of serotonin 5-HT2C receptor induced by interferon: implications for depression associated with cytokine therapy. Brain Res Mol Brain Res. 2004;124:70–78. doi: 10.1016/j.molbrainres.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Zhou M, Morais-Cabral JH, Mann S, MacKinnon R. Potassium channel receptor site for the inactivation gate and quaternary amine inhibitors. Nature. 2001;411:657–661. doi: 10.1038/35079500. [DOI] [PubMed] [Google Scholar]