Abstract
Immunoglobulin A nephropathy (IgAN) is the most common form of glomerulonephritis throughout the world. A majority (approx. 60%) of patients with IgAN experience disease exacerbations associated with an acute respiratory or gastrointestinal illness that appears to represent a viral infection. However, the exact mechanism of the disease exacerbation by viral infection is not understood, especially at the cellular and molecular levels. Here we report that glomerular podocytes express the major sensors for double-stranded RNA (dsRNA), a common byproduct of viral replication. In addition to these receptors, Toll-like receptor 3 (TLR3) and retinoic acid-inducible gene 1 (RIG-I)-like helicases (RLHs), podocytes express the collateral proteins required to support intracellular signaling. The pathways that mediate responses to dsRNA are fully functional in podocytes. The transcription factor interferon regulatory factor 3 (IRF3) and nuclear factor kappa B (NF-ĸB) are phosphorylated and translocate to the nucleus, and dsRNA increases synthesis of proteins driven by IRF3 (P54, P56 and P60) or NF-ĸB (interleukin 8 and A20). Furthermore, dsRNA suppresses podocyte cell migration, alters the expression of a panel of podocyte essential proteins (nephrin, podocin and CD2-associated protein or CD2AP) and changes transepithelial albumin flux. These effects are dsRNA sensor-specific: TLR3−/− podocytes do not respond to extracellular dsRNA, while intracellular dsRNA has no effect on podocytes bearing a dominant negative form of the major active RLH. These results demonstrate that innate responses to viruses can disturb podocyte cell function in vitro.
Key Words: Glomerulopathy, Immunoglobulin A nephropathy, Viral infection, Podocyte cell function
Introduction
The innate immune system is the first line of defense against microbial infections [1, 2]. Most pathogenic microbes produce one or more common motifs; such pathogen-associated molecular patterns (PAMPs) can interact with various receptors or adaptor proteins that are anchored within cellular membranes or free in the cytosol. Upon ligation to the relevant PAMP, these receptors or adaptor proteins transmit intracellular signals that evoke acute inflammation and innate cellular responses to either blunt or eliminate the infection [3, 4].
Among the sensors of PAMPs particularly relevant to viral infection, several interact with double-stranded RNA (dsRNA). Most viral pathogens for mammals produce dsRNA incidental to viral replication. Membrane-bound Toll-like receptor 3 (TLR3) and a family of cytosolic helicases bind to dsRNA. As the first recognized cytosolic sensor for dsRNA, retinoic acid-inducible gene 1 (RIG-I) lent its name to the family of helicases, termed RIG-I-like helicases (RLHs); Mda-5 and LGP2 are the only other known RLHs at present [5]. Depending on cell lineage, TLR3 is present mainly on endosomal membrane, but also on plasma membrane in some types of cells. Whereas TLR3 binds primarily to dsRNA either after endocytosis or in extracellular fluids, RLHs recognize cytoplasmic dsRNA. Importantly, both dsRNA sensors eventually induce antiviral and inflammatory responses primarily through the activation of two transcription factors, interferon regulatory factor 3 (IRF3) and nuclear factor kappa B (NF-ĸB) [6, 7].
Some forms of glomerulonephritis, i.e. inflammation of the filtering apparatus in kidneys, are associated with viral infection. Notably, immunoglobulin A nephropathy (IgAN), the most common form of glomerulonephritis throughout the world, develops and is exacerbated in close temporal association with viral infections. Glomerulonephritis is a recognized consequence of hepatitis C infection; glomerulonephritis instigated by hepatitis B or by systemic lupus erythematosus or other autoimmune diseases might also be caused or intensified by innate responses to viruses [8]. On the other hand, the glomerulopathy instigated by human immunodeficiency virus I is induced by the cytopathic effects of proteins produced by the retrovirus and is unrelated to viral replication. Notably, the onset and episodic flares of clinical IgAN and the derangements in glomerular function in a murine model of IgAN induced by immunization and challenge with murine paramyxovirus are closely related to viral replication. We hypothesized that glomerular dysfunction arises from synergy between the glomerular deposition of immune complexes and innate responses to viral replication. We focused on dsRNA because this PAMP depends upon replication of, rather than simply the presence of a virus.
In patients with IgAN, proteinuria, excretion of protein in the urine is an important predictor of the course of disease and a marker for response to therapy. Proteinuria is also an index of the severity of glomerulonephritis in animal models. Visceral glomerular epithelial cells, those that line the glomerular capillary tufts at the interface with the urinary (Bowman's) space, have become well recognized as a determinant of glomerular permselectivity. These cells, also known as podocytes, are highly specialized epithelial cells that extend a series of actin-rich projections known as foot processes, which interdigitate to form a sheath around the glomerular capillaries. A highly organized multiprotein complex formed between juxtaposed foot processes establishes a distinct porous cell junction known as the slit diaphragm serves as the final sieve of the glomerular filter [9]. Injury to or loss of podocytes causes proteinuria.
Accordingly, we investigated the innate responses of podocytes to dsRNA, reasoning that such responses might contribute to glomerular dysfunction, and especially to proteinuria, in glomerulonephritis associated with viral infection. We report that TLR3 and RLH signalings in podocytes lead to structural and functional changes that might influence glomerular permeability to serum proteins and/or glomerular response to injury instigated by immune complexes. These observations might elucidate the molecular mechanisms operative in glomerulonephritis, especially those associated with viral infection, and might ultimately offer novel strategies for therapy of these diseases.
Materials and Methods
Cell Cultures and Stimuli
Wild-type murine podocytes, a generous gift from Dr. Peter Mundel (Boston, Mass., USA), and TLR3−/− podocytes, generated from TLR3−/− ‘immorto-mice’ [10, 11], were propagated on collagen I-coated dishes at 33°C (permissive temperature) in RPMI supplemented with 10% FBS and 10 U/ml of recombinant mouse IFN-γ (Invitrogen). Murine podocytes expressing RIG-Ic, a dominant negative form of RIG-I, were generated by transfection with pBos-Flag-RIG-Ic (a generous gift from Dr. Takashi Fujita, University of Kyoto, Kyoto, Japan) and pBABE-puro (Addgene, Cambridge, Mass., USA) selected by puromycin (1 µg/ml). RIG-Ic expression was confirmed by Western blot with Flag antibody (Sigma, St. Louis, Mo., USA). To induce differentiation, the medium was changed to RPMI with 10% FBS without IFN-γ, and the cells were shifted to 37°C (nonpermissive temperature) for 7-14 days. Under these conditions, cells underwent growth arrest, increased in size, and developed elongated cell processes. Immortalized human podocytes, generously provided by Dr. Moin Saleem (University of Bristol, Bristol, UK) through Dr. Jeffrey Kopp (NIH, Bethesda, Md., USA), were cultured as described previously [12] and used for experiments under permissive conditions. Mouse embryonic fibroblasts (MEFs), either wild-type or lacking TLR3, TRIF, IRF3, RIG-I, Mda-5, IPS-1 or TBK-1, Raw 264.7, and human fibrosarcoma HT1080 cells were all maintained in DMEM supplemented with 10% FBS (Atlanta Biologicals, Lawrenceville, Ga., USA), 100 U/ml penicillin and 100 µg/ml streptomycin [13, 14]. Polyinosinic-polycytidylic acid [Poly(I:C)], a synthetic dsRNA, was purchased from GE Healthcare (Piscataway, N.J., USA), and FuGENE 6 was obtained from Roche Applied Science (Indianapolis, Ind., USA). Staurosporine was purchased from Sigma and was used at 1 µM. Sendai virus (SeV) was obtained from ATCC (Manassas, Va., USA) and used at a multiplicity of infection (MOI) of 10.
RT-PCR and Immunoblotting
RT-PCR was performed as previously described [15], with various primer pairs (table 1). Amplified bands were separated on agarose gels and detected with ethidium bromide. As detailed elsewhere [13], for immunoblotting, cells were subjected to lysis in a buffer (150 mM NaCl, 20 mM HEPES [pH 7.4], 1.5 mM MgCl2, 2 mM DTT, 2 mM EGTA, 10 mM NaF, 12.5 mM β-glycerophosphate and 1 mM Na3VO4) supplemented with complete EDTA-free protease inhibitor (Roche Applied Science) with 0.5% (v/v) Triton X-100. Cytosolic and nuclear fractions of cellular proteins were isolated by procedures described earlier [14]. After separation on PAGE gels, proteins were transferred to PDVF membranes. The blots were incubated with antibodies specific for mouse or human TLR3 (Abcam, Cambridge, Mass. and IMGENEX, San Diego, Calif., USA, respectively), P54, P56, P60 (generated in our lab), IRF3 (Invitrogen, Camarillo, Calif., USA), pS396 IRF3 and cleaved PARP (Cell Signaling Technology, Danvers, Mass., USA), NF-ĸB P65 (Santa Cruz, Calif., USA), pS32 IĸBα, IĸBα, CD2AP, RIG-I, IPS-1/MAVS (Cell Signaling Technology), A20 (IMGENEX) nephrin, podocin (Abcam), DRBP76 (BD Biosciences, San Jose, Calif., USA) or tubulin (MD Bioscience, San Diego, Calif., USA).
Table 1.
Primer sequences and product sizes
| Forward primer | Reverse primer | Product size, bp | |
|---|---|---|---|
| Mouse | |||
| TLR3 | ttgtcttctgcacgaacctg | cgcaacgcaaggattttatt | 206 |
| TRIF | cactgcctccagtctcttcc | gatcagtcagagggcccata | 213 |
| RIG-I | atctcaacaacggagccatc | gcggtcttagcatctccaac | 199 |
| Mda-5 | gcagtggctcaggagttacc | gcagtggctcaggagttacc | 244 |
| IPS-1 | ggacacactctggggactct | ggtcagggatgttgtgacct | 161 |
| TBK-1 | atggagttttgtccctgtgg | tgatgttgcctggcttgata | 169 |
| IRF3 | ctggctagagcatggaaacc | gatgccaaagtcagccatct | 170 |
| GAPDH | gaatgggaagcttgtcatcaa | ctaagcagttggtggtgcag | 288 |
| Human | |||
| TLR3 | acccataccaacatccctga | gccctcaaagtggatgagaa | 192 |
| TRIF | actgtgtcatccccttcctg | tgtcctgttccttcctccac | 186 |
| RIG-I | agagcacttgtggacgcttt | tgcaatgtcaatgccttcat | 213 |
| Mda-5 | catctgattggagctggaca | tgtgagcaaccaggacgtag | 250 |
| IPS-1 | ataagtccgagggcaccttt | gtgactaccagcacccctgt | 208 |
| TBK-1 | agcggcagagttaggtgaaa | tgagtgccttcttgatgtgc | 156 |
| IRF3 | gaggtgacagccttctaccg | tgcctcacgtagctcatcac | 176 |
| GAPDH | gtcagtggtggacctgacct | tgctgtagccaaattcgttg | 245 |
Plasmid Construct and Recombinant Protein
The plasmid encoding the cytoplasmic domain of human TLR3 (CTD, AA726-904, Swiss-Prot: O15455) was generated by PCR amplification using full-length TLR3 as a template and cloned into pHis parallel vector (Roche). Escherichia coli BL21(DE3) cells were transformed with pHis parallel vectors. TLR3-CTD was induced by 1 mM isopropyl β-D-thiogalactopyranoside (IPTG) for 8 h at 30°C. Protein purification was achieved using Ni-NTA superflow beads (Qiagen) as described previously [16].
Immunocytochemistry and ELISA
Human or murine podocytes were cultured on type I collagen-coated coverslips for 7–10 days under nonpermissive condition. Cells were fixed with 3% paraformaldehyde in PBS for 20 min at room temperature, were rinsed 3 times with PBS, and then permeabilized by incubating with 100% methanol for 5 min. Cells were again rinsed 3 times with PBS and then blocked with 10% goat serum in PBS for 1 h. Cells were incubated with the primary antibodies against human or murine TLR3 (Abcam or IMGENEX, respectively), IRF3 (Invitrogen) or NF-ĸB P65 (Santa Cruz) overnight at 4°C. After washing 3 times with PBS, cells were incubated with the Alexa 488-labeled appropriate secondary antibody (Invitrogen) for 1 h at room temperature. Cells were again rinsed 3 times with PBS, mounted using mounting medium and then visualized using a fluorescent microscope. To detect interleukin (IL)-8 protein in culture supernatants, we employed a commercial ELISA kit (human IL-8 DuoSet; R&D Systems, Minneapolis, Minn., USA), following the manufacturer's instructions.
Wound Healing Assay
The wound healing assay has been described elsewhere [13]. Briefly, podocytes were grown on type I collagen-coated 6-well plates for 7–10 days to be differentiated. Wounds were created by scratching the monolayer with the tip of a micropipette. After the cells were washed with warm media, they were maintained in regular media with 10% FBS, with or without stimulus, for 24 h. To analyze cell migration, the same area of the culture surface was photographed at 0 h and the end of the incubation time. Cells migrating into the defect were enumerated in at least 8 fields for each condition, and normalized to the number of migrating cells without any stimulation (defined as 100%).
Transepithelial Albumin Flux
To evaluate the filtration barrier function of podocytes [17, 18], differentiated human podocytes were incubated in growth-permissive conditions (as above), then split at 95% confluence and seeded onto 5.0-µm Transwell® permeable supports (CoSTART, Corning, N.Y., USA) with RPMI (no phenol red) containing 10% FBS. The basolateral chamber was supplemented with 500 µg/ml of bovine serum albumin conjugated to fluorescein (Invitrogen). Triplicate wells were incubated at 37°C with or without stimulation. At selected times, a 50-µl aliquot of apical medium was transferred to a clean well in a 96-well assay plate (BD Falcon Optiflux black/clear bottom plates), and replaced with fresh prewarmed medium. The fluorescent albumin concentration in each well at each time point was read on a Victor Fluorescence Multiplate Reader (Perkin Elmer).
Statistics
Statistical analyses were performed using Prism 5 (GraphPad Software, La Jolla, Calif., USA). Error bars represent SEM, and differences were analyzed using ANOVA with the Bonferroni post hoc test.
Results
TLR3 and RLH Signaling Molecules in Murine and Human Podocytes
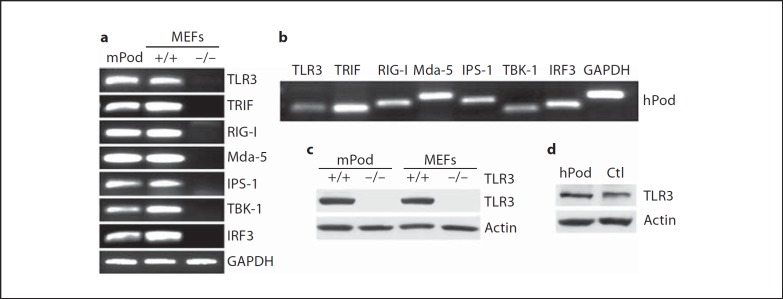
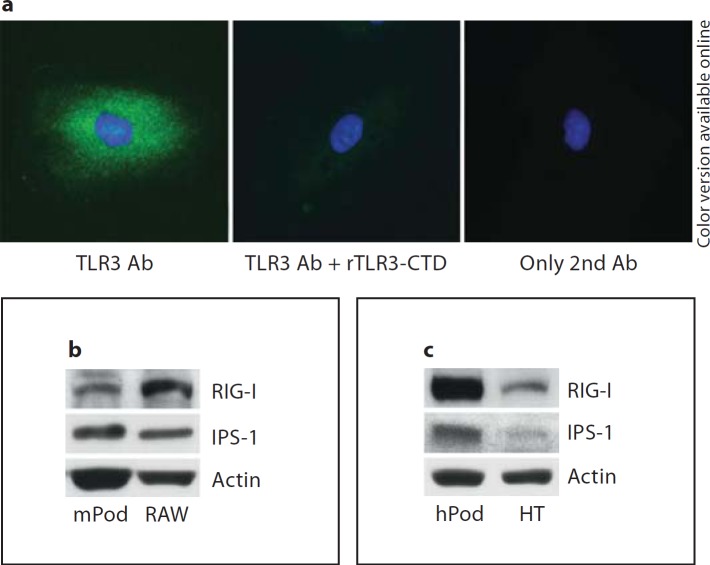
We examined the expression of various signaling molecules in podocytes. Both murine podoyctes (fig. 1a) and human podocytes (fig. 1b) express TLR3, TRIF, RIG-I, Mda-5, IPS-1, TBK-1 and IRF3 at the messenger RNA (mRNA) level. However, in human podocytes, the level of expression of TLR3, RIG-I and TBK1 relative to GAPDH appears lower than that in murine podocytes. Murine podocytes (fig. 1c) and human podocytes (fig. 1d) produce demonstrable TLR3 protein. Murine podocytes express TLR3 at levels comparable to wild-type (+/+) MEFs, whereas neither TLR3−/− podocytes nor TLR3−/− MEFs have detectable protein. Likewise, human podocytes express similar or higher amounts of TLR3 compared to the human fibrosarcoma cell-line HT1080 used as a positive control [19]. By immunocytochemistry, human podocytes exhibited a punctate pattern of staining for TLR3 throughout the cytoplasm but predominantly in the perinuclear areas (fig. 2a, left panel), which is suggestive of localization within endosomes. Like TLR3, murine podocytes as well as human podocytes express RIG-I and IPS-1 proteins, as detected by Western blot (fig. 2b, c).
Fig. 1.
TLR3 and RLH signaling molecules in podocytes. a mRNA was isolated from mouse podocytes (mPod), wild-type MEFs (+/+), or knock-out MEFs (−/−) as indicated in the figure. The expression of each molecule at the mRNA level was tested by RT-PCR. GAPDH bands are representative bands from IRF3 RT-PCR. b mRNA from human podocytes (hPod) was tested for each signaling molecule as indicated, by RT-PCR. c Expression of TLR3 protein in mouse podocytes (mPod) or MEFs was tested by Western blot; for both mPod and MEFs, cells from wild-type (+/+) mice were compared to cells from mice with targeted deletion of the TLR3 gene (−/−). d Expression of TLR3 protein was tested in human podocytes (hPod) by Western blot. As a positive control (Ctl), lysate from the human fibrosarcoma cell line HT1080 was loaded.
Fig. 2.
Podocytes express TLR3, RIG-I, and IPS-1. a Human podocytes were incubated with anti-TLR3-CTD mouse IgM Ab without (left panel, TLR3 Ab) or with (middle panel, TLR3 Ab + rTLR3-CTD) preincubation with recombinant hTLR3-CTD. Control cells were incubated with buffer (right panel, only 2nd Ab). All cultures were then stained with Alexa 488-anti-mouse IgM (TLR3 Ab) and DAPI. b The expression levels of RIG-I and IPS-1 proteins in murine podocytes (mPod) were analyzed by Western blot. Raw 264.7 (RAW), a murine macrophage cell line, was used as positive control. c RIG-I and IPS-1 expression levels in human podocytes (hPod) were tested by Western blot. HT1080 (HT), a human fibrosarcoma cell line, was used as positive control.
IRF3 Pathway Activation by TLR3 or RLH Stimulation
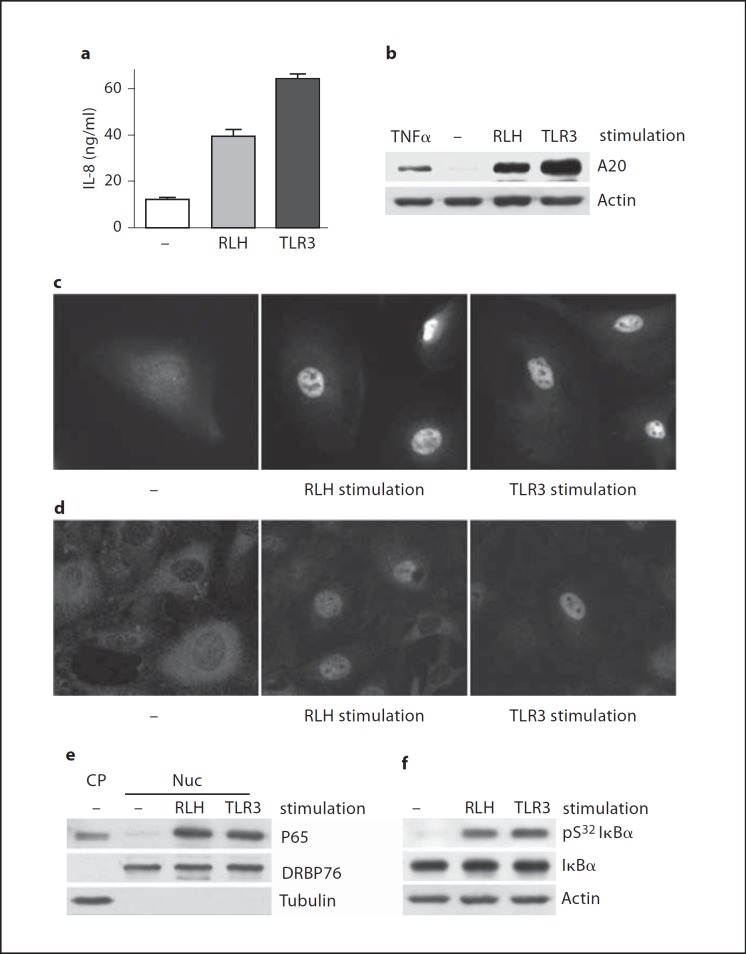
To determine whether TLR3 and RLH function to transmit intracellular signals in podocytes, we examined the expression of P54, P56 and P60. Synthesis of these proteins is driven by IRF3 as a common transcription factor [4]. Stimulation by TLR3 or RLH agonists strongly induced P56 and P60 in human podocytes after 8 h (fig. 3a). Mouse podocytes with a homozygous deletion of TLR3 (TLR3−/−) produced P54 in response to RLH stimulation, but not in response to TLR3 stimulation (fig. 3b). Addition of as little as 1 µg/ml of extracellular dsRNA to human podocytes induced P56 and P60 (via TLR3) by 8 h (fig. 3c), and 50 µg/ml of extracellular dsRNA elicited P56 and P60 protein after 4 or 16 h, respectively (fig. 3d).
Fig. 3.
IRF3 is activated by TLR3 or RLH stimulation. a Human podocytes were left unstimulated (-) or stimulated by Poly(I:C) transfection [RLH, 2 µg/ml Poly(I:C) + 3 µl/ml FuGENE6] or by adding 50 µg/ml Poly(I:C) to the culture medium (TLR3) for 8 h. The cell lysates were probed by Western blot for human P56 and P60. b Podocytes from TLR3−/− mice were left unstimulated (-) or treated by adding (TLR3) or transfecting (RLH) Poly(I:C); after 8 h, cell lysates were tested for mouse P54 by Western blot. c Human podocytes were stimulated by adding varying concentrations (0–100 µg/ml) of Poly(I:C) as indicated for 8 h; blots from cell lysates were probed for P56 or P60. d Human podocytes were stimulated by adding 50 µg/ml Poly(I:C) for varying incubation times (0–24 h), as indicated; blots were stained for P56 or P60. Immunoblotting for actin served as a loading control for all Western blots. e Human podocytes, grown on coverslips, were left unstimulated (-) or stimulated by transfection with (RLH) or addition of (TLR3) Poly(I:C) for 3 h, as above. Cells were then stained with anti-IRF3 Ab. f Mouse podocytes were grown on coverslips and left untreated (-) or transfected (RLH) or added (TLR3) Poly(I:C) for 3 h, as above and stained with anti-IRF3 Ab. g Cytosolic (CP) or nuclear (Nuc) proteins fractionated from human podocytes were collected 3 h after no addition of dsRNA (-) or after RLH or TLR3 stimulation. IRF3 in the fractions was detected by Western blot. The membranes were also probed for DRBP76 and tubulin as markers for nuclear and cytoplasmic proteins, respectively. h Phosphorylation of IRF3 at serine 396 (pS396 IRF3) was tested by Western blots applied to lysates from human podocytes left untreated or stimulated for 3 h by RLH or TLR3 ligands (as above). Immunostains for total IRF3 and actin were used as loading controls.
The transcription factor IRF3 can be activated by either TLR3 or RLH stimulation. In both human (fig. 3e) and murine (fig. 3f) podocytes, IRF3 was translocated into the nucleus in response to either RLH or TLR3 stimulation (middle or right panels, respectively); in unstimulated cells, IRF3 is localized mainly in the cytoplasm (left panels). In human podocytes, IRF3 was present in nuclear as well as cytosolic fractions within 3 h after RLH or TLR3 stimulation, but was confined to the cytosolic fraction in the unstimulated cells (fig. 3g). Transcriptional activation of gene products driven by IRF3 requires phosphorylation of IRF3 at serine 396 [14]. After stimulation via RLH or TLR3 for 3 h, IRF3 phosphorylated at serine 396 was readily detected in human podocytes, whereas the phosphorylated form was not detected in the unstimulated cells (fig. 3h). These data demonstrate that the IRF3 pathway in podocytes is activated by TLR3 or RLH signaling.
NF-ĸB Pathway Activation by TLR3 or RLH Stimulation
In addition to IRF3, NF-ĸB is a major transcription factor that regulates inflammatory responses to TLR3 and RLH signaling. After 24 h, culture supernatants from human podocytes contained double or triple the amount of the chemokine IL-8, 24 h after transfection with (RLH stimulation) or extracellular addition of (TLR3 stimulation) dsRNA (fig. 4a). Likewise, the regulatory protein A20 was upregulated in human podocytes after 8 h of RLH or TLR3 stimulation (fig. 4b). Synthesis of these proteins, among others, is driven by the activation of NF-ĸB. To assess activation of NF-ĸB directly, we examined NF-ĸB P65 nuclear translocation in human and murine podocytes by immunocytochemistry. Both human podocytes (fig. 4c) and murine podocytes (fig. 4d) translocated P65 to the nucleus within 3 h of RLH or TLR3 stimulation. Furthermore, whereas P65 was confined to the cytosol in the unstimulated human podocytes, stimulation via RLH or TLR3 promoted accumulation of P65 in the nuclear fraction (fig. 4e). Finally, IkBα, the regulatory inhibitor of NF-ĸB, was phosphorylated at serine 32 in human podocytes within 3 h of RLH or TLR3 stimulation (fig. 4f). This phosphorylation leads to proteasomal degradation of IĸBα, releasing P65 for heterodimer formation and nuclear translocation. Thus, functional activation of NF-ĸB occurs in podocytes after TLR3 and RLH ligation.
Fig. 4.
NF-ĸB is activated by TLR3 or RLH stimulation. a Culture supernatants from human podocytes were collected after 24 h incubation in medium (-), or transfection with (RLH) or addition of (TLR3) Poly(I:C) as detailed for figure 3. Release of human IL-8 was measured by ELISA. b Western blotting of lysates from human podocytes stimulated for 8 h with 20 ng/ml TNFα as a positive control, left unstimulated (-) or stimulated by transfection with (RLH) or addition of (TLR3) Poly(I:C) disclosed production of A20, an NF-ĸB-driven gene product in response to dsRNA. c Human podocytes, grown on coverslips, were left unstimulated (-) or stimulated by transfection with (RLH) or addition of (TLR3) Poly(I:C) for 3 h. Cells were then stained with anti-NF-ĸB P65 Ab. d Similarly, mouse podocytes were grown on coverslips and left untreated (-) or transfected (RLH) or added (TLR3) Poly(I:C) for 3 h, and stained with anti-P65 Ab. e Nuclear translocation of P65 in human podocytes was tested as in figure 3g. Tubulin and DRBP76 marked the cytoplasmic and nuclear fractions, respectively. f Phosphorylation of IĸBα at serine 32 (pS32 IĸBα) was detected by Western blot in human podocytes 3 h after RLH or TLR3 stimulation. Protein loading was evaluated by blotting for total IĸBα and actin.
Innate Responses to Viral Infection Disturb Cell Migration of Podocytes
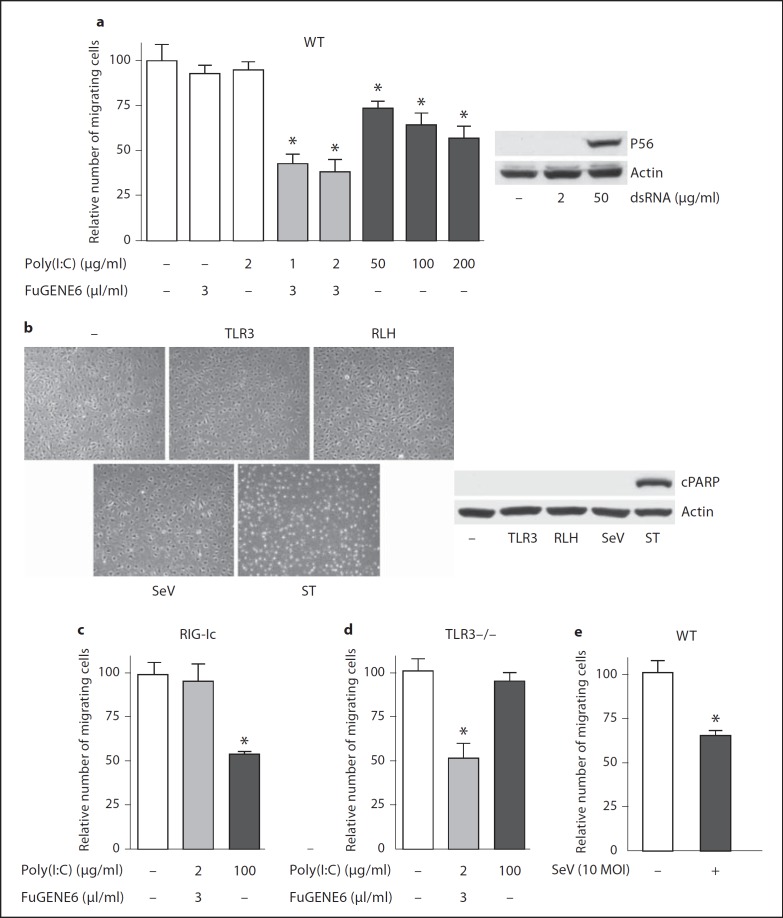
Cell migration is a complex and highly regulated cellular function that is important in a wide variety of physiological and pathologic conditions [20, 21, 22]. To evaluate the functional effects of innate podocyte responses to viruses, we measured cell migration in a wound-healing assay. Stimulation of wild-type murine podocytes by transfection with (via RLH signaling) or the addition of (via TLR3 signaling) dsRNA strongly suppressed migration of cells into a standardized defect introduced into the culture monolayer (fig. 5a, left). In contrast to RLH signaling, TLR3 stimulation by 2 µg/ml of Poly(I:C) was not enough to induce the cell migration effect or expression of the IRF3-dependent gene product, P56 (fig. 5a, right). The suppressed cell migration was not due to cell death by dsRNA stimulation; cell monolayers appeared intact (fig. 5b, left panel) and did not exhibit cleavage of PARP, a marker of apoptotic cells (fig. 5b, right panel). As expected, treatment with staurospoine (1 µM), a strong inducer of mitochondrial dysfunction, triggered rapid, morphologically evident cell death and the generation of cleaved PARP in murine podocytes (fig. 5b). Addition of, but not transfection with, dsRNA suppressed wound healing in murine podocytes that bore a dominant negative RIG-I construct and were therefore defective in RLH signaling (fig. 5c). Reciprocally, transfected, but not added, dsRNA suppressed wound healing in TLR3−/− murine podocytes (fig. 5d). These data indicate that either cytosolic or extracellular dsRNA, dependent on RLH or TLR3 signaling, respectively, exerts significant effects on podocyte function. Likewise, infection of wild-type murine podocytes by SeV leads to defective wound healing (fig. 5e), without triggering cell death (fig. 5b).
Fig. 5.
Antiviral innate immunity suppresses cell migration. a Wild-type (WT) murine podocytes were stimulated as indicated in the abscissa. Migration of cells into a defect in the monolayer in a wound-healing assay was quantified 24 h after the stimulation (left panel). Cells left untreated, incubated in FuGENE6 alone, or incubated in the presence of 2 µg/ml Poly(I:C) are in white bars; cells subject to Poly(I:C) transfection (with FuGENE6 and low-dose Poly(I:C) are in gray bars, and cells treated by adding (high-dose, extracellular) Poly(I:C) are in black bars. Murine P56 induction in WT murine podocytes was analyzed by Western blot (right panel). Cells were treated by adding extracellular Poly(I:C) at indicated concentrations for 16 h. b Murine podocytes were stimulated with added Poly(I:C) (50 µg/ml, TLR3) or transfected Poly(I:C) (RLH), infected by SeV (10 MOI) or treated with staurosporine (1 µM, ST) for 24 h. Culture fields were photographed (left) and cell lysates were analyzed for cleaved PARP (cPARP) by Western blot (right). c Migration of murine podocytes expressing a dominant negative RIG-I (RIG-Ic) was measured by a wound-healing assay as described above in a. d Migration of podocytes isolated from TLR3−/− mice was tested as described above in a. e Migration of WT murine podocytes was tested by a wound-healing assay after 24-hour incubation in culture medium (white bar) or infection by SeV (10 MOI, black bar). * p < 0.05, versus untreated cells in culture medium.
TLR3 or RLH Activation Alters the Expression Levels of Podocyte-Specific Proteins and Changes Transepithelial Albumin Flux
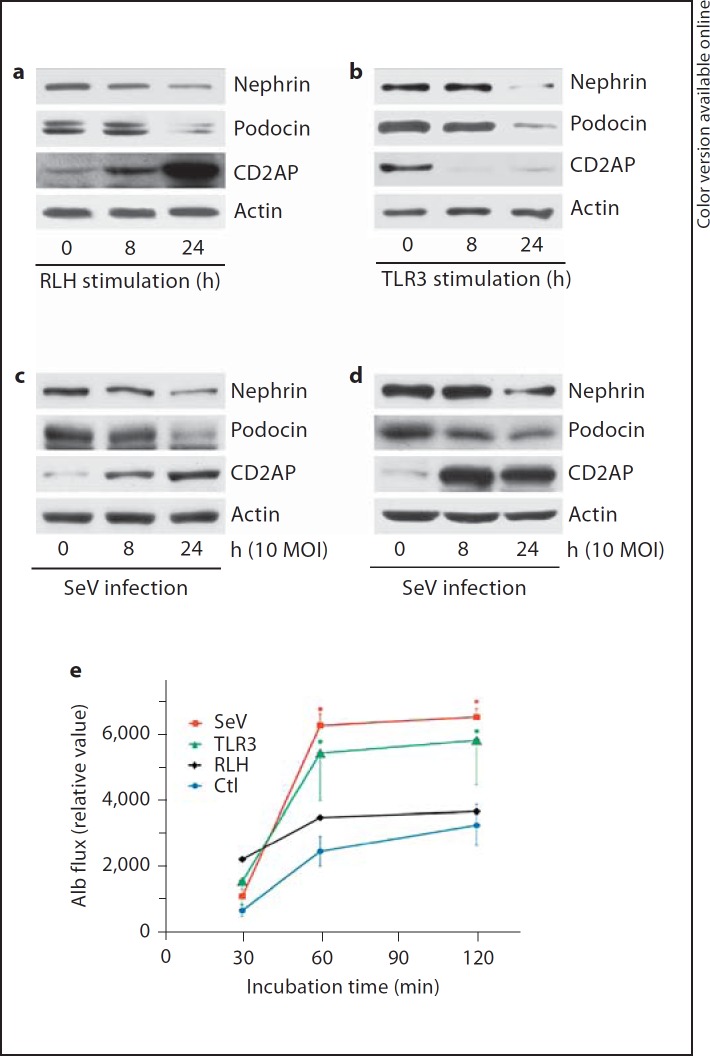
Nephrin, podocin and CD2AP are proteins that are widely recognized as essential for podocyte function. Mutation of any of these molecules causes nephrotic syndrome, leading to end-stage renal failure, and the level of these proteins is altered in a variety of glomerular diseases [23, 24, 25]. To ascertain if innate responses to viruses influence the expression of these molecules, we performed Western blotting on lysates from podocytes. RLH stimulation decreased nephrin and podocin expression within 24 h and strongly increased CD2AP levels within 8 h (fig. 6a). On the other hand, TLR3 stimulation of human podocytes had decreased CD2AP after 8 h and nephrin and podocin expression after 24 h (fig. 6b). Although dsRNA represents a surrogate of virus infection, the subsequent outcomes vary with virus and cell types. We therefore tested the expression of podocyte marker proteins after viral infection. The murine paramyxovirus SeV downregulated the expression of nephrin and podocin in both human (fig. 6c) and murine (fig. 6d) podocytes, but increased the expression of CD2AP. Taken together, innate immune responses to dsRNA or SeV infection perturbs expression of proteins that are specific to and functionally important for podocytes.
Fig. 6.
TLR3 or RLH activation by dsRNA, or SeV infection, alters the expression levels of podocyte-specific proteins and changes transepithelial albumin flux. a Human podocytes were transfected with Poly(I:C) using FuGENE6 for the indicated time. Cell lysates were analyzed for nephrin, podocin and CD2AP by Western blot. b Human podocytes were stimulated by added Poly(I:C) for the indicated time; cell lysates were probed for nephrin, podocin and CD2AP by Western blot. For all blots, actin was used as a loading control. Human (c) or murine (d) podocytes were infected with SeV at 10 MOI; cell lysates were collected at the indicated time, and analyzed for nephrin, podocin or CD2AP by Western blot. e Differentiated human podocytes grown on Transwell® permeable supports were infected by SeV, TLR3 stimulation (TLR3) or RLH stimulation (RLH), or were left untreated (Ctl) for the indicated times. The content of fluoresceinated albumin in the apical chambers (relative units on the ordinate) indicates transepithelial flux from the basolateral medium across the monolayer. The error bars indicate 1 SEM. * p < 0.05, versus cells with no stimulus at the corresponding time point. For clarity, error for SeV is indicated only in the positive direction and error for TLR3 stimulation is indicated only in the negative direction. The (large) errors associated with RLH stimulation (p = n.s. vs. no stimulus) are not plotted.
Monolayers of differentiated podocytes cultured on cell well inserts polarize, form the organized junctions observed in vivo and restrict the flux of macromolecules between basolateral and apical surfaces. Transcellular macromolecular flux across podocyte monolayers has been employed as an in vitro homologue to proteinuria [17, 18]. Therefore, in addition to assessment of the in vitro wound healing and expression of the proteins intrinsic to the slit diaphragm (fig. 5, 6), we measured transcellular flux of fluoresceinated albumin across differentiated human podocyte monolayers on culture inserts. Infection of polarized human podocytes by SeV doubled or tripled the passage of albumin from the basolateral to the apical chamber, compared to the flux observed with cells left untreated in culture medium (fig. 6e). Human podocytes also increased paracellular albumin permeability after the addition of extracellular dsRNA (TLR3 stimulation), whereas transfection with dsRNA (RLH stimulation) exerts an intermediate effect on albumin transport with considerable variability. Addition of FuGENE6 without dsRNA exerted no significant effect compared to cells incubated in medium (data not shown). None of these stimuli leads to cell death or apoptosis (fig. 5b). The effects we observed are similar in magnitude and kinetics to those reported in several other systems focused on elucidating the contribution of injury to podocytes on proteinuria.
Discussion
Clinical onset of the most common form of glomerulonephritis, IgAN, is often associated with respiratory or gastrointestinal syndromes that are apparently viral infections. Moreover, exacerbations of disease in the episodic pattern of IgAN are closely associated with viral disease. Up to one third of patients with IgAN follow a clinical course of progressive loss of renal function, culminating over decades in glomerulosclerosis and end-stage renal failure. Progression of IgAN to irreversible nephron loss is best predicted by the severity of proteinuria at the time of onset. Patients in whom initial proteinuria subsides are much less likely to progress to end-stage renal disease than individuals with similar initial proteinuria that persists or increases. Therefore, amelioration of proteinuria is widely considered to be a therapeutic goal.
In a murine model of IgAN induced by immunization and subsequent challenge with SeV, clinical signs of glomerulonephritis are provoked only after challenge with the infectious virus; challenge of immunized mice with inactivated SeV leads to accretions of immune complexes in glomeruli, but does not produce any measurable derangement in glomerular function, even at higher doses intended to compensate for the lack of viral replication [26, 27, 28]. Conversely, challenge of nonimmune mice with the infectious virus does not elicit nephritis. Accordingly, we hypothesized that the replication of a virus in mice with a specific antiviral immune response is required for glomerular dysfunction in this mouse model; presumably, there is synergy between the glomerular deposition of immune complexes and (glomerular or extraglomerular) innate responses to viral replication. We focused on dsRNA as an important intermediate of viral replication because other PAMPs associated with viral exposure are not limited to infectious viruses and therefore would likely be invoked subsequent to challenge with an inactivated as well as an infectious virus. This report clearly shows that innate responses to dsRNA via TLR3 or RLH signaling perturb podocyte cell function and alter podocyte marker expression levels.
Podocytes are highly specialized and terminally differentiated epithelial cells that play an essential role in glomerular ultrafiltration. Proteinuria of glomerular origin, caused by leakage of protein from the blood across the glomerular capillary wall into the urinary space, can arise from injury to glomerular endothelial cells, the glomerular basement membrane, podocytes or any combination of these structures. The stereotypical morphologic response of podocytes to injury is effacement of the glomerular foot processes, characterized by loss of interdigitation and disruption of the slit diaphragm; these changes are closely associated with clinical proteinuria [29], and often lead to renal failure. Proteinuria cannot be determined directly at the cell culture level. Surrogates in vitro for foot process effacement or proteinuria in vivo include altered migration of cultured podocytes [30, 31], defective expression of proteins specific to podocytes such as nephrin [23] or podocin [24] or those limited to a few cell lineages including podocytes such as CD2AP [25], and increased transepithelial albumin flux [17, 18]. We report (fig. 5, 6) that podocytes exhibit changes in all these features in response to dsRNA or infection by SeV. These observations corroborate the principle that dsRNA can contribute to glomerular injury associated with immune complex deposition. Podocytes reside at the glomerular filtration barrier, and are subject to infection during viremia; these cells are also highly exposed to circulating viral products. Indeed, in a passive mouse model, we observed that parenteral injection of dsRNA into mice given injections of preformed IgA immune complexes intensifies proteinuria relative to animals given only the immune complexes [Inoshita et al., unpubl. data].
Whereas both TLR3 and RLH signalings reduced expression of nephrin and podocin in podocytes, only TLR3 stimulation decreased CD2AP content. Stimulation of podocytes via RLH paradoxically increased CD2AP expression. The mechanism underlying this dichotomous response is unclear at present; most intracellular signals downstream of TLR3 and RLH are common to both pathways. This antithetical response to extracellular versus intracellular dsRNA may be a linchpin for the long-term consequences of glomerular injury. Decreases in or loss of CD2AP, a scaffolding protein for dendrin, induce TGF-β1-dependent translocation of dendrin from the cytosol to the nucleus, leading to cathepsin L-mediated cell injury [32]. Therefore, although RLH activation might lead to proteinuria by reducing the expression of other proteins intrinsic to the slit diaphragm, the effect of such signaling might promote the repair of podocytes by increasing CD2AP and thereby blunting cathepsin L-mediated podocyte injury. On the other hand, TLR3 activation diminishes CD2AP expression, and might favor the progression of podocyte injury to glomerulosclerosis and end-stage renal disease.
Although originally recognized as a sensor for viral dsRNA, TLR3 was recently found to detect RNA from necrotic cells as well [33]. Therefore, TLR3 might play an important role not only in glomerulonephritis associated with viral infection, but also in conjunction with necrosis associated with severe inflammation, such as the crescentic and necrotizing forms of glomerulonephritis secondary to microscopic angiitis, vasculitides and systemic lupus erythemaotosus. The potential for TLR3 signaling to suppress angiogenesis [34] might also influence the balance between the repair of glomerular capillaries and the evolution of glomerulosclerosis.
In summary, our study demonstrates that podocytes express functional TLR3 and RLH signaling pathways and that intraglomerular innate responses can contribute to glomerular dysfunction and shape the long-term consequences of podocyte injury. Deeper investigation of these effects and their potential synergy with responses to immune complexes in glomeruli might foster improved diagnosis and treatment of IgAN and other forms of glomerulonephritis.
Acknowledgements
This study was supported by a Veterans Affairs grant (BX000376, S.N.E.) and an NIH grant (AI073303, G.C.S.).
References
- 1.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 2.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 3.Sen GC, Sarkar SN. Transcriptional signaling by double-stranded RNA: role of TLR3. Cytokine Growth Factor Rev. 2005;16:1–14. doi: 10.1016/j.cytogfr.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 4.Sen GC, Sarkar SN. The interferon-stimulated genes: targets of direct signaling by interferons, double-stranded RNA, and viruses. Curr Top Microbiol Immunol. 2007;316:233–250. doi: 10.1007/978-3-540-71329-6_12. [DOI] [PubMed] [Google Scholar]
- 5.Baccala R, Hoebe K, Kono DH, Beutler B, Theofilopoulos AN. TLR-dependent and TLR-independent pathways of type I interferon induction in systemic autoimmunity. Nat Med. 2007;13:543–551. doi: 10.1038/nm1590. [DOI] [PubMed] [Google Scholar]
- 6.Sarkar SN, Elco CP, Peters KL, Chattopadhyay S, Sen GC. Two tyrosine residues of Toll-like receptor 3 trigger different steps of NF-kappa B activation. J Biol Chem. 2007;282:3423–3427. doi: 10.1074/jbc.C600226200. [DOI] [PubMed] [Google Scholar]
- 7.Ozato K, Tailor P, Kubota T. The interferon regulatory factor family in host defense: mechanism of action. J Biol Chem. 2007;282:20065–20069. doi: 10.1074/jbc.R700003200. [DOI] [PubMed] [Google Scholar]
- 8.Older SA, Battafarano DF, Enzenauer RJ, Krieg AM. Can immunization precipitate connective tissue disease? Report of five cases of systemic lupus erythematosus and review of the literature. Semin Arthritis Rheum. 1999;29:131–139. doi: 10.1016/s0049-0172(99)80024-9. [DOI] [PubMed] [Google Scholar]
- 9.Tryggvason K, Patrakka J, Wartiovaara J. Hereditary proteinuria syndromes and mechanisms of proteinuria. N Engl J Med. 2006;354:1387–1401. doi: 10.1056/NEJMra052131. [DOI] [PubMed] [Google Scholar]
- 10.Mundel P, Reiser J, Zuniga Mejia Borja A, Pavenstadt H, Davidson GR, Kriz W, Zeller R. Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp Cell Res. 1997;236:248–258. doi: 10.1006/excr.1997.3739. [DOI] [PubMed] [Google Scholar]
- 11.Kajiyama H, Titus S, Austin CP, Chiotos K, Matsumoto T, Sakairi T, Kopp JB. Tetracycline-inducible gene expression in conditionally immortalized mouse podocytes. Am J Nephrol. 2009;29:153–163. doi: 10.1159/000151770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saleem MA, O'Hare MJ, Reiser J, Coward RJ, Inward CD, Farren T, Xing CY, Ni L, Mathieson PW, Mundel P. A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J Am Soc Nephrol. 2002;13:630–638. doi: 10.1681/ASN.V133630. [DOI] [PubMed] [Google Scholar]
- 13.Yamashita M, Chattopadhyay S, Fensterl V, Zhang Y, Sen GC. A TRIF-independent branch of TLR3 signaling. J Immunol. 2012;188:2825–2833. doi: 10.4049/jimmunol.1103220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chattopadhyay S, Marques JT, Yamashita M, Peters KL, Smith K, Desai A, Williams BR, Sen GC. Viral apoptosis is induced by IRF-3-mediated activation of BAX. EMBO J. 2010;29:1762–1773. doi: 10.1038/emboj.2010.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mundel P, Heid HW, Mundel TM, Kruger M, Reiser J, Kriz W. Synaptopodin: An actin-associated protein in telencephalic dendrites and renal podocytes. J Cell Biol. 1997;139:193–204. doi: 10.1083/jcb.139.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Terenzi F, Saikia P, Sen GC. Interferon-inducible protein, P56, inhibits HPV DNA replication by binding to the viral protein E1. EMBO J. 2008;27:3311–3321. doi: 10.1038/emboj.2008.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hunt JL, Pollak MR, Denker BM. Cultured podocytes establish a size-selective barrier regulated by specific signaling pathways and demonstrate synchronized barrier assembly in a calcium switch model of junction formation. J Am Soc Nephrol. 2005;16:1593–1602. doi: 10.1681/ASN.2004080679. [DOI] [PubMed] [Google Scholar]
- 18.Macconi D, Abbate M, Morigi M, Angioletti S, Mister M, Buelli S, Bonomelli M, Mundel P, Endlich K, Remuzzi A, Remuzzi G. Permselective dysfunction of podocyte-podocyte contact upon angiotensin II unravels the molecular target for renoprotective intervention. Am J Pathol. 2006;168:1073–1085. doi: 10.2353/ajpath.2006.050701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elco CP, Sen GC. Stat1 required for interferon-inducible but not constitutive responsiveness to extracellular DSRNA. J Interferon Cytokine Res. 2007;27:411–424. doi: 10.1089/jir.2006.0172. [DOI] [PubMed] [Google Scholar]
- 20.Appel D, Kershaw DB, Smeets B, Yuan G, Fuss A, Frye B, Elger M, Kriz W, Floege J, Moeller MJ. Recruitment of podocytes from glomerular parietal epithelial cells. J Am Soc Nephrol. 2009;20:333–343. doi: 10.1681/ASN.2008070795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ronconi E, Sagrinati C, Angelotti ML, Lazzeri E, Mazzinghi B, Ballerini L, Parente E, Becherucci F, Gacci M, Carini M, Maggi E, Serio M, Vannelli GB, Lasagni L, Romagnani S, Romagnani P. Regeneration of glomerular podocytes by human renal progenitors. J Am Soc Nephrol. 2009;20:322–332. doi: 10.1681/ASN.2008070709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Asanuma K, Yanagida-Asanuma E, Faul C, Tomino Y, Kim K, Mundel P. Synaptopodin orchestrates actin organization and cell motility via regulation of RHOA signalling. Nat Cell Biol. 2006;8:485–491. doi: 10.1038/ncb1400. [DOI] [PubMed] [Google Scholar]
- 23.Kestila M, Lenkkeri U, Mannikko M, Lamerdin J, McCready P, Putaala H, Ruotsalainen V, Morita T, Nissinen M, Herva R, Kashtan CE, Peltonen L, Holmberg C, Olsen A, Tryggvason K. Positionally cloned gene for a novel glomerular protein - nephrin - is mutated in congenital nephrotic syndrome. Mol Cell. 1998;1:575–582. doi: 10.1016/s1097-2765(00)80057-x. [DOI] [PubMed] [Google Scholar]
- 24.Boute N, Gribouval O, Roselli S, Benessy F, Lee H, Fuchshuber A, Dahan K, Gubler MC, Niaudet P, Antignac C. NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat Genet. 2000;24:349–354. doi: 10.1038/74166. [DOI] [PubMed] [Google Scholar]
- 25.Shih NY, Li J, Karpitskii V, Nguyen A, Dustin ML, Kanagawa O, Miner JH, Shaw AS. Congenital nephrotic syndrome in mice lacking CD2-associated protein. Science. 1999;286:312–315. doi: 10.1126/science.286.5438.312. [DOI] [PubMed] [Google Scholar]
- 26.Chintalacharuvu SR, Nagy NU, Sigmund N, Nedrud JG, Amm ME, Emancipator SN. T cell cytokines determine the severity of experimental IgA nephropathy by regulating IgA glycosylation. Clin Exp Immunol. 2001;126:326–333. doi: 10.1046/j.1365-2249.2001.01678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amore A, Coppo R, Nedrud JG, Sigmund N, Lamm ME, Emancipator SN. The role of nasal tolerance in a model of IgA nephropathy induced in mice by Sendai virus. Clin Immunol. 2004;113:101–108. doi: 10.1016/j.clim.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 28.Jessen RH, Emancipator SN, Jacobs GH, Nedrud JG. Experimental IgA-IgG nephropathy induced by a viral respiratory pathogen. Dependence on antigen form and immune status. Lab Invest. 1992;67:379–386. [PubMed] [Google Scholar]
- 29.Kelley VE, Cavallo T. An ultrastructural study of the glomerular slit diaphragm in New Zealand black/white mice. Lab Invest. 1976;35:213–220. [PubMed] [Google Scholar]
- 30.Reiser J, Oh J, Shirato I, Asanuma K, Hug A, Mundel TM, Honey K, Ishidoh K, Kominami E, Kreidberg JA, Tomino Y, Mundel P. Podocyte migration during nephrotic syndrome requires a coordinated interplay between cathepsin L and alpha3 integrin. J Biol Chem. 2004;279:34827–34832. doi: 10.1074/jbc.M401973200. [DOI] [PubMed] [Google Scholar]
- 31.Kistler AD, Altintas MM, Reiser J. Podocyte GTPases regulate kidney filter dynamics. Kidney Int. 2012;81:1053–1055. doi: 10.1038/ki.2012.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yaddanapudi S, Altintas MM, Kistler AD, Fernandez I, Moller CC, Wei C, Peev V, Flesche JB, Forst AL, Li J, Patrakka J, Xiao Z, Grahammer F, Schiffer M, Lohmuller T, Reinheckel T, Gu C, Huber TB, Ju W, Bitzer M, Rastaldi MP, Ruiz P, Tryggvason K, Shaw AS, Faul C, Sever S, Reiser J. CD2AP in mouse and human podocytes controls a proteolytic program that regulates cytoskeletal structure and cellular survival. J Clin Invest. 2011;121:3965–3980. doi: 10.1172/JCI58552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cavassani KA, Ishii M, Wen H, Schaller MA, Lincoln PM, Lukacs NW, Hogaboam CM, Kunkel SL. TLR3 is an endogenous sensor of tissue necrosis during acute inflammatory events. J Exp Med. 2008;205:2609–2621. doi: 10.1084/jem.20081370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kleinman ME, Yamada K, Takeda A, Chandrasekaran V, Nozaki M, Baffi JZ, Albuquerque RJ, Yamasaki S, Itaya M, Pan Y, Appukuttan B, Gibbs D, Yang Z, Kariko K, Ambati BK, Wilgus TA, DiPietro LA, Sakurai E, Zhang K, Smith JR, Taylor EW, Ambati J. Sequence- and target-independent angiogenesis suppression by siRNA via TLR3. Nature. 2008;452:591–597. doi: 10.1038/nature06765. [DOI] [PMC free article] [PubMed] [Google Scholar]