Abstract
Objective
Minimal clinical research has investigated the significance of different blood pressure monitoring techniques in the ICU and whether systolic vs. mean blood pressures should be targeted in therapeutic protocols and in defining clinical study cohorts. The objectives of this study are to compare real-world invasive arterial blood pressure with noninvasive blood pressure, and to determine if differences between the two techniques have clinical implications.
Design
We conducted a retrospective study comparing invasive arterial blood pressure and noninvasive blood pressure measurements using a large ICU database. We performed pairwise comparison between concurrent measures of invasive arterial blood pressure and noninvasive blood pressure. We studied the association of systolic and mean invasive arterial blood pressure and noninvasive blood pressure with acute kidney injury, and with ICU mortality.
Setting
Adult intensive care units at a tertiary care hospital.
Patients
Adult patients admitted to intensive care units between 2001 and 2007.
Interventions
None.
Measurements and Main Results
Pairwise analysis of 27,022 simultaneously measured invasive arterial blood pressure/noninvasive blood pressure pairs indicated that noninvasive blood pressure overestimated systolic invasive arterial blood pressure during hypotension. Analysis of acute kidney injury and ICU mortality involved 1,633 and 4,957 patients, respectively. Our results indicated that hypotensive systolic noninvasive blood pressure readings were associated with a higher acute kidney injury prevalence (p = 0.008) and ICU mortality (p < 0.001) than systolic invasive arterial blood pressure in the same range (≤70 mm Hg). Noninvasive blood pressure and invasive arterial blood pressure mean arterial pressures showed better agreement; acute kidney injury prevalence (p = 0.28) and ICU mortality (p = 0.76) associated with hypotensive mean arterial pressure readings (≤60 mm Hg) were independent of measurement technique.
Conclusions
Clinically significant discrepancies exist between invasive and noninvasive systolic blood pressure measurements during hypotension. Mean blood pressure from both techniques may be interpreted in a consistent manner in assessing patients’ prognosis. Our results suggest that mean rather than systolic blood pressure is the preferred metric in the ICU to guide therapy. (Crit Care Med 2013;41:0–0)
Keywords: arterial blood pressure monitoring, blood pressure, hemodynamic monitoring, hypotension, medical devices
Blood pressure monitoring is essential in managing hemo-dynamically unstable ICU patients. Invasive measurement from an arterial line (invasive arterial blood pressure [IAP]) is generally considered to be the gold standard (1, 2), despite recognition that errors may be introduced by over- or underdamping, calibration errors, and movement artifacts (1, 3, 4). Automated noninvasive blood pressure systems (NIBP) using oscillometric techniques (5, 6) have advantages over invasive arterial lines as they avoid bleeding and infection risk, and can be used outside the ICU. Both theoretical (7) and existing clinical studies (5, 8–12) have suggested that NIBP measurements may differ from intra-arterial estimates. Clinical data comparing the two techniques in the ICU are sparse, however, particularly at the extremes of blood pressure when the absolute value of blood pressure is most critical. Although both invasive and noninvasive methods report systolic, mean, and diastolic pressures, consensus statements regarding patient management and ICU acuity scores have often recommended systolic rather than mean pressures as targets, and the modality-dependence (invasive vs. noninvasive) of pressure measurement has not been appreciated (13–19).
We used a large ICU database to test several hypotheses. First, we hypothesized that the mean arterial pressure (MAP) corresponds well between invasive and noninvasive techniques but that the systolic pressure readings are discordant. Second, we hypothesized that the discrepancy between invasive and noninvasive systolic blood pressure measurement is clinically significant, and use of noninvasive systolic readings may fail to recognize end-organ hypoperfusion, for example, acute kidney injury (AKI). Finally, we hypothesized that ICU mortality associated with particular hypotensive thresholds may be significantly different using the invasive vs. noninvasive measurement techniques.
MATERIALS AND METHODS
The Multiparameter Intelligent Monitoring for Intensive Care–II Database
Adult patients were selected from the publicly available Multiparameter Intelligent Monitoring for Intensive Care (MIMIC)-II Database (20, 21). The database, collected between 2001 and 2007, includes clinical (laboratory values, IV medications, etc.) and physiological data (heart rate, blood pressure, oxygen saturation, etc.) from bedside monitors (Component Monitoring System Intellivue MP-70; Philips Healthcare, Andover, MA) in seven ICUs of the Beth Israel Deaconess Medical Center in Boston. Vital signs derived from the monitors were validated and recorded by ICU nurses approximately every hour. In addition, the MIMIC II waveform database (version 2) includes approximately 4000 sets of high-resolution physiological waveforms with associated minute-by-minute vital sign trends.
Pairwise Analysis of Agreement Between IAP and NIBP
We performed pairwise comparison between concurrent measures of IAP and NIBP using once-per-minute blood pressure trends. NIBP measurements were recorded from either Philips M1008A or M3000A NIBP modules (Philips Healthcare). These oscillometric devices determine MAP based on peak cuff pressure oscillations and derive systolic and diastolic values using proprietary algorithms. IAP measurements (typically measured at the radial artery) were used in pairwise comparison to NIBP measurements with concurrent time stamps. Nonphysiologic outliers and damped waveforms were filtered out (see supplementary Appendix, Supplemental Digital Content 1, http://links.lww.com/CCM/A595). Trend records with fewer than two concurrently measured IAP/NIBP sample pairs were excluded.
Pairwise comparison was performed using a regression-based Bland–Altman technique (22), which models the mean and SD of the blood pressure differences as a function of the averaged measurements. A total of 27,022 concurrently measured IAP/NIBP sample pairs from 852 patient trend records were included in the Bland–Altman analysis. Regression coefficients were derived from a bootstrap procedure (23) involving 10,000 resamples. Each bootstrap sample was constructed by randomly selecting one IAP/NIBP sample pair from each of 852 randomly selected (with replacement) patient trend records. Thus, each bootstrap sample consisted of 852 randomly drawn IAP/NIBP sample pairs, one from each of the randomly selected patient trend records. Statistical significance of regression coefficients was determined based on 95% confidence intervals.
To investigate whether our results were dependent on use of vasopressors, a separate Bland–Altman analysis was conducted. IAP/NIBP measurement pairs obtained during pressor treatment formed the pressor group, with the remaining measurements forming the no-pressor group. The differences between NIBP and IAP were analyzed for the two groups, respectively, using the same regression-based Bland–Altman technique described above.
Clinical Studies
The second analysis sought to determine if differences between IAP and NIBP led to clinically significant discrepancies in estimating risks in an ICU setting. We compared the association of hypotensive levels of systolic and mean IAP and NIBP with AKI and ICU mortality. Patients were selected from among the 19,742 patients with clinical records in MIMIC II (see text below and supplemental Appendices C and D, Supplemental Digital Content 1, http://links.lww.com/CCM/A595). Hourly nurse-verified invasive and NIBP samples with concurrent timestamps were analyzed. For patients with multiple ICU stays, the first ICU stay that met the specified criterion was used.
Association Between Blood Pressure and AKI
We examined patients with at least 2 serum creatinine values within 48 hrs during their ICU stays. We excluded patients with an admission creatinine >5 mg/dL, evidence of prior renal replacement therapy, the ICD-9 code for ESRD (585.6), or a diagnosis of ESRD in discharge summaries. Based on existing literature (24, 25), AKI was defined as an increase in creatinine of ≥50% within 48 hrs, with resulting creatinine ≥1.2 mg/dL. The onset of AKI was defined as the time when the rise in creatinine criterion was first met. The control group included patients who did not develop AKI during their ICU stays.
For each ICU stay, we identified the minimum blood pressure from among the available concurrently time-stamped IAP and NIBP measurements during a target window of 48 hrs immediately preceding AKI onset, and compared the performance of IAP and NIBP measurements in assessing patients’ risks for developing AKI. For the controls, the target window was the 48-hr period prior to the end of the third day after ICU admission. This window was chosen so that its end time corresponded with the average AKI onset time (2.99 days after ICU admission). Patients with fewer than six concurrently time-stamped IAP and NIBP measurements in the target window were excluded.
Association Between Blood Pressure and ICU Mortality
The minimum blood pressure from among the available concurrently time-stamped IAP/NIBP sample pairs during an entire ICU stay was extracted. We included patients who had at least six nurse-verified, concurrently time-stamped IAP/NIBP sample pairs during their ICU stays regardless of pressor treatment or resuscitation status.
Statistical Analyses
Continuous variables were expressed as mean with SD or median with interquartile range where applicable. Categorical variables were described as proportions and compared with the chi-square test; 95% confidence interval for proportions was calculated based on binomial distributions. Univariate logistic regression was performed to investigate the correlations between blood pressure and AKI onset. Two-sided p values of <.05 were considered statistically significant. All data analyses were performed using MATLAB 7.10 and supporting statistical toolboxes.
RESULTS
IAP/NIBP Pairwise Analysis of Agreement
Figure 1A and B plots the biases and 95% limits of agreement from the regression-based Bland–Altman analysis using a total of 27,022 concurrently measured systolic and MAP IAP/NIBP sample pairs from 852 patient trend records (see supplemental Appendix Fig. 1 for diastolic measurements, Supplemental Digital Content 1, http://links.lww.com/CCM/A595). Results from Figure 1A indicate that NIBP was higher than systolic IAP at pressures <95 mm Hg, and was lower than systolic IAP at pressures ≥95 mm Hg. Hypotensive patients with systolic blood pressure of 60 mm Hg showed a marked discrepancy between the two techniques with an average offset of 10.05 mm Hg and 95% limits of agreement ranging from −15.32 to +35.42 mm Hg. The biases (with the 95% limits of agreement in parentheses) between noninvasive and invasive systolic blood pressure measurements in the hypotensive range from 70 to 90 mm Hg were 7.04 (−19.37, 33.46), 4.03 (−23.43, 31.49), and 1.02 (−27.48, 29.53) mm Hg, respectively, in each of the 10-mm Hg intervals.
Figure 1.
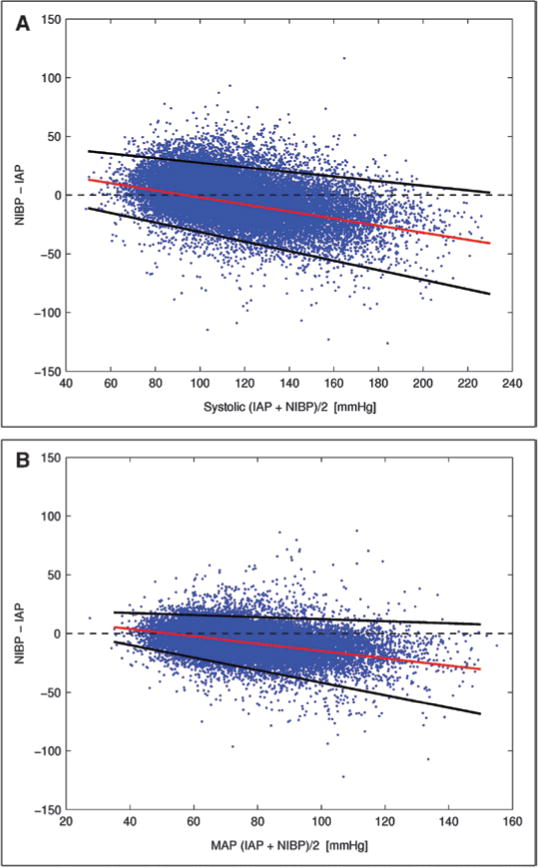
A, Bias and 95% limits of agreement between concurrently measured systolic invasive arterial blood pressure/noninvasive blood pressure (IAP/NIBP). The differences between noninvasive and invasive systolic measurements tended to be positive when the blood pressures were low (<95 mm Hg), and negative when blood pressures were high (≥95 mm Hg). B, Bias and 95% limits of agreement between concurrently measured invasive and noninvasive mean arterial pressure (MAP).
Noninvasive MAP conformed reasonably well to its invasive counterpart in the hypotensive range (Fig. 1B). The biases and the 95% limits of agreement (in parentheses) between invasive and noninvasive MAP in the hypotensive range were 3.90 (−9.72, 17.52), 0.79 (−15.06, 16.64), and −2.32 (−20.41, 15.76) mm Hg, respectively, in each of the 10-mm Hg intervals from 40 to 60 mm Hg.
Further analysis based on 20,399 (of the 27,022) pairwise IAP/NIBP measurements with available clinical information (from 609 unique patients) confirmed that similar pressure-dependent IAP/NIBP biases were observed regardless of the use of vasopressor medications (see supplemental Appendix E for details, Supplemental Digital Content 1, http://links.lww.com/CCM/A595).
Blood Pressure and AKI Prevalence
Analysis was performed on 1,633 patients (300 in the AKI cohort and 1,333 controls) who had at least six concurrently time-stamped IAP and NIBP measurements in the target window (Table 1). Univariate logistic regression analysis indicated that low values in all four types of blood pressure measurements (systolic and mean IAP and NIBP) were significant risk factors for AKI (p < .001). All four types of blood pressure measurements (systolic and mean IAP and NIBP) had similar prognostic performance in assessing patients’ risk in developing AKI with area under curves between 0.65 and 0.69 (see supplemental Appendix F for sensitivity/specificity analysis, Supplemental Digital Content 1, http://links.lww.com/CCM/A595). However, the prevalence of AKI as a function of blood pressure depended significantly on the method of measurement. The prevalence of AKI associated with systolic NIBP diverged from that of systolic IAP for pressures <100 mm Hg, and the divergence widened at lower blood pressure values (Fig. 2A). In the hypotensive range, AKI prevalence at a particular systolic NIBP threshold was significantly greater than when measured inva-sively. Specifically, when systolic NIBP values were 70 mm Hg or less, 51.3% (39/76 patients) developed AKI, whereas when pressures were measured using the IAP technique only 34.3% (82/239 patients) developed AKI (AKI prevalence significantly different, p = 0.008). In contrast, AKI prevalence based on MAP (Fig. 2B) was not significantly different between the invasive vs. the noninvasive techniques (e.g., p = 0.28 when MAP ≤60 mm Hg). These results are consistent with our findings in the pairwise analysis.
TABLE 1.
Characteristics of the Study Population
| AKI (n = 300) | Control (No AKI) (n = 1333) |
p | Missing | |
|---|---|---|---|---|
| Age | 68.15 (54.84, 77.51) | 68.33 (55.43, 77.88) | 0.92 | 0 |
| Male sex (n, %) | 198 (66%) | 775 (58%) | 0.002 | 0 |
| Weight (kg) | 83 (70, 94) | 77 (66, 90) | <0.001 | AKI: 0% (1), Control: 2% (31) |
| Simplified Acute Physiology Score–I | 18 (14, 22) | 15 (12, 22) | <0.001 | AKI: 1% (2), Control: 1% (18) |
| First service type | Coronary care unit 8% (24), Cardiac surgery recovery unit 25% (76), Medical ICU 29% (86), Surgical intensive care unit 38% (114) |
Coronary care unit 6% (84), Cardiac surgery recovery unit 36% (476), Medical ICU 18% (241), Surgical ICU 40% (532) |
– | 0 |
| Length of stay (days) | 8.07 (3.47, 15.00) | 4.21 (2.92, 8.02) | <0.001 | 0 |
| Mortality | 33% | 12% | <0.001 | – |
| Presence of vasoactive medications | 67% | 41% | <0.001 | – |
| AKI Mean (SD), mm Hg |
Control (No AKI) Mean (SD), mm Hg |
p | Odds Ratio (95% Confidence Interval) | |
|---|---|---|---|---|
| Minimum systolic invasive arterial blood pressure | 81.84 (19.17) | 94.00 (22.68) | <0.001 | 1.93 (1.65–2.25) |
| Minimum systolic noninvasive blood pressure | 86.28 (17.03) | 98.37 (17.88) | <0.001 | 2.26 (1.92–2.65) |
| Minimum mean arterial pressure invasive arterial blood pressure | 58.10 (12.43) | 65.15 (13.64) | <0.001 | 1.86 (1.59–2.16) |
| Minimum mean arterial pressure noninvasive blood pressure | 55.28 (11.76) | 61.85 (11.31) | <0.001 | 1.94 (1.67–2.25) |
AKI = acute kidney injury.
AKI vs. control patient characteristics. Continuous variables (age, weight, Simplified Acute Physiology Score–I, and length of stay) are reported as median and interquartile range; p values are from the Wilcoxon’s rank sum test. Minimum blood pressure measurements (in mm Hg) from the study window are reported as mean and SD; p values and odds ratios for blood pressure measurements are from univariate logistic regression. Odds ratios for the presence of AKI are calculated per one SD decrease in blood pressure. The average number of concurrent invasive arterial blood pressure/noninvasive blood pressure samples in the study window is 16.1 for the AKI cohort, and 16.88 for the controls.
Figure 2.
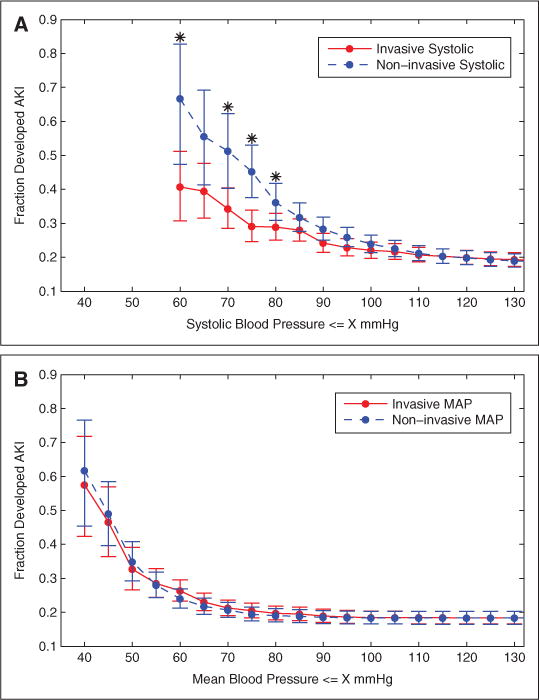
Prevalence of acute kidney injury (AKI) as a function of minimum (concurrently time-stamped) systolic (A) and mean (B) blood pressure. Patients were divided into cumulative bins based on blood pressure values less than equal to specified thresholds. Error bars show 95% confidence intervals. Data points with * indicate that the differences between invasive and noninvasive measurements were significant (p < 0.05) based on chi-square test. Number of patients is 1633. MAP = mean arterial pressure.
Furthermore, when the traditional threshold for hypotension (≤90 mm Hg) was used, systolic NIBP had a significantly lower sensitivity (NIBP 0.60, IAP 0.71, p = 0.004) than systolic IAP in assessing patients’ risk of developing AKI (supplemental Appendix F, Supplemental Digital Content 1, http://links.lww.com/CCM/A595). In contrast, noninvasive and invasive MAP had similar sensitivities in assessing patients’ risk of developing AKI with thresholds of 60 mm Hg (NIBP 0.69, IAP 0.63, p = 0.10) and 65 mm Hg (NIBP 0.82, IAP 0.76, p = 0.07).
ICU Mortality as a Function of Minimum IAP/NIBP
The association between ICU mortality and blood pressure was assessed in 4,957 patients (873 expired). ICU mortality associated with systolic NIBP diverged from that of systolic IAP in hypotensive patients (≤90 mm Hg, p = 0.04), with increasing discrepancy between the two techniques as the blood pressure threshold decreased (Fig. 3A). For example, ICU mortality associated with minimum systolic blood pressure ≤70 mm Hg was 33.7% (383/1138 patients expired) for the invasive measurements and 43.4% (215/495 expired) for the noninvasive measurements (p < 0.001). Systolic NIBP had a significantly lower sensitivity than systolic IAP in assessing patients’ risk of ICU mortality with thresholds of 90 mm Hg (NIBP 0.74, IAP 0.81, p = 0.001), 80 mm Hg (NIBP 0.52, IAP 0.65, p < 0.001), and 70 mm Hg (NIBP 0.25, IAP 0.44, p < 0.001). There were no significant differences (p = 0.76) between hypotensive (≤60 mm Hg) invasive and noninvasive MAP readings in terms of their association with ICU mortality (Fig. 3B).
Figure 3.
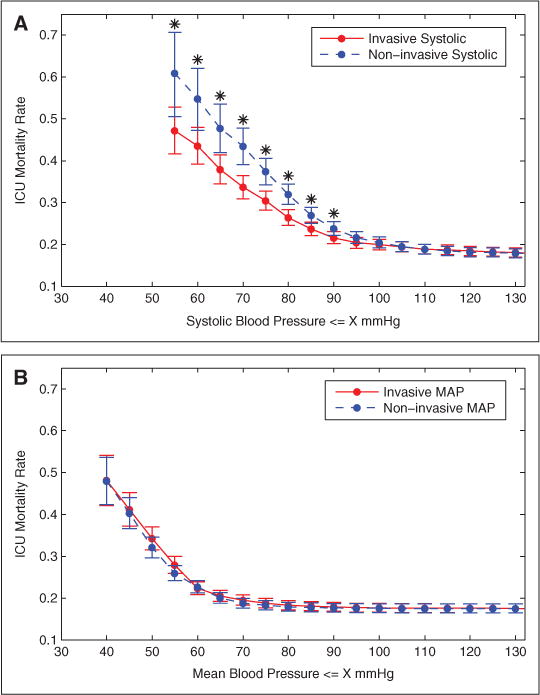
ICU mortality as a function of minimum (concurrently time-stamped) invasive and noninvasive systolic blood pressure (A) and mean blood pressure (B). Error bars show 95% confidence intervals of the mortality rates. Data points with * indicate that the differences between invasive and noninvasive blood pressure measurements in terms of their ICU mortality are statistically significant (p < 0.05) based on chi-square test. Number of patients is 4957. MAP = mean arterial pressure.
DISCUSSION
Our results are clinically important as we have analyzed invasive and NIBP trends in a large and diverse ICU database including patients with significant hemodynamic instability and organ failure. First, we observed that systematic deviations are present between systolic blood pressure values derived from noninvasive devices and invasive arterial line measurements. Furthermore, our results suggest that the bias between systolic NIBP and IAP is highly dependent upon the underlying actual blood pressure. Most strikingly, NIBP tends to be higher than systolic IAP at low blood pressures and to be lower than systolic IAP at high blood pressures.
Second, our analysis confirms that the discrepancies between invasive and noninvasive systolic blood pressure readings are clinically significant. Systolic IAP and NIBP gave inconsistent assessment of patients’ risks of AKI and ICU mortality. Specifically, hypotensive systolic NIBP values (≤70 mm Hg) were associated with significantly higher AKI prevalence (p = 0.008) and higher ICU mortality (p < 0.001) in comparison to invasive systolic blood pressure with the same absolute values. Noninvasive systolic blood pressures were not as sensitive as intra-arterial measurements in assessing patients’ risk of developing AKI (p = 0.004) when traditional hypotension thresholds (≤90 mm Hg) were used; a higher threshold should be considered for systolic NIBP to avoid failure in recognizing hypoperfusion. These findings suggest that clinical interpretation of hypotensive systolic blood pressure readings should be made cautiously in a device-dependent manner.
In contrast, MAPs are similar when assessed by oscillometry and invasive techniques over a range of pressures, including commonly accepted upper bound thresholds for hypotension (MAP between 60 and 65 mm Hg). Clinically, the risks of AKI (p = 0.28) and ICU mortality (p = 0.76) associated with hypotensive MAP readings (≤60 mm Hg) are not dependent on measurement technique.
The mean pressure is the true driving pressure for peripheral blood flow (1, 26), and taken together with measures of cardiac output permits estimation of peripheral resistance. However, ever since the advent of manual blood pressure assessment, clinicians have traditionally relied upon the noninvasive auscultatory method to measure systolic and diastolic blood pressure. In contrast, MAP constitutes the sole parameter physically measured by oscillometric techniques. Current practice guidelines have been slow to integrate NIBP MAP in vital sign monitoring. For example, the American Heart Association definition of hypertension is based on systolic and diastolic blood pressure only (27). The Society for Critical Care Medicine has utilized both systolic and mean arterial blood pressure for defining sepsis-induced hypotension, whereas MAP was used in setting therapeutic goals (13). Different measurement techniques were not considered. Our results confirm that mean blood pressure is the most consistent metric for monitoring blood pressure in the ICU, and is independent of measurement modality. Patients with particular underlying pathologies might also require tracking of systolic, diastolic, or pulse pressures (e.g., arterial aneurysm, coronary disease, valvular insufficiency, etc.).
Our study has a number of strengths including a large patient population (much larger than any prior ICU study of blood pressure), and our focus on clinically relevant outcomes. However, we acknowledge several weaknesses. First, the MIMIC II database relies on clinical nurse verification of data, which has variable accuracy considering the competing priorities in a busy ICU. However, we supplemented nurse verification by algorithmic rejection of data outliers. Furthermore, our findings are clinically relevant as they represent “real-world” nonideal conditions in which pressure measurements are performed at a major academic hospital, and the blood pressure trends we utilized would be typical of the data utilized in advanced monitoring systems for clinical decision support. Second, our data are relevant to the equipment being used during the time of study enrollment. Thus, equipment made by other companies or different algorithms from the same companies may perform better or worse than our current findings. Thus, our findings are limited to the population and equipment studied. However, prior studies using rigorous mathematical models (7) to characterize the oscillometric technique, along with animal and human studies (5, 8–12), have suggested similar limitations in the utility of the oscillometric method to measure systolic blood pressure. Our findings are also consistent with a prior study that demonstrated the utility of NIBP MAP in tracking hypotension in patients (28).
Third, our analyses regarding blood pressure and incident AKI were limited by not accounting for nephrotoxic drugs, contrast agents, and concomitant diseases (e.g., sepsis) that have been causally linked to AKI. Because hypotension may influence the AKI risk by these other mechanisms (29), we do not feel it is appropriate to exclude (or control for) other etiologies of renal failure. Furthermore, if other etiologies of renal failure were contributing nonspecific variability to our results, one would expect such “random noise” to bias toward the null hypothesis because neither measurement technique would have predictive value if blood pressure was irrelevant.
Fourth, the mortality statistics in this study are not representative of overall ICU mortality, but reflect the outcomes of patients with invasive IAP monitoring, which selects for a hemodynamically unstable cohort with high expected mortality. Fifth, our study focused on the clinical effect of discrepancies between IAP and NIBP in low blood pressure values. The discrepancies between the two devices at high blood pressure ranges might possibly become clinically important under conditions different from those considered in this study. Finally, clinicians should be cautioned against applying the observed statistical bias between IAP and NIBP to individuals due to the wide 95% limits of agreement between the two devices.
Despite these limitations, we believe our findings are robust and have important clinical implications for the care of critically ill patients. In particular, our study suggests that MAP-based thresholds are more consistent across measurement modalities than systolic blood pressures, and are the preferred basis for therapeutic protocols and clinical trial designs.
CONCLUSIONS
Clinically significant discrepancies were observed between invasive and noninvasive oscillometric methods in measuring systolic blood pressure during hypotension. Mean blood pressure from both techniques can be interpreted in a consistent manner in assessing patients’ prognosis.
Our results suggest that mean rather than systolic blood pressure is the preferred metric in an ICU, and should be used for diagnostic and treatment decisions, although we support further research in this area.
Supplementary Material
Acknowledgments
This work was supported by award number R01EB001659 from the National Institute of Biomedical Imaging and Bioengineering. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Biomedical Imaging and Bioengineering or the National Institutes of Health. Dr. Malhotra is funded by NIH R01 HL090897, R01 HL085188, AHA 0840159N1, NIH P01 HL 095491, and NIH K24 HL 093218.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (http://journals.lww.com/ccmjournal).
The authors have not disclosed any potential conflicts of interest.
References
- 1.Marino P, Sutin K. The ICU Book. Philadelphia, PA: Lippincott, Williams & Wilkins; 2007. [Google Scholar]
- 2.Antonelli M, Levy M, Andrews PJ, et al. Hemodynamic monitoring in shock and implications for management. International Consensus Conference, Paris, France, 27–28 April 2006. Intensive Care Med. 2007;33:575–590. doi: 10.1007/s00134-007-0531-4. [DOI] [PubMed] [Google Scholar]
- 3.McGhee BH, Bridges EJ. Monitoring arterial blood pressure: What you may not know. Crit Care Nurse. 2002;22:60–79. [PubMed] [Google Scholar]
- 4.Gardner RM. Direct arterial pressure monitoring. Curr Anaesth Crit Care. 1990;1:239–246. [Google Scholar]
- 5.Bur A, Herkner H, Vlcek M, et al. Factors influencing the accuracy of oscillometric blood pressure measurement in critically ill patients. Crit Care Med. 2003;31:793–799. doi: 10.1097/01.CCM.0000053650.12025.1A. [DOI] [PubMed] [Google Scholar]
- 6.Geddes LA, Voelz M, Combs C, et al. Characterization of the oscillometric method for measuring indirect blood pressure. Ann Biomed Eng. 1982;10:271–280. doi: 10.1007/BF02367308. [DOI] [PubMed] [Google Scholar]
- 7.Ursino M, Cristalli C. A mathematical study of some biomechanical factors affecting the oscillometric blood pressure measurement. IEEE Trans Biomed Eng. 1996;43:761–778. doi: 10.1109/10.508540. [DOI] [PubMed] [Google Scholar]
- 8.Bur A, Hirschl MM, Herkner H, et al. Accuracy of oscillometric blood pressure measurement according to the relation between cuff size and upper-arm circumference in critically ill patients. Crit Care Med. 2000;28:371–376. doi: 10.1097/00003246-200002000-00014. [DOI] [PubMed] [Google Scholar]
- 9.Araghi A, Bander JJ, Guzman JA. Arterial blood pressure monitoring in overweight critically ill patients: Invasive or noninvasive? Crit Care. 2006;10:R64. doi: 10.1186/cc4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loubser PG. Comparison of intra-arterial and automated oscillometric blood pressure measurement methods in postoperative hypertensive patients. Med Instrum. 1986;20:255–259. [PubMed] [Google Scholar]
- 11.Pytte M, Dybwik K, Sexton J, et al. Oscillometric brachial mean artery pressures are higher than intra-radial mean artery pressures in intensive care unit patients receiving norepinephrine. Acta Anaesthesiol Scand. 2006;50:718–721. doi: 10.1111/j.1399-6576.2006.01045.x. [DOI] [PubMed] [Google Scholar]
- 12.Manios E, Vemmos K, Tsivgoulis G, et al. Comparison of noninvasive oscillometric and intra-arterial blood pressure measurements in hyperacute stroke. Blood Press Monit. 2007;12:149–156. doi: 10.1097/MBP.0b013e3280b083e2. [DOI] [PubMed] [Google Scholar]
- 13.Dellinger RP, Levy MM, Carlet JM, et al. International Surviving Sepsis Campaign Guidelines Committee. American Association of Critical-Care Nurses. American College of Chest Physicians. American College of Emergency Physicians. Canadian Critical Care Society. European Society of Clinical Microbiology and Infectious Diseases. European Society of Intensive Care Medicine. European Respiratory Society. International Sepsis Forum. Japanese Association for Acute Medicine. Japanese Society of Intensive Care Medicine. Society of Critical Care Medicine; Society of Hospital Medicine. Surgical Infection Society World Federation of Societies of Intensive and Critical Care Medicine: Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36:296–327. [Google Scholar]
- 14.Knaus WA, Draper EA, Wagner DP, et al. APACHE II: A severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 15.Knaus WA, Wagner DP, Draper EA, et al. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest. 1991;100:1619–1636. doi: 10.1378/chest.100.6.1619. [DOI] [PubMed] [Google Scholar]
- 16.Zimmerman JE, Kramer AA, McNair DS, et al. Acute Physiology and Chronic Health Evaluation (APACHE) IV: Hospital mortality assessment for today’s critically ill patients. Crit Care Med. 2006;34:1297–1310. doi: 10.1097/01.CCM.0000215112.84523.F0. [DOI] [PubMed] [Google Scholar]
- 17.Bruns B, Gentilello L, Elliott A, et al. Prehospital hypotension redefined. J Trauma. 2008;65:1217–1221. doi: 10.1097/TA.0b013e318184ee63. [DOI] [PubMed] [Google Scholar]
- 18.Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270:2957–2963. doi: 10.1001/jama.270.24.2957. [DOI] [PubMed] [Google Scholar]
- 19.Chobanian AV, Bakris GL, Black HR, et al. Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute National High Blood Pressure Education Program Coordinating Committee: Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 20.Saeed M, Villarroel M, Reisner AT, et al. Multiparameter intelligent monitoring in intensive care II: A public-access intensive care unit database. Crit Care Med. 2011;39:952–960. doi: 10.1097/CCM.0b013e31820a92c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.MIMIC II Database. Available at: http://www.physionet.org/mimic2/. Accessed November 6, 2011.
- 22.Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8:135–160. doi: 10.1177/096228029900800204. [DOI] [PubMed] [Google Scholar]
- 23.Efron B. Bootstrap methods: Another look at the jackknife. Ann Statist. 1979;7:1–26. [Google Scholar]
- 24.Chertow GM, Burdick E, Honour M, et al. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365–3370. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 25.Barrantes F, Tian J, Vazquez R, et al. Acute kidney injury criteria predict outcomes of critically ill patients. Crit Care Med. 2008;36:1397–1403. doi: 10.1097/CCM.0b013e318168fbe0. [DOI] [PubMed] [Google Scholar]
- 26.Pinsky MR, Payen D. Functional hemodynamic monitoring. Crit Care. 2005;9:566–572. doi: 10.1186/cc3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosendorff C, Black HR, Cannon CP, et al. American Heart Association Council for High Blood Pressure Research. American Heart Association Council on Clinical Cardiology. American Heart Association Council on Epidemiology and Prevention Treatment of hypertension in the prevention and management of ischemic heart disease: A scientific statement from the American Heart Association Council for High Blood Pressure Research and the Councils on Clinical Cardiology and Epidemiology and Prevention. Circulation. 2007;115:2761–2788. doi: 10.1161/CIRCULATIONAHA.107.183885. [DOI] [PubMed] [Google Scholar]
- 28.Lakhal K, Ehrmann S, Runge I, et al. Tracking hypotension and dynamic changes in arterial blood pressure with brachial cuff measurements. Anesth Analg. 2009;109:494–501. doi: 10.1213/ane.0b013e3181a8d83a. [DOI] [PubMed] [Google Scholar]
- 29.Abuelo JG. Normotensive ischemic acute renal failure. N Engl J Med. 2007;357:797–805. doi: 10.1056/NEJMra064398. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


