Abstract
Purpose
To determine whether taurine transporter (TauT) activity and expression are regulated by hyperosmolarity in RPE, ganglion and Müller cells.
Methods
Uptake of taurine was measured in ARPE-19 cells cultured in DMEM:F12 medium without or with the addition of 50 mM NaCl or 100 mM mannitol. The kinetics of the transport were analyzed. RT-PCR, northern and western blot analyses were used to assess TauT mRNA and protein levels. The influence of hyperosmolarity on the uptake of taurine, myo-inositol and GABA was studied in RPE, RGC-5 and rMC1 cells.
Results
TauT activity was abundant in RPE and was stimulated (3.5-fold) when the cells were exposed to hyperosmolar conditions (DMEM:F12 culture medium plus 50 mM NaCl or 100 mM mannitol). Peak stimulation of taurine uptake occurred after 17 h exposure to hyperosmolar medium. Kinetic analysis revealed that the hyperosmolarity-induced stimulation was associated with an increase in Vmax of TauT with no change in Km. TauT mRNA and protein levels increased in RPE cells exposed to hyperosmolar conditions. Hyperosmolarity also stimulated the uptake of myo-inositol (~15-fold); GABA uptake was influenced less markedly. Immunofluorescence and functional studies showed that TauT is present in cultured RGC-5 and rMC1 cells. TauT activity was robust in these cells under normal osmolar conditions and increased by ~2-fold under hyperosmolar conditions.
Conclusions
These studies provide the first evidence that hyperosmolarity regulates TauT activity and expression in RPE; and that TauT is present in ganglion and Müller cells and is regulated by hypertonicity. The data are relevant to diseases such as diabetes, macular degeneration and neurodegeneration, in which retinal cell volumes may fluctuate dramatically.
Keywords: taurine, taurine transporter, RPE, retinal ganglion cells, Müller cells
INTRODUCTION
Taurine, a β-aminosulfonic acid, is the most abundant retinal amino acid [1] and is essential for maintenance of retinal structure and function [2,3]. Although its role in retina is uncertain, several functions have been ascribed to taurine including antioxidant defense, protein stabilization, stress responses and osmoregulation. Regarding osmoregulation, there is evidence that taurine is involved in cell volume homeostasis. Cells achieve osmotic equilibrium by accumulating or losing small organic solutes, such as taurine and myo-inositol, accompanied by the flux of osmotically obligated water. Studies in astrocytes showed that exposure to hypotonic conditions resulted in massive efflux of taurine [4,5] and myo-inositol [6]. Conversely, hypertonic conditions caused a significant influx of these solutes [7]. In retinal pigment epithelium (RPE), Yokoyama et al demonstrated that hypertonic stress increased NaK-ATPase activity and led to accumulation of taurine and myo-inositol [8].
The influx and efflux of solutes, such as taurine and myo-inositol, are mediated via transporter proteins. Hypertonicity upregulates the taurine transporter, abbreviated TauT (SLC6A6), in several cell types including astrocytes [7], Caco-2 cells [9], and hepatocytes [10]. Recent studies in ocular tissues demonstrated that hyperosmolar stress results in an increased expression of TauT in corneal [11] and lens [12] epithelial cells. The cDNAs for human [13,14], rat [15] and mouse [16] TauT have been cloned and sequence homology places TauT within the gene family of Na+- and Cl−- dependent neurotransmitter transporters. Human TauT cDNA encodes a protein of 619 amino acids with 12 putative transmembrane domains. It is noteworthy that a mouse model with a disrupted gene coding for TauT (taut−/− mice), reported recently by Heller-Stilb et al [17], exhibits severe retinal degeneration, suggesting that TauT is critical for normal retinal development and function.
In situ hybridization analysis of mouse retina showed that TauT is expressed in several retinal cell types including RPE and photoreceptor cells [16]. Our studies localized TauT protein to RPE, ganglion cells and the inner nuclear layer [18]. In the present study, we were interested in determining whether hyperosmotic conditions altered the function and expression of TauT in RPE. RPE maintains the adjacent, highly metabolically active photoreceptor cells by transporting nutrients, removing waste, and phagocytosing shed photoreceptor disks [19]. While TauT has been characterized in RPE [20-23], the regulation of TauT by hyperosmolarity in RPE has not been studied. The question is relevant because fluctuations in cell volume are associated with pathologies such as ischemia and reperfusion during diabetic retinopathy, macular edema and neurodegeneration [24]. Using functional and molecular biological assays, we investigated TauT regulation under hyperosmotic conditions in ARPE-19 cells, a well-characterized human retinal pigment epithelial cell line [25]. In addition, we examined TauT activity in retinal ganglion and Müller cells. Ganglion cells are the second order neurons of the visual pathway and are susceptible to apoptotic death in glaucoma and diabetic retinopathy [26-28, Moore et al unpublished observations]. Müller cells are the major glial cells of the retina. They subserve metabolic, ionic and extracellular buffering requirements of adjacent neurons and play a key role in clearing toxic substances. Müller cell function is altered in diabetic retinopathy [29,30]. With the recent development of a rat ganglion cell line, RGC-5 [31] and a rat Müller cell line, rMC1 [32], we were able to study TauT function under normal and hyperosmolar conditions. Our data show that TauT is stimulated under hyperosmotic conditions in RPE, RGC-5 and rMC1 cells.
MATERIALS AND METHODS
Materials
Reagents were obtained from the following sources: [1,2-3H]-Taurine, [α-32P]-dCTP (Amersham-Pharmacia, Piscataway, NJ); [2,3-3H(N)]-γ aminobutyric acid (GABA) (Dupont/NEN, Boston, MA); [3H(N)]-carnitine, [4,5-3H]-leucine, [2-3H]-myo-inositol (Moravek Biochemicals, Brea, CA); taurine, myo-inositol, GABA, succinyl Conconavalin A (sConA), anti-β actin antibody (Sigma, St. Louis, MO); DMEM:F12 medium (Cat. Number 12320), TRIzol, penicillin-streptomycin (Gibco-Invitrogen Corp., Grand Island, NY); fetal bovine serum (FBS) (Atlanta Biologicals, Atlanta, GA); ARPE-19 cells (ATCC, Manassas, VA); rat ganglion cells (RGC-5) (Dr. N. Agarwal, UNT Health Science Center, Fort Worth, TX); rat Müller cells (rMC-1) (Dr. V. Sarthy, Northwestern University, Chicago, IL); antibody against rat TauT and the immunogenic control peptide (Alpha Diagnostics, Inc., San Antonio, TX); FITC-conjugated AffiniPure anti-rabbit IgG (Jackson ImmunoResearch Laboratories, West Grove, PA); HRP-conjugated goat anti-rabbit IgG, ECL detection kit (Santa Cruz Biotechnology, Santa Cruz, CA); Complete Mini Protease Inhibitor Cocktail tablets (Roche Applied Science, Indianapolis, IN); RNA PCR core kit (Perkin-Elmer, Boston, MA); ExpressHyb hybridization solution (Clontech, Palo Alto, CA), Ultrasensitive hybridization buffer ULTRAHyb™ (Ambion, Austin, TX).
Cell Culture
ARPE-19 and rMC1 cells were cultured in DMEM:F12 medium, supplemented with 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin at 37°C in a humidified chamber with 5% CO2. The culture medium was replaced every other day. Upon confluence, cultures were passaged by dissociation in 0.05% (w/v) trypsin in 0.01M PBS. RGC-5 cells were passaged initially in DMEM:F12. For differentiation, the method of Krishnamoorthy et al. [31] was followed in which cells were cultured in the absence of serum for 24 h, after which they were cultured in DMEM:F12 (1% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin) supplemented with 50 μg/ml sConA for 6-7 days.
Determination of hyperosmolarity effects on taurine uptake
Cells were grown in 24-well plates and exposed to varying concentrations of NaCl (10, 25, 50, or 75 mM) or mannitol (20, 50, 100, 150 mM) in the normal culture medium, which increased the osmolarity of the medium by 20, 50, 100 and 150 mOsm, respectively. For uptake experiments, culture medium was removed and cells were washed twice with uptake buffer (25 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES)/Tris, 140 mM NaCl, 5.4 mM KCl, 1.8 mM CaCl2, 0.8 mM MgSO4, and 5 mM glucose, pH 7.5). For experiments assessing the influence of Na+ and Cl− on the transport process, the uptake buffer was modified by replacing KCl and CaCl2 with isoosmolar concentrations of respective gluconate salts and by replacing NaCl with isoosmolar concentrations of either sodium gluconate or N-methyl-D-glucamine (NMDG)-chloride. Uptake was initiated by adding 250 μl of uptake buffer containing radiolabeled substrates. Uptake measurements were performed with 15 min incubation at 37°C. At the end of the incubation, uptake was terminated by removal of the medium followed by two washes with ice-cold uptake buffer without the radiolabeled substrates. Cells were solubilized in 0.5 ml of 1% SDS in 0.2 N NaOH; radioactivity was quantified by scintillation.
RT-PCR and northern blot analysis of steady-state levels of TauT mRNA
The expression of TauT mRNA in RPE cells, exposed for 17 h to normal culture medium or hyperosmolar medium in which the osmolarity was increased by 100 mOsm by addition of either 50 mM NaCl or 100 mM mannitol, was analyzed by semiquantitative RT-PCR and northern hybridization. For RT-PCR, total RNA was prepared using the TRIzol reagent. RT-PCR was carried out using primer pairs specific for human TauT (5′-GCTAGCTGCATAGTAGTC-3′ (sense); 5′-TGGAACACAC CTCACTGC-3′ (antisense), nucleotide positions 872-889 and 1751-1769 of human TauT cDNA (14)) and human GAPDH (5′-AAGGCTGAGAACGGGAAGCTTGTCATCAAT-3′ (sense); 5′-TTCCCGTTCAGCTCAGGATGACCTTGCCC-3′ (antisense), nucleotide positions 241-270 and 711-740 in human GAPDH cDNA [34]). PCR following reverse transcription was performed over a range of cycles (9-30). The products were size-fractionated on agarose gels, transferred onto nylon membranes and subjected to Southern hybridization with probes specific for TauT or GAPDH. These probes were generated by labeling the respective subcloned RT-PCR products with [α-32P]dCTP. The intensity of hybridization signals was quantified using the STORM phosphorimaging system (Molecular Dynamics, Sunnyvale, CA). The relationship between the signal intensity and PCR cycle number was analyzed to determine the linear range for the PCR product formation, which was then used for data analysis. For northern analysis, poly(A)+ mRNA was isolated using a commercially available kit MACS (Miltenyi Biotec, Auburn, CA) and size-fractionated on a denaturing agarose gel. A 1.1 kb cDNA fragment, derived from human TauT cDNA [14], was labeled with [α-32P]-dCTP and used as the probe. Hybridization was carried out in ULTRAHyb™ hybridization solution for 18 h at 42°C. The blots were washed two times (30 min each) at 60°C in a solution containing 2x SSC (saline-sodium citrate buffer; 0.15 M NaCl and 15 mM sodium citrate, pH 7) and 0.5 % SDS (sodium dodecyl sulphate). The same blot was stripped and reprobed with [32P]-GAPDH cDNA [34] as an internal control. Hybridization signals were quantified using the STORM PhosphorImaging System. The intensity of the TauT mRNA signal was normalized with that of the GAPDH mRNA signal to correct for potential differences in RNA loading. The TauT mRNA/GAPDH mRNA signal ratio in control cells exposed to normal culture medium was taken as 1 and the changes in TauT mRNA in cells exposed to hyperosmolar conditions were compared to this value.
Western blot analysis of TauT protein levels in RPE cells
Confluent cultures of ARPE-19 cells were exposed for 17 h to normal culture medium without or with the addition of 50 mM NaCl or 100 mM mannitol (an increase of 100 mOsm in the osmolarity of the medium). Cells were washed with 0.01 M PBS and lysed in cold lysis buffer (50 mM Tris-HCl, pH 7.4, containing 1% Triton-X-100, 10 mM EDTA, 2 mM Na3VO4, 0.5% deoxycholate, 10 mM sodium pyrophosphate and 50 mM NaF) containing the Complete Mini Protease Inhibitor Cocktail (1 tablet/10 ml lysis buffer) and were sonicated for 30 sec. Cell debris was removed by centrifugation at 20,000 x g for 15 min at 4°C. Protein concentration in the supernatant was determined [34]. Equivalent amounts of protein (40 μg) from total cell lysates were boiled in Laemmli’s buffer for 5 min and analyzed by 7.5% SDS-PAGE [35]. Separated proteins were transferred to nitrocellulose membranes and were blocked for 1.5 h with TBS-0.1% Tween-20 containing 5% non-fat milk. Membranes were incubated overnight with anti-TauT antibody, and were probed with HRP secondary antibody (1:3000) for 1.5 h, washed and proteins visualized using the ECL system. Membranes were washed and reprobed with anti-β actin antibody. Densitometric scans of the membranes were performed using the AlphaImager 2200 digital imaging system (Alpha Innotech Corporation, San Leandro, CA).
Immunocytochemical detection of TauT in cultured retinal cells
ARPE-19, RGC-5 and rMC-1 cells were cultured on chamber slides. Cells were fixed with ice-cold methanol, blocked with 10% goat serum and incubated overnight at 4°C with anti-TauT antibody (1:100). As a negative control, the primary antibody was neutralized with an excess of antigenic peptide prior to use. Additional negative controls included using buffer only and 0.1% normal rabbit serum in place of the primary antibody. Samples were incubated overnight at 4°C with FITC-conjugated anti-rabbit IgG (1:100). Slides were examined using a Zeiss Axioskop 2 flourescent microscope (Carl Zeiss Inc., Germany) equipped with a Spot Camera and Spot Software version 3.4.5 (Diagnostic Instruments, Inc., Sterling Heights, MI).
Data Analysis
Data were analyzed using the NCSS 97 statistical package (Kaysville, UT). In cases of multiple comparisons, ANOVA was used followed by Tukey-Kramer’s paired comparison test. A p-value < 0.05 was considered significant.
RESULTS
Influence of hyperosmolarity on taurine uptake
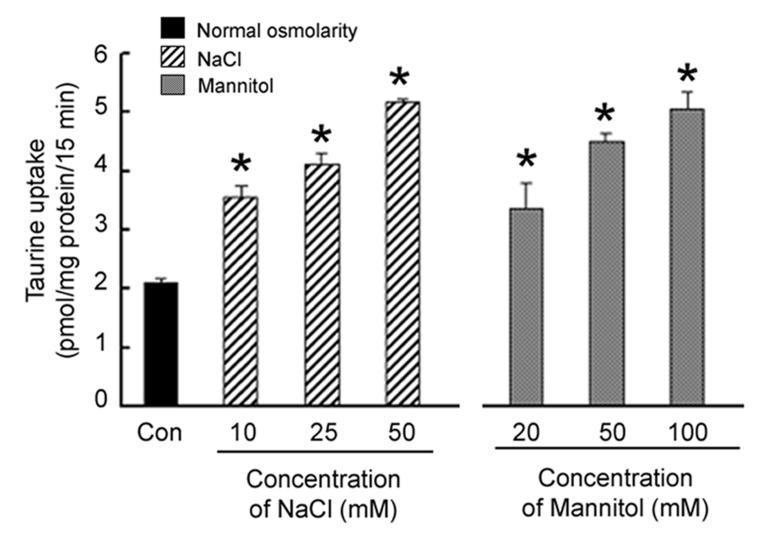
Earlier reports demonstrated an active TauT in RPE cells [20-23]. To determine whether TauT function is regulated in RPE by hyperosmotic conditions, ARPE-19 cells were exposed for 8 h to varying concentrations of NaCl (ranging from 10 mM – 50 mM) or mannitol (ranging from 20 mM to 100 mM)added to normal culture medium. An increase of 50 mOsm in the osmolarity of the culture medium (addition of 25 mM NaCl or 50 mM mannitol) stimulated taurine uptake significantly by 100% and 120%, respectively (Fig. 1). An increase of 100 mOsm in the medium osmolarity (addition of 50 mM NaCl or 100 mM mannitol) stimulated taurine uptake to a greater extent (150% in both cases). Unless otherwise stated, for the remainder of the experiments, cells were exposed to normal culture medium without or with the addition of 50 mM NaCl or 100 mM mannitol.
Fig. 1. Influence of increasing osmolarity on taurine uptake by RPE cells.
Confluent ARPE-19 cells were exposed for 8 h to varying concentrations of NaCl or mannitol added to the normal culture medium and the uptake of taurine (80 nM) was determined. Values represent mean ± SEM for 6 determinations from two independent experiments. *Significantly different from control (p < 0.05).
Influence of exposure time to hyperosmotic conditions on taurine uptake
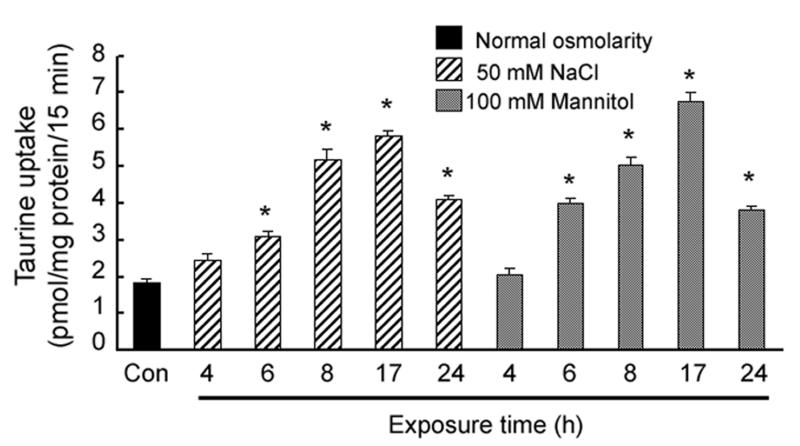
ARPE-19 cells were incubated in normal or hyperosmolar medium for varying periods of time (0-24 h) and taurine uptake was measured. By 6 h, there was a significant increase in taurine uptake in cells exposed to medium containing 50 mM NaCl or 100 mM mannitol (an increase of 100 mOsm in medium osmolarity) (Fig. 2). Increasing the exposure time resulted in an increase in taurine uptake through 17 h, when the greatest stimulation in taurine uptake was observed. By 24 h, taurine uptake under hyperosmolar conditions had decreased compared to the 17 h uptake, although the levels were significantly greater than in cells exposed to normal osmolar (control) conditions.
Fig. 2. Influence of exposure time to hyperosmotic conditions on taurine uptake in RPE cells.
Confluent ARPE-19 cells were exposed for varying lengths of time (0 - 24 h) to either 50 mM NaCl or 100 mM mannitol added to the normal culture medium and the uptake of taurine (80 nM) determined. Values represent mean ± SEM for 6 determinations from two independent experiments. *Significantly different from control (p < 0.05).
Specificity of hyperosmolarity effects on taurine uptake
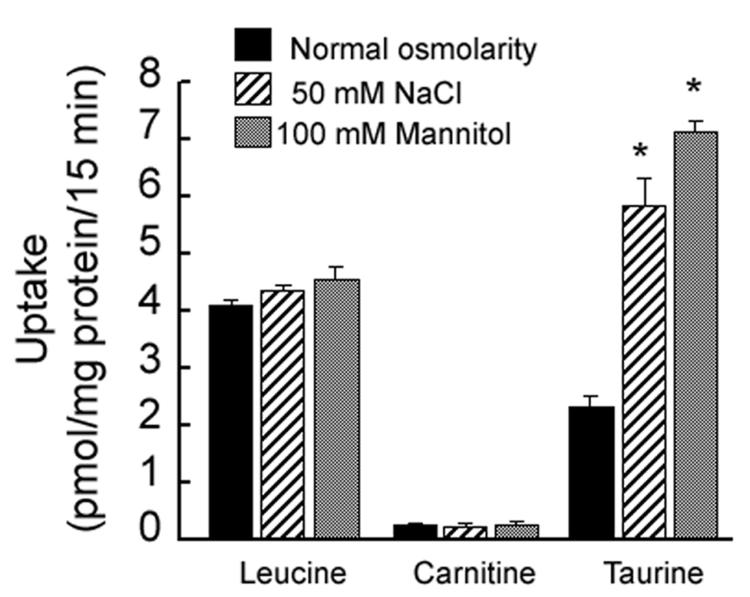
The hyperosmolarity-induced stimulation of taurine uptake in RPE was not a non-specific effect as the uptake of other selected nutrients was not affected in RPE cells cultured in hyperosmotic conditions. Incubation of ARPE-19 cells for 17 h in hyperosmotic medium had no significant effect on the uptake of leucine (30 nM) or carnitine (30 nM) (Fig. 3). Taurine uptake increased markedly under identical conditions.
Fig. 3. Specificity of the stimulation of taurine uptake in RPE cells by hyperosmolarity.
Confluent ARPE-19 cells were exposed for 17 h to either 50 mM NaCl or 100 mM mannitol added to the normal culture medium and the uptake of leucine (30 nM), carnitine (30 nM) or taurine (80 nM) was determined. Values represent mean ± SEM for 6 determinations from two independent experiments. *Significantly different from cells incubated under normal osmolar conditions (p < 0.05).
Influence of hyperosmolarity on the kinetic parameters of taurine uptake
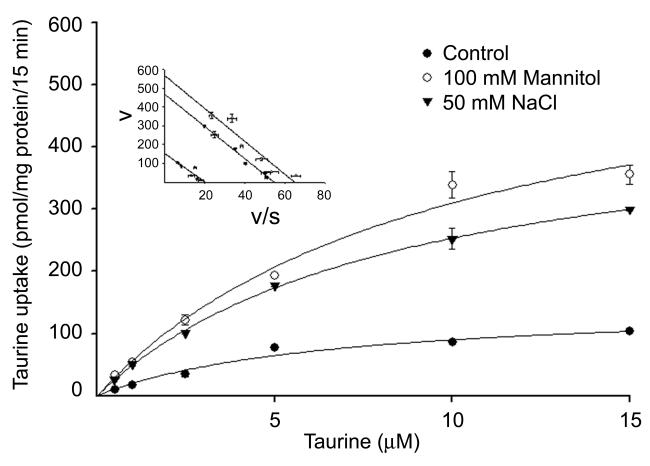
We analyzed the kinetics of TauT uptake by RPE cells maintained in normal osmolar conditions compared to those incubated for 17 h in hyperosmolar conditions. Taurine uptake was measured over a concentration range of 0.025 – 15 μM (Fig. 4). The analysis showed that the increase in TauT activity observed under hyperosmolar conditions was associated with an increase in the maximal velocity of the transporter with no significant change in substrate affinity. The maximal velocity of taurine uptake was significantly greater in cells exposed to hyperosmolar conditions than in controls cells (471 ± 13 for 50 mM NaCl and 615 ± 83 for 100 mM mannitol versus 148 ± 21 pmol/mg protein/15 min in control cells). The Michaelis-Menten constant for taurine remained almost the same in controls cells and those incubated under hyperosmolar conditions (8.6 ± 0.5 μM for 50 mM NaCl, 9.9 ± 2.6 μM for 100 mM mannitol and 6.5 ± 2.2 μM in control cells).
Fig. 4. Kinetic analysis of taurine uptake in RPE cells cultured under normal and hyperosmolar conditions.
Confluent ARPE-19 cells were incubated for 17 h in normal culture medium with or without (control) the addition of 100 mM mannitol or 50 mM NaCl. Uptake of taurine was measured over a taurine concentration range of 0.5 – 15 μM. Values are means ± SEM for 6 determinations from 2 independent experiments. Results are presented as plots describing the relationship between taurine concentration and taurine uptake rate. Inset: Eadie-Hofstee plots (v/s vs v, where v is taurine uptake in pmol/mg protein/15 min and s is taurine concentration in μM).
Influence of hyperosmolarity on steady-state levels of TauT mRNA
RNA samples isolated from ARPE-19 cells that had been incubated in normal or hyperosmolar culture medium (increase of 100 mOsm by addition of 50 mM NaCl or 100 mM mannitol) for 17 h were used for semi-quantitative RT-PCR (total RNA) and for northern analysis (mRNA). As an internal control, the steady-state levels of GAPDH mRNA were determined in the samples in parallel. The steady-state levels of TauT mRNA increased significantly compared to control cells as assessed by both methods (Fig. 5). The steady-state levels of TauT mRNA, after normalizing with the internal control, were ~2-fold higher in cells exposed to hyperosmolar medium compared to control cells exposed to normal culture medium. These results demonstrate that the hyperosmolarity-induced increase in TauT activity is likely due to increased expression of the gene encoding TauT.
Fig. 5. Analysis of steady-state levels of TauT mRNA in RPE cells cultured under hyperosmolar conditions.
Confluent cells were exposed for 17 h to normal culture medium with or without (control) the addition of 100 mM mannitol or 50 mM NaCl. RNA was isolated from these cells and used for semiquantitative RT-PCR (total RNA) and northern analysis (mRNA). (A) Data from Southern hybridization with 32P-labeled cDNA probes specific for TauT and GAPDH. (B) Data shown in panel A were subjected to phosphorimage analysis and the ratios of the TauT-specific band to the GAPDH-specific band under varying osmolar conditions are shown; the ratio in control cells was taken as 1. (C) Northern blot.
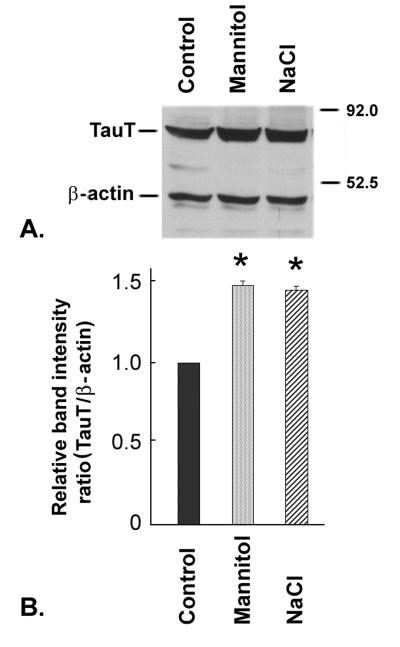
Influence of hyperosmolarity on TauT protein levels
Confluent ARPE-19 cells were incubated either in normal culture medium or hyperosmolar culture medium (increase of 100 mOsm by addition of 50 mM NaCl or 100 mM mannitol) for 17 h and subjected to western blot analysis using an antibody specific for TauT or β-actin (Fig. 6A). Densitometric scans of membranes (Fig. 6B) showed that cells incubated in hyperosmolar medium had a 45% increase in TauT protein compared to control cells. The β-actin band was used as an internal control for protein loading to normalize the values for TauT protein levels.
Fig. 6. Analysis of TauT protein levels in RPE cells cultured under hyperosmolar conditions.
Confluent ARPE-19 cells were exposed for 17 h to normal culture medium with or without (control) the addition of 100 mM mannitol or 50 mM NaCl. Protein was isolated and subjected to immunoblotting. (A) Representative blot of proteins from RPE cells cultured under varying osmolar conditions. TauT has a molecular mass of 70 kDa and β-actin (internal control) has a molecular mass of 45 kDa. (B) Quantitation of the ratio of the TauT band to β-actin band. The ratio in control cells was taken as 1.
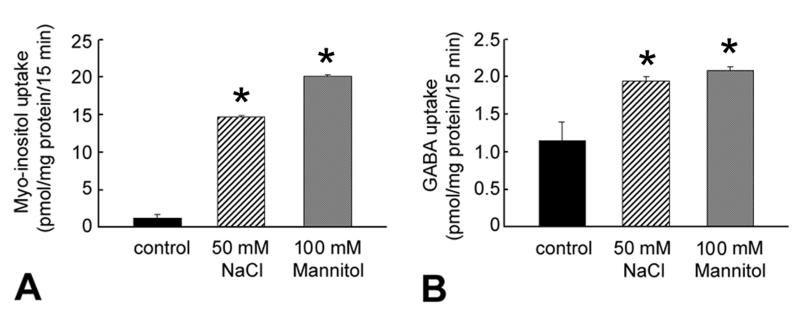
Influence of hyperosmolarity on myo-inositol and GABA uptake in RPE
Hyperosmolar conditions upregulate the uptake of other osmolytes such as myo-inositol in cell types other than RPE [6]. Therefore, we investigated the influence hyperosmolarity on the uptake of myo-inositol in RPE cells. Our previous studies showed that a GABA transporter expressed in RPE apical membrane vesicles can transport taurine to a significant extent [36]. Hence, we tested whether hyperosmolarity influences the uptake of GABA in RPE cells. Cells were incubated for 17 h in normal or hyperosmolar medium and the uptake of myo-inositol (100 nM) or GABA (65 nM) determined. An increase in medium osmolarity of 100 mOsm (by addition of 50 mM NaCl or 100 mM mannitol) caused a marked stimulation of myo-inositol uptake (~15-fold increase) (Fig. 7). GABA uptake was also stimulated under hyperosmolar conditions, but to a much lesser extent.
Fig. 7. Influence of hyperosmolarity on the uptake of myo-inositol and GABA in RPE cells.
Confluent ARPE-19 cells were maintained for 17 h in normal culture medium with or without (control) the addition of 50 mM NaCl, or 100 mM mannitol. Uptake of myo-inositol (100 nM) (A) or GABA (65 nM) (B) was determined in these cells. Values represent mean ± SEM for 6 determinations from two independent experiments. *Significantly different from control. (p < 0.05).
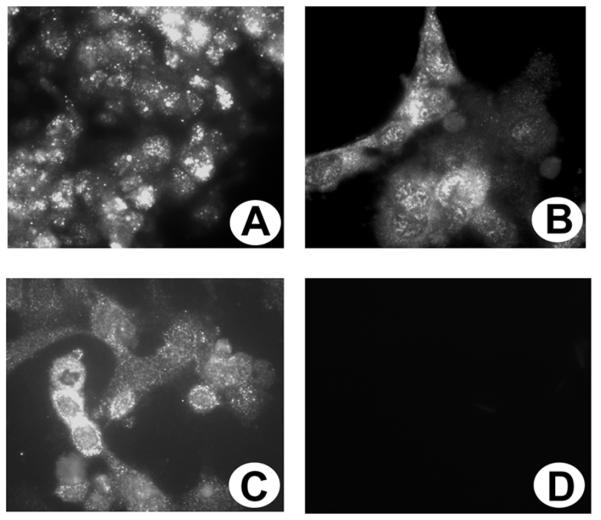
Immunocytochemical localization of TauT in cultured RPE, RGC-5 and rMC1 cells
ARPE-19, RGC-5 and rMC1 cell lines were grown in chamber slides and immunocytochemical methods were used to determine whether TauT was present. TauT was detected in ARPE-19 cells (Fig. 8A), RGC-5 cells (Fig. 8B) and rMC1 cells (Fig. 8C). Incubation of cells with antibody that had been incubated with an excess of the antigenic peptide showed no positive immunofluorescence (Fig. 8D). These immunofluorescence data show that TauT is present abundantly in the cultured RPE, ganglion and Müller cells.
Fig. 8. Immunolocalization of TauT in cultured human ARPE-19, rat ganglion (RGC-5) and Müller (rMC1) cells.
ARPE-19 (A), RGC-5 (B) and rMC1cells (C) were cultured on laminin-coated chamber slides and processed for immunohistochemistry using a primary antibody against TauT followed by an FITC-labeled secondary antibody. (D) ARPE-19 cells incubated with antibody that had been preincubated with the peptide (negative control) showing no positive staining. (Magnifications 600X)
Analysis of TauT activity in RGC-5 and rMC1 cells
To determine whether RGC-5 and rMC1 cells have a functional Na+- and Cl−-dependent TauT, cells were incubated under various buffer conditions and taurine uptake assessed (Table 1). RGC-5 and rMC1 cells demonstrated significant taurine uptake when cells were incubated with taurine (40 nM) in buffer containing NaCl, but virtually no uptake of taurine in the absence of Na+ but the presence of Cl− (NMDG chloride buffer), or in the presence of Na+ but the absence of Cl− (sodium gluconate buffer). In addition, cells incubated in NaCl buffer containing β-alanine, a known competitive inhibitor of taurine uptake [14], showed minimal uptake of taurine. These data provide strong evidence of a functional TauT in these retinal cell lines.
Table I.
Demonstration that RGC-5 and rMC1 cells have a functional TauT.
| Taurine Uptake pmol/mg protein/15 min | ||
|---|---|---|
| Buffer | Ganglion cells | Müller cells |
| NaCl | 14.50 ± 0.70 | 20.95 ± 0.48 |
| NMDG-Cl | 0.06 ± 0.001 | 0.09 ± 0.01 |
| Sodium gluconate | 0.12 ± 0.01 | 2.72 ± 0.12 |
| NaCl + β-alanine | 0.33 ± 0.01 | 0.79 ± 0.09 |
Values are means ±SE. RGC-5 and rMC1 cells were cultured as described in the text and the uptake of [3H]taurine (80 nM) was measured. Uptake buffer consisted of 20 mM HEPES-Tris (pH 7.5) containing 140 mM NaCl, sodium gluconate or N-methyl-D-glucamine (NMDG) chloride. Inhibition of taurine transport in the presence of 2.5 mM β-alanine (in NaCl buffer) was also measured.
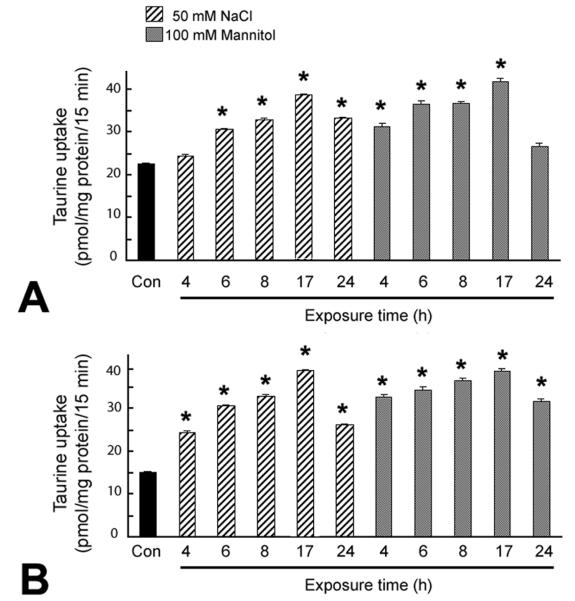
Influence of hyperosmolarity on taurine uptake in RGC-5 and rMC1 cells
Confluent RGC-5 and rMC1 cells were incubated in normal or hyperosmolar culture medium (increase of 100 mOsm by addition of 50 mM NaCl or 100 mM mannitol) for varying periods of time (0-24 h) and the uptake of taurine (80 nM) assessed. RGC-5 and rMC1 cells demonstrated an increased TauT activity under hyperosmolar conditions (Fig. 9). Within 6 h of exposure to hyperosmolar conditions, RGC-5 cells demonstrated a marked increase in TauT activity which peaked at 17 h. Müller cells showed an even more rapid response to hyperosmolar conditions by increasing TauT activity within 4 h exposure to hyperosmolar conditions. Interestingly, the basal activity (activity under normal osmolar conditions) of TauT was more robust in RGC-5 and rMC1 cells than in RPE. When incubated in normal culture medium, the uptake of taurine by RPE was about 2.5 pmol/mg protein/15 min, whereas for ganglion and Müller cells it was about 15-20 pmol/mg protein/15 min.
Fig. 9. Influence of exposure time to hyperosmotic conditions on taurine uptake in RGC-5 and rMC-1 cells.
Confluent RGC-5 (A) and rMC1 (B) cells were exposed for varying lengths of time (0 -24 h) to either 50 mM NaCl or 100 mM mannitol added to the normal culture medium (an increase of 100 mOsm in the osmolarity of the medium) and the uptake of taurine (80 nM) determined. Values represent mean ± SEM for 6 determinations from two independent experiments. *Significantly different from control (p < 0.05).
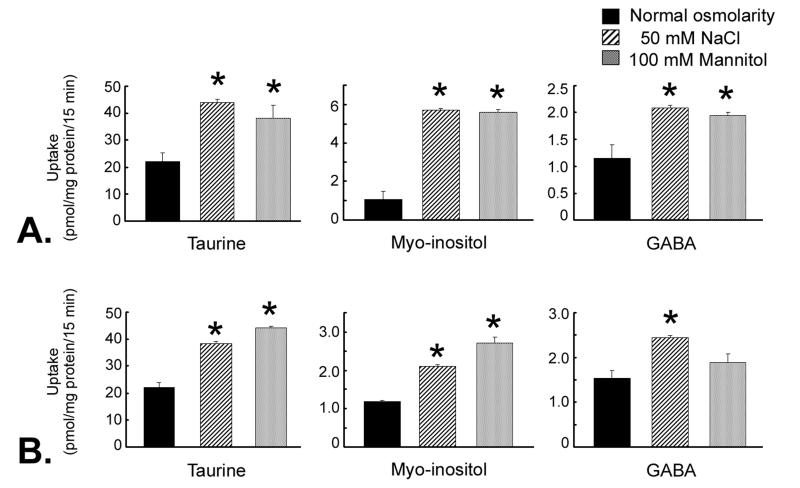
Influence of hyperosmolarity on myo-inositol and GABA uptake in RGC-5 and rMC1 cells
To determine whether uptake of myo-inositol and GABA in RGC-5 and rMC1 cells was regulated also by hyperosmotic conditions, cells were cultured for 17 h in normal or hyperosmolar medium. The osmolarity of the medium was increased by 100 mOsm by the addition of 50 mM NaCl or 100 mM mannitol. The uptake of myo-inositol increased dramatically in RGC-5 cells incubated in hyperosmolar medium compared to cells incubated in normal medium (Fig. 10A). GABA uptake was also enhanced by hyperosmolarity, but to a lesser extent. In rMC1 cells (Fig. 10B) there was a modest, but significant, increase in myo-inositol uptake in cells exposed to hyperosmolar conditions. GABA uptake in rMC1 cells was stimulated slightly, but significantly, under identical conditions.
Fig. 10. Uptake of taurine, myo-inositol and GABA in ganglion cells and Müller cells cultured under hyperosmolar conditions.
Confluent RGC-5 cells (A) or rMC1 cells (B) were maintained for 17 h in normal culture medium with no addition (normal osmolarity), 50 mM NaCl, or 100 mM mannitol (an increase of 100 mOsm in the osmolarity of the culture medium). Uptake of taurine (80 nM), myo-inositol (100 nM) or GABA (65 nM) was determined. Values represent mean ± SEM for 6 determinations from two independent experiments. *Significantly different from control (normal osmolarity) (p < 0.05).
DISCUSSION
Our previous studies demonstrated that oxidative stress upregulates TauT in RPE [18], providing evidence that taurine may act as an antioxidant in these cells. The present study assessed the effects of hyperosmolarity on taurine transport by RPE. Changes in RPE cell volume in vivo may alter the volume and composition of the extracellular (subretinal) space surrounding photoreceptors, thus isosmotic volume regulation could play a key physiological role in maintaining the integrity and health of the neural retina under normal and pathophysiological conditions [37]. One mechanism used by cells to ensure proper volume homeostasis is by accumulation or release of osmolytes such as taurine. When cultured under hyperosmolar conditions, Yokoyama reported an increase in taurine content in RPE cells [8]. These studies did not reveal whether the increase in taurine content was due to increased taurine synthesis or increased influx of extracellular taurine via TauT.
To determine whether TauT activity was increased by hyperosmolar conditions in RPE, we used ARPE-19 cells, which retain many features characteristic of RPE cells in vivo [25, 38-39]. Our studies were performed by increasing the osmolarity of the culture medium with the addition of either NaCl or mannitol and determining the ability of the cells to take up taurine. Addition of either 50 mM NaCl or 100 mM mannitol to the normal culture medium (an increase of 100 mOsm in the osmolarity of the medium) led to a marked increase in taurine uptake. The effects of hyperosmolarity on TauT activity were not immediate. Rather, a significant increase in TauT activity was observed after 8 h incubation under hyperosmolar conditions and peaked at 17 h. Incubation of cells for 17 h under hyperosmolar conditions led to a 2.5 - 3.5-fold stimulation of TauT activity. The effects of hyperosmolarity were not non-specific, as uptake of carnitine and leucine was not affected by a change in osmolarity.
Kinetic analysis showed that the increased TauT activity under hyperosmolar conditions was due to an increase in the maximal velocity of taurine uptake. The substrate affinity was not affected under these conditions. This suggests that transporter density in the plasma membrane may be increased by hyperosmolarity. RT-PCR and northern analysis provided evidence that the steady-state levels of TauT mRNA increased ~2-fold under hyperosmolar conditions. Thus, the hyperosmolarity-induced increase in TauT activity is likely due to increased expression of the gene coding for TauT. Western blot analysis demonstrated an increase in TauT protein in cells exposed to hyperosmolar conditions.
We determined whether uptake of myo-inositol and GABA in RPE was regulated by hyperosmotic conditions. Myo-inositol belongs to the polyol class of chemicals and, like taurine, is a small organic solute that can be lost or accumulated by cells accompanied by the flux of osmotically obligated water. GABA is an amino acid that is abundant in retina and can act as an osmolyte. Exposure of RPE cells to hyperosmolar conditions led to a marked stimulation of myo-inositol uptake (15-fold) and a 2-fold increase in the uptake of GABA.
Osmoregulation of TauT has been studied in other ocular epithelial cells. Shioda et al [11] studied the activity and expression of TauT in corneal epithelial cells. Exposure to hypertonic medium for 12 h led to a 4-fold increase in TauT activity, which was associated with an increase in gene expression, just as we found for RPE. Similarly, Cammarata and colleagues [12] studied taurine uptake in lens epithelial cells and found that hyperosmolar conditions led to an increase in the maximum velocity of the transporter, but no change in substrate affinity. They reported an upregulation of TauT mRNA following exposure to hyperosmolar conditions. Our studies provide the first evidence that TauT activity in RPE, like corneal and lens epithelial cells, is regulated by hyperosmolarity. These findings are important because there are significant changes in extracellular osmolarity in a broad range of RPE disorders including retinitis pigmentosa, retinal detachment, macular degeneration [24,40,41] and in systemic disease such as diabetic retinopathy [40-42].
In addition to RPE, we asked whether TauT activity in other retinal cell types would respond similarly to hyperosmolar stress. We were interested in ganglion and Müller cells, both of which are affected in diabetes [26,27,29,43]. Since in diabetes elevated glucose levels disturb cellular osmoregulation [44], it was of interest to determine whether ganglion and Müller cells demonstrate TauT activity. TauT activity has not been reported in either of these cell types, although TauT activity was reported in neurons [45] and astrocytes [46] derived from brain. Our earlier immunohistochemical studies in intact retinal tissue detected TauT in retinal ganglion cells and the inner nuclear layer [18], which contains Müller cells. It is not feasible to study TauT activity of specific cells using intact retinas, therefore we obtained the ganglion cell line, RGC-5 [31] and the Müller cell line, rMC1 [32] to study taurine uptake in these specific cell types. Our immunocytochemical analyses detected TauT in both cell lines. We demonstrated that RGC-5 and rMC1 cells have a NaCl-dependent TauT whose taurine transport function is inhibited completely by β-alanine, a known substrate of TauT.
Exposure of RGC-5 and rMC1 cells to hyperosmolar conditions resulted in a marked increase in taurine uptake. Interestingly, the basal level of taurine uptake in these cells was significantly greater than in RPE. Under normal osmolar conditions, RPE cells took up taurine (80 nM) at an average rate of about 2 pmol/mg/protein/15 min, whereas the rates for ganglion and Müller cells were 22 pmol/mg protein/15 min and 15 pmol/mg protein/15 min, respectively under similar conditions. Exposing RGC-5 and rMC1 cells to hyperosmolar conditions stimulated TauT and the time course of stimulation was similar to that in RPE cells with the peak stimulation occurring at 17 h. The increase in TauT activity was about 2-fold, which is slightly less than that observed in RPE (~3.5 fold). As with the RPE, incubation of RGC-5 and rMC1 cells in hyperosmolar conditions did not affect all transport systems. For example, the uptake of carnitine and leucine was not affected. Interestingly, while ARPE-19 cells demonstrated robust uptake of myo-inositol, neither RGC-5 nor rMC1 cells had such marked activity. Both cell types appeared to preferentially activate TauT under hyperosmolar conditions rather than take up myo-inositol. As with RPE, the induction of GABA uptake under hyperosmolar conditions was not extensive.
These studies represent the first demonstration that TauT is functional in ganglion and Müller cells and they provide the first evidence that TauT is regulated by hyperosmolar conditions in these cells and RPE. It is noteworthy that in many call types, failure to adapt to osmotic stress can result in apoptotic cell death. Moreover, the ability of cells to resist osmotic shrinkage by cell volume regulation parallels their resistance to apoptosis after osmotic shock [47]. A recent study indicated that chronic taurine supplementation ameliorates oxidative stress in diabetic rat retina [48]. Taurine supplementation may have therapeutic potential for the treatment of derangement in osmoregulation characteristic of diabetes. Future analysis of the effects of hyperglycemia on the activity of TauT in these retinal cell types should provide insights about the function of this transporter under diabetic conditions. The present study assessed the activity of the transporters for taurine and myoinositol using tracer concentrations of substrate in vitro. It is possible that in an in vivo situation with physiological concentrations of taurine and myoinositol, the alterations in transporter expression might lead to sufficient accumulation of these osmolytes inside of cells, which matches the changes in osmolarity in the extracellular space. Future comprehensive studies to address this issue should establish whether this is indeed the case.
Acknowledgements
This research was supported by a CIGP award from the Medical College of Georgia Research Institute.
Literature cited
- 1.Pasantes-Morales H, Klethi J, Ledig M, Mandel P. Free amino acids of chicken and rat retina. Brain Res. 1972;41:494–497. doi: 10.1016/0006-8993(72)90523-9. [DOI] [PubMed] [Google Scholar]
- 2.Obrosova IG, Minchenko AG, Marinescu V, Fathallah L, Kennedy A, Stockert CM, Frank RN, Stevens MJ. Antioxidants attenuate early up regulation of retinal vascular endothelial growth factor in streptozotocin-diabetic rats. Diabetologia. 2001;44:1102–1110. doi: 10.1007/s001250100631. [DOI] [PubMed] [Google Scholar]
- 3.Hayes KC, Carey RE, Schmidt SY. Retinal degeneration associated with taurine deficiency in the cat. Science. 1975;188:949–951. doi: 10.1126/science.1138364. [DOI] [PubMed] [Google Scholar]
- 4.Pasantes-Morales H, Schousboe A. Volume regulation in astrocytes: a role for taurine as an osmoeffector. J Neurosci Res. 1988;20:503–509. doi: 10.1002/jnr.490200415. [DOI] [PubMed] [Google Scholar]
- 5.Kimelberg HK, Goderie SK, Higman S, Pang S, Waniewski RA. Swelling-induced release of glutamate, aspartate, and taurine from astrocyte cultures. J Neurosci. 1990;10:1583–1591. doi: 10.1523/JNEUROSCI.10-05-01583.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strange K, Morrison R, Shrode L, Putnam R. Mechanism and regulation of swelling-activated inositol efflux in brain glial cells. Am J Physiol. 1993;265:C244–256. doi: 10.1152/ajpcell.1993.265.1.C244. [DOI] [PubMed] [Google Scholar]
- 7.Bitoun M, Tappaz M. Gene expression of taurine transporter and taurine biosynthetic enzymes in hyperosmotic states: a comparative study with the expression of the genes involved in the accumulation of other osmolytes. Adv Exp Med Biol. 2000;483:239–248. doi: 10.1007/0-306-46838-7_26. [DOI] [PubMed] [Google Scholar]
- 8.Yokoyama T, Lin LR, Chakrapani B, Reddy VN. Hypertonic stress increases NaK ATPase, taurine, and myo-inositol in human lens and retinal pigment epithelial cultures. Invest Ophthalmol Vis Sci. 1993;34:2512–2517. [PubMed] [Google Scholar]
- 9.Satsu H, Miyamoto Y, Shimizu M. Hypertonicity stimulates taurine uptake and transporter gene expression in Caco-2 cells. Biochim Biophys Acta. 1999;1419:89–96. doi: 10.1016/s0005-2736(99)00058-9. [DOI] [PubMed] [Google Scholar]
- 10.Warskulat U, Wettstein M, Haussinger D. Osmoregulated taurine transport in H4IIE hepatoma cells and perfused rat liver. Biochem J. 1997;321:683–690. doi: 10.1042/bj3210683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shioda R, Reinach PS, Hisatsune T, Miyamoto Y. Osmosensitive taurine transporter expression and activity in human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2002;43:2916–2922. [PubMed] [Google Scholar]
- 12.Cammarata PR, Schafer G, Chen SW, Guo Z, Reeves RE. Osmoregulatory alterations in taurine uptake by cultured human and bovine lens epithelial cells. Invest Ophthalmol Vis Sci. 2002;3:425–433. [PubMed] [Google Scholar]
- 13.Jhaing SM, Fithian L, Smanik P, McGill J, Tong Q, Mazzaferri EL. Cloning of the human taurine transporter and characterization of taurine uptake in thyroid cells. FEBS Lett. 1993;318:139–144. doi: 10.1016/0014-5793(93)80008-i. [DOI] [PubMed] [Google Scholar]
- 14.Ramamoorthy S, Leibach FH, Mahesh VB, Han H, Yang-Feng T, Blakely RD, Ganapathy V. Functional characterization and chromosomal localization of a cloned taurine transporter from human placenta. Biochem. J. 1994;300:893–900. doi: 10.1042/bj3000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith KE, Borden LA, Wang CH, Hartig PR, Branchek TA, Weinshank RL. Cloning and expression of a high affinity taurine transporter from rat brain. Mol Pharmacol. 1992;42:563–569. [PubMed] [Google Scholar]
- 16.Vinnakota S, Qian X, Egal H, Sarthy V, Sarkar HK. Molecular characterization and in situ localization of a mouse retinal taurine transporter. J Neurochem. 1997;69:2238–2250. doi: 10.1046/j.1471-4159.1997.69062238.x. [DOI] [PubMed] [Google Scholar]
- 17.Heller-Stilb B, van Roeyen C, Rascher K, Hartwig HG, Huth A, Seeliger MW, Warskulat U, Haussinger D. Disruption of the taurine transporter gene (TauT) leads to retinal degeneration in mice. FASEB J. 2002;16:231–233. doi: 10.1096/fj.01-0691fje. [DOI] [PubMed] [Google Scholar]
- 18.Bridges CC, Ola MS, Prasad PD, El-Sherbeny A, Ganapathy V, Smith SB. Regulation of taurine transporter gene expression by nitric oxide in cultured human retinal pigment epithelial cells. Am J Physiol. 2001;281:C1825–836. doi: 10.1152/ajpcell.2001.281.6.C1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hughes BA, Gallemore RP, Miller SS. Transport mechanisms in the retinal pigment epithelium. In: Marmor MF, Wolfensberger TJ, editors. The Retinal Pigment Epithelium: Function and Disease. Oxford; New York: 1998. pp. 103–134. [Google Scholar]
- 20.Ganapathy V, Ramamoorthy JD, Del Monte MA, Leibach FH, Ramamoorthy S. Cyclic AMP-dependent up-regulation of the taurine transporter in a human retinal pigment epithelial cell line. Curr. Eye Res. 1995;14:843–850. doi: 10.3109/02713689508995807. [DOI] [PubMed] [Google Scholar]
- 21.Miller SS, Steinberg RH. Potassium modulation of taurine transport across the frog retinal pigment epithelium. J Gen. Physiol. 1979;74:237–259. doi: 10.1085/jgp.74.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miyamoto Y, Kulanthaivel P, Leibach FH, Ganapathy V. Taurine uptake in apical membrane vesicles from the bovine retinal pigment epithelium. Invest. Ophthalmol. Vis. Sci. 1991;32:2542–2551. [PubMed] [Google Scholar]
- 23.Leibach JW, Cool DR, Del Monte MA, Ganapathy V, Leibach FH, Miyamoto Y. Properties of taurine transport in a human retinal pigment epithelial cell line. Curr Eye Res. 1993;12:29–36. doi: 10.3109/02713689308999493. [DOI] [PubMed] [Google Scholar]
- 24.Gallemore RP, Maruiwa F, Marmor MF. Clinical electrohyisology of the retinal pigment epithelium. In: Marmor MF, Wolfensberger TJ, editors. The Retinal Pigment Epithelium: Function and Disease. Oxford; New York: 1998. pp. 199–224. [Google Scholar]
- 25.Dunn KC, Aotaki-Keen AE, Putkey FR, Hjelmeland LM. ARPE-19, a human retinal pigment epithelial cell line with differentiated properties. Exp. Eye Res. 1996;62:155–169. doi: 10.1006/exer.1996.0020. [DOI] [PubMed] [Google Scholar]
- 26.Barber AJ, Lieth E, Khin SA, Antonetti DA, Buchanan AG, Gardner TW, The Penn State Retina Research Group Neural apoptosis in the retina during experimental and human diabetes: early onset and effect of insulin. J. Clin. Invest. 1998;102:783–791. doi: 10.1172/JCI2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang L, Inoue M, Dong K, Yamamoto M. Retrograde axonal transport impairment of large- and medium-sized retinal ganglion cells in diabetic rat. Curr. Eye Res. 2000;20:131–136. [PubMed] [Google Scholar]
- 28.Wein FB, Levin LA. Current understanding of neuroprotection in glaucoma. Curr Opin Ophthalmol. 2002;13:61–7. doi: 10.1097/00055735-200204000-00002. [DOI] [PubMed] [Google Scholar]
- 29.Mizutani M, Gerhardinger C, Lorenzi M. Muller cell changes in human diabetic retinopathy. Diabetes. 1998;47:445–449. doi: 10.2337/diabetes.47.3.445. [DOI] [PubMed] [Google Scholar]
- 30.Li Q, Puro DG. Diabetes-induced dysfunction of the glutamate transporter in retinal Muller cells. Invest Ophthalmol Vis Sci. 2002;43:3109–3116. [PubMed] [Google Scholar]
- 31.Krishnamoorthy RR, Agarwal P, Prasanna G, Vopat K, Lambert W, Sheedlo HJ, Pang IH, Shade D, Wordinger RJ, Yorio T, Clark AF, Agarwal N. Characterization of a transformed rat retinal ganglion cell line. Mol. Brain Res. 2001;86:1–12. doi: 10.1016/s0169-328x(00)00224-2. [DOI] [PubMed] [Google Scholar]
- 32.Sarthy VP, Brodjian SJ, Dutt K, Kennedy, French RP, Crabb JW. Establishment and characterization of a retinal Müller cell line. Invest. Ophthalmol. Vis. Sci. 1998;39:212–216. [PubMed] [Google Scholar]
- 33.Tokunaga K, Nakamura Y, Sakata K, Fujimori K, Ohkubo M, Sawada K, Sakiyama S. Enhanced expression of a glyceraldehyde-3-phosphate dehydrogenase gene in human lung cancers. Cancer Res. 1987;47:5616–5619. [PubMed] [Google Scholar]
- 34.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 35.Laemmli UK. Cleavage of structural properties during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 36.Sivakami S, Ganapathy V, Leibach FH, Miyamoto Y. The gamma-aminobutyric acid transporter and its interaction with taurine in the apical membrane of the bovine retinal pigment epithelium. Biochem J. 1992;283:391–397. doi: 10.1042/bj2830391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adorante JS. Regulatory volume decrease in frog retinal pigment epithelium. Am J Physiol. 1995;268:C89–100. doi: 10.1152/ajpcell.1995.268.1.C89. [DOI] [PubMed] [Google Scholar]
- 38.Dunn KC, Marmorstein AD, Bonilha VL, Rodriguez-Boulan E, Giordano F, Hjelmeland LM. Use of the ARPE-19 cell line as a model of RPE polarity: Basolateral secretion of FGF5. Invest. Ophthalmol. Vis. Sci. 1998;39:2744–2749. [PubMed] [Google Scholar]
- 39.Finnemann SC, Bonilha VL, Marmorstein AD, Rodriguez-Boulan E. Phagocytosis of rod outer segments by retinal pigment epithelial cells requires alpha(v)beta5 integrin for binding but not for internalization. Proc. Natl. Acad. Sci. USA. 1997;94:12932–12937. doi: 10.1073/pnas.94.24.12932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yonemura D, Kawasaki K, Madachi-Yamamoto S. Hyperosmolarity response of ocular standing potential as a clinical test for retinal pigment epithelium activity. Chorioretinal dystrophies. Doc Ophthalmol. 1984;57:163–73. doi: 10.1007/BF00143080. [DOI] [PubMed] [Google Scholar]
- 41.Mori T, Marmor MF, Miyoshi K, Tazawa Y. Combined photic and nonphotic electro-oculographic responses in the clinical evaluation of the retinal pigment epithelium. Doc Ophthalmol. 1991;76:315–22. doi: 10.1007/BF00142669. [DOI] [PubMed] [Google Scholar]
- 42.Winkler BS. Hyperosmolarity and electroretinogram (ERG) potentials in isolated rat retinas: possible implications in diabetic models. Exp Eye Res. 2003;77:115–6. doi: 10.1016/s0014-4835(03)00085-x. [DOI] [PubMed] [Google Scholar]
- 43.Lieth E, Barber AJ, Xu B, Dice C, Ratz MJ, Tanase D, Strother JM. Glial reactivity and impaired glutamate metabolism in short-term experimental diabetic retinopathy. Diabetes. 1998;47:815–820. doi: 10.2337/diabetes.47.5.815. [DOI] [PubMed] [Google Scholar]
- 44.Hansen SH. The role of taurine in diabetes and the development of diabetic complications. Diabetes Metab Res Rev. 2001;17:330–346. doi: 10.1002/dmrr.229. [DOI] [PubMed] [Google Scholar]
- 45.Huxtable RJ. Taurine in the central nervous system and the mammalian actions of taurine. Prog Neurobiol. 1989;32:471–453. doi: 10.1016/0301-0082(89)90019-1. [DOI] [PubMed] [Google Scholar]
- 46.Bitoun M, Tappaz M. Gene expression of the transporters and biosynthetic enzymes of the osmolytes in astrocytes primary cultures exposed to hyperosmotic conditions. Glia. 2000;32:165–176. [PubMed] [Google Scholar]
- 47.Bortner CD, Cidlowski JA. Uncoupling cell shrinkage from apoptosis reveals that Na+ influx is required for volume loss during programmed cell death. J Biol Chem. 2003;278:39176–84. doi: 10.1074/jbc.M303516200. [DOI] [PubMed] [Google Scholar]
- 48.Di Leo MA, Santini SA, Cercone S, Lepore D, Gentiloni Silveri N, Caputo S, Greco AV, Giardina B, Franconi F, Ghirlanda G. Chronic taurine supplementation ameliorates oxidative stress and Na(+)K(+)ATPase impairment in the retina of diabetic rats. Amino Acids. 2002;23:401–406. doi: 10.1007/s00726-002-0202-2. [DOI] [PubMed] [Google Scholar]