Abstract
Cyclophosphamide (CP) is widely used in the treatment of cancer and non-malignant disease states such as rheumatoid arthritis. Hemorrhagic cystitis is a major dose-limiting side effect of CP. The incidence of this side effect is related to the dosage and can be as high as 75%. Elimination of the side effects of CP can lead to better tolerance of the drug, and a more efficient therapy can be achieved for patients in need of CP treatment. Several studies have demonstrated that oxidative stress and neutrophil infiltration play important roles in CP-induced bladder damage. Glutamine is utilized under clinical conditions for preventing chemotherapeutic drug-induced side effects, based on its ability to attenuate oxidative stress. The aim of the study is to verify whether glutamine prevents CP-induced oxidative stress and bladder damage using a rat model. Adult male rats were administered 150 mg/kg body weight of CP intraperitoneally. Glutamine pretreated rats were administered 1 g/kg body weight of glutamine orally 2 h before the administration of CP. Vehicle/glutamine-treated rats served as controls. All the rats were killed 16 h after the dose of CP/vehicle. The urinary bladders were removed and used for light microscopic and biochemical studies. The markers of oxidative stress including malondialdehyde content, protein carbonyl content, protein thiol, and myeloperoxidase activity, a marker of neutrophil infiltration, were measured in bladder homogenates. CP treatment induced hemorrhagic cystitis in the rats. Pretreatment with glutamine significantly reduced CP-induced lipid peroxidation (p < 0.01), protein oxidation (p < 0.01), and increase in myeloperoxidase activity (p < 0.05). However, it did not prevent CP-induced bladder damage. The results of the present study show that glutamine pretreatment does not attenuate CP-induced hemorrhagic cystitis, although it prevents CP-induced oxidative stress and neutrophil infiltration significantly. It is therefore necessary to clarify the utility of glutamine as a chemoprotective agent before it is recommended in the market as a nutrient supplement.
Keywords: Cyclophosphamide, Hemorrhagic cystitis, Oxidative stress, Glutamine, Rat
Introduction
Cyclophosphamide (CP) is a drug with a wide spectrum of clinical uses, and it has been proved to be effective in the treatment of cancer (lymphoma, acute and chronic leukemias, and multiple myeloma) and non-malignant disease states such as rheumatoid arthritis [1]. However, this drug may induce acute inflammation of the urinary bladder (cystitis) [2, 3]. Hemorrhagic cystitis (HC) is a major dose-limiting side effect of CP [1]. The incidence of this side effect can be great (75%) in patients receiving a high intravenous dose. Elimination of the urotoxicity of CP could lead to better tolerance of the drug, and more efficient and comfortable therapy could be achieved for patients in need of CP treatment.
Recent studies have shown that reactive oxygen species (ROS) play an important role in CP-induced HC. These studies have shown that the indicator of oxidative stress, namely malondialdehyde (MDA), increases in the bladder after the administration of CP. The role of ROS in the pathogenesis of CP-induced HC is supported by the findings that antioxidants such as tocopherol, taurine, carotene, and melatonin protect against CP-induced HC [4–6]. Sadir et al. [4] have demonstrated that MDA levels increase significantly in bladder tissue after CP injection and the antioxidants ameliorated this increase. Abd-Allah et al. [6] demonstrated a significant decrease in the endogenous antioxidant compound glutathione and elevation of lipid peroxidation in rat urinary bladder tissue after the administration of CP. They have also shown that taurine pretreatment resulted in a significant decrease in lipid peroxide and restoration of glutathione content in urinary bladder tissue.
Glutamine (GLN) is the most abundant amino acid found in blood and in the free amino acid pool of the human body [7]. Besides its role as a constituent of proteins and its importance as metabolic fuel, glutamine has nonnutritive effects, including regulation of the cellular redox balance [8], cell volume [9], glutathione metabolism [10], and attenuation of oxidative stress [11–13].
Several studies have shown that glutamine administration attenuates oxidative stress and protects against chemotherapeutic agent induced organ injury. These include anthracycline-induced mucositis/stomatitis in breast cancer patients [14], fluorouracil-induced stomatitis in patients with advanced or metastatic cancer [15], and oxaliplatin-induced peripheral neuropathy in colorectal cancer patients [16]. In rats, oral glutamine has been shown to attenuate oxidative stress in cyclophosphamide-induced cardiotoxicty[17].
Because of the high turnover rate of glutamine, it is suggested that even high amounts of glutamine can be given without any important side effects. It is suggested that if glutamine is shown to prevent CP-induced oxidative stress and urotoxicity, it can lead to better tolerance of the drug. Thus, a more efficient therapy can be achieved for patients in need of CP treatment. Therefore, the purpose of the present study was to investigate whether the administration of the glutamine has any protective effect against cyclophosphamide-induced oxidative stress and urotoxicity in rats.
Materials and Methods
Chemicals
Glutamine powder, 1-chloro-2,4-dinitrobenzene, bovine serum albumin powder, 5,5-dithio-bis-(2-nitrobenzoic acid) (DTNB), reduced glutathione 2-thiobarbituric acid, and 1,1,3,3 tetramethoxy propane were obtained from Sigma Chemical Co., St. Louis, MO, USA. All other chemicals were of analytical grade.
Animals and Treatments
Adult male Wistar rats (200–250 g) were used for the experiments. The study was approved by the animal ethics Committee for the Purpose of Control and Supervision of Experimentation on Animals (CPCSEA), Government of India. The guidelines of CPCSEA were followed. Dosage and route of administration of CP were determined from that described in literature the [18]. Todora et al. have recently demonstrated that GLN at the dose of 1 g/kg body weight protects against CP-induced cardiotoxicity in rats [17]. Therefore, we used the same dose for our study. CP was dissolved in 0. 9% saline, and glutamine was dissolved in water.
Experimental Design
The rats were divided into four groups and were treated as follows.
Group I (Vehicle Control)
The rats in this group (n = 6) received saline (the vehicle for CP) and water (the vehicle for glutamine).
Group II (Glutamine)
The rats (n = 6) in this group received 1 g/kg body weight glutamine dissolved in water orally.
Group III (CP Alone)
The rats in this group (n = 8) received a single intraperitoneal injection of CP in saline at the dose of 150 mg/kg body weight.
Group IV (CP + Glutamine)
The rats in this group (n = 8) received 1 g/kg body weight of glutamine orally. Two hours later, they received a single intraperitoneal injection of CP in saline at the dose of 150 mg/kg body weight.
The rats were killed 16 h after the administration of CP or saline. The urinary bladders were removed and blotted dry before weighing. A part of the urinary bladder was used for biochemical assays and another part for histological assessment by light microscopy.
Light Microscopy of the Urinary Bladder
The tissues were fixed overnight in 10% buffered neutral formalin, processed to paraffin wax, sectioned at 5 μm, and stained with hematoxylin and eosin for examination by light microscopy.
Biochemical Assays
The biochemical assays were carried out on 10% (w/v) of bladder tissue homogenates.
Malondialdehyde
Malondialdehyde content was measured as described by Ohkawa et al. [19]. The mixture consisted of 0.8 ml of sample, 0.2 ml of 8.1% SDS, 1.5 ml of 20% glacial acetic acid adjusted to pH 3.5, and 1.5 ml of 0.8% aqueous solution of TBA. The mixture was made up to 4 ml with distilled water and heated at 95°C for 60 min using a glass ball as condenser. After cooling with tap water, 1 ml distilled water and 5 ml n-butanol and pyridine mixture (15:1) were added, and the solution was shaken vigorously. After centrifugation at 2,000×g for 10 min, the absorbance of the organic layer was measured at 532 nm. Amount of thiobarbituric reacting substances formed is calculated from standard curve prepared using 1,1′,3,3′ tetramethoxy propane, and the values were expressed as nanomoles per milligram protein
Protein Carbonyl Content
Protein carbonyl content was measured using DNPH as described by Sohal et al. [20]. To 0.5 ml of sample, an equal volume of 10 mM DNPH in 2 N HCl was added and incubated for 1 h shaking intermittently at room temperature. Corresponding blank was carried out by adding only 2 N HCl to the sample. After incubation, the mixture was precipitated with 10% TCA (final concentration) and centrifuged. The precipitate was washed twice with ethanol/ethylacetate (1:1) and finally dissolved in 1 ml of 6 M guanidine HCl and centrifuged at low speed, and the supernatant was read at 366 nm. The difference in absorbance between the DNPH- and HCl-treated sample was determined and expressed as nanomoles of carbonyl groups per milligram of protein, using extinction coefficient of 22 mM−1 cm−1.
Protein Thiol Groups
Thiol groups were measured as described by Habeeb [21]. To 1 ml of the sample suspension, 1 ml of 10% TCA containing 1 mM EDTA was added. The protein precipitate was separated by high-speed centrifugation (10,000×g) for 10 min. For total thiol estimation, the sample was taken directly without precipitation. To this, 1 ml of solution I and 0.5% SDS were added followed by 2 ml of solution II and 30 μl of DTNB. The tubes were mixed well and kept in the dark for 15 min at room temperature. The intense yellow color of the nitromercaptobenzoate anion formed from the DTNB reaction with the thiol was read at 412 nm, which has a molar absorption of 13,600 m−1 cm−1. The results are expressed in micromole per milligram protein.
Myeloperoxidase Activity
Myeloperoxidase activity was measured with O-dianisidine-H2O2 by Diaz-Granados method [22]. The rate of decomposition of H2O2 by myeloperoxidase was determined by measuring the rate of color development at 460 nm. To 10 μl of sample, 11 μl of H2O2, 17 μl of O-dianisidine, and 962 μl of phosphate buffer were added, and the color was read at 460 nm at an interval of 30 s for 4 min, and the rate of change per minute was determined. Extinction coefficient of 1.13 × 104 cm−1 was used for the calculation. One unit is the amount of enzyme decomposing 1 μmol of peroxide per minute.
Protein was measured by the Lowry method [23].
Statistical Analysis
A power analysis was done. A sample size of minimum six was to be used for our study. The data represent mean values ± SD. Means of the four groups were compared by ANOVA. In the case of a significant F, Student’s t test with Bonferroni correction was used to compare individual means. A P value of <0.05 was considered to be statistically significant.
Results
Light Microscopic Studies
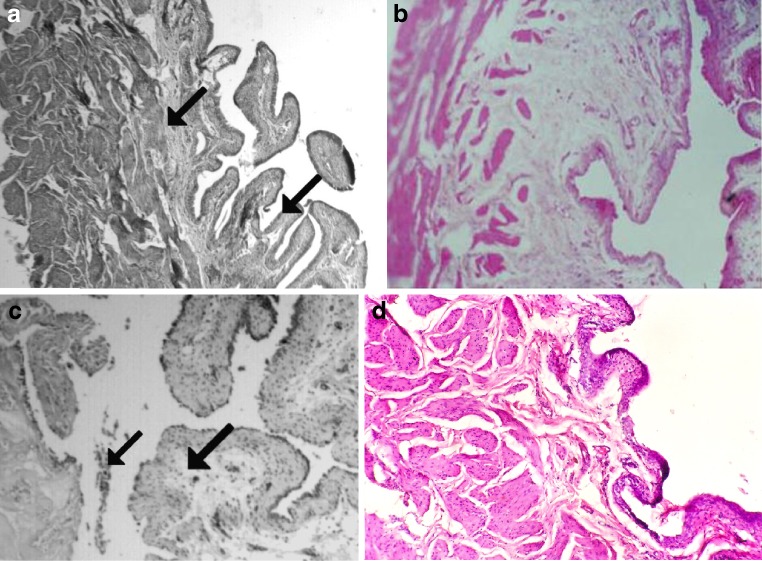
In the control rats (both vehicle treated and glutamine treated), the bladders had normal structure. The urinary bladder had the urothelium formed by tightly packed cells with little intercellular space. The basement membrane that separates the epithelium from the underlying lamina propria was intact. There was no breach in it. The connective tissue that constituted the lamina propria was dense with normal vascular supply. The mucosa was thrown into folds, and there were subepithelial crypts. The smooth muscle coat underlying the lamina propria displayed circular and longitudinal muscle coat with minimal connective tissue packing. There were no exudates in the lumen (Fig. 1a).
Fig. 1.
Light microscopy of the bladders of control rats and experimental rats. Original magnification, ×100. a Saline control rats with normal morphology. Arrow points to folded mucosa, with epithelium showing tight packing of cells and dense connective tissue in lamina propria. Subepithelial crypts also seen. No hemorrhage found. b Glutamine-treated rats with normal epithelium and lamina propria. c Bladder from cyclophosphamide-treated rat showing edema of epithelium, and it appears that epithelium has been breached. Vascular changes in lamina propria are apparent. d Bladder from GLN + CP-treated rat showing degeneration of the epithelium, edema in the lamina propria, and erosion of the mucosa
Contrary to the normal picture presented above, the bladder wall in CP-treated animals showed severe damage. The mucosa was edematous, and the cells of the urothelium were not compact. Cellular exudates were seen in the lumen. Mucosal content formed follicular cystitis (Fig. 1c). Edema of lamina propria with epithelial and subepithelial hemorrhage was seen (arrows in Fig. 1c). The epithelium showed patches of ulceration (arrow in Fig. 1c). The lamina propria was edematous, and there were exudates in the lumen (arrow in Fig. 1c).
Pretreatment with glutamine did not prevent CP-induced hemorrhagic cystitis (Fig. 1d). The epithelia were detached from the lamina propria and appear degenerated. The mucosa was eroded, and severe edema was observed.
Biochemical Parameters
The biochemical parameters are shown in Figs. 2, 3, 4, and 5.
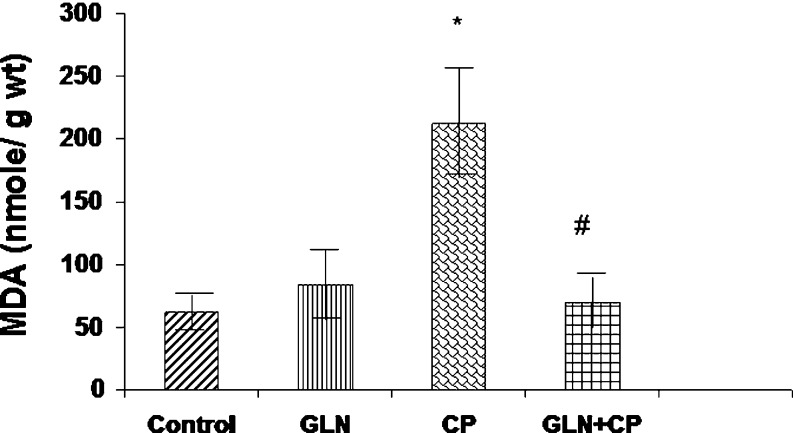
Fig. 2.
MDA levels in the bladders of control rats and experimental rats. Data represent mean ± SD of five to seven rats. *P < 0.01 vs control, GLN, #P < 0.01 vs CP
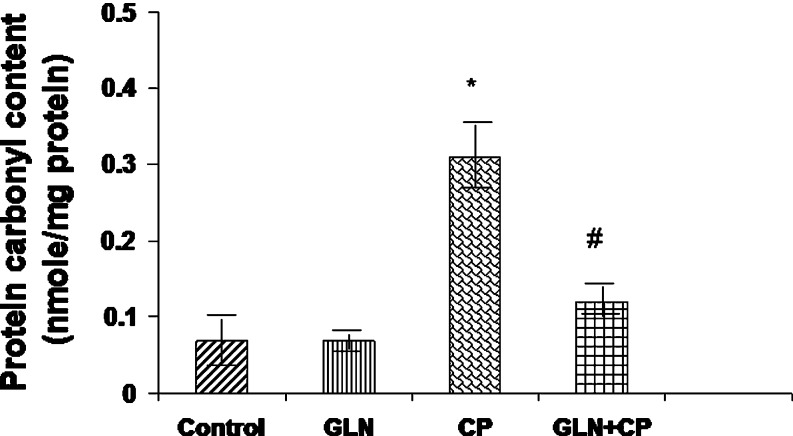
Fig. 3.
Protein carbonyl content in the bladders of control rats and experimental rats. Data represent mean ± SD of five to seven rats. *P < 0.01 vs control, GLN, #P < 0.01 vs CP
Fig. 4.
Protein thiol in the bladders of control rats and experimental rats. Data represent mean ± SD of 5–7 rats. *P < 0.01 vs control, $P < 0.01 vs GLN
Fig. 5.
MPO activity in the bladders of control rats and experimental rats. Data represent mean ± SD of five to seven rats. *P < 0.01 vs control, GLN, #P < 0.05 vs CP
MDA Level
The MDA level in the bladder of CP-treated rats was elevated by 292% as compared with the control (p < 0.01). Pretreatment with glutamine completely prevented CP-induced increased MDA level (P < 0.01) (Fig. 2).
Protein Carbonyl Content
The bladder protein carbonyl content in the CP-treated rats was increased by 219% as compared with the control (P < 0.01). Pretreatment with glutamine partially but significantly prevented CP-induced increase in protein carbonyl content (P < 0.01) (Fig. 3).
Protein Thiol
Protein thiol was increased by 61% in the bladders of CP-treated rats as compared with control (P < 0.01). Pretreatment with glutamine had no effect on protein thiol level (Fig. 4).
Myeloperoxidase Activity
A threefold increase in myeloperoxidase (MPO) activity was observed in the bladders of CP-treated rats as compared with control (P < 0.01). Pretreatment with glutamine partially but significantly reduced CP-induced elevation in MPO activity (P < 0.05) (Fig. 5).
Discussion
In the present study, the treatment of rats with CP resulted in enhanced oxidative stress and neutrophil infiltration in the bladder as evidenced by enhanced MDA level, protein thiol level, protein carbonyl content, and MPO activity. Pretreatment with glutamine significantly attenuated CP-induced oxidative stress and neutrophil infiltration as evidenced by decrease in MPO activity, which is one of the markers of neutrophil infiltration [24]. However, surprisingly, glutamine did not prevent CP-induced bladder damage.
Several antioxidants and oxidative stress inhibitors have been shown to protect against CP-induced oxidative stress and hemorrhagic cystitis. These include α-tocopherol, β-carotene, taurine, and melatonin [4–6]. In a recent study, we have shown that aminoguanidine pretreatment attenuates CP-induced oxidative stress, decrease in the activities of antioxidant enzymes, and reduced bladder damage [25].
Neutrophils are suggested to be one of the sources of reactive oxygen species that cause enhanced lipid peroxidation [26]. Recent studies demonstrate that glutamine prevents neutrophil recruitment and infiltration and attenuates liposaccharide-induced acute lung injury. This effect is suggested to be related to its antioxidant property [27–29]. Yeh et al. [28] have demonstrated that under septic conditions, GLN administration enhances lymphocyte function, attenuate interactions between polymorphonuclear lymphocytes and endothelium, and thus decreases neutrophil infiltration into tissues. In the present study, CP treatment resulted in increased MPO activity in the bladders of rats. GLN pretreatment prevented CP-induced neutrophil infiltration as reflected by the MPO activity, but did not prevent the cystitis.
Protein thiol level was elevated in the bladders of rat that were treated with CP. This is probably a defense mechanism in the bladder in response to increased oxidative stress [30].
Todora et al. [17] have recently demonstrated that GLN pretreatment (1 g/kg body weight) attenuates CP-induced oxidative stress and protects against CP-induced cardiotoxicity in rats. However, we could not demonstrate the protective effects of GLN on the bladder, although it attenuated CP-induced oxidative stress. We suggest that CP-induced cardiotoxicity and urotoxicity may involve different mechanisms.
In the present study, although glutamine pretreatment significantly attenuated CP-induced oxidative stress and neutrophil infiltration, it was not effective in preventing CP-induced bladder damage. These observations suggest that oxidative stress may not be the only mechanism by which CP induces bladder damage. While antioxidants such as tocopherol, carotene, taurine, and melatonin protect against CP-induced HC, glutamine does not do so. The reason is not clear. It is noteworthy to mention here that the agents that were shown to be protective against CP-induced OS and bladder damage, namely α tocopherol, β carotene, melatonin, and aminoguanidine, are not only potent antioxidants/ROS scavengers but are also strong inhibitors of nitrosative stress. Nitrosative stress is overproduction of reactive nitrogen species (RNS) such as nitric oxide and peroxynitrite. There is a very close relationship between ROS and RNS. To be precise, superoxide anion is an important ROS that reacts with nitric oxide and forms peroxynitrite radical. Peroxynitrite is a strong oxidant and nitrating species that can cause destruction of host cellular constituents. Peroxynitrite can oxidize and covalently modify all types of biomolecules, such as membrane lipids, thiols, proteins, and DNA [31–33]. Therefore, the protective effect of alpha tocopherol, beta carotene, melatonin, and aminoguanidine on CP-induced HC may be attributed to their inhibition of not only oxidative stress but also nitrosative stress. Thus far, glutamine has not been shown to have any effect on nitrosative stress to the best of our knowledge. This may explain why GLN does not protect against CP-induced hemorrhagic cystitis although it attenuated CP-induced oxidative stress significantly while the other antioxidants had a protective effect.
In summary, the present study shows that glutamine attenuates CP-induced oxidative stress but does prevent CP-induced bladder damage. This suggests that mechanisms other than oxidative stress such as nitrosative stress may also be involved. We suggest that it is necessary to clarify the utility of glutamine as a uroprotective agent.
Acknowledgement
The study was supported by the Department of Science and Technology (DST), New Delhi, India.
References
- 1.Dollery C. Cyclophosphamide. In: Dollery C, editor. Therapeutic drugs. Edinburg: Churchill Livingstone; 1999. pp. 349–353. [Google Scholar]
- 2.Wong TM, Yeo W, Chan LW, Mok TS. Hemorrhagic pyelitis, ureteritis, and cystitis secondary to cyclophosphamide: case report and review of literature. Gynecol Oncol. 2000;76:223–225. doi: 10.1006/gyno.1999.5680. [DOI] [PubMed] [Google Scholar]
- 3.Walker RD, Sommerkamp H. Hemorrhagic cystitis after high dose chemotherapy. An interdisciplinary problem. Urologie A. 1998;37:516–521. doi: 10.1007/s001200050211. [DOI] [PubMed] [Google Scholar]
- 4.Sadir S, Deveci S, Korkmaz A. Alpha tocopherol, beta carotene, and melatonin administration protects cyclophosphamide induced oxidative damage to bladder tissue in rats. Cell Biochem Funct. 2007;25:521–552. doi: 10.1002/cbf.1347. [DOI] [PubMed] [Google Scholar]
- 5.Topal P, Oztas Y, Korkmaz A. Melatonin ameliorates bladder damage induced by cyclophosphamide in rats. J Pineal Res. 2005;38:272–277. doi: 10.1111/j.1600-079X.2004.00202.x. [DOI] [PubMed] [Google Scholar]
- 6.Abd-Allah AR, Gado AM, Al-Majed AA, et al. Protective effect of taurine against cyclophosphamide-induced urinary bladder toxicity in rats. Clin Exp Pharmacol Physiol. 2005;32:167–172. doi: 10.1111/j.1440-1681.2004.04129.x. [DOI] [PubMed] [Google Scholar]
- 7.Roth E. Nonnutritive effects of glutamine. J Nutr. 2008;138:2025S–2031S. doi: 10.1093/jn/138.10.2025S. [DOI] [PubMed] [Google Scholar]
- 8.Matés JM, Pérez-Gómez C, Núñez de Castro I, Asenjo M, Márquez J. Glutamine and its relationship with intracellular redox status, oxidative stress and cell proliferation/death. Int J Biochem Cell Biol. 2002;34:439–458. doi: 10.1016/S1357-2725(01)00143-1. [DOI] [PubMed] [Google Scholar]
- 9.Häussinger D, Roth E, Lang F, Gerok W. Cellular hydration state: an important determinant of protein catabolism in health and disease. Lancet. 1993;341:1330–1332. doi: 10.1016/0140-6736(93)90828-5. [DOI] [PubMed] [Google Scholar]
- 10.Hong RW, Rounds JD, Helton WS, Robinson MK, Wilmore DK. Glutamine preserves liver glutathione after lethal hepatic injury. Ann Surg. 1992;215:114–119. doi: 10.1097/00000658-199202000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amores-Sánchez MI, Medina MA. Glutamine, as a precursor of glutathione, and oxidative stress. Mol Genet Metab. 1999;67(2):100–105. doi: 10.1006/mgme.1999.2857. [DOI] [PubMed] [Google Scholar]
- 12.Heyland DK, Dhaliwal R, Day AG, Muscedere J, Drover J, Suchner U, Cook D. Reducing Deaths due to OXidative Stress (The REDOXS Study): rationale and study design for a randomized trial of glutamine and antioxidant supplementation in critically-ill patients. Proc Nutr Soc. 2006;65:250–263. doi: 10.1079/PNS2006505. [DOI] [PubMed] [Google Scholar]
- 13.Kul M, Vurucu S, Demirkaya E, Tunc T, Aydinoz S, Meral C, Kesik V, Alpay F. Enteral glutamine and/or arginine supplementation have favorable effects on oxidative stress parameters in neonatal rat intestine. J Pediatr Gastroenterol Nutr. 2009;49:85–89. doi: 10.1097/MPG.0b013e318198cd36. [DOI] [PubMed] [Google Scholar]
- 14.Peterson DE, Jones JB, Petit RG., II Randomized, placebo controlled trial of saforis for prevention and treatment of oral mucositis in breast cancer patients receiving anthracycline-based chemotherapy. Cancer. 2007;109:322–331. doi: 10.1002/cncr.22384. [DOI] [PubMed] [Google Scholar]
- 15.Choi K, Lee SS, Oh SJ, Lim SY, Jeon WK, Oh TY, Kim JW. The effect of oral glutamine on 5-fluorouracil/leucovorin-induced mucositis/stomatitis assessed by intestinal permeability test. Clin Nutr. 2007;26:57–62. doi: 10.1016/j.clnu.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 16.Wang WS, Lin JK, Chen WS, Jiang JK, Wang HS, Chiou TJ, Liu JH, Yen CC, Chen PM. Oral glutamine is effective for preventing oxaliplatin-induced neuropathy in colorectal cancer patients. Oncologist. 2007;12:312–319. doi: 10.1634/theoncologist.12-3-312. [DOI] [PubMed] [Google Scholar]
- 17.Todorova V, Vanderpool D, Blossom S, Nwokedi E, Hennings L, Mrak R, Klimberg VS. Oral glutamine protects against cyclophosphamide-induced cardiotoxicity in experimental rats through increase of cardiac glutathione. Nutrition. 2009;25:812–817. doi: 10.1016/j.nut.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 18.Ahluwalia A, Maggi CA, Santicioli P, Lecci A, Giuliani S. Characterization of the capsaicin-sensitive component of cyclophosphamide induced inflammation in the rat urinary bladder. Br J Pharmacol. 1994;111:1017–1022. doi: 10.1111/j.1476-5381.1994.tb14845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 20.Sohal RS, Agarwal S, Dubey A, Orr WC. Protein oxidative damage is associated with life expectancy of houseflies. Proc Natl Acad Sci USA. 1993;90:7255–7259. doi: 10.1073/pnas.90.15.7255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonproteinsulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem. 1968;25:192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- 22.Doan T, Massarotti E. Rheumatoid arthritis, an overview of new and emerging therapies. J Clin Pharmacol. 2005;45:751–762. doi: 10.1177/0091270005277938. [DOI] [PubMed] [Google Scholar]
- 23.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;93:265–275. [PubMed] [Google Scholar]
- 24.Perkins GD, Nathani N, McAuley DF, Gao F, Thickett DR. In vitro and in vivo effects of salbutamol on neutrophil function in acute lung injury. Thorax. 2007;62:36–42. doi: 10.1136/thx.2006.059410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abraham P, Rabi S, Selvakumar D. Protective effect of aminoguanidine against oxidative stress and bladder injury in cyclophosphamide-induced hemorrhagic cystitis in rat. Cell Biochem Funct. 2009;27:56–62. doi: 10.1002/cbf.1534. [DOI] [PubMed] [Google Scholar]
- 26.Al Laham F, Kälsch AI, Heinrich L, Birck R, Kallenberg CG, Heeringa P, Yard B (2010) Inhibition of neutrophil-mediated production of reactive oxygen species (ROS) by endothelial cells is not impaired in anti-neutrophil cytoplasmic autoantibodies (ANCA)-associated vasculitis patients. Clin Exp Immunol (in press) [DOI] [PMC free article] [PubMed]
- 27.Ohno Y, Ormstad K. Formation, toxicity and inactivation of acrolein during biotransformation of cyclophosphamide as studied in freshly isolated cells from rat liver and kidney. Arch Toxicol. 1985;57:99–103. doi: 10.1007/BF00343118. [DOI] [PubMed] [Google Scholar]
- 28.Yeh CL, Hsu CS, Yeh SL, Lin MT, Chen WJ. Dietary glutamine supplementation reduces cellular adhesion molecule expression and tissue myeloperoxidase activity in mice with gut-derived sepsis. Nutrition. 2006;22:408–413. doi: 10.1016/j.nut.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 29.Zhang F, Wang X, Wang W, Li N, Li J. Glutamine reduces TNF-alpha by enhancing glutathione synthesis in lipopolysaccharide-stimulated alveolar epithelial cells of rats. Inflammation. 2008;31:344–350. doi: 10.1007/s10753-008-9084-0. [DOI] [PubMed] [Google Scholar]
- 30.Kon T, Tanigawa T, Hayamizu K, Shen M, Tsuji T, Naito Y, Yoshikawa T. Singlet oxygen quenching activity of human serum. Redox Rep. 2004;9(6):325–330. doi: 10.1179/135100004225006821. [DOI] [PubMed] [Google Scholar]
- 31.Radi R, Beckman JS, Bush KM. Peroxynitrite-inducedmembrane lipid peroxidation: the cytotoxic potential of superoxide and nitric oxide. Arch Biochem Biophys. 1991;288:481. doi: 10.1016/0003-9861(91)90224-7. [DOI] [PubMed] [Google Scholar]
- 32.Szabo C, Zingarelli B, O’Connor M. DNA strandbreakage, activation of poly (ADP-ribose) synthase and cellularenergy depletion are involved in the cytotoxicity of macrophages, and smooth muscle cell exposed to peroxynitrite. Proc Natl Acad Sci. 1996;93:1753–1758. doi: 10.1073/pnas.93.5.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Radi R. Nitric oxide, oxidants, and protein tyrosine nitration. Proc Nat Acad Sci USA. 2004;101:4003–4008. doi: 10.1073/pnas.0307446101. [DOI] [PMC free article] [PubMed] [Google Scholar]